The Lipids and Volume in Satiation and Satiety (LIVES) Hypothesis: A Proposed Alternative Model for the Pathogenesis of Obesity
Abstract
:1. Introduction
2. Regulation of Dietary Energy Intake
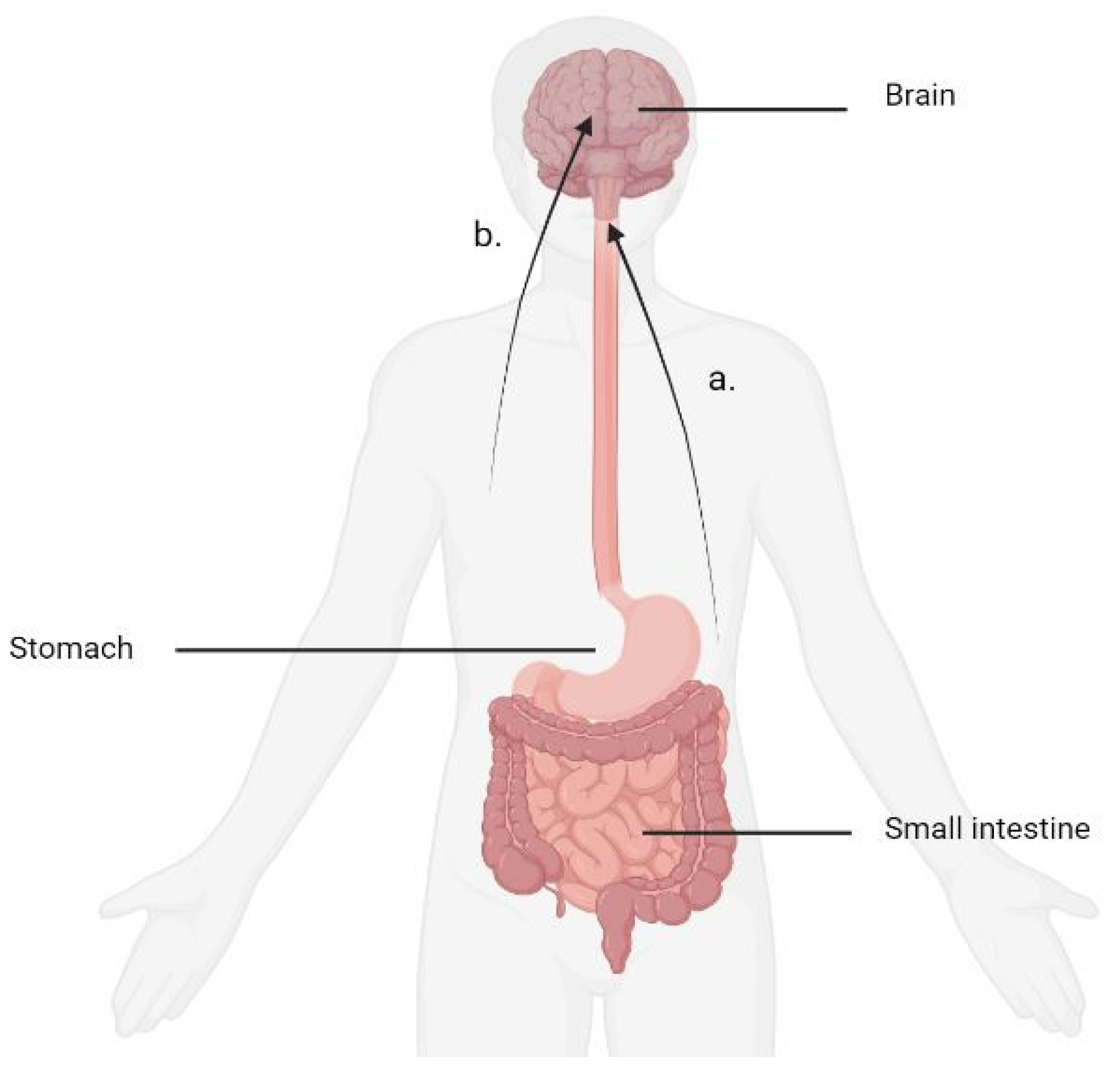
2.1. Satiation and Satiety
2.2. Physiological Regulation of Dietary Energy Intake
2.3. Biochemical Signalling
2.4. Macronutrients and Regulation of Dietary Intake
2.4.1. Protein and Dietary Energy Intake
2.4.2. Carbohydrate and Dietary Energy Intake
2.4.3. Fat and Dietary Energy Intake
3. Discussion
3.1. LIpids, Volume in Satiation and Satiety (LIVES) Hypothesis Proposal
3.2. Macronutrient Combinations-Satiation Phase
3.3. Macronutrient Combinations-Satiety Phase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeFronzo, R.A.; Abdul-Ghani, M. Type 2 diabetes can be prevented with early pharmacological intervention. Diabetes Care 2011, 34 (Suppl. S2), S202–S209. [Google Scholar]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar]
- Friedrich, M.J. Global Obesity Epidemic Worsening. JAMA 2017, 318, 603. [Google Scholar]
- WHO. Mean Fasting Blood Glucose. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/mean-fasting-blood-glucose-age-standardized-estimate (accessed on 24 December 2022).
- Lim, E.; Hollingsworth, K.; Aribisala, B.; Chen, M.; Mathers, J.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar]
- Taylor, R. Pathogenesis of type 2 diabetes: Tracing the reverse route from cure to cause. Diabetologia 2008, 51, 1781–1789. [Google Scholar]
- Gerstein, D.E.; Woodward-Lopez, G.; Evans, A.E.; Kelsey, K.; Drewnowski, A. Clarifying concepts about macronutrients’ effects on satiation and satiety. J. Am. Diet. Assoc. 2004, 104, 1151–1153. [Google Scholar]
- Benelam, B. Satiation, satiety and their effects on eating behaviour. Nutr. Bull. 2009, 34, 126–173. [Google Scholar]
- Berthoud, H.R. Vagal and hormonal gut–brain communication: From satiation to satisfaction. Neurogastroenterol. Motil. 2008, 20, 64–72. [Google Scholar]
- Neary, N.M.; Goldstone, A.P.; Bloom, S.R. Appetite regulation: From the gut to the hypothalamus. Clin. Endocrinol. 2004, 60, 153–160. [Google Scholar]
- Blevins, J.E.; Baskin, D.G. Hypothalamic-brainstem circuits controlling eating. Forum. Nutr. 2010, 63, 133–140. [Google Scholar]
- Berthoud, H.R. The vagus nerve, food intake and obesity. Regul. Pept. 2008, 149, 15–25. [Google Scholar]
- Moriarty, P.; Dimaline, R.; Thompson, D.G.; Dockray, G.J. Characterization of cholecystokininA and cholecystokininB receptors expressed by vagal afferent neurons. Neuroscience 1997, 79, 905–913. [Google Scholar]
- Ekblad, E.; Sundler, F. Distribution of pancreatic polypeptide and peptide YY. Peptides 2002, 23, 251–261. [Google Scholar]
- Sloth, B.; Holst, J.J.; Flint, A.; Gregersen, N.T.; Astrup, A. Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1062–E1068. [Google Scholar]
- Ueno, H.; Yamaguchi, H.; Mizuta, M.; Nakazato, M. The role of PYY in feeding regulation. Regul. Pept. 2008, 145, 12–16. [Google Scholar]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A. Gut hormone PYY3-36 physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar]
- Batterham, R.L.; Heffron, H.; Kapoor, S.; Chivers, J.E.; Chandarana, K.; Herzog, H.; Le Roux, C.W.; Thomas, E.L.; Bell, J.D.; Withers, D.J. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar]
- Batterham, R.L.; Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Withers, D.J.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Inhibition of food intake in obese subjects by peptide YY3–36. N. Engl. J. Med. 2003, 349, 941–948. [Google Scholar]
- Fu-Cheng, X.; Anini, Y.; Chariot, J.; Castex, N.; Galmiche, J.-P.; Rozé, C. Mechanisms of peptide YY release induced by an intraduodenal meal in rats: Neural regulation by proximal gut. Pflügers Arch. 1997, 433, 571–579. [Google Scholar]
- Higgins, S.C.; Gueorguiev, M.; Korbonits, M. Ghrelin, the peripheral hunger hormone. Ann. Med. 2007, 39, 116–136. [Google Scholar]
- Woods, S.C. Gastrointestinal Satiety Signals I. An overview of gastrointestinal signals that influence food intake. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 286, G7–G13. [Google Scholar]
- Murdolo, G.; Lucidi, P.; Di Loreto, C.; Parlanti, N.; De Cicco, A.; Fatone, C.; Fanelli, C.G.; Bolli, G.B.; Santeusanio, F.; De Feo, P. Insulin is Required for Prandial Ghrelin Suppression in Humans. Diabetes 2003, 52, 2923–2927. [Google Scholar]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Rodin, J.; Wack, J.; Ferrannini, E.; DeFronzo, R.A. Effect of insulin and glucose on feeding behavior. Metabolism 1985, 34, 826–831. [Google Scholar]
- Lavin, J.H.; Wittert, G.; Sun, W.M.; Horowitz, M.; Morley, J.E.; Read, N.W. Appetite regulation by carbohydrate: Role of blood glucose and gastrointestinal hormones. Am. J. Physiol.-Endocrinol. Metab. 1996, 271, E209–E214. [Google Scholar]
- Miller, S.; Petocz, P. Interrelationships among postprandial satiety, glucose and insulin responses and changes in subsequent food intake. Eur. J. Clin. Nutr. 1996, 50, 788–797. [Google Scholar]
- Yutaka, S.; Mitsuo, F.; Daisuke, Y. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar]
- Holst, J.J. Incretin hormones and the satiation signal. Int. J. Obes. 2013, 37, 116. [Google Scholar]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar]
- Zander, M.; Madsbad, S.; Madsen, J.L.; Holst, J.J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: A parallel-group study. Lancet 2002, 359, 824–830. [Google Scholar]
- Flint, A.; Raben, A.; Astrup, A.; Holst, J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Investig. 1998, 101, 515–520. [Google Scholar]
- Gutzwiller, J.; Göke, B.; Drewe, J.; Hildebrand, P.; Ketterer, S.; Handschin, D.; Winterhalder, R.; Conen, D.; Beglinger, C. Glucagon-like peptide-1: A potent regulator of food intake in humans. Gut 1999, 44, 81–86. [Google Scholar]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar]
- Verdich, C.; Toubro, S.; Buemann, B.; Lysgård Madsen, J.; Juul Holst, J.; Astrup, A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—Effect of obesity and weight reduction. Int. J. Obes. 2001, 25, 1206–1214. [Google Scholar]
- Vozzo, R.; Baker, B.; Wittert, G.A.; Wishart, J.M.; Morris, H.; Horowitz, M.; Chapman, I. Glycemic, hormone, and appetite responses to monosaccharide ingestion in patients with type 2 diabetes. Metab. -Clin. Exp. 2002, 51, 949–957. [Google Scholar]
- Gautier, J.F.; Choukem, S.P.; Girard, J. Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes. Diabetes Metab. 2008, 34, S65–S72. [Google Scholar]
- Rehfeld, J.F. Cholecystokinin. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 569–586. [Google Scholar]
- Gibbs, J.; Young, R.C.; Smith, G.P. Cholecystokinin decreases food intake in rats. J. Comp. Physiol. Psychol. 1973, 84, 488–495. [Google Scholar]
- Liddle, R.A.; Goldfine, I.D.; Rosen, M.S.; Taplitz, R.A.; Williams, J.A. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J. Clin. Investig. 1985, 75, 1144–1152. [Google Scholar]
- Koulischer, D.; Moroder, L.; Deschodt-Lanckman, M. Degradation of cholecystokinin octapeptide, related fragments and analogs by human and rat plasma in vitro. Regul. Pept. 1982, 4, 127–139. [Google Scholar]
- Rehfeld, J.F.; Sennels, H.P.; Jørgensen, H.L.; Fahrenkrug, J. Circadian variations in plasma concentrations of cholecystokinin and gastrin in man. Scand. J. Clin. Lab. Investig. 2020, 80, 546–551. [Google Scholar]
- Geary, N. Endocrine controls of eating: CCK, leptin, and ghrelin. Physiol. Behav. 2004, 81, 719–733. [Google Scholar]
- Schwizer, W.; Borovicka, J.; Kunz, P.; Fraser, R.; Kreiss, C.; D’Amato, M.; Fried, M. Role of cholecystokinin in the regulation of liquid gastric emptying and gastric motility in humans: Studies with the CCK antagonist loxiglumide. Gut 1997, 41, 500–504. [Google Scholar]
- Gilliam-Vigh, H.; Jorsal, T.; Rehfeld, J.F.; Pedersen, J.; Poulsen, S.S.; Vilsbøll, T.; Knop, F.K. Expression of Cholecystokinin and its Receptors in the Intestinal Tract of Type 2 Diabetes Patients and Healthy Controls. J. Clin. Endocrinol. Metab. 2021, 106, 2164–2170. [Google Scholar]
- Noble, F.; Wank, S.A.; Crawley, J.N.; Bradwejn, J.; Seroogy, K.B.; Hamon, M.; Roques, B.P. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharm. Rev. 1999, 51, 745–781. [Google Scholar]
- Kissileff, H.R.; Pi-Sunyer, F.X.; Thornton, J.; Smith, G.P. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am. J. Clin. Nutr. 1981, 34, 154–160. [Google Scholar]
- Muurahainen, N.E.; Kissileff, H.R.; Lachaussee, J.; Pi-Sunyer, F.X. Effect of a soup preload on reduction of food intake by cholecystokinin in humans. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1991, 260, R672–R680. [Google Scholar]
- Lieverse, R.J.; Jansen, J.B.M.J.; van de Zwan, A.; Samson, L.; Masclee, A.A.M.; Lamers, C.B.H.W. Effects of a physiological dose of cholecystokinin on food intake and postprandial satiation in man. Regul. Pept. 1993, 43, 83–89. [Google Scholar]
- Schwartz, G.J.; Berkow, G.; McHugh, P.R.; Moran, T.H. Gastric branch vagotomy blocks nutrient and cholecystokinin-induced suppression of gastric emptying. Am. J. Physiol. 1993, 3 Pt 2, R630–R637. [Google Scholar]
- Schwartz, G.J.; McHugh, P.R.; Moran, T.H. Gastric loads and cholecystokinin synergistically stimulate rat gastric vagal afferents. Am. J. Physiol 1993, 4 Pt 2, R872–R876. [Google Scholar]
- Melton, P.M.; Kissileff, H.R.; Pi-Sunyer, F.X. Cholecystokinin (CCK-8) affects gastric pressure and ratings of hunger and fullness in women. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1992, 263, R452–R456. [Google Scholar]
- Blundell, J.E.; MacDiarmid, J.I. Passive Overconsumption Fat Intake and Short-Term Energy Balancea. Ann. New York Acad. Sci. 1997, 827, 392–407. [Google Scholar]
- Gautron, L.; Elmquist, J.K. Sixteen years and counting: An update on leptin in energy balance. J. Clin. Investig. 2011, 121, 2087–2093. [Google Scholar]
- Klok, M.D.; Jakobsdottir, S.; Drent, M. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar]
- Latner, J.D.; Schwartz, M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite 1999, 33, 119–128. [Google Scholar]
- Lejeune, M.P.; Westerterp, K.R.; Adam, T.C.; Luscombe-Marsh, N.D.; Westerterp-Plantenga, M.S. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am. J. Clin. Nutr. 2006, 83, 89–94. [Google Scholar]
- Porrini, M.; Santangelo, A.; Crovetti, R.; Riso, P.; Testolin, G.; Blundell, J.E. Weight, protein, fat, and timing of preloads affect food intake. Physiol. Behav. 1997, 62, 563–570. [Google Scholar]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar]
- Morell, P.; Fiszman, S. Revisiting the role of protein-induced satiation and satiety. Food Hydrocoll. 2017, 68, 199–210. [Google Scholar]
- Holt, S.H.; Brand Miller, J.C.; Petocz, P.; Farmakalidis, E. A satiety index of common foods. Eur. J. Clin. Nutr. 1995, 49, 675–690. [Google Scholar]
- Journel, M.; Chaumontet, C.; Darcel, N.; Fromentin, G.; Tomé, D. Brain responses to high-protein diets. Adv. Nutr. 2012, 3, 322–329. [Google Scholar]
- Tannous dit El Khoury, D.; Obeid, O.; Azar, S.T.; Hwalla, N. Variations in Postprandial Ghrelin Status following Ingestion of High-Carbohydrate, High-Fat, and High-Protein Meals in Males. Ann. Nutr. Metab. 2006, 50, 260–269. [Google Scholar]
- Stubbs, J.; Ferres, S.; Horgan, G. Energy density of foods: Effects on energy intake. Crit. Rev. Food Sci. Nutr. 2000, 40, 481–515. [Google Scholar]
- Booth, D.A.; Nouwen, A. Satiety. No way to slim. Appetite 2010, 55, 718–721. [Google Scholar]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar]
- Clark, M.J.; Slavin, J.L. The effect of fiber on satiety and food intake: A systematic review. J. Am. Coll. Nutr. 2013, 32, 200–211. [Google Scholar]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar]
- Wanders, A.J.; van den Borne, J.J.; de Graaf, C.; Hulshof, T.; Jonathan, M.C.; Kristensen, M.; Mars, M.; Schols, H.A.; Feskens, E.J. Effects of dietary fibre on subjective appetite, energy intake and body weight: A systematic review of randomized controlled trials. Obes. Rev. 2011, 12, 724–739. [Google Scholar]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar]
- Burton-Freeman, B. Dietary fiber and energy regulation. J. Nutr. 2000, 130, 272S–275S. [Google Scholar]
- Johnson, L.; Mander, A.P.; Jones, L.R.; Emmett, P.M.; Jebb, S.A. Energy-dense, low-fiber, high-fat dietary pattern is associated with increased fatness in childhood. Am. J. Clin. Nutr. 2008, 87, 846–854. [Google Scholar]
- Howarth, N.C.; Huang, T.T.-K.; Roberts, S.B.; McCrory, M.A. Dietary fiber and fat are associated with excess weight in young and middle-aged US adults. J. Am. Diet. Assoc. 2005, 105, 1365–1372. [Google Scholar]
- Dickman, S.R. Why we store fat. Am. Sci. 1958, 46, 285–293. [Google Scholar]
- Krauss, R.M.; Deckelbaum, R.J.; Ernst, N.; Fisher, E.; Howard, B.V.; Knopp, R.H.; Kotchen, T.; Lichtenstein, A.H.; McGill, H.C.; Pearson, T.A. Dietary guidelines for healthy American adults. Circulation 1996, 94, 1795–1800. [Google Scholar]
- Aranceta, J.; Pérez-Rodrigo, C. Recommended dietary reference intakes, nutritional goals and dietary guidelines for fat and fatty acids: A systematic review. Br. J. Nutr. 2012, 107 (Suppl. S2), S8–S22. [Google Scholar]
- Cordain, L.; Miller, J.B.; Eaton, S.B.; Mann, N.; Holt, S.H.; Speth, J.D. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am. J. Clin. Nutr. 2000, 71, 682–692. [Google Scholar]
- O’keefe, J.H.; Cordain, L. Cardiovascular disease resulting from a diet and lifestyle at odds with our Paleolithic genome: How to become a 21st-century hunter-gatherer. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands; pp. 101–108.
- Blundell, J.E. What foods do people habitually eat? A dilemma for nutrition, an enigma for psychology. Am. J. Clin. Nutr. 2000, 71, 3–5. [Google Scholar]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar]
- Misra, A.; Singhal, N.; Khurana, L. Obesity, the Metabolic Syndrome, and Type 2 Diabetes in Developing Countries: Role of Dietary Fats and Oils. J. Am. Coll. Nutr. 2010, 29 (Suppl. S3), 289S–301S. [Google Scholar]
- Austin, G.L.; Ogden, L.G.; Hill, J.O. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am. J. Clin. Nutr. 2011, 93, 836–843. [Google Scholar]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar]
- West, D.B.; York, B. Dietary fat, genetic predisposition, and obesity: Lessons from animal models. Am. J. Clin. Nutr. 1998, 67, 505S–512S. [Google Scholar]
- Bray, G.A.; Paeratakul, S.; Popkin, B.M. Dietary fat and obesity: A review of animal, clinical and epidemiological studies. Physiol. Behav. 2004, 83, 549–555. [Google Scholar]
- Astrup, A. The Role of Dietary Fat in Obesity. In Seminars in Vascular Medicine; Thieme Medical Publishers Inc.: New York, NY, USA, 2005. [Google Scholar]
- Ravussin, E.; Tataranni, P.A. Dietary Fat and Human Obesity. J. Am. Diet. Assoc. 1997, 97 (Suppl. S7), S42–S46. [Google Scholar]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar]
- Blundell, J.E.; Burley, V.J.; Cotton, J.R.; Lawton, C.L. Dietary fat and the control of energy intake: Evaluating the effects of fat on meal size and postmeal satiety. Am. J. Clin. Nutr. 1993, 57, 772S–777S. [Google Scholar]
- Blundell, J.E.; MacDiarmid, J.I. Fat as a risk factor for overconsumption: Satiation, satiety, and patterns of eating. J. Am. Diet. Assoc. 1997, 97, S63–S69. [Google Scholar]
- Rolls, B.J.; Bell, E.A.; Castellanos, V.H.; Chow, M.; Pelkman, C.L.; Thorwart, M.L. Energy density but not fat content of foods affected energy intake in lean and obese women. Am. J. Clin. Nutr. 1999, 69, 863–871. [Google Scholar]
- Warrilow, A.; Mellor, D.; McKune, A.; Pumpa, K. Dietary fat, fibre, satiation, and satiety—A systematic review of acute studies. Eur. J. Clin. Nutr. 2018, 73, 333–344. [Google Scholar]
- Powley, T.L.; Phillips, R.J. Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol. Behav. 2004, 82, 69–74. [Google Scholar]
- Warrilow, A.; Turner, M.; Naumovski, N.; Somerset, S. Role of cholecystokinin in satiation: A systematic review and meta-analysis. Br. J. Nutr. 2022, 1–25. [Google Scholar] [CrossRef]
- Williams, D.L.; Cummings, D.E.; Grill, H.J.; Kaplan, J.M. Meal-Related Ghrelin Suppression Requires Postgastric Feedback. Endocrinology 2003, 144, 2765–2767. [Google Scholar]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of Fat on Gastric Emptying of and the Glycemic, Insulin, and Incretin Responses to a Carbohydrate Meal in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar]
- Hellström, P.M.; Samuelsson, B.; Al-Ani, A.N.; Hedström, M. Normal gastric emptying time of a carbohydrate-rich drink in elderly patients with acute hip fracture: A pilot study. BMC Anesth. 2017, 17, 23. [Google Scholar]
- Celiker, H. A new proposed mechanism of action for gastric bypass surgery: Air hypothesis. Med. Hypotheses 2017, 107, 81–89. [Google Scholar]
- Chan, J.L.; Mun, E.C.; Stoyneva, V.; Mantzoros, C.S.; Goldfine, A.B. Peptide YY levels are elevated after gastric bypass surgery. Obesity 2006, 14, 194–198. [Google Scholar]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar]
- Lindström, J.; Peltonen, M.; Eriksson, J.G.; Louheranta, A.; Fogelholm, M.; Uusitupa, M.; Tuomilehto, J. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: The Finnish Diabetes Prevention Study. Diabetologia 2006, 49, 912–920. [Google Scholar]
- Schulz, M.; Nöthlings, U.; Hoffmann, K.; Bergmann, M.M.; Boeing, H. Identification of a food pattern characterized by high-fiber and low-fat food choices associated with low prospective weight change in the EPIC-Potsdam cohort. J. Nutr. 2005, 135, 1183–1189. [Google Scholar]
- Pastorino, S.; Richards, M.; Pierce, M.; Ambrosini, G.L. A high-fat, high-glycaemic index, low-fibre dietary pattern is prospectively associated with type 2 diabetes in a British birth cohort. Br. J. Nutr. 2016, 115, 1632–1642. [Google Scholar]
| Macronutrient Combination | Satiation Phase | Satiety Phase | Outcome |
|---|---|---|---|
| Fat + F&V 1 | CCK 2 signalling optimised with F&V. | PYY 3 signal not optimised due to energy intake; rapid emptying of carbohydrate. | Non-excessive. |
| Fat and Protein | Strong satiation signal due to the action of CCK but potential for energy intake to be excessive depending on fat content. | PYY signal optimised due to slow gastric emptying during PYY release and resultant volume signal from the stomach. | Non-excessive energy intake. |
| Fat and N-F&V 4 | CCK signal not optimised with N-F&V. | PYY signal not optimised due to rapid gastric emptying of carbohydrate. | Excessive energy intake. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warrilow, A.; Pumpa, K.; Somerset, S.; Naumovski, N. The Lipids and Volume in Satiation and Satiety (LIVES) Hypothesis: A Proposed Alternative Model for the Pathogenesis of Obesity. Diabetology 2023, 4, 64-75. https://doi.org/10.3390/diabetology4010008
Warrilow A, Pumpa K, Somerset S, Naumovski N. The Lipids and Volume in Satiation and Satiety (LIVES) Hypothesis: A Proposed Alternative Model for the Pathogenesis of Obesity. Diabetology. 2023; 4(1):64-75. https://doi.org/10.3390/diabetology4010008
Chicago/Turabian StyleWarrilow, Andrew, Kate Pumpa, Shawn Somerset, and Nenad Naumovski. 2023. "The Lipids and Volume in Satiation and Satiety (LIVES) Hypothesis: A Proposed Alternative Model for the Pathogenesis of Obesity" Diabetology 4, no. 1: 64-75. https://doi.org/10.3390/diabetology4010008
APA StyleWarrilow, A., Pumpa, K., Somerset, S., & Naumovski, N. (2023). The Lipids and Volume in Satiation and Satiety (LIVES) Hypothesis: A Proposed Alternative Model for the Pathogenesis of Obesity. Diabetology, 4(1), 64-75. https://doi.org/10.3390/diabetology4010008









