Diabetes Mellitus Management: An Extensive Review of 37 Medicinal Plants
Abstract
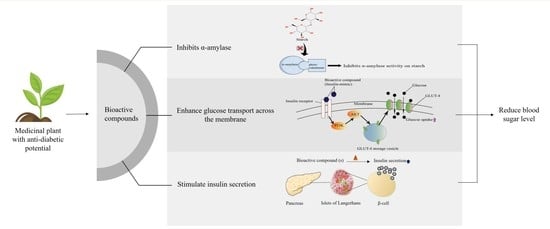
1. Introduction
2. Retrieval of Published Research
3. Plants in the Management of Diabetes Mellitus
3.1. Achyranthes aspera
3.2. Aloe barbadensis
3.3. Amaranthus tricolor
3.4. Anacardium occidentale
3.5. Andrographis paniculata
3.6. Annona squamosa
3.7. Argemone mexicana
3.8. Asparagus racemosus
3.9. Azadirachta indica
3.10. Blumea lacera
3.11. Boerhaavia diffusa
3.12. Bombax ceiba
3.13. Bryophyllum pinnatum
3.14. Cajanus cajan
3.15. Catharanthus roseus Linn.
3.16. Coccinia cordifolia
3.17. Cocculus hirsutus Linn.
3.18. Cocos nucifera
3.19. Costus speciosus
3.20. Ficus benghalensis L.
3.21. Ficus hispida Linn.
3.22. Ficus racemosa
3.23. Kalanchoe pinnata
3.24. Mangifera indica
3.25. Mikania cordata
3.26. Momordica charantia
3.27. Moringa oleifera
3.28. Murraya koenigii
3.29. Nelumbo nucifera
3.30. Neolamarckia cadamba
3.31. Piper betle Leaves
3.32. Punica granatum
3.33. Syzygium cumini (L.)
3.34. Tamarindus indica
3.35. Terminalia catappa
3.36. Terminalia chebula
3.37. Wedelia chinensis
4. Plant-Based Treatments: Current Progress and Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Tantawy, W.H.; Temraz, A. Management of diabetes using herbal extracts: Review. Arch. Physiol. Biochem. 2017, 124, 383–389. [Google Scholar] [CrossRef]
- Nash, R.J.; Kato, A.; Yu, C.-Y.; Fleet, G.W. Iminosugars as therapeutic agents: Recent advances and promising trends. Futur. Med. Chem. 2011, 3, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Chennaiah, A.; Dahiya, A.; Dubbu, S.; Vankar, Y.D. A Stereoselective Synthesis of an Imino Glycal: Application in the Synthesis of (-)-1-epi-Adenophorine and a Homoimindosugar. Eur. J. Org. Chem. 2018, 2018, 6574–6581. [Google Scholar] [CrossRef]
- Deyno, S.; Eneyew, K.; Seyfe, S.; Wondim, E. Efficacy, safety and phytochemistry of medicinal plants used for the management of diabetes mellitus in Ethiopia: A systematic review. Clin. Phytoscience 2021, 7, 16. [Google Scholar] [CrossRef]
- Malode, L.L.; Manwar, J.V.; Panchale, W.A.; Bartere, S.A.; Bakal, R.L. Potential of medicinal plants in management of diabetes: An updates. GSC Adv. Res. Rev. 2021, 8, 149–169. [Google Scholar] [CrossRef]
- Izzo, A.; Massimino, E.; Riccardi, G.; Della Pepa, G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.B.; Rao, C.V.; Ojha, S.K.; Vijayakumar, A.; Verma, A.; Alok, S. An analytical review of plants for anti diabetic activity with their phytoconstituent & mechanism of action. Int. J. Pharm. Sci. Res. 2010, 1, 29–46. [Google Scholar]
- Abdel-Aziz, S.M.; Aeron, A.; Kahil, T.A. Health Benefits and Possible Risks of Herbal Medicine. Microbes Food Health 2016, 97–116. [Google Scholar] [CrossRef]
- Nawaz, A.H.M.M.; Hossain, M.; Karim, M.; Khan, M.; Jahan, R.; Rahamatullah, M. An ethnobotanical survey of Rajshahi district in Rajshahi division, Bangladesh. Am. Eurasian J. Sustain. Agric. 2009, 3, 143–150. [Google Scholar]
- Srivastav, S.; Singh, P.; Mishra, G.; Jha, K.K.; Khosa, R.L. Achyranthes aspera—An important medicinal plant: A review. J. Nat. Prod. Plant Resour. 2011, 1, 1–14. [Google Scholar]
- Vijayaraj, R.; Kumar, K.N.; Mani, P.; Senthil, J.; Jayaseelan, T.; Kumar, G.D. Hypoglycemic and antioxidant activity of Achyranthes aspera seed extract and its effect on Streptozotocin induced diabetic rats. Int. J. Biol. Pharm. Res. 2016, 7, 23–28. [Google Scholar]
- Kumar, S.S.; Gnananath, K.; Saibana, G.; Rajasekhar, G.E.; Rajesh, P.; Nagarjuna, S. Anti diabetic Activity of Ethanolic Extract of Achyranthes aspera Leaves in Streptozotocin induced diabetic rats. J. Pharm. Res. 2011, 4, 3124–3125. [Google Scholar]
- Sadashiv, P.; Krishna, A. Acute toxicity study for Achyranthes aspera leaves. J. Pharmacol. Res. 2011, 4, 2221–2222. [Google Scholar]
- Joseph, B.; Raj, S.J. Pharmacognostic and phytochemical properties of Aloe vera Linn—An overview. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 106–110. [Google Scholar]
- Lanjhiyana, S.; Garabadu, D.; Ahirwar, D.; Bigoniya, P.; Rana, A.C.; Patra, K.C.; Lanjhiyana, S.K.; Karuppaih, M. Antihyperglycemic potential of Aloe vera gel in experimental animal model. Ann. Biol. Res. 2011, 2, 17–31. [Google Scholar]
- Kim, J.H.; Cho, C.W.; Lee, J.I.; Vinh, L.B.; Kim, K.T.; Cho, I.S. An investigation of the inhibitory mechanism of α-glucosidase by chysalodin from Aloe vera. Int. J. Biol. Macromol. 2020, 147, 314–318. [Google Scholar] [CrossRef]
- Prasannaraja, C.; Kamalanathan, A.S.; Vijayalakshmi, M.A.; Venkataraman, K. A dipyrrole derivative from Aloe vera inhibits an anti-diabetic drug target Dipeptidyl Peptidase (DPP)-IV in vitro. Prep. Biochem. Biotechnol. 2020, 50, 511–520. [Google Scholar] [CrossRef]
- Rahman, A.H.M.M.; Gulshana, M.I.A. Taxonomy and Medicinal Uses on Amaranthaceae Family of Rajshahi, Bangladesh. Appl. Ecol. Environ. Sci. 2014, 2, 54–59. [Google Scholar] [CrossRef]
- Aneja, S.; Vats, M.; Aggarwal, S.; Sardana, S. Phytochemistry and hepatoprotective activity of aqueous extract of Amaranthus tricolor Linn. roots. J. Ayurveda Integr. Med. 2013, 4, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Kunyanga, C.N.; Imungi, J.K.; Okoth, M.W.; Biesalski, H.K.; Vadivel, V. Antioxidant and Type 2 Diabetes Related Functional Properties of Phytic Acid Extract from Kenyan Local Food Ingredients: Effects of Traditional Processing Methods. Ecol. Food Nutr. 2011, 50, 452–471. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Desai, P. Evaluation of the Hematological, Hypoglycemic, Hypolipidemic and Antioxidant Properties of Amaranthus tricolor Leaf Extract in Rat. Trop. J. Pharm. Res. 2011, 10, 595–602. [Google Scholar] [CrossRef]
- Kumar, S.; Saini, M.; Kumar, V.; Prakash, O.; Arya, R.; Rana, M.; Kumar, D. Traditional medicinal plants curing diabetes: A promise for today and tomorrow. Asian J. Tradit. Med. 2012, 7, 178–188. [Google Scholar]
- De Sousa Leite, A.; Islam, T.; Gomes, A., Jr.; de Castro e Sousa, J.M.; de Alencar, M.V.A.B.; Fernanda Correia Jardim Paz, M.; Rolim, H.M.L.; das Graças Freire de Medeiros, M.; de Carvalho Melo-Cavalcante, A.A.; Lopes, J.A.D. Pharmacological properties of cashew (Anacardium occidentale). Afr. J. Biotechnol. 2016, 15, 1855–1863. [Google Scholar]
- Ukwenya, V.O.; Ashaolu, J.O.; Adeyemi, D.O.; Akinola, O.A.; Caxton-Martins, E.A. Antihyperglycemic activities of methanolic leaf extract of Anacardium occidentale (Linn.) on the pancreas of streptozotocin-induced diabetic rats. J. Cell Anim. Biol. 2012, 6, 207–212. [Google Scholar] [CrossRef]
- Tedong, L.; Madiraju, P.; Martineau, L.C.; Vallerand, D.; Arnason, J.T.; Desire, D.D.P.; Lavoie, L.; Kamtchouing, P.; Haddad, P.S. Hydro-ethanolic extract of cashew tree (Anacardium occidentale) nut and its principal compound, anacardic acid, stimulate glucose uptake in C2C12 muscle cells. Mol. Nutr. Food Res. 2010, 54, 1753–1762. [Google Scholar] [CrossRef]
- Konan, N.A.; Bacchi, E.M.; Lincopan, N.; Varela, S.D.; Varanda, E.A. Acute, subacute toxicity and genotoxic effect of a hydroethanolic extract of the cashew (Anacardium occidentale L.). J. Ethnopharmacol. 2007, 110, 30–38. [Google Scholar] [CrossRef]
- Jiang, M.; Sheng, F.; Zhang, Z.; Ma, X.; Gao, T.; Fu, C.; Li, P. Andrographis paniculata (Burm.f.) Nees and its major constituent andrographolide as potential antiviral agents. J. Ethnopharmacol. 2021, 272, 113954. [Google Scholar] [CrossRef]
- Akter, R.; Mahabub-uz-Zaman, M.; Rahman, S.; Abdullah, A.; Ahmed, N.U.; Islam, F. Comparative Studies on Antidiabetic effect with phytochemical screening of Azadirachta indicia and Andrographis paniculata. IOSR J. Pharm. Biol. Sci. 2013, 5, 122–128. [Google Scholar] [CrossRef][Green Version]
- Augustine, A.W.; Narasimhan, A.; Vishwanathan, M.; Karundevi, B. Evaluation of antidiabetic property of Andrographis paniculata powder in high fat and sucrose-induced type-2 diabetic adult male rat. Asian Pac. J. Trop. Dis. 2014, 4, S140–S147. [Google Scholar] [CrossRef]
- Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Sasi, M.; Maheshwari, C.; Singh, S.; Dhumal, S.; et al. Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Biological Activities. Biomolecules 2021, 11, 614. [Google Scholar] [CrossRef]
- Rout, S.P.; Kar, D.M.; Mohapatra, S.B.; Swain, S.P. Anti-hyperglycemic effect Annona reticulata L. leaves on experimental diabetic rat model. Asian J. Pharm. Clin. Res. 2013, 6, 56–60. [Google Scholar]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H. Evaluation of the Antidiabetic and Insulin Releasing Effects of A. squamosa, Including Isolation and Characterization of Active Phytochemicals. Plants 2020, 9, 1348. [Google Scholar] [CrossRef]
- Onwusonye, J.C.; Uwakwe, A.; Patrick, A.; Iwuanyanwu, K.P. Acute and sub-acute toxicity studies of methanol leaf extracts of Annona squamosa Linn. In mice. Sky J. Biochem. Res. 2014, 3, 53–59. [Google Scholar]
- Mandal, S.C.; Mukherjee, P.K.; Saha, K.; Das, J.; Pal, M.; Saha, B.P. Hypoglycemic activity of Ficus racemosa L. (Moraceae) leaves in streptozotocin-induced diabetic rats. Nat. Prod. Sci. 1997, 3, 38–41. [Google Scholar]
- Vadivelan, R.; Dipanjan, M.; Umasankar, P.; Dhanabal, S.P.; Satishkumar, M.N.; Antony, S.; Elango, K. Hypoglycemic, antioxidant and hypolipidemic activity of Asparagus racemosus on streptozotocin-induced diabetic in rats. Adv. Appl. Sci. Res. 2011, 2, 179–185. [Google Scholar]
- Bhavsar, C.; Talele, G.S. Potential anti-diabetic activity of Bombax ceiba. Bangladesh J. Pharmacol. 2013, 8, 102–106. [Google Scholar] [CrossRef]
- Nahar, L.; Nasrin, F.; Zahan, R.; Haque, A.; Haque, E.; Mosaddik, A. Comparative study of antidiabetic activity of Cajanus cajan and Tamarindus indica in alloxan-induced diabetic mice with a reference to in vitro antioxidant activity. Pharmacogn. Res. 2014, 6, 180–187. [Google Scholar] [CrossRef]
- Islam, M.A.; Khan, M.D.; Hossain, M.S.; Alam, A.K.; Wahed, M.I.; Rahman, B.M.; Anisuzzaman, A.S.; Shaheen, S.; Ahmed, M. Antidiabetic and hypolipidemic effects of different fractions of Coccinia cordifolia L. on normal and streptozotocin-induced diabetic rats. Pak. J. Pharm. Sci. 2011, 24, 331–338. [Google Scholar]
- Husna, R.N.; Noriham, A.; Nooraain, H.; Azizah, A.H.; Amna, O.F. Acute oral toxicity effects of Momordica charantia in sprague dawley rats. Int. J. Biochem. Bioinforma. 2013, 3, 408. [Google Scholar]
- Gupta, R.; Saxena, A.M. Hypoglycemic and anti-hyperglycemic activities of Syzygium cumini (Linn.) skeels whole fruit, in normal and streptozotocin-induced diabetic rats. Asian J. Pharm. Biol. Res. 2011, 8, 88–93. [Google Scholar]
- Schoenfelder, T.; Warmlin, C.Z.; Manfredini, M.S.; Pavei, L.L.; Réus, J.V.; Tristão, T.C.; Fernandes, M.S.; Costa-Campos, L. Hypoglycemic and hypolipidemic effect of leaves from Syzygium cumini (L.) Skeels, Myrtaceae. in diabetic rats. Rev. Bras. Farm. 2010, 20, 222–227. [Google Scholar] [CrossRef]
- Ahmed, F.; Rahman, S.; Ahmed, N.; Hossain, M.; Biswas, A.; Sarkar, S.; Banna, H.; Khatun, A. Evaluation of Neolamarckia cadamba (Roxb.) bosser leaf extract on glucose tolerance in glucose-induced hyperglycemic mice. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 79–81. [Google Scholar] [CrossRef]
- Madhuri, A.S.; Mohanvelu, R. Evaluation of Antidiabetic Activity of Aqueous Extract of Mangifera Indica Leaves in Alloxan Induced Diabetic Rats. Biomed. Pharmacol. J. 2017, 10, 1029–1035. [Google Scholar] [CrossRef]
- Naskar, S.; Mazumder, U.K.; Pramanik, G.; Gupta, M.; Kumar, R.S.; Bala, A.; Islam, A. Evaluation of antihyperglycemic activity of Cocos nucifera Linn. on streptozotocin induced type 2 diabetic rats. J. Ethnopharmacol. 2011, 138, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Parvin, A.; Alam, M.; Haque, A.; Bhowmik, A.; Ali, L.; Rokeya, B. Study of the Hypoglycemic Effect of Tamarindus indica Linn. Seeds on Non-Diabetic and Diabetic Model Rats. Br. J. Pharm. Res. 2013, 3, 1094–1105. [Google Scholar] [CrossRef]
- Pottathil, S.; Nain, P.; Morsy, M.A.; Kaur, J.; Al-Dhubiab, B.E.; Jaiswal, S.; Nair, A.B. Mechanisms of Antidiabetic Activity of Methanolic Extract of Punica granatum Leaves in Nicotinamide/Streptozotocin-Induced Type 2 Diabetes in Rats. Plants 2020, 9, 1609. [Google Scholar] [CrossRef]
- Gharib, E.; Kouhsari, S.M. Study of the antidiabetic activity of Punica granatum L. fruits aqueous extract on the allox-an-diabetic wistar rats. Iran. J. Pharm. Res. 2019, 18, 368. [Google Scholar]
- Bisht, S.; Sisodia, S.S. Anti-hyperglycemic And antidyslipidemic potential of Azadirachta indica leaf extract in STZ- induced diabetes mellitus. J. Pharm. Sci. Res. 2010, 2, 622. [Google Scholar]
- Revathy, J.; Abdullah, S.S.; Kumar, P.S. Antidiabetic effect of Costus speciosus rhizome Extract in alloxan induced albino rats. J. Chem. Biochem. 2014, 2, 13–22. [Google Scholar]
- Bavarva, J.H.; Narasimhacharya, A.V.R.L. Antihyperglycemic and hypolipidemic effects of Costus speciosus in alloxan induced diabetic rats. Phytotherapy Res. 2008, 22, 620–626. [Google Scholar] [CrossRef]
- Bamagous, G.A.; Al Ghamdi, S.S.; Ibrahim, I.A.A.; Mahfoz, A.M.; Afify, M.A.; Alsugoor, M.H.; Shammah, A.A.; Arulselvan, P.; Rengarajan, T. Antidiabetic and antioxidant activity of ethyl acetate extract fraction of Moringa oleifera leaves in streptozotocin-induced diabetes rats via inhibition of inflammatory mediators. Asian Pac. J. Trop. Biomed. 2018, 8, 320. [Google Scholar] [CrossRef]
- Saraswathi, S.; Senthamarai, R.; Sundari, S. Antidiabetic activity of leaves extract of Ficus benghalensis linn on alloxan induced diabeteic rats. Int. J. Pharmacol. Biol. Sci. 2013, 7, 47–51. [Google Scholar]
- Kasireddy, G.R.; Kumar, K.S.; Nadithe, L.R.; Chinnam, P. Experimental evaluation of hypoglycemic effect of bark extract of Ficus benghalensis in streptozotocin-induced diabetic rats. Natl. J. Physiol. Pharm. Pharmacol. 2021, 11, 320–325. [Google Scholar] [CrossRef]
- Nalamolu, R.K.; Boini, K.M.; Nammi, S. Effect of chronic administration of Boerhaavia diffusa Linn. leaf extract on experimental diabetes in rats. Trop. J. Pharm. Res. 2007, 3, 305–309. [Google Scholar] [CrossRef]
- Nammi, S.; Boini, M.K.; Lodagala, S.D.; Behara, R.B.S. The juice of fresh leaves of Catharanthus roseus Linn. reduces blood glucose in normal and alloxan diabetic rabbits. BMC Complement. Altern. Med. 2003, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Bodhankar, S.; Jain, B.; Bhardwaj, S.; Badole, S.; Patel, N. Antihyperglycemic activity of aqueous extract of leaves of Cocculus hirsutus (L.) Diels in alloxan-induced diabetic mice. Indian J. Pharmacol. 2006, 38, 49. [Google Scholar] [CrossRef]
- Ghosh, R.; Sharatchandra, K.H.; Rita, S.; Thokchom, I.S. Hypoglycemic activity of Ficus hispida (bark) in normal and diabetic albino rats. Indian J. Pharmacol. 2004, 36, 222. [Google Scholar]
- Kumar, G.P.S.; Arulselvan, P.; Kumar, D.S.; Subramanian, S.P. Anti-Diabetic Activity of Fruits of Terminalia chebula on Streptozotocin Induced Diabetic Rats. J. Heal Sci. 2006, 52, 283–291. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Swamy, V.B.M.; Gopkumar, P.; Dhanapal, R.; Chandrashekara, V.M. Anti-diabetic activity of Terminalia catappa Linn. leaf extracts in alloxan-induced diabetic rats. Iran. J. Pharmacol. Ther. 2005, 4, 36–39. [Google Scholar]
- Hasan, N.; Rahman, M.H.; Guo, R.; Hirashima, A. Hypoglycemic activity of methanolic leaf extract of Blumea lacera in Swiss-albino mice. Asian Pac. J. Trop. Dis. 2015, 5, 195–198. [Google Scholar] [CrossRef]
- Arambewela, L.; Arawwawala, L.; Ratnasooriya, W. Antidiabetic activities of aqueous and ethanolic extracts of Piper betle leaves in rats. J. Ethnopharmacol. 2005, 102, 239–245. [Google Scholar] [CrossRef]
- Dd, I. Antidiabetic activity of ethanolic extract of Kalanchoe pinnata leaves in alloxan induced hyperglycaemic rats. Indones. J. Pharm. 2016, 27, 139. [Google Scholar] [CrossRef]
- Sakuljaitrong, S.; Buddhakala, N.; Chomko, S.; Talubmook, C. Effects of flower extract from Lotus (Nelumbo nucifera) on hypoglycemic and hypolipidemic in streptozotocin-induced diabetic rats. Int. J. Sci. Eng. Res. 2013, 4, 1441–1446. [Google Scholar]
- Nasrin, F.; Hakim, M.L.; Hassan, M.R.; Afroz, N.; Ikbal, M.H.; Azam, S. Hypoglycemic study of ethanolic extract of Mikania cordata leaf. World J. Pharm. Res. 2016, 4, 1–9. [Google Scholar]
- Bari, W.; Islam, M.; Khatun, M.; Sultana, M.J.; Ahmed, R.; Islam, A.; Hossain, I.; Rahman, M.; Islam, M.A. Antidiabetic effect of Wedelia chinensis leaf extract in alloxan induced Swiss albino diabetic mice. Clin. Phytoscience 2020, 6, 58. [Google Scholar] [CrossRef]
- Husna, F.; Suyatna, F.D.; Arozal, W.; Poerwaningsih, E.H. Anti-Diabetic Potential of Murraya koenigii (L.) and its Antioxidant Capacity in Nicotinamide-Streptozotocin Induced Diabetic Rats. Drug Res. 2018, 68, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Casmir, U.E.; Joshua, P.E.; Ukegbu, C.Y.; Ezeagu, C.U.; Nwodo, O.F.C. Antidiabetic potential of ethanol leaf extract of Bryophyllum pinnatum on alloxan-induced diabetic rats and their haematological profiles. Afr. J. Pharm. Pharmacol. 2017, 11, 526–533. [Google Scholar] [CrossRef]
- Nayak, P.; Kar, D.M.; Maharana, L. Antidiabetic activity of aerial parts of Argemone mexicana linn. In alloxan induced hyperglycaemic rats. Pharmacologyonline 2011, 1, 889–903. [Google Scholar]
- Rahman, A.H.; Biswas, M.C.; Islam, A.K.; Zaman, A.T. Assessment of traditional medicinal plants used by local people of monirampur thana under Jessore district of Bangladesh. Wudpecker J. Med. Plans 2013, 2, 99–109. [Google Scholar]
- Rahman, A.M.; Kabir, E.Z.M.F.; Islam, A.R.; Zaman, A.T.M.N. Medico-botanical investigation by the tribal people of Naogaon district, Bangladesh. J. Med. Plants 2013, 1, 147–196. [Google Scholar]
- Priya, C.L.; Rao, K.V.B. Ethanobotanical and current ethanopharmacological aspects of Argemone mexicana Linn: An overview. Int. J. Pharm. Sci. Res. 2012, 3, 2143. [Google Scholar]
- Chanda, P.; Gupta, N.; Kumari, A.; Bhattacharya, S. A review on pharmacological potential of Argemone mexicana in management of wound healing & antidiabetic activity. Pharmacol. Potential Med. Plants Manag. Differ. Dis. 2022, 11, 21–27. [Google Scholar]
- Prasad, M.; Venugopal, S.P. Preliminary phytochemical analysis and oral acute toxicity study of the root of Argemone mexicana linn. Int. J. Res. Dev. Pharm. Life Sci. 2016, 5, 2010–2017. [Google Scholar]
- Sarkar, K.K.; Mitra, T.; Acharyya, R.N.; Sadhu, S.K. Phytochemical screening and evaluation of the pharmacological activities of ethanolic extract of Argemone mexicana Linn. aerial parts. Orient. Pharm. Exp. Med. 2019, 19, 91–106. [Google Scholar] [CrossRef]
- Pahwa, R.; Chatterjee, V.C. The toxicity of Mexican poppy (Argemone mexicana L.) seeds to rats. Veter-Hum. Toxicol. 1989, 31, 555–558. [Google Scholar]
- Verma, S.; Dev, G.; Tyagi, A.; Goomber, S.; Jain, G. Argemone mexicana poisoning: Autopsy findings of two cases. Forensic Sci. Int. 2000, 115, 135–141. [Google Scholar] [CrossRef]
- Pingale, S.S. Toxicity study for Argemone mexicana L. World J. Pharm. Sci. 2013, 1, 151–155. [Google Scholar]
- Singh, R.; Geetanjali. Asparagus racemosus: A review on its phytochemical and therapeutic potential. Nat. Prod. Res. 2015, 30, 1896–1908. [Google Scholar] [CrossRef] [PubMed]
- Vadivelan, R.; Krishnan, R.G.; Kannan, R. Antidiabetic potential of Asparagus racemosus Willd leaf extracts through inhibition of α-amylase and α-glucosidase. J. Tradit. Complement. Med. 2018, 9, 1–4. [Google Scholar] [CrossRef]
- Selvaraj, K.; Sivakumar, G.; Veeraraghavan, V.P.; Dandannavar, V.S.; Veeraraghavan, G.R.; Rengasamy, G. Asparagus racemosus—A Review. Syst. Rev. Pharm. 2019, 10, 87–89. [Google Scholar]
- Hannan, J.M.A.; Ali, L.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Antihyperglycaemic activity of Asparagus racemosus roots is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of cellular insulin action. Br. J. Nutr. 2012, 107, 1316–1323. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Marenah, L.; Ali, L.; Begum, R.; Peter, R.F.; Yasser, H.A.W. Insulin secretory actions of extracts of Asparagus racemosus root in perfused pancreas, isolated islets and clonal pancreatic beta-cells. J. Endocrinol. 2007, 192, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.C.S.; Udupa, A.L.; Sammodavardhana, K.; Rathnakar, U.P.; Shvetha, U.; Kodancha, G.P. Acute toxicity and diuretic studies of the roots of Asparagus racemosus Willd in rats. West Indian Med. J. 2010, 59, 3–6. [Google Scholar] [PubMed]
- Goyal, R.K.; Singh, J.; Lal, H. Asparagus racemosus—An update. Indian J. Med. Sci. 2003, 57, 408–414. [Google Scholar] [PubMed]
- Rahmatullah, M.; Mollik, M.A.H.; Khatun, A.; Jahan, R.; Chowdhury, A.R.; Seraj, S.; Hossain, M.; Nasrin, D.; Khatun, Z. A survey on the use of medicinal plants by folk medicinal practitioners in five villages of Boalia sub-district, Rajshahi district, Bangladesh. Adv. Nat. Appl. Sci. 2010, 4, 39–44. [Google Scholar]
- Islas, J.F.; Acosta, E.; G-Buentello, Z.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.; Moreno-Cuevas, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods 2020, 74, 104171. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Almatroudi, A.; Alrumaihi, F.; Khan, A.A. Pharmacological and therapeutic potential of neem (Azadirachta indica). Pharmacogn. Rev. 2018, 12, 250. [Google Scholar] [CrossRef]
- Pandey, G.; Verma, K.K.; Singh, M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta indica (neem) leaves. Int. J. Pharm. Pharm. Sci. 2014, 6, 444–447. [Google Scholar]
- Arumugam, A.; Agullo, P.; Boopalan, T.; Nandy, S.; Lopez, R.; Gutierrez, C.; Narayan, M.; Rajkumar, L. Neem leaf extract inhibits mammary carcinogenesis by altering cell proliferation, apoptosis, and angiogenesis. Cancer Biol. Ther. 2014, 15, 26–34. [Google Scholar] [CrossRef]
- Kanagasanthosh, K.; Shanmugapriyan, S.; Kavirajan, V. Evaluation of acute toxicity, anti-inflammatory activity and phytochemical screening of ethanolic extract of Azadirachta indica leaves. Int. J. Res. Dev. Pharm. Life Sci. 2015, 4, 1737–1742. [Google Scholar]
- Satyanarayana, K.; Sravanthi, K.; Shaker, I.; Ponnulakshmi, R. Molecular approach to identify antidiabetic potential of Azadirachta indica. J. Ayurveda Integr. Med. 2015, 6, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gutierrez, R.M.; Damian-Guzman, M. Meliacinolin: A Potent α-Glucosidase and α-Amylase Inhibitor Isolated from Azadirachta indica Leaves and In Vivo Antidiabetic Property in Streptozotocin-Nicotinamide-Induced Type 2 Diabetes in Mice. Biol. Pharm. Bull. 2012, 35, 1516–1524. [Google Scholar] [CrossRef]
- Rahman, A.H.M.M.; Hossain, M.M.; Islam, A.K.M.R. Taxonomy and Medicinal Uses of Angiosperm Weeds in the Wheat Field of Rajshahi, Bangladesh. Front. Biol. Life Sci. 2014, 2, 8. [Google Scholar] [CrossRef]
- Kundu, P.; Debnath, S.L.; Sadhu, S.K. Exploration of Pharmacological and Toxicological Properties of Aerial Parts of Blumea lacera, a Common Weed in Bangladesh. Clin. Complement. Med. Pharmacol. 2022, 2, 10038. [Google Scholar] [CrossRef]
- Hossen, A.; Reza, A.A.; Abu Ahmed, A.; Islam, K.; Jahan, I.; Hossain, R.; Khan, M.F.; Alam Maruf, M.R.; Haque, A.; Rahman, A. Pretreatment of Blumea lacera leaves ameliorate acute ulcer and oxidative stress in ethanol-induced Long-Evan rat: A combined experimental and chemico-biological interaction. Biomed. Pharmacother. 2021, 135, 111211. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Aeri, V.; Gaur, P.K.; Jachak, S.M. Phytochemical, Therapeutic, and Ethnopharmacological Overview for a Traditionally Important Herb:Boerhavia diffusa Linn. BioMed Res. Int. 2014, 2014, 808302. [Google Scholar] [CrossRef] [PubMed]
- Oyebode, O.A.; Erukainure, O.L.; Chukwuma, C.I.; Ibeji, C.U.; Koorbanally, N.A.; Islam, S. Boerhaavia diffusa inhibits key enzymes linked to type 2 diabetes in vitro and in silico; and modulates abdominal glucose absorption and muscle glucose uptake ex vivo. Biomed. Pharmacother. 2018, 106, 1116–1125. [Google Scholar] [CrossRef]
- Hiruma-Lima, C.A.; Gracioso, J.S.; Bighetti, E.J.; Robineou, L.G.; Brito, A.S. The juice of fresh leaves of Boerhaavia diffusa L. (Nyctaginaceae) markedly reduces pain in mice. J. Ethnopharmacol. 2000, 71, 267–274. [Google Scholar] [CrossRef]
- Chaudhary, P.H.; Khadabadi, S.S. Bombax ceiba Linn.: Pharmacognosy, ethnobotany and phyto-pharmacology. Pharmacogn. Commun. 2012, 2, 2–9. [Google Scholar] [CrossRef]
- Wanjari, M.M.; Gangoria, R.; Dey, Y.N.; Gaidhani, S.N.; Pandey, N.K.; Jadhav, A.D. Hepatoprotective and antioxidant activity of Bombax ceiba flowers against carbon tetrachloride-induced hepatotoxicity in rats. Hepatoma Res. 2016, 2, 144. [Google Scholar] [CrossRef]
- Jan, H.T.U.; Zahra, S.S.; Nasir, B.; Baig, M.W.; Ahmed, M. Divulging the Antimicrobial and Antidiabetic Potential of Bombax ceiba L. J. Bioresour. Manag. 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Xu, G.-K.; Qin, X.-Y.; Wang, G.-K.; Xie, G.-Y.; Li, X.-S.; Sun, C.-Y.; Liu, B.-L.; Qin, M.-J. Antihyperglycemic, antihyperlipidemic and antioxidant effects of standard ethanol extract of Bombax ceiba leaves in high-fat-diet- and streptozotocin-induced Type 2 diabetic rats. Chin. J. Nat. Med. 2017, 15, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Yang, J.; Wang, F.; Wang, Z.; Xiang, P.; Xie, X.; Sun, J.; He, X.; Zhang, X. A preliminary study of the chemical composition and bioactivity of Bombax ceiba L. flower and its potential mechanism in treating type 2 diabetes mellitus using ultra-performance liquid chromatography quadrupole-time-flight mass spectrometry and network pharmacology analysis. Front. Nutr. 2022, 9, 2519. [Google Scholar] [CrossRef]
- Kushwaha, P.S.; Raj, V.; Singh, A.K.; Keshari, A.K.; Saraf, S.A.; Mandal, S.C.; Yadav, R.K.; Saha, S. Antidiabetic effects of isolated sterols from Ficus racemosa leaves. RSC Adv. 2015, 5, 35230–35237. [Google Scholar] [CrossRef]
- Ca, J.; Dai, W.; Zhang, N. Advance on chemical constituents and pharmacological activities of Cajanus cajan (L.) Millsp. Nat. Prod. Res. Dev. 2020, 32, 515. [Google Scholar] [CrossRef]
- Khatun, S.; Pervin, F.; Karim, M.R.; Ashraduzzaman, M.; Rosma, A. Phytochemical screening and antimicrobial activity of Coccinia cordifolia L. plant. Pak. J. Pharm. Sci. 2012, 25, 25. [Google Scholar]
- Grover, J.; Yadav, S. Pharmacological actions and potential uses of Momordica charantia: A review. J. Ethnopharmacol. 2004, 93, 123–132. [Google Scholar] [CrossRef]
- Chhikara, N.; Kaur, R.; Jaglan, S.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds and pharmacological and food applications of Syzygium cumini—A review. Food Funct. 2018, 9, 6096–6115. [Google Scholar] [CrossRef] [PubMed]
- Bandiola, T.M.; Ignacio, G.B.; Yunson, E.G.; Bandiola, P.D. Syzygium cumini (L.) skeels: A review of its phytochemical constituents, toxicity studies, and traditional and pharmacological uses. Int. J. Appl. Pharm. Biol. Res. 2017, 2, 15–23. [Google Scholar]
- Gurjar, H.; Jain, S.K.; Irchhaiya, R.; Nandanwar, R.; Sahu, V.K.; Saraf, H. Hypoglycemic effects of methanolic extract of Anthocephalus cadamba bark in alloxan induced diabetic rats (Rox B) Miq. Int. J. Pharm. Sci. Res. 2010, 1, 79–83. [Google Scholar]
- Pandey, A.; Negi, P.S. Traditional uses, phytochemistry and pharmacological properties of Neolamarckia cadamba: A review. J. Ethnopharmacol. 2016, 181, 118–135. [Google Scholar] [CrossRef]
- Dubey, A.; Nayak, S.; Goupale, D.C. A review on phytochemical, pharmacological and toxicological studies on Neo-lamarckia cadamba. Pharm. Lett. 2011, 3, 45–54. [Google Scholar]
- Shah, K.; Patel, M.; Patel, R.; Parmar, P. Mangifera indica (Mango). Pharmacogn. Rev. 2010, 4, 42–48. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A Review on Ethnopharmacological Applications, Pharmacological Activities, and Bioactive Compounds of Mangifera indica (Mango). Evid.-Based Complement. Altern. Med. 2017, 2017, 6949835. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.B.C.; Sousa, C.N.S.; Meneses, L.N.; Ximenes, N.C.; Júnior, M.A.S.; Vasconcelos, G.S.; Lima, N.B.C.; Patrocínio, M.C.A.; Macedo, D.; Vasconcelos, S.M.M. Cocos nucifera (L.) (Arecaceae): A phytochemical and pharmacological review. Braz. J. Med. Biol. Res. 2015, 48, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Meher, B.; Dash, D.K.; Roy, A. A review on: Phytochemistry, phamracology and tradiional uses of Tamarindus indica L. World J. Pharm. Pharm. Sci. 2014, 3, 229–240. [Google Scholar]
- Moga, M.; Dimienescu, O.; Bălan, A.; Dima, L.; Toma, S.; Bîgiu, N.; Blidaru, A. Pharmacological and Therapeutic Properties of Punica granatum Phytochemicals: Possible Roles in Breast Cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Shaheen, H.M.; Alsenosy, A.W.; El-Sayed, Y.S.; Al Jaouni, S.K.; Mousa, S. Costus speciosus: Traditional uses, phytochemistry, and therapeutic potentials. Pharmacogn. Rev. 2018, 12, 120–127. [Google Scholar] [CrossRef]
- Kumar, S.; Bhattacharya, A.; Tiwari, P.; Sahu, P. A review of the phytochemical and pharmacological characteristics of Moringa oleifera. J. Pharm. Bioallied Sci. 2018, 10, 181–191. [Google Scholar] [CrossRef]
- Xu, Y.B.; Chen, G.L.; Guo, M.Q. Antioxidant and anti-inflammatory activities of the crude extracts of Moringa oleifera from Kenya and their correlations with flavonoids. Antioxidants 2019, 8, 296–318. [Google Scholar] [CrossRef]
- Zainab, B.; Ayaz, Z.; Alwahibi, M.S.; Khan, S.; Rizwana, H.; Soliman, D.W.; Alawaad, A.; Abbasi, A.M. In-silico elucidation of Moringa oleifera phytochemicals against diabetes mellitus. Saudi J. Biol. Sci. 2020, 27, 2299–2307. [Google Scholar] [CrossRef]
- Gopukumar, S.T.; Praseetha, P.K. Ficus benghalensis Linn—The sacred indian medicinal tree with potent pharmacological remedies. Int. J. Pharm. Sci. Rev. Res. 2015, 32, 223–227. [Google Scholar]
- Gabhe, S.; Tatke, P.; Khan, T. Evaluation of the immunomodulatory activity of the methanol extract of Ficus benghalensis roots in rats. Indian J. Pharmacol. 2006, 38, 271. [Google Scholar] [CrossRef]
- Nisar, A.; Mamat, A.S.; Hatim, M.I.; Aslam, M.S.; Syarhabil, M. An updated review on Catharanthus roseus: Phytochemical and pharmacological analysis. Indian Res. J. Pharm. Sci. 2016, 3, 631–653. [Google Scholar]
- Logesh, R.; Das, N.; Adhikari-Devkota, A.; Devkota, H.P. Cocculus hirsutus (L.) W.Theob. (Menispermaceae): A Review on Traditional Uses, Phytochemistry and Pharmacological Activities. Medicines 2020, 7, 69. [Google Scholar] [CrossRef]
- Majumder, S.C.; Islam, K.; Hossain, M.M. Potential anti-diabetic medicinal plants in bangladesh: A comprehensive review. World J. Pharm. Res. 2019, 8, 140–150. [Google Scholar]
- Cheng, J.-X.; Zhang, B.-D.; Zhu, W.-F.; Zhang, C.-F.; Qin, Y.-M.; Abe, M.; Akihisa, T.; Liu, W.-Y.; Feng, F.; Zhang, J. Traditional uses, phytochemistry, and pharmacology of Ficus hispida L.f.: A review. J. Ethnopharmacol. 2019, 248, 112204. [Google Scholar] [CrossRef]
- Gupta, P.C. Biological and pharmacological properties of Terminalia chebula Retz. (Haritaki). Int. J. Pharm. Pharm. Sci. 2012, 4, 62–68. [Google Scholar]
- Anand, A.; Divya, N.; Kotti, P. An updated review of Terminalia catappa. Pharmacogn. Rev. 2015, 9, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Azahar, N.I.; Mokhtar, N.M.; Arifin, M.A. Piper betle: A review on its bioactive compounds, pharmacological properties, and extraction process. IOP Conf. Series Mater. Sci. Eng. 2020, 991, 12044. [Google Scholar] [CrossRef]
- Majaz, A.Q.; Khurshid, M.; Nazim, S. The miracle plant (Kalanchoe pinnata ): A phytochemical and pharmacological review. Int. J. Res. Ayurveda Pharm. 2011, 2, 1478–1482. [Google Scholar]
- Paudel, K.R.; Panth, N. Phytochemical Profile and Biological Activity of Nelumbo nucifera. Evid.-Based Complement. Altern. Med. 2015, 2015, 789124. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.R.; Patel, E.P.; Patani, P.V.; Shah, B. Nelumbo nucifera (Lotus): A Review on Ethanobotany, Phytochemistry and Pharmacology. Indian J. Pharm. Biol. Res. 2013, 1, 152–167. [Google Scholar] [CrossRef]
- Dewi, K.; Ridwanuloh, A.; Rahmat, J. Phytochemical screening, antibacterial and cytotoxic activity of Mikania cordata extracts. In Proceedings of the 2nd International Seminar on Chemistry; 2021; pp. 217–221. [Google Scholar]
- Gahlawat, D.K.; Jakhar, S.; Dahiya, P. Murraya koenigii (L.) Spreng: An ethnobotanical, phytochemical and pharmaco-logical review. J. Pharmacogn. Phytochem. 2014, 3, 109–119. [Google Scholar]
- Latif, A.; Ashiq, K.; Qayyum, M.; Ashiq, S.; Ali, E.; Anwer, I. Phytochemical and pharmacological profile of the medicinal herb: Bryophyllum pinnatum. J. Anim. Plant Sci. 2019, 29, 295–301. [Google Scholar]
- Ibitoye, O.B.; Olofinsan, K.A.; Teralı, K.; Ghali, U.M.; Ajiboye, T.O. Bioactivity-guided isolation of antidiabetic principles from the methanolic leaf extract of Bryophyllum pinnatum. J. Food Biochem. 2018, 42, e12627. [Google Scholar] [CrossRef]
- Afzal, M.; Kazmi, I.; Anwar, F. Antineoplastic potential of Bryophyllum pinnatum lam. on chemically induced hepatocarcinogenesis in rats. Pharmacogn. Res. 2013, 5, 247–253. [Google Scholar] [CrossRef]
- Tang, R.; Tian, R.-H.; Cai, J.-Z.; Wu, J.-H.; Shen, X.-L.; Hu, Y.-J. Acute and sub-chronic toxicity of Cajanus cajan leaf extracts. Pharm. Biol. 2017, 55, 1740–1746. [Google Scholar] [CrossRef]
- Ezike, A.C.; Akah, P.A.; Okoli, C.C.; Okpala, C.B. Experimental evidence for the antidiabetic activity of Cajanus cajan leaves in rats. J. Basic Clin. Pharm. 2010, 1, 81–84. [Google Scholar] [PubMed]
- Ohadoma, S.C.; Akpan, J.L.; Odey, P.A.; Okoro, E.P. Mechanistic considerations of Catharanthus roseus on the hypo-glycemic activity of alpha glucosidase inhibitors and biguanides: A review. J. Pharm. Adv. Res. 2021, 4, 1390–1398. [Google Scholar]
- Al-Shaqha, W.M.; Khan, M.; Salam, N.; Azzi, A.; Chaudhary, A.A. Anti-diabetic potential of Catharanthus roseus Linn. and its effect on the glucose transport gene (GLUT-2 and GLUT-4) in streptozotocin induced diabetic wistar rats. BMC Complement. Altern. Med. 2015, 15, 379. [Google Scholar] [CrossRef] [PubMed]
- Vutukuri, V.R.; Das, M.C.; Reddy, M.; Prabodh, S.; Sunethri, P. Evaluation of Acute Oral Toxicity of Ethanol Leaves Extract of Catharanthus roseus in Wistar Albino Rats. J. Clin. Diagn. Res. 2017, 11, FF01–FF04. [Google Scholar] [CrossRef] [PubMed]
- Balaraman, A.K.; Singh, J.; Dash, S.; Maity, T.K. Antihyperglycemic and hypolipidemic effects of Melothria maderaspatana and Coccinia indica in Streptozotocin induced diabetes in rats. Saudi Pharm. J. 2010, 18, 173–178. [Google Scholar] [CrossRef]
- Putra, I.M.W.A.; Fakhrudin, N.; Nurrochmad, A.; Wahyuono, S. Antidiabetic activity of Coccinia grandis (L.) Voigt: Bioactive constituents, mechanisms of action, and synergistic effects. J. Appl. Pharm. Sci. 2021, 12, 41–54. [Google Scholar] [CrossRef]
- Jha, U.; Asad, M.; Asdaq, S.M.B.; Das, A.K.; Prasad, V.S. Fertility inducing effect of aerial parts of Coccinia cordifolia L. in female rats. J. Ethnopharmacol. 2010, 127, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Bothara, S.; Marya, B.; Saluja, A.K. Antiarthritic activity of root extracts of Cocculus hirsutus. Int. J. Pharm. Pharm. Sci. 2011, 3, 175–177. [Google Scholar]
- Obidoa, O.; Joshua, P.E.; Eze, N.J. Phytochemical analysis of Cocos nucifera L. J. Pharm. Res. 2010, 3, 280–286. [Google Scholar]
- Salil, G.; Nevin, K.; Rajamohan, T. Arginine rich coconut kernel protein modulates diabetes in alloxan treated rats. Chem. Biol. Interact. 2011, 189, 107–111. [Google Scholar] [CrossRef]
- Paul, N.; Roy, R.; Bhattacharya, S.; Biswas, M. Acute and sub-chronic toxicity study of Cocos nucifera leaf extracts in mice. J. Adv. Pharm. Educ. Res. 2012, 2, 74–81. [Google Scholar]
- Waisundara, V.; Watawana, M.; Jayawardena, N. Costus speciosus and Coccinia grandis: Traditional medicinal remedies for diabetes. S. Afr. J. Bot. 2015, 98, 1–5. [Google Scholar] [CrossRef]
- Rani, A.S.; Sulakshana, G.; Patnaik, S. Costus speciosus, An antidiabetic plant-review. FS J. Pharm. Res. 2012, 1, 51–53. [Google Scholar]
- Muthukumaran, P.; Thiyagarajan, G.; Babu, R.A.; Lakshmi, B.S. Raffinose from Costus speciosus attenuates lipid synthesis through modulation of PPARs/SREBP1c and improves insulin sensitivity through PI3K/AKT. Chem. Interact. 2018, 284, 80–89. [Google Scholar] [CrossRef]
- Ahmed, F.; Chavan, S.; Satish, A.; Punith, K.R. Inhibitory activities of Ficus benghalensis bark against carbohydrate hydrolyzing enzymes—An in vitro study. Pharmacogn. J. 2011, 3, 33–37. [Google Scholar] [CrossRef]
- Khanal, P.; Patil, B.M. Consolidation of network and experimental pharmacology to divulge the antidiabetic action of Ficus benghalensis L. bark. 3 Biotech 2021, 11, 238. [Google Scholar] [CrossRef]
- Swathi, N.; Sreedevi, A.; Bharathi, K. Evaluation of Nephroprotective Activity of Fruits of Ficus hispida on Cisplatin-Induced Nephrotoxicity. Pharmacogn. J. 2011, 3, 62–68. [Google Scholar] [CrossRef]
- Ahmed, F.; Urooj, A. In vitro studies on the hypoglycemic potential of Ficus racemosa stem bark. J. Sci. Food Agric. 2009, 90, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.B.; Murugesan, T.; Sinha, S.; Saha, B.P.; Pal, M.; Mandal, S.C. Glucose lowering efficacy of Ficus racemosa bark extract in normal and alloxan diabetic rats. Phytotherapy Res. 2002, 16, 590–592. [Google Scholar] [CrossRef]
- Morshed, A.; Hossain, M.H.; Shakil, S.; Nahar, K.; Rahman, S.; Ferdausi, D.; Hossain, T.; Ahmad, I.; Chowdhury, M.H.; Rahmatullah, M. Evaluation of antinociceptive activity of two Bangladeshi medicinal plants, Kalanchoe pinnata (Lam.) Pers. and Lagerstroemia speciosa (L.). Pers. Adv. Nat. Appl. Sci. 2010, 4, 193–197. [Google Scholar]
- George, L.O.; Radha, H.R.; Somasekariah, B.V. In vitro anti-diabetic activity and GC-MS analysis of bioactive compounds present in the methanol extract of Kalanchoe pinnata. Indian J. Chem.-Sect. B 2019, 57, 1213–1221. [Google Scholar]
- John, O.R.; Yahaya, A.A.; Emmanuel, A. Aqueous ethanolic extract of Mangifera indica stem bark effect on the biochemical and haematological Parameters of Albino Rats. Arch. Appl. Sci. Res. 2012, 4, 1618–1622. [Google Scholar]
- Katagiri, H.; Asano, T.; Ishihara, H.; Inukai, K.; Shibasaki, Y.; Kikuchi, M.; Yazaki, Y.; Oka, Y. Overexpression of Catalytic Subunit p110α of Phosphatidylinositol 3-Kinase Increases Glucose Transport Activity with Translocation of Glucose Transporters in 3T3-L1 Adipocytes. J. Biol. Chem. 1996, 271, 16987–16990. [Google Scholar] [CrossRef]
- Sangeetha, K.N.; Sujatha, S.; Muthusamy, V.S.; Anand, S.; Nithya, N.; Velmurugan, D.; Balakrishnan, A.; Lakshmi, B.S. 3β-taraxerol of Mangifera indica, a PI3K dependent dual activator of glucose transport and glycogen synthesis in 3T3-L1 adipocytes. Biochim. Biophys. Acta-Gen. Subj. 2010, 1800, 359–366. [Google Scholar] [CrossRef]
- Deb, L.; Bhattacharjee, C.; Shetty, S.R.; Dutta, A. Evaluation of anti-diabetic potential of the Syzygium cumini (Linn) Skeels by reverse pharmacological approaches. Bull. Pharm. Res. 2012, 3, 135–145. [Google Scholar]
- Abukakar, M.G.; Ukwuani, A.N.; Shehu, R.A. An evaluation of the toxic effects of Tamarindus indica pulp extract in albino rats. J. Pharmacol. Toxicol. 2008, 3, 111–118. [Google Scholar] [CrossRef]
- Bhandary, B.S.K.; Sharmila, K.P.; Kumari, N.S.; Bhat, S.V. Acute and subacute toxicity study of the ethanol extracts of Punica granatum (linn). Whole fruit and seeds and synthetic ellagic acid in swiss albino mice. Asian J. Pharm. Clin. Res. 2013, 6, 192–198. [Google Scholar]
- Kasolo, J.N.; Bimenya, G.S.; Ojok, L.; Ogwal-okeng, J.W. Phytochemicals and acute toxicity of Moringa oleifera roots in mice. J. Pharmacogn. Phyther. 2011, 3, 38–42. [Google Scholar]
- Panunto, W.; Jaijoy, K.; Lerdvuthisopon, N.; Lertprasertsuke, N.; Jiruntanat, N.; Soonthornchareonnon, N.; Sireeratawong, S. Acute and chronic toxicity studies of the water extract from dried fruits of Terminalia chebula Rezt. in rats. Int. J. Appl. Res. Nat. Prod. 2010, 3, 36–43. [Google Scholar]
- Lokman, M.A.; Arshad, A.M.; Ahmad, W.M.A.W.; Effendy, A.W.M. Determination of toxicological effects of Terminalia catappa leaves on Sprague-Dawley white rats in short-term period. Int. J. Toxicol. Appl. Pharm. 2013, 3, 44–47. [Google Scholar]
- Al-Adhroey, A.H.; Nor, Z.M.; Al-Mekhlafi, H.M.; Amran, A.A.; Mahmud, R. Antimalarial Activity of Methanolic Leaf Extract of Piper betle L. Molecules 2010, 16, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Umasankar, K.; Suresh, V.; Kumar, R.M.; Suresh, A.; Kumar, N.S.; Arunachalam, G. CNS Activity of Ethanol Extract of Wedelia chinensis in Experimental Animals. Int. J. Pharm. Sci. Nanotechnol. 2010, 3, 881–886. [Google Scholar] [CrossRef]
- Darvekar, V.M.; Patil, V.R.; Choudhari, A.B. Anti-inflammatory activity of Murraya koenigii Spreng on experimental animals. J. Nat. Prod. Plant Resour. 2011, 1, 65–69. [Google Scholar]
- Sathishsekar, D.; Subramanian, S. Beneficial Effects of Momordica charantia Seeds in the Treatment of STZ-Induced Diabetes in Experimental Rats. Biol. Pharm. Bull. 2005, 28, 978–983. [Google Scholar] [CrossRef]
- Oyelere, S.F.; Ajayi, O.H.; Ayoade, T.E.; Pereira, G.B.S.; Owoyemi, B.C.D.; Ilesanmi, A.O.; Akinyemi, O.A. A detailed review on the phytochemical profiles and anti-diabetic mechanisms of Momordica charantia. Heliyon 2022, 8, e09253. [Google Scholar] [CrossRef] [PubMed]
- Anwer, T.; Safhi, M.M.; Makeen, H.A.; Alshahrani, S.; Siddiqui, R.; Sivakumar, S.; Shaheen, E.S.; Alam, M.F. Antidiabetic potential of Moringa oleifera Lam. leaf extract in type 2 diabetic rats, and its mechanism of action. Trop. J. Pharm. Res. 2021, 20, 95–103. [Google Scholar] [CrossRef]
- Ahmad, J.; Khan, I.; Blundell, R. Moringa oleifera and glycemic control: A review of current evidence and possible mechanisms. Phytotherapy Res. 2019, 33, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Kim, M.S.; Rhyu, D.Y. Nelumbo nucifera leaf extract attenuated pancreatic ß-cells toxicity induced by interleukin-1ß and interferon-γ, and increased insulin secrection of pancreatic ß-cells in streptozotocin-induced diabetic rats. J. Tradit. Chin. Med. 2016, 36, 71–77. [Google Scholar] [CrossRef]
- Avijit, B.; Zerin, T.; Rajia, S. Comparative Phytochemical and Antibacterial Properties of Piper betle Leave Extracts from Barguna and Moheshkhali, Bangladesh. Iran. J. Med. Microbiol. 2020, 14, 125–132. [Google Scholar] [CrossRef]
- Yogeswari, S.; Bindu, K.H.; Kamalraj, S.; Ashokkumar, V.; Jayabaskaran, C. Antidiabetic, Antithrombin and Cytotoxic bioactive compounds in five cultivars of Piper betle L. Environ. Technol. Innov. 2020, 20, 101140. [Google Scholar] [CrossRef]
- Ocvirk, S.; Kistler, M.; Khan, S.; Talukder, S.H.; Hauner, H. Traditional medicinal plants used for the treatment of diabetes in rural and urban areas of Dhaka, Bangladesh—An ethnobotanical survey. J. Ethnobiol. Ethnomedicine 2013, 9, 43. [Google Scholar] [CrossRef]
- Huang, T.H.; Peng, G.; Kota, B.P.; Li, G.; Yamahara, J.; Roufogalis, B.; Li, Y. Anti-diabetic action of Punica granatum flower extract: Activation of PPAR-γ and identification of an active component. Toxicol. Appl. Pharmacol. 2005, 207, 160–169. [Google Scholar] [CrossRef]
- Tang, D.; Liu, L.; Ajiakber, D.; Ye, J.; Xu, J.; Xin, X.; Aisa, H.A. Anti-diabetic Effect of Punica granatum Flower Polyphenols Extract in Type 2 Diabetic Rats: Activation of Akt/GSK-3β and Inhibition of IRE1α-XBP1 Pathways. Front. Endocrinol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Bharti, S.; Kumar, R.; Krishnamurthy, B.; Bhatia, J.; Kumari, S.; Arya, D.S. Syzygium cumini Ameliorates Insulin Resistance and β-Cell Dysfunction via Modulation of PPARγ, Dyslipidemia, Oxidative Stress, and TNF-α in Type 2 Diabetic Rats. J. Pharmacol. Sci. 2012, 119, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kuru, P. Tamarindus indica and its health related effects. Asian Pac. J. Trop. Biomed. 2014, 4, 676–681. [Google Scholar] [CrossRef]
- Sole, S.S.; Srinivasan, B. Aqueous extract of tamarind seeds selectively increases glucose transporter-2, glucose transporter-4, and islets’ intracellular calcium levels and stimulates β-cell proliferation resulting in improved glucose homeostasis in rats with streptozotocin-induced diabetes mellitus. Nutr. Res. 2012, 32, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.; Lima, M.; Medeiros, A.; Bezerra, L.; Santos, P.; Serquiz, A.; Lima, M.; Oliveira, G.; Santos, E.; Maciel, B.; et al. An Insulin Receptor-Binding Multifunctional Protein from Tamarindus indica L. Presents a Hypoglycemic Effect in a Diet-Induced Type 2 Diabetes—Preclinical Study. Foods 2002, 11, 2207. [Google Scholar] [CrossRef]
- Mallik, J.; AI, F.A.; Kumar, B.R. A comprehensive review on Pharmacological activity of Terminalia Catappa (Com-bretaceae)—An Update. Asian J. Pharm. Res. Dev. 2010, 1, 65–70. [Google Scholar]
- Iheagwam, F.N.; Iheagwam, O.T.; Onuoha, M.K.; Ogunlana, O.O.; Chinedu, S.N. Terminalia catappa aqueous leaf extract reverses insulin resistance, improves glucose transport and activates PI3K/AKT signalling in high fat/streptozotocin-induced diabetic rats. Sci. Rep. 2022, 12, 10711. [Google Scholar] [CrossRef]
- Biswas, K.R.; Ishika, T.; Rahman, M.; Swarna, A.; Khan, T.; Monalisa, M.N.; Rahmatullah, M. Antidiabetic plants and formulations used by folk medicinal practitioners of two villages in Narail and Chuadanga districts, Bangladesh. Am. J. Sustain. Agric. 2011, 5, 158–167. [Google Scholar]
- Borgohain, R.A.; Lahon, K.I.; Das, S.W.; Gohain, K.A. Evaluation of mechanism of anti-diabetic activity of Terminalia chebula on alloxan and adrenaline-induced diabetic albino rats. Drugs 2012, 3, 256–266. [Google Scholar]
- Das, M.P.; Kumar, S.S. Preliminary phytochemical analysis of Illicium verum and Wedelia chinensis. Int. J. Pharmtech Res. 2013, 5, 324–329. [Google Scholar]
- Thao, N.P.; Binh, P.T.; Luyen, N.T.; Hung, T.M.; Dang, N.H.; Dat, N.T. α-Amylase and α-Glucosidase Inhibitory Activities of Chemical Constituents from Wedelia chinensis (Osbeck.) Merr. Leaves. J. Anal. Methods Chem. 2018, 2018, 2794904. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H.A. Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef]
- Ghafouri, A.; Hajiluian, G.; Karegar, S.J.; Hosseini, S.; Shidfar, S.; Kamalinejad, M.; AghaHosseini, F.; Heydari, I.; Shidfar, F. The effect of Aqueous, Ethanolic extracts of Rheum ribeson insulin sensitivity, inflammation, oxidative stress in patients with type 2 diabetes mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Herb. Med. 2020, 24, 100389. [Google Scholar] [CrossRef]
- Aziz, T.A.; Hussain, S.A.; Mahwi, T.O.; Ahmed, Z.A.; Rahman, H.S.; Rasedee, A. The efficacy and safety of Ginkgo biloba extract as an adjuvant in type 2 diabetes mellitus patients ineffectively managed with metformin: A double-blind, randomized, placebo-controlled trial. Drug. Des. Dev. Ther. 2018, 12, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, A.S.D.; Kalansuriya, P.; Attanayake, A.P. Nanoformulation of Plant-Based Natural Products for Type 2 Diabetes Mellitus: From Formulation Design to Therapeutic Applications. Curr. Ther. Res. 2022, 96, 100672. [Google Scholar] [CrossRef]
- Márquez-Escobar, V.A.; Rosales-Mendoza, S.; Beltrán-López, J.I.; González-Ortega, O. Plant-based vaccines against respiratory diseases: Current status and future prospects. Expert. Rev. Vaccines 2016, 16, 137–149. [Google Scholar] [CrossRef]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chinese Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]





































| Scientific Name | Common Name | Family | Parts Used | Model Organism | Solvent | Dosage | Reference Drug | Result | References |
|---|---|---|---|---|---|---|---|---|---|
| Ficus racemosa | Cluster fig, Dumur | Moraceae | Leaves | STZ-induced diabetic rats | Petroleum ether | 200 mg/kg body weight and 400 mg/kg body weight | Glibenclamide | Reduction in blood glucose levels (p < 0.001) | [34] |
| Asparagus racemosus | Satamuli | Liliaceae | Whole plant | STZ-induced diabetic Wistar rats | Ethanol | 200 and 400 mg/kg body weight | Glibenclamide 10 mg/kg body weight | Decreased blood glucose level (p < 0.05) | [35] |
| Bombax ceiba | Shimul, red silk-cotton tree | Bombacaceae | Bark | STZ-induced diabetic rats | Ethyl-acetate extract | 600 mg/kg body weight | Glibenclamide (10 mg/kg body weight) | Displayed significant hypoglycemic activity (p < 0.001) | [36] |
| Cajanus cajan | Arhar, pigeon pea | Fabaceae | Root | Alloxan-induced diabetic mice | Methanol | 200 mg/kg body weight or 400 mg/kg body weight | Metformin (50 mg/kg body weight) | Significantly lowered fasting serum glucose (p < 0.001) and blood glucose level (p < 0.001) | [37] |
| Coccinia cordifolia | Telakucha, Ivy gourd | Cucurbitaceae | Leaves | STZ-induced diabetic rats | Petroleum ether, ethyl acetate | 150 mg/kg body weight | Metformin HCl (150 mg/kg body weight) | Reduced blood glucose levels by 50.39% and 50% at the 10th and 24th hours, respectively (p < 0.001) | [38] |
| Momordica charantia | Korola, bitter gourd | Cucurbitaceae | Seed | STZ-induced diabetic rats | Water | 150 mg/kg body weight | Glibenclamide (600 μg/kg) | Significantly reduced plasma glucose (p < 0.05) | [39] |
| Syzygium cumini | Kalojam, Java plum | Myrtaceae | Whole fruit, Leaves | STZ-induced diabetic albino Wistar rats STZ-induced diabetic rats | Ethanol Ethanol | 200 mg/kg body weight 250 mg/kg body weight | Glibenclamide (200 mg/kg body weight) | Decreased blood glucose levels (p < 0.01) Significantly decreased blood glucose concentration, increased muscle glycogen store (p < 0.001) | [40,41] |
| Neolamarckia cadamba | Burflower tree, Kodom | Rubiaceae | Leaves | Glucose-loaded hyperglycemic mice | Methanol | 200 mg/kg body weight and 400 mg/kg body weight | 10 mg/kg body weight of glibenclamide | Reduced elevated blood glucose (p < 0.05) | [42] |
| Mangifera indica | Mango, Aam | Anacardiaceae | Leaves | Alloxan-induced diabetic rats | Water | 200 mg/kg body weight and 400 mg/kg body weight | Gliclazide 2 mg/kg body weight and 4 mg/kg body weight | Significantly lowered the fasting blood glucose (p < 0.0001) | [43] |
| Cocos nucifera | Coconut | Arecaceae | Spadix | STZ (STZ)-induced diabetic rats | Hydromethanol | 250 mg/kg body weight and 500 mg/kg body weight | 0.5 mg/kg body weight glibenclamide | Significantly lowered fasting blood glucose (p < 0.001) | [44] |
| Tamarindus indica | Tamarind | Fabaceae | Seed | STZ-induced diabetic Long–Evans rats | - | 1.25 g/kg | Glibenclamide (5 mg/kg body weight) | Demonstrated anti-hyperglycemic properties (p < 0.03) | [45] |
| Punica granatum | Pomegranate | Lythraceae | Leaves Fruit | STZ-induced type 2 diabetic rats Alloxan-induced diabetic Wistar rats | Methanol Water | 100, 200, 400 and 600 mg/kg body weight 100 mg/kg, 200 mg/kg and 350 mg/kg body weight | Glibenclamide (1 mg/kg body weight) - | Demonstrated anti-diabetic activity(p < 0.05) Reduced fasting blood glucose (p < 0.001) | [46,47] |
| Azadirachta indica | Neem | Meliaceae | Leaves | STZ (STZ)-induced diabetic rats | Ethanol | 200 mg/kg body weight | Glibenclamide (0.25 mg/kg body weight) | Normalized glucose levels after STZ-induced hyperglycemia (p < 0.05) | [48] |
| Costus speciosus | Crepe Ginge | Costaceae | Rhizome Root | Alloxan-induced diabetic rats | Ethanol | 200 mg/kg body weight 300 and 450 mg/kg body weight | Glibenclamide (2.5 mg/kg body weight) Glibenclamide (600 μg/kg) | Enhanced insulin secretion lowered blood glucose concentration, decreased glyconeogenesis, increased glycogenesis | [49,50] |
| Moringa oleifera | Drumstick tree | Moringaceae | Leaves | STZ-induced Sprague-Dawley rats | Ethyl acetate | 200 mg/kg body weight | Glibenclamide (5 mg/kg body weight) | Significantly reverses the effects of STZ on serum glucose and insulin, demonstrating anti-diabetic activity (p < 0.05) | [51] |
| Andrographis paniculata | Green chiretta | Acanthaceae | leaves | Alloxan-induced diabetic Wistar rats | Ethanol | 1000 mg/kg body weight | Glimepiride (4 mg/kg) body weight | Significantly reduced blood glucose (p < 0.01) | [28] |
| Ficus benghalensis | Banyan | Moraceae | Leaves Bark | Alloxan-induced diabetic albino rats STZ-induced diabetic rats | Ethanol Ethanol | 200 mg/kg body weight and 400 mg/kg body weight 150 mg/kg body weight, 300 mg/kg body weight, 500 mg/kg body weight | Glibenclamide (5 mg/kg body weight) Glibenclamide 0.5 mg/kg body weight | Reduced triglyceride, cholesterol and glucose levels Reduction in blood glucose levels by stimulating insulin secretion (p < 0.01) | [52,53] |
| Anacardium occidentale | Cashew nut, kaju badam | Sumac | Leaves | STZ-induced diabetic Wistar rats | Methanol | 300 mg/kg body weight | 1 IU/kg body weight insulin | Significantly reduced blood glucose (p < 0.05) | [24] |
| Annona squamosa | Custard apple | Annonaceae | Leaves | STZ-induced rats | Water and ethanol | 200 mg/kg body weight, 400 mg/kg body weight | Metformin (300 mg/kg body weight) | Reduced blood glucose (p < 0.05) | [31] |
| Boerhaavia diffusa | Hogweed | Nyctaginaceae | Leaves | STZ-induced diabetic rats | Chloroform | 50, 100, 200 mg/kg body weight | Glibenclamide9 (25 µg/kg body weight) | Reduced blood glucose, possibly through rejuvenation of pancreatic β-cells | [54] |
| Catharanthus roseus | Nayantara | Apocynaceae | Leaf | Alloxan-induced diabetic rat | Juice | 0.5, 0.75, 1.0 mL/kg body weight | Glibenclamide (40 μg/kg body weight) | Lowered blood glucose (p < 0.01) | [55] |
| Cocculus hirsutus | Broom creeper, Daikhali, Jalajmani | Menispermaceae | Leaf | Alloxan-induced diabetic rat | Water | 250, 500, 1000 mg/kg body weight | Glyburide (10 mg/kg body weight) | Reduced serum glucose level (p < 0.01) | [56] |
| Ficus hispida | Hairy fig | Moraceae | Bark | Alloxan-induced diabetic albino rat | Ethanol | 1.25 g/kg body weight | Glibenclamide (0.5 mg/kg body weight) | Reduced blood glucose (p < 0.001) | [57] |
| Terminalia chebula | Myrobalan | Combretaceae | Fruit | STZ-induced diabetic rats | Ethanol | 200 mg/kg body weight | Glibenclamide (600 μg/kg body weight) | Stimulated insulin and lowered blood glucose (p < 0.05) | [58] |
| Terminilia catappa | Bangla badam | Combretaceae | Leaf | Alloxan-induced diabetic Wistar albino rats | Water, cold extract | Aqueous extract (43 mg/kg body weight) Cold extract (46 mg/kg body weight) | Glibenclamide (10 mg/kg body weight) | Regenerated pancreas | [59] |
| Amaranthus tricolor | Edible amaranth | Amaranthaceae | Leaf | Alloxan-induced diabetic male albino rats | Water | 400 mg/kg body weight | - | Reduced serum glucose levels (p < 0.001) | [21] |
| Blumea lacera | Lettuce leaf blumea | Asteraceae | Leaf | Swiss albino mice | Methanol | 50–400 mg/kg body weight | Glibenclamide (10 mg/kg body weight) | Reduced blood glucose (p < 0.0001) | [60] |
| Piper betle leaves | Betel leaf | Piperaceae | Leaf | STZ-induced diabetic rats | Ethanol | 100, 200, 300, 1500 mg/kg body weight | Tolbutamide (22.5 mg/kg body weight) | Reduced blood glucose significantly (p < 0.050) | [61] |
| Achyranthes aspera | Devil’s horsewhip | Amaranthaceae | Seed | STZ-induced diabetic rat | Ethanol | 300, 600 mg/kg body weight for 24 days | Glibenclamide (5 mg/kg body weight) | Reduced blood glucose level (p < 0.001) | [11] |
| Kalanchoe pinnata | Cathedral bells, life plant | Crassulaceae | Leaf | Alloxan-induced diabetic rats | Ethanol | 5.8, 11.6 and 33.2 mg/kg body weight | Glibenclamide (1.35 mg/kg body weight), acarbose (13.5 mg/kg body weight) | Significantly powered fasting blood glucose (p < 0.05), increased number of pancreatic β-cells | [62] |
| Nelumbo nucifera | Indian lotus | Nymphaeaceae | Flower | STZ-induced diabetic male Wistar rat | Ethanol | 250 mg/kg body weight | Glibenclamide (0.25 mg/kg body weight) | Lowered fasting blood glucose | [63] |
| Mikania cordata | Bitter vine | Asteraceae | Leaf | Alloxan-induced diabetic rat | Ethanol | 200 mg/kg, 400 mg/kg body weight | Metformin hydrochloride (100 mg/kg body weight) | Reduced blood glucose (p < 0.05, p < 0.01) | [64] |
| Wedelia chinensis | Wedelia | Asteraceae | Leaf | Alloxan-induced diabetic Swiss albino mice | Methanole | 100 mg/kg, 200 mg/kg body weight | Glibenclamide (5 mg/kg body weight) | Reduced blood glucose levels (p < 0.01) | [65] |
| Murraya koenigii | Curry tree | Rutaceae | Leaf | STZ-induced and nicotinamide-induced hyperglycemic rats | Ethaol | 200 mg/kg, 400 mg/kg body weight | Glibenclamide (1 mg/kg body weight) | Decreased blood glucose (p < 0.01) | [66] |
| Aloe barbadensis | Aloe | Asphodelaceae | Gel | Alloxan-induced diabetic rat | Methanol | 300 mg/kg body weight | Glibenclamide (0.25 mg/kg body weight) | Reduced blood glucose level (p < 0.05) | [15] |
| Bryophyllum pinnatum | Goethe | Crassulaceae | Leaf | Alloxan-induced diabetic rat | Ethanol | 200 mg/kg, 400 mg/kg body weight | Glibenclamide (2.5 mg/kg body weight) | Reduced blood glucose level (p < 0.05) | [67] |
| Agremone mexican | Mexican prickly poppy, Sialkanta | Papaveraceae | Aerial parts | Alloxan-induced diabetic rat | Ethanol, water | 200 mg/kg, 400 mg/kg body weight | Glibenclamide (5 mg/kg body weight) | Demonstrated significant anti-diabetic effect (p < 0.05) | [68] |
| Name of Plant | Potential Hypoglycemic Compounds | References |
|---|---|---|
| Ficus racemosa | β-Sitosterol, stigmasterol, lanosterol, flavonoids (gluanol acetate and racemosic acid) | [104] |
| Asparagus racemosa | Flavonoids, Saponins-shatavarin IV (asparanin B), shatavarin V, shatavarin VI, shatavarin VII, shatavarin VIII, immunoside, schidigerasaponin D5 (asparanin A), racemoside A, racemoside B and racemoside C, triterpenoids, alkaloids-asparagamine A, quercetin, quercetin glycosides- quercetin-3-O-rutinoside, quercetin 3-O-galactoside, isoflavones, sapogenins | [78] |
| Bombax ceiba | alkaloids, flavonoids, glycosides, β-sitosterol, kaempferol, hentriacontane, hentriacontanol, quercetin, shamimin (2-(2,4,5-trihydroxyphenyl)-3,5,7-trihydroxy-6-C-glucopyranosyloxy-4H-1-benzopyran-4-one) | [99] |
| Cajanus cajan | Β-sitosterol, flavonoids- luteonin, vitexin, apigenin, genistic, ononin, sissiotrin, 2′-hydroxygenistein, stilbenes-cajanusin A, cajanusin B, cajanusin C, cajanusin D, cajanstilbene H, cajanolactone A, cajanonic acid A, canjanotone | [105] |
| Coccinia cordifolia | saponin, glycoside, alkaloid compounds (catharanthin, leurosine, lochnerine, vindoline and vindolinine), flavonoids, tannins, phenols, terpenoids | [38,106] |
| Momordica charantia | Diosgenin, saponins, alkaloids, trtiterpenes, proteins, steroids | [107] |
| Syzygium cumini | alkaloids, tanins, flavonoids, carotenoids, glycosides, saponins, steroids, triterpenoids, anthocyanins, phenols, oxalic acid, phytosterols, myrcetin, gallic acid | [108,109] |
| Neolamarckia cadama | Phenolics, alkaloids, Cadambine, Chlorogenic acid, Dihydrocinchonine, flavonoids, saponins, triterpenes, indole alkaloids, triterpenoid glycosides, 3α-dihydrocadambine, isodihydrocadambine, isocadamine | [110,111,112] |
| Mangifera indica | Mangiferin, flavonoids, phenolic acids, xanthones, gallic acid, catechins, kaempferol, carotenoids-luteoxanthine, zeaxantine, β-carotene, terpenoids- careen, myrcene, terpinoline, terpenoid saponins- indicoside A and B | [113,114] |
| Cocos nucifera | phenols, tannins, β-sitosterol, flavonoids, noctinic acid, folic acid, riboflavin, biotin, triterpenes, alkaloids, steroids, saponins, tannins, catechins, epicatechins | [115] |
| Tamarindus indica | phenolic compounds, β-sitosterol, proanthocyanins, apigenins tartaric acid, cardiac glycosides, mucilage, pectin, eicosanoic acid, β-sitosterol, palmitic acid, oleic acid, succininc acid, formic acid, β-amyrin, apigenin, epicatechins, catechins, taxifolin, eriodictoyl, naringenin | [116] |
| Punica granatum | flavonols, triterpenoids, fatty acids, organic acids- citric acid, malic acid, ascorbic acid, tannins- gallagic acid, punicalin, punicalagin, flavonoids-luteolin, quercetin, kaempferol, magnesium, alkaloids, catechin, gallic acid | [117] |
| Azadirachta indica | phenols, flavonoids, saponins, tannins, alkaloids, glycosides, carbohydrates, triterpenoids, β-sitosterol, ferulic acid | [87,88] |
| Costus speciosus | alkaloids, glycosides, flavonoids, steroids, polyphenols, tannins, β-sitosterol, gracillin, dioscin, diosgenin | [118] |
| Moringa oleifera | β-sitosterol, saponins, steroids, alkaloids- moringine and moringinine, flavonoids- rhamnetin, isoquercitrin, kaempferitrin, saccharides, phenolic acids, tannins, terpenoid, alpha-carotene | [119,120,121] |
| Andrographis paniculata | terpenoids, flavonoids, iridoids, ferulic acid, diterpenoid lactones, andrographolide, 14-deoxyandrographolide, 14-Deoxy-11,12-dehydroandrographolide, fersulic acids | [27] |
| Ficus benghalensis | anthocyanidin derivatives, aliphatic long-chain ketones, glycosides, flavonoids, amino acids, steroids, saponins, carbohydrates, tannins | [122,123] |
| Anacardium occidentale | phenolics, saponin, flavonoids, alkaloids, anthocyanidins, tannins, essential oils, glycosides, myricetin, pentoside, lactone, quercetin hexoside, canthones, chalcones, 4-hydroxydodecanoic acid, palmitate, sitosterol, stigmasterol, 3-O-βD-galactopyranoside | [23] |
| Annona squamosa | flavonoids, alkaloids, phenols, saponins, tannins, glycosides, diterpenes | [30] |
| Boerhaavia diffusa | steroids, ecdysteroid, alkaloids, lignan glycosides, phenolic glycosides, flavonoids, isoflavonoids, rotenoids | [96] |
| Catharanthus roseus | alkaloids, catharanthine, tetrahydroalstonine, vindoline, kaempferol, lochnerine, flavonoids | [124] |
| Cocculus hirsutus | β-sitosterol, ginnol, flavonoids such as luteolin, kaempferol and quercetin, glycosides, carbohydrates, tannins, saponins, steroids | [125] |
| Ficus hispida | flavonoids, saponins, steroids, glycosides, alkaloids, alkanes | [126,127] |
| Terminalia chebula | Β-sitosterol, flavonoids, tannins, sterols, gallic acid, chebulanin, corilagin, ellagic acid, chebulinic acid, amino acids, fructose, resin, triterpenoids, glycosides | [128] |
| Terminilia catappa | tannins, saponins, phenolics, flavonoids, triterpenoids, kaempferol, geraniin, punicalin, quercetin, gentisic acid, tercatain, tergallagin, β-carotene, cyanidin-3-glucoside, ellagic acid, gallic acid | [59,129] |
| Amaranthus tricolor | flavonoids, amino acids, alkaloids, carbohydrates, saponins, phenolic compounds, tannins, phytic acid | [19,20] |
| Blumea lacera | β-sitosterol, artemesinin, lupeol, β-caryophyllene, protocatechuic acid, alkaloids, flavonoids, tannins, terpenoids, flavones, triterpenes | [94] |
| Piper betle leaves | glycosides, quercitin, alkaloids, saponins, steroids, tannins, diterpenes, eugenol, chavibetol, flavonoids, hydroxychavicol | [130] |
| Achyranthes aspera | Alkaloids, oleanolic acid, saponins, D-Glucuronic Acid, dihydroxy ketones, quercitin, β-sitosterol, aliphatic alcohol, benzoquinone, hydroquinone, asarone and eugenol | [10] |
| Kalanchoe pinnata | β-sitosterol, flavonoids, kaempferol, quercetin, alkaloids, tannins, phenolic compounds, caffeic acid, syringic acid, luteolin, rutin, para-coumaric acid, ferulic acid, stigmaterol, astragalin, campesterol | [131] |
| Nelumbo nucifera | asteroidal triterpenoid, alkaloids, phenolic bases, flavonoids, quercetin, glycoside, kaempferol, nuciferin, roemerin, armepavine, β-sitosterol glucopyranoside | [132,133] |
| Mikania cordata | saponins, alkaloids, flavonoids, tannins, steroids | [134] |
| Wedelia chinensis | flavonoids, alkaloids, saponins, phytosterols, mucilage, tannins | |
| Murraya koenigii | carbazole alkaloids, mahanimbine, flavonoids, sterols, koenimbine, koenine, girinimbine | [135] |
| Aloe barbadensis | flavonoids, terpenoids, polysaccharides, pectins, hemicelluloses, glucomannan, sterols β-sitosterol, lupeol, tannins | [14] |
| Bryophyllum pinnatum | flavonoids, alkaloids, reducing sugars, tannins, bufadienolides, glycosaponins, polyphenols, steroidal glycosides | [136] |
| Agremone mexicana | Berberine, cheilanthifoline, coptisine, cryptopine, sanguinarine, stylopine, tetrahydroberberine, protopine, sanguinarine, dihydrosanguinarine, palmitic acid, oleic acid, myristic acid, linoleic acids, β-sitosterol, amino acids, fatty acids, tannin, saponins, flavonoids, phytosterols | [71,72,73] |
| Name of Plant | Solvent | Part of Plant | Model | Highest Safe Dose with No Toxicity (mg/kg Body Weight) | LD50 (Median Lethal Dose) (mg/kg Body Weight) | References |
|---|---|---|---|---|---|---|
| Ficus racemosa | Methanol | Bark | Normal and alloxan-induced diabetic rat | 3200 mg/kg | 3200 mg/kg | [158] |
| Asparagus racemosus | Water | Roots | Rats | 3200 mg/kg | [83] | |
| Bombax ceiba | Water | Flower | Wistar rats | 2000 mg/kg | [100] | |
| Cajanus cajan | Water, ethanol | Leaf | Sprague–Dawley rats | 6000 mg/kg | [139] | |
| Coccinia cordifolia | Water | Aerial parts | Wistar rats | 5000 mg/kg | [146] | |
| Momordica charantia | Ethanol | Fruit | Sprague–Dawley rats | 2000 mg/kg | [39] | |
| Syzygium cumini | Methanol, water | Bark, root, seed, leaf | Albino mice | 2000 mg/kg (seed extract-200 mg/kg) | [164] | |
| Neolamarckia cadama | Methanol | Bark | Mouse | 3000 mg/kg | [112] | |
| Mangifera indica | Water | Bark | Rat | >5000 mg/kg | [161] | |
| Cocos nucifera | Petroleum ether, chloroform, methanol | Leaf | Swiss albino rat | 2000 mg/kg | [150] | |
| Tamarindus indica | Water | Pulp | Albino rats | 4500 mg/kg | [165] | |
| Punica granatum | Ethanol | Whole fruit, seeds | Swiss albino mice | 2000 mg/kg | [166] | |
| Azadirachta indica | Ethanol | Leaf | Swiss albino mice | 2000 mg/kg | [90] | |
| Costus speciosus | Ethanol | Rhizome | Albino mice | 5000 mg/kg | [57] | |
| Moringa oleifera | Ethanol, water | Roots | Swiss albino mice | Ethanolic extract—1780 mg/kg; aqueous extract—1590 mg/kg | [167] | |
| Andrographis paniculata | Ethanol | Leaf | Wistar rats | 4000 mg/kg | [28] | |
| Ficus benghalensis | Methanol | Roots | Rats | >2000 mg/kg | [123] | |
| Anacardium occidentale | Water, ethanol | Leaf | Rats | 2000 mg/kg | [26] | |
| Annona squamosa | Methanol | Leaf | Rat | >5000 mg/kg | [33] | |
| Boerhaavia diffusa | Juice | Leaf | Mice | 5000 mg/kg | [98] | |
| Catharanthus roseus | Ethanol | Leaf | Wistar rat | 2000 mg/kg | [143] | |
| Cocculus hirsutus | Water, methanol | Root | Wistar albino rat | >3000 mg/kg | [147] | |
| Ficus hispida | Methanol | Fruit | Wistar albino rat | 1000 mg/kg | [156] | |
| Terminalia chebula | Water | Fruit | Rat | 5000 mg/kg | [168] | |
| Terminilia catappa | Water | Leaf | Sprague-Dawley rats | 3000 mg/kg | [169] | |
| Amaranthus tricolor | Water | Root | Wistar albino rat | 2000 mg/kg | [19] | |
| Blumea lacera | Methanol | Leaf | Rat | 5000 mg/kg | [95] | |
| Piper betle leaves | Methanol | Leaf | Mice | 5000 mg/kg | [170] | |
| Achyranthes aspera | Methanol | Leaf | Swiss albino mice | 8000 mg/kg | [13] | |
| Kalanchoe pinnata | Ethanol | Leaf | Wistar rat | >2000 mg/kg | [131] | |
| Nelumbo nucifera | Ethanol | Flower | Wistar rat | 2000 mg/kg | [63] | |
| Mikania cordata | Ethanol | Leaf | Rat | 2000 mg/kg | [64] | |
| Wedelia chinensis | Ethanol | Whole plant | Mice | >1600 mg/kg | [171] | |
| Murraya koenigii | Petroleum ether, chloroform, ethanol | Leaf | Rat | 2500 mg/kg | [172] | |
| Aloe barbadensis | Methanol | Gel | Rat | 2000 mg/kg | [15] | |
| Bryophyllum pinnatum | Ethanol, water | Aerial parts | Swiss albino mice | 2000 mg/kg | [138] | |
| Agremone mexicana | Aqueous slurry of root-bark powder | Root bark | Swiss albino mice | 7000 mg/kg | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanzabil, K.Z.; Hossain, M.S.; Hasan, M.K. Diabetes Mellitus Management: An Extensive Review of 37 Medicinal Plants. Diabetology 2023, 4, 186-234. https://doi.org/10.3390/diabetology4020019
Zanzabil KZ, Hossain MS, Hasan MK. Diabetes Mellitus Management: An Extensive Review of 37 Medicinal Plants. Diabetology. 2023; 4(2):186-234. https://doi.org/10.3390/diabetology4020019
Chicago/Turabian StyleZanzabil, Khwaja Zohura, Md. Sabbir Hossain, and Md. Kamrul Hasan. 2023. "Diabetes Mellitus Management: An Extensive Review of 37 Medicinal Plants" Diabetology 4, no. 2: 186-234. https://doi.org/10.3390/diabetology4020019
APA StyleZanzabil, K. Z., Hossain, M. S., & Hasan, M. K. (2023). Diabetes Mellitus Management: An Extensive Review of 37 Medicinal Plants. Diabetology, 4(2), 186-234. https://doi.org/10.3390/diabetology4020019