Abstract
Introduction: Metabolic Syndrome (MS) is a pathologic condition characterized by Type 2 diabetes mellitus (T2DM), insulin resistance, abdominal obesity, hypertension, and hyperlipidemia. Until now, specific drugs such as metformin (MET) have been used to address its individual components; however, according to the recommendation of WHO, various plant extracts might be used as alternative medicines due to the side effects of pharmacologic agents. Policaptil Gel Retard® (PGR), a macromolecule complex based on polysaccharides which slows down the absorption rates of carbohydrates and fats, proved effective against glucose abnormalities. Our study aimed to verify the short-term efficacy and safety of PGR under real-life conditions. Methods: We evaluated both the 6-month changes in metabolic parameters in Italian patients with MS and T2DM, and the 10-year CV risk score (10-y-CV-RS) from the CUORE equation, competitively randomized to Policaptil Gel Retard (2172 mg before each main meal); Group A, n = 75, or Metformin (1500–2000 mg/day equally divided between the two main meals), and Group B, n = 75. Results: Fasting plasma glucose and HbA1c decreased significantly and similarly (p < 0.001) in the two groups. A significant decrease in BMI (−20% in the PGR group (p < 0.01), −14.3% in the MET group (p < 0.05)), % visceral fat, and UA levels was also apparent in both groups (p < 0.01). The opposite occurred for lipid profile, which improved significantly in the PGR group but remained unchanged in the MET group. Consequently, only the PGR group experienced a significant decrease in the 10-y-CV-RS (31.4 ± 8.0 vs. 19.7 ± 5.2, p < 0.0001), whereas this remained unchanged in the MET group (32.2 ± 3.3 vs. 30.5 ± 8.7; p n.s.). Conclusions: PGR could represent a suitable alternative to MET as a first-line treatment option, especially now that an ever-increasing number of people prefer natural products based on plant extracts. This is particularly pertinent given that, besides trying to avoid gastrointestinal side-effects as much as possible, patients might be sensitive to ecotoxicology-related problems involving plants and animals caused by the worldwide spread of environmental MET metabolites.
1. Introduction
The prevalence of metabolic syndrome (MS) has risen for the last two decades and is now approaching global epidemic proportions [1,2]. Metabolic Syndrome is a pathologic condition characterized by Type 2 diabetes, insulin resistance, abdominal obesity, hypertension, and hyperlipidemia [3]. Due to the high mortality and morbidity of metabolic syndrome, it is essential that these diseases are prevented or managed. Lifestyle changes, including adequate exercise and a balanced diet, often prove effective in stopping the progression of and treating metabolic syndrome. Nevertheless, much of the published data indicate that it is difficult to change lifestyle permanently and achieve and maintain weight loss for a long time.
Besides, suitable pharmacological and non-pharmacological treatment strategies are necessary for managing metabolic syndrome, including fibrates, lipid-lowering drugs, and anti-diabetes and anti-gout agents. The WHO recommend that various plant extracts might be alternative medicines due to the side effects of chemical drugs [4].
Policaptil Gel Retard® (PGR) is a new macromolecule complex based on polysaccharides which slows down the absorption rates of carbohydrates and fats. Recent studies indicate that, when used in conjunction with a low glycemic index diet (LGID), PGR significantly reduces acanthosis nigricans expression, HbA1c levels, and glucose metabolism abnormalities, such as impaired glucose tolerance (IGT) and Type 2 Diabetes Mellitus (T2DM) in obese patients [5,6]. The effect of PGR is related to a reduction in post-meal glycemic and insulinemic peaks [7].
The attenuated pancreatic insulin response is likely due to slowed absorption rates of glucose absorption, given that the latter directly regulates pancreatic insulin release [6,7,8]. Metformin is the most used, first-step hypoglycemic, oral medication for T2DM [9], and is often used off-label as a preventive strategy against any insulin-resistance states, including obesity. In children and adolescents with MS [8], Metformin + PGR treatment induced a further reduction in BMI, HbA1c, and HOMA-IR index, as well as Matsuda disposition and insulinogenic indices compared to Metformin alone [9]. More recently, in adults with MS, we achieved similar glucometabolic results with PGR and Metformin and a better lipid profile with PGR than Metformin. However, the gastrointestinal side effects study aimed to verify the short-term efficacy and safety of PGR under real-life conditions in an attempt to provide appropriate answers to the need for adequate care for MS. To do that, we evaluated 6-month changes in metabolically relevant parameters in patients with MS and T2DM, as well as the 10-year CV risk score (10-y-CV-RS) from the Italian CUORE equation [10,11,12,13,14], which fits the population under study better than others based on populations characterized by different eating habits, lifestyles, and CV mortality rates.
2. Methods
The present report is a randomized, double-blind comparison study carried out in an outpatient setting. Randomization was done by the generation of random numbers by the website www.randomizer.org (accessed on 15 July 2020), attributing treatment PGR to even numbers and treatment Metformin to odd numbers. The two products being compared were contained in perfectly identical boxes containing a code number and detailed instructions on how to take the tablets.
We analyzed a series of consecutive outpatients with MS and T2DM who met the inclusion criteria and were followed up by a network of eight outpatient diabetes centers (DCs) from the University Hospital of Naples “Luigi Vanvitelli” (coordinator center) and the Nefrocenter Research network.
The study was carried out in compliance with good clinical practice standards and the ethical guidelines of the 1964 Declaration of Helsinki and its subsequent amendments. The University of Campania “Luigi Vanvitelli” served as the central reference Ethical Committee (EC) for the participating diabetes centers, which all belonged to the same private consortium associated with the University mentioned above. The EC approved the present study on 30 June 2019, with the registration number n. 1287/bis as an extension of a previously approved protocol (reference n.10, Protocol registration trial n.1287, 23 June 2019) which had been performed on different patients, and later by the Institutional Review Board (IRB Min. no. 10,712, dated 5 June 2020). The study participants were different from those enrolled in another recent investigation of ours which had a similar experimental design but different endpoints [10]. The study participants’ data were processed anonymously according to good clinical practice guidelines. Informed consent was obtained from all subjects involved in the study.
By participating in the so-called Associazione Medici Diabetologi (AMD) Annals Initiative, all Centers involved shared the same organizational structure, adopted the same software for everyday outpatient management, and were documented to attain the same performance levels. They were therefore considered to be part of a single institution [15]. A dedicated software package (AMD Data File) allowed data extraction for further analysis [16].
The diagnosis of MS was made/confirmed according to the criteria proposed by the World Heart Federation and the International Association for the Study of Obesity [17].
The International Classification of Diseases, Clinical Modification (ICD-9-CM, V82.9 2014) was used to assign T2DM comorbidities/complications to individual subjects [18]. The diagnosis of type 2 diabetes was made/confirmed according to the ADA Standards of Medical Care in Diabetes 2021 criteria [19].
The CUORE project was an epidemiological and ischemic heart disease prevention project launched in 1998 and validated in non-pregnant persons aged 35 to 69 without previous major cardiovascular accidents [11]. The individual 10-year CV risk score (10-y CVrs) was assessed using the CUORE project calculator (http://www.cuore.iss.it/altro/cuore (accessed on 15 July 2020) to estimate the probability of experiencing any CV events (mainly myocardial infarction or stroke) for the first time over the next ten years based on eight CV risk factors, including age, gender, systolic BP (SBP), TC, HDL-C, diabetes mellitus, smoking habit, and use of antihypertensive medications [11,12,13,14]. It cannot be used in case of extreme risk factor values, including systolic blood pressure (SBP) higher than 200 mmHg or less than 90 mmHg, TC higher than 320 mg/dL or less than 130 mg/dL, HDL less than 20 mg/dL or higher than 100 mg/dL.
The sample sizes were estimated by using a single proportion formula and calculated as follows: n = Z2Pq/d2 = 68
Z: 1.96 (standard score for the 95% confidence level)
P: the highest percentage of population MS prevalence in Italy in 2015 [20]
q: percentage of failure (1 − P)
d: 5% (proportion of sampling error)
Due to possible expected drop-out, we increased the sample to 75 patients per treatment group. Keeping in mind the CUORE risk calculator’s requirements, the enrollment criteria were defined as follows:
- MS (defined according to the consensus document 2009) [17];
- Age > 39 and <69 years;
- Body mass index (BMI) > 30 kg/m2;
- No previous major CV events (MACE);
- T2DM, known for no more than 1 year (±0.5) (ADA criteria 2021) [19];
- Altered lipid profile (TC ≥ 200 mg/dL, LDL- C ≥ 100 mg/dL);
- Reliability (visiting the clinic regularly);
- Acceptance of informed consent;
- Normal estimated glomerular filtration rate (eGFR) (60–90 mL/min/1.73 m2);
- No micro-macro-albuminuria.
- Exclusion criteria:
- Blood pressure and plasma lipid levels exceeding the above-mentioned range applicable to the individual CV risk calculator of the Heart Project;
- Previous bariatric or coronary surgery interventions;
- Pregnancy or breastfeeding;
- Disabling conditions, severe liver, kidney or neoplastic diseases, dementia and/or inability to regularly comply with prescriptions;
- Known hypersensitivity/intolerance to treatment or history of drug allergy or known allergic disease;
- Irritable bowel disease or dyspepsia.
Informed consent was obtained from all subjects involved in the study.
After a 12-week-run-in period, the patients were competitively randomized among all participating centers to Policaptil Gel Retard (2350 mg/sachet twice a day, taken 30 min before each main meal), Group A, n = 75; or Metformin (2000 mg/day equally divided between the two main meals to minimize side effects), Group B, n = 75 (see Figure 1). Metformin was individually titrated during the follow-up to its maximal tolerated doses (1500 to 2000 mg a day), which was not necessary for any patient, so everyone took 2000 mg/day.
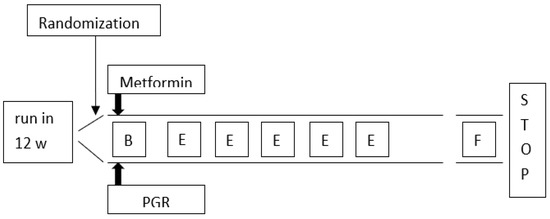
Figure 1.
Flow chart of the study protocol. B = baseline evaluation: clinical records, anthropometry, biochemistry, diet, physical activity, prescription, education; E = educational reinforcement on diet, physical activity, treatment adherence: weekly call; F = final evaluation at the end of 24-week follow-up: evaluations were same as at baseline; w = wick; PGR = Policaptil Gel Retard.
The parameters under study included: (i) anthropometry: body mass index (BMI), waist circumference (WC), and visceral fat percentage (% VF); (ii) blood chemistry: Tg, TC, LDL- C, HDL-C, AST, ALT, gamma-GT, and alkaline phosphatase; (iii) adherence to treatment + count of pills/sachets found in the drug packs used during each study period; (iv) side effects (specific Q sections); (v) adverse toxic effect: clinical history, smoking habits + circulating AST, ALT, gamma-GT, alkaline phosphatase, and blood creatinine levels.
All blood tests were performed using the high-standard automatic biochemistry analyzers in laboratories successfully participating in nationwide quality control programs.
All the subjects had their 10-year individual CV risk score calculated according to the “Progetto Cuore” [11,12,13,14] at baseline and the end of follow-up (see the Supplementary Material, Table S1).
All parameters were measured at baseline (T-0) and after three (T-3) and six months (T-6), respectively. Visceral fat was measured with Body Metrix BX2000, IntelaMetrix, Inc. Brentwood, CA, USA), a validated instrument based on ultrasound technology, allowing direct and immediate measurement of subcutaneous fat thickness to avoid inconvenient and operator-dependent manual plication maneuvers [21].
During the study, all patients received a low-calorie diet (20–25% less than calories required to maintain current weight) varying in percentages of proteins (10–20%), fat (20–30%, saturated ones being less than 10%), and carbohydrates (50–60%, sucrose being less than 5%). A specifically trained diet specialist prepared dietary regimens to manage overweight/obese people with T2DM to meet each patient’s wishes, tastes, and needs. All subjects completed a daily logbook by recording food changes regarding prescriptions and the number of daily tablets/sachets to verify adherence and any side effects throughout the whole treatment period. Diet adherence allowed the rate of nutritional advice breaches in excess carbohydrates and calories to be checked.
Treatment adherence was assessed by counting the missing pills (Metformin)/sachets (PGR) from the drug packs used by the patients (>80% adherence to treatment being accepted), and treatment undesirable effects were monitored through questionnaires completed by patients at the end of the study, as previously described (10). Patients were encouraged to perform daily aerobic physical activity, e.g., walking, for at least 3 METs (Metabolic Equivalent of Task).
The side effect logbook section consisted of ten dichotomic questions (yes/no) concerning gastrointestinal disorders. The diary mentioned above had been previously tested for validity and reliability in a sample of 10 healthcare workers by verifying the concordance of the answers given three times in two weeks by the same subject (mean concordance being 95 ± 5%), as previously described [22]. During follow-up, all patients received weekly motivational phone support on diet, physical activity, and treatment adherence.
Statistical Analysis. Data are presented as mean values ± standard deviation (M ± SD). Categorical variables are given as frequencies and percentages. Repeated measures ANOVA was applied for intergroup and intragroup comparisons. p values < 0.05 were considered statistically significant. All analyses were performed using the STATA software, version 14 (Stata-Corp LP, College Station, TX, USA).
3. Results
Seventy-two subjects from Group A and 70 from Group B completed the follow-up (Table 1). Drop-outs occurred because of town change (three cases) or referral to closer DCUs (five cases). Adherence to physical activity and diet was as high as 85% for nutritional prescriptions on 89% of treatment days in all patients and did not differ between groups. Similarly, 89% of subjects from the two groups were responsive to treatment, while in the remaining 11% the parameters under study improved only slightly and non-significantly. General side effects were mild and similar across groups, ranging between 1% and 3%, and including drowsiness, acid regurgitation, post-prandial nausea/vomiting, itching, headache, dizziness and/or fainting, cold sweat with/without hunger pangs, palpitations, and tachycardia. However, gastrointestinal side effects were more frequent in Group B than in Group A, including meteorism (3.5% vs. 20.6%; p < 0.05), flatulence (4.4% vs. 22.2%; p < 0.05), diarrhea (0% vs. 8.3%; p < 0.05), and long and tiring digestion (1.0% vs. 8.3%; p < 0.05). In general, side effects tended to resolve or decline spontaneously during treatment. Slight percent variations in biochemical safety parameters were within the reference range, ranging 0.2–0.5%, and were superimposable between groups during the study period.

Table 1.
Baseline clinically relevant parameters from all participants. As shown, no significant differences were apparent between the two groups. Absolute, percent, or M ± SD values are reported, as appropriate.
Table 1 reports general parameters of all participants, being virtually superimposable between groups, especially in terms of gender and smoking habits. Because of this, we refrained from classifying CVR scores based on those two conditions. Likewise, other CVR factors, including % visceral fat, hypertension, and familial history of premature heart disease, were similar in the two groups.
Table 2 reports parameters contributing to the individual 10-year CVR score as recorded immediately before and six months after PGR or MET treatment onset. It clearly shows that BMI, % visceral fat, fasting plasma glucose, HbA1c, and UA levels decreased significantly (p < 0.05 to <0.001) in both groups (p < 0.01) (Figure 2). The opposite occurred in the case of the lipid profile, which improved significantly in the PGR group while remaining unchanged in the MET group. Consequently, only the PGR group experienced a significant decrease in the 10-y-CV-RS, which, conversely, remained virtually unchanged in the MET group.

Table 2.
Parameters contributing to the calculation of the individual risk of cardio-vascular events at 10 years, and inter-group and intra-group differences. Other metabolic parameters are reported.
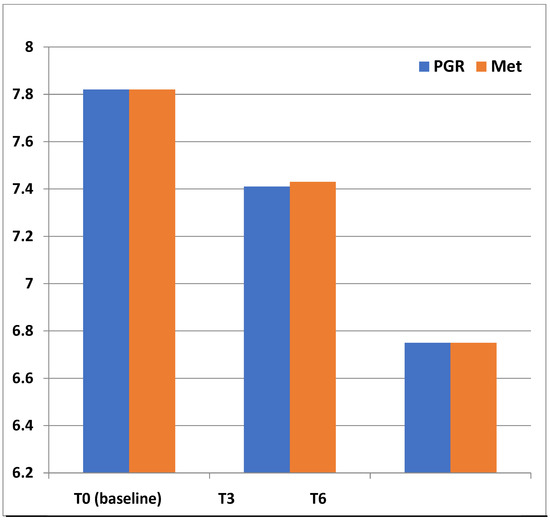
Figure 2.
Superimposable HbA1c (%) changes between treatment groups during follow-up. (T + 3 vs. baseline p < 0.001; T6 vs both T3 and T0 (baseline) p < 0.001).
Table 3 refers to the Multivariate Cox Regression Analysis of all parameters contributing to the 10-y-CV-RS, including LDL-Cholesterol, BMI, Visceral fat, HbA1c, and Uric Acid.

Table 3.
Multivariate Cox Regression Analysis of all parameters contributing to the Final 10-y-CV-RS (10-y Cardiovascular risk score) in our population, and of all other parameters commonly involved in CV risk (CVR). As expected, despite all being significantly associated with CVR increase, lipids and visceral fat were even more so.
Figure 3 classifies 10-y-CV-RS by group and total cholesterol levels below and above 200 mg/dL: 57 subjects were below such cutoff at baseline vs. 11 a T6 in the PGR group, against the 66 at baseline vs. 62 a T6 recorded in the MET group. Moreover, the PGR group displayed a significant risk decrease at baseline and even more so at T6 vs. the MET group, which kept constant 10-y-CV-RS levels during follow-up.
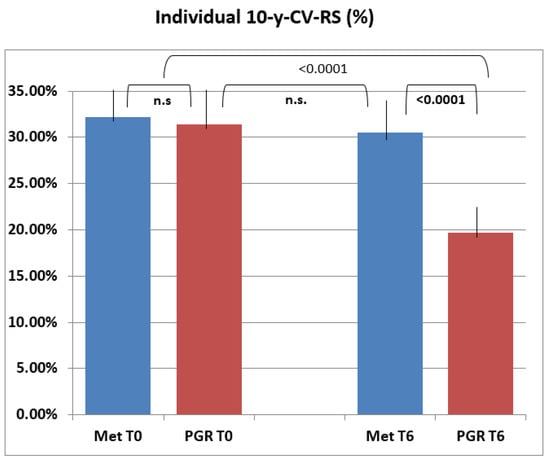
Figure 3.
Individual 10-y-CV-RS (10-y Cardiovascular risk score) in the two treatment groups. The final mean score in the PGR group was significantly lower than baseline (T0) and differed significantly from final and baseline ones in the MET group (p < 0.0001). n.s.: no significance.
HDL-C followed a similar improving trend, as shown in Figure 4 and Supplementary Figure S1.
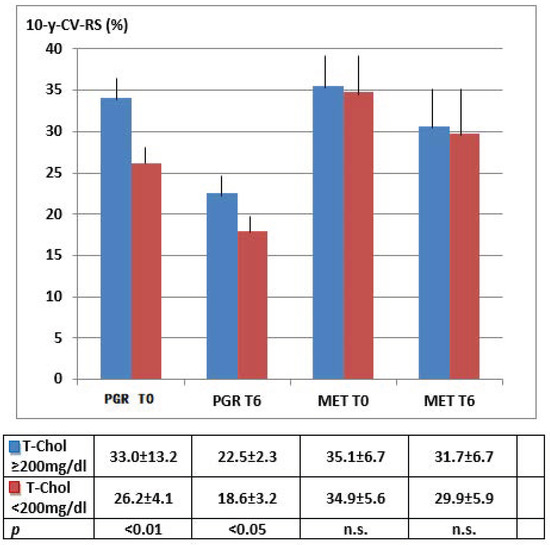
Figure 4.
Individual 10-y-CV-RS changes based on related Total Cholesterol levels (≥ or < 200 mg/dL). Differences between T0 (baseline) and T6 (end of follow-up): PGR p < 0.01; MET p n.s.
4. Discussion
MS is independently associated with an increased risk of incident cardiovascular morbidity and mortality in the European population. In clinical practice, a more informed assessment can come from the number of individual risk factors involved [23]. Its prevalence is still debated due to different diagnostic criteria, target populations, and clinical settings. According to the criteria of the Adult Treatment Panel (ATP) III definition [24], in a study from Middle Italy GP databases [25], MS was present in 1456 (33.0%) out of 4513 outpatients. There is no universal therapeutic option for MS, especially when associated with T2DM, as different risk factors characterizing an individual specific clinical picture have to be addressed each time. Many strategies have been used so far worldwide, involving different agents classified as inorganic/organic/pharmaceutical. The inorganic ones include zinc complexes with garlic derivatives as insulin-mimetics; selenium as an antioxidant; and copper, zinc, and manganese as microcomponents of antioxidant enzymes. An organic agent, i.e., glycine, has proved helpful against high blood pressure, hypertriglyceridemia, lipid oxidation, and visceral fat accretion in sucrose-fed rats. Widely used pharmaceutical products include fibrates, lipid- and glucose-lowering drugs, anti-gout agents, antioxidants, and omega-3-oils (fish oils). Finally, despite being less effective than drugs, frequently used antioxidant and lipid-lowering plant derivatives include digitalis pupurea (a century-old cardiovascular medication), magnolia officinalis, spirulina maxima, prickly pear cactus (Opuntia), ficus-indica, and cochlospermum vitifolium [26].
We would also like to stress another finding from our previous paper [10], i.e., that PGR, a new macromolecule complex based on polysaccharides slowing the down absorption rates of carbohydrates and fats, is non-inferior to metformin against insulin-resistance and high HbA1c or fasting blood glucose levels, and—opposed to metformin—significantly reduces blood lipids in adult subjects with MS and/or T2DM (10).
Guidelines for cardiovascular disease (CVD) prevention recommend using risk scores to identify adults at high CVD risk expected to benefit the most from preventive therapy. Several scoring systems exist to help clinicians assess related 10-year risk, the Framingham Score being the most widely used among them [27,28].
Framingham Study researchers [28] also proposed a new model for use in the primary care setting, clearly differentiated by gender. This “reclassification” of CVR was based on three sound ideas: first, it was easier to use four parameters recognized as the main risk factors (RF) in cardiovascular epidemiology, i.e., cholesterol, blood pressure (BP), diabetes mellitus, and smoking; second, the model could predict all CVD events [coronary heart disease (CHD), cerebrovascular events (CVE), peripheral arterial disease (PAD) and heart failure (HF)], and provided physicians with calibration factors for each entity of interest; and third, it included the concept of vascular or heart age, as calculated from the model.
Moreover, according to the 2009 Canadian Cholesterol Guidelines [29], despite all cardiovascular risk assessment calculators being defective per se, the Framingham Risk Score (FRS) is recommended for total CVD anticipation. It was validated in Canada with the Cardiovascular Life Expectancy Model and increased adherence to therapeutic measures. However, the FRS underestimates the risk in specific patients, including young, female subjects and those with MS.
By adding just two measurements to the Framingham model (a family history of premature CHD and elevated highly-sensitive C-reactive protein levels [+hsCRP]), the Reynolds Risk Score (RRS) seemed to improve the physician’s CVD risk prediction ability further, particularly for people previously classified as those at moderate risk, and was validated in men and women in the American population [30,31]. However, neither the FRS nor the RRS may be appropriate to Southern Europe or Mediterranean populations, whose CVD prevalence is lower than observed in the ones from Northern, Central, and Eastern Europe [31]. A simple risk score algorithm was developed and validated within the CUORE project [11,12,13,14] to fill such a gap by assessing the individual probability of undergoing a major cardiovascular event (myocardial infarction or stroke) over the next ten years in the Italian population. It requires information concerning eight risk factors, including sex, age, presence of diabetes or need for antihypertensive treatment, smoking habits, systolic blood pressure, TC, and HDL-C. Interestingly, the score provides a more accurate assessment than risk cards produced by the Italian Institute of Health (ISS) under the same CUORE Project [32].
Besides confirming superimposable PGR and MET effects on glucose levels, our results offer inspiration for a role of PGR against individual 10-year risk for heart or brain ischemic events through the improvement in lipid parameters already shown in adult people with MS [10] and in obese children [5,6,8].
Despite pathophysiological mechanisms underlying the cardio-protective role of metformin in T2DM remaining controversial, a recent meta-analysis [33,34] confirms: (i) the anti-inflammatory and antioxidative properties of metformin might also indirectly improve endothelial function; (ii) experimental and clinical data suggest direct effects on cardiac metabolism, structure, and function; (iii) clinical trials indicate a protective effect of metformin on both coronary events and progression to heart failure.
Since then, all guidelines have considered MET as the first-step treatment choice, together with diet and physical activity, in T2DM. At present, this approach is debated; therefore 2019 ESC/EASD guidelines [35] suggest Sodium-glucose Cotransporter-2 Inhibitors (SGLT-2is) and Glucagon-like peptide-1 receptor agonists (GLP1-RAs) as first-line options per se or equally to MET in patients at very high CVR. Indeed, PGR appears to be a viable stand-alone solution against combined MS glucose and lipid metabolism defects based on the more pronounced effects on the 10-y-CV-RS than MET at a much lower rate and intensity of troublesome gastrointestinal symptoms eventually responsible for poor treatment adherence [10]. Moreover, we would also like to stress another finding from our paper, i.e., the PGR-related visceral fat redistribution—which contributes to the observed 10-y-CV-RS reduction—as one of high relevance for patients with MS.
Therefore, PGR could represent a suitable alternative to MET as a first-line treatment option, especially now that an ever-increasing number of people prefer natural products based on plant extracts. This is especially the case given that, besides trying to avoid gastrointestinal side-effects as much as possible, patients might be sensitive to ecotoxicology-related problems involving plants and animals caused by the worldwide spread of environmental MET metabolites [36].
5. Limitations
Despite being aware of the relatively small number of subjects analyzed, we are confident that our findings, being perfectly in line with those previously reported by our and other groups, provide the scientific community with original and helpful information on how to safely and efficiently treat Metabolic Syndrome associated with overt diabetes. Further studies are needed involving a larger number of subjects to confirm our results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diabetology3020022/s1, Figure S1: 10-y-CV-RS changes based on related HDL-Cholesterol level (≥ or <40 mg/dL, cut-off arbitrarily chosen independently of gender), Table S1: Interpretation of the CV risk score.
Author Contributions
Conceptualization, methodology, and validation S.G. and F.S., software, T.D.-C., formal analysis S.G., F.S. and G.G.; investigation, E.S., C.R., C.A., G.C. (Gerardo Corigliano), M.C., G.C. (Giuseppe Cozzolino), C.B., C.M., D.O., C.L., A.V., L.F.; resources, G.G.; data curation, T.D.-C.; writing—original draft preparation, A.V. and F.S.; writing—review and editing, S.G.; visualization, S.G., F.S. and G.G.; supervision, S.G.; project administration, S.G., F.S. and G.G.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ours was a spontaneous, unconditioned study. The EC of Vanvitelli University of Naples, Italy, approved the present study as an extension of a previously approved protocol (reference n.10, Protocol registration trial n.1287, 23 June 2019), with the approval protocol registration n. 1287/bis, 30 June 2019, and by the Institutional Review Board (IRB Min. no. 10,712, dated 05.06.2020). All participants signed informed consent and data were processed anonymously according to good clinical practice guidelines. This study was conducted in conformance with good clinical practice standards. The study was led in accordance with the Declaration of Helsinki 1975, as subsequent amendments. All followed procedures were in accordance with the ethical standards of the responsible committee on human experimentation (both institutional and national), and in accordance with usual clinical practice.
Informed Consent Statement
Written informed consent was obtained from all participants before enrollment.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Special thanks are due to Paola Murano, General Manager of the Nefrocenter Research Network, for the effective and continuous support offered as complimentary for the realization of the study. Thanks are due to all the patients participating in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scheen, A.J. Management of the metabolic syndrome. Minerva Endocrinol. 2004, 29, 31–45. [Google Scholar] [PubMed]
- Bianchi, C.; Penno, G.; Romero, F.; Del Prato, S.; Miccoli, R. Treating the metabolic syndrome. Expert Rev. Cardiovasc. Ther. 2007, 5, 491–506. [Google Scholar] [CrossRef]
- Gogia, A.; Agarwal, P.K. Metabolic syndrome. Indian J. Med. Sci. 2006, 60, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Baños, G.; Perez-Torres, I.; El Hafidi, M. Medicinal Agents in the Metabolic Syndrome. Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 237–252. [Google Scholar] [CrossRef]
- Fornari, E.; Morandi, A.; Piona, C.; Tommasi, M.; Corradi, M.; Maffeis, C. Policaptil Gel Retard Intake Reduces Postprandial Triglycerides, Ghrelin and Appetite in Obese Children: A Clinical Trial. Nutrients 2020, 12, 214. [Google Scholar] [CrossRef] [Green Version]
- Stagi, S.; Lapi, E.; Seminara, S.; Pelosi, P.; Del Greco, P.; Capirchio, L.; Strano, M.; Giglio, S.; Chiarelli, F.; De Martino, M. Policaptil Gel Retard significantly reduces body mass index and hyperinsulinism and may decrease the risk of type 2 diabetes mellitus (T2DM) in obese children and adolescents with family history of obesity and T2DM. Ital. J. Pediatr. 2015, 41, 10. [Google Scholar] [CrossRef] [Green Version]
- Ellis, P.R.; Roberts, F.G.; Low, A.G.; Morgan, L.M. The effect of high-molecular-weight guar gum on net apparent glucose absorption and net apparent insulin and gastric inhibitory polypeptide production in the growing pig: Relationship to rheological changes in jejunal digesta. Br. J. Nutr. 1995, 74, 539–556. [Google Scholar] [CrossRef] [Green Version]
- Stagi, S.; Ricci, F.; Bianconi, M.; Sammarco, M.A.; Municchi, G.; Toni, S.; Lenzi, L.; Verrotti, A.; De Martino, M. Retrospective Evaluation of Metformin and/or Metformin Plus a New Polysaccharide Complex in Treating Severe Hyperinsulinism and Insulin Resistance in Obese Children and Adolescents with Metabolic Syndrome. Nutrients 2017, 9, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dludla, P.V.; Nyambuya, T.M.; Johnson, R.; Silvestri, S.; Orlando, P.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Mxinwa, V.; Mokgalaboni, K.; Tiano, L.; et al. Metformin and heart failure-related outcomes in patients with or without diabetes: A systematic review of randomized controlled trials. Heart Fail. Rev. 2021, 26, 1437–1445. [Google Scholar] [CrossRef]
- Guarino, G.; Della Corte, T.; Strollo, F.; Gentile, S.; Nefrocenter Research Study Group. Policaptil Gel Retard in adult subjects with the metabolic syndrome: Efficacy, safety, and tolerability compared to metformin. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 901–907. [Google Scholar] [CrossRef]
- Giampaoli, S.; Palmieri, L.; Donfrancesco, C.; Noce, C.L.; Pilotto, L.; Vanuzzo, D.; Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey Research Group. Cardiovascular health in Italy. Ten-year surveillance of cardiovascular diseases and risk factors: Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey 1998–2012. Eur. J. Prev. Cardiol. 2015, 22 (Suppl. S2), 9–37. [Google Scholar] [CrossRef] [PubMed]
- Giampaoli, S.; Palmieri, L.; Panico, S.; Vanuzzo, D.; Ferrario, M.; Chiodini, P.; Pilotto, L.; Donfrancesco, C.; Cesana, G.; Sega, R.; et al. Favorable Cardiovascular Risk Profile (Low Risk) and 10-Year Stroke Incidence in Women and Men: Findings from 12 Italian Population Samples. Am. J. Epidemiol. 2006, 163, 893–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, L.; Donfrancesco, C.; Giampaoli, S.; Trojani, M.; Panico, S.; Vanuzzo, D.; Pilottoc, L.; Cesana, G.; Ferrario, M.M.; Chiodini, P.; et al. Favorable cardiovascular risk profile and 10-year coronary heart disease incidence in women and men: Results from the Progetto CUORE. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, M.; Chiodini, P.; Chambless, L.E.; Cesana, G.; Vanuzzo, D.; Panico, S.; Sega, R.; Pilotto, L.; Palmieri, L.; Giampaoli, S.; et al. Prediction of coronary events in a low incidence population. Assessing accuracy of the CUORE Cohort Study prediction equation. Int. J. Epidemiol. 2005, 34, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Giorda, C.B.; Nicolucci, A.; Pellegrini, F.; Kristiansen, C.K.; Hunt, B.; Valentine, W.J.; Vespasiani, G. Improving quality of care in people with Type 2 diabetes through the Associazione Medici Diabetologi-annals initiative: A long-term cost-effectiveness analysis. Diabet. Med. 2014, 31, 615–623. [Google Scholar] [CrossRef]
- Manicardi, V.; Clemente, G.; De Cosmo, S.; Manti, R.; Mazzucchelli, C.; Pisanu, P.; Rocca, A. Valutazione Degli Indicatori AMD di Qualità Dell’assistenza al Diabete Tipo 1 e 2 in Italia. The Monograph of AMD Annals 2018; Idelson Gnocchi: Naples, Italy; Available online: https://aemmedi.it/wp-content/uploads/2018/11/Annali_AMD-_2018_prot.pdf (accessed on 22 December 2021).
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- National Center for Health Statistics. International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Available online: http://www.cdc.gov/nchs/icd/icd9cm.htm (accessed on 15 March 2022).
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care 2021, 44 (Suppl. S1), S15–S33, Erratum in Diabetes Care 2021, 44, 2182. [Google Scholar] [CrossRef]
- Cicero, A.F.; Nascetti, S.; Noera, G.; Gaddi, A.V.; Massa Lombarda Project team. Metabolic syndrome prevalence in Italy. Nutr. Metab. Cardiovasc. Dis. 2006, 16, e5–e6. [Google Scholar] [CrossRef]
- Baranauskas, M.N.; Johnson, K.E.; Juvancic-Heltzel, J.A.; Kappler, R.M.; Richardson, L.; Jamieson, S.; Otterstetter, R. Seven-site versus three-site method of body composition using Body Metrix ultrasound compared to dual-energy X-ray absorptiometry. Clin. Physiol. Funct. Imaging 2017, 37, 317–321. [Google Scholar] [CrossRef]
- Guarino, G.; Ragozzino, G.; Della Corte, T.; Fontana, S.; Strollo, F.; Cecaro, M.; Gentile, S. Selenium supplementation in obese patients with subclinical hypothyroidism and type 2 diabetes. J. Nutr. Health Sci. 2018, 5, 202. Available online: http://www.annexpublishers.co/articles/JNH/5202-Selenium-Supplementation-in-Obese-Patients-with-Subclinical-Hypothyroidism-and-Type-2-Diabetes.pdf (accessed on 22 December 2021).
- Dekker, J.M.; Girman, C.; Rhodes, T.; Nijpels, G.; Stehouwer, C.D.A.; Bouter, L.M.; Heine, R.J. Metabolic Syndrome and 10-Year Cardiovascular Disease Risk in the Hoorn Study. Circulation 2005, 112, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef]
- Tocci, G.; Ferrucci, A.; Bruno, G.; Mannarino, E.; Nati, G.; Trimarco, B.; Volpe, M. Prevalence of metabolic syndrome in the clinical practice of general medicine in Italy. Cardiovasc. Diagn. Ther. 2015, 5, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Ruiz, M.; El Hafidi, M.; Perez-Torres, I.; Banos, G.; Guarner, V. Medicinal agents and metabolic syndrome. Curr. Med. Chem. 2013, 20, 2626–2640. [Google Scholar] [CrossRef]
- Rodondi, N.; Locatelli, I.; Aujesky, D.; Butler, J.; Vittinghoff, E.; Simonsick, E.; Satterfield, S.; Newman, A.B.; Wilson, P.W.F.; Pletcher, M.J.; et al. Framingham Risk Score and Alternatives for Prediction of Coronary Heart Disease in Older Adults. PLoS ONE 2012, 7, e34287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genest, J.; McPherson, R.; Frohlich, J.; Anderson, T.; Campbell, N.; Carpentier, A.; Couture, P.; Dufour, R.; Fodor, G.; Francis, G.A.; et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult—2009 recommendations. Can. J. Cardiol. 2009, 25, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Buring, J.E.; Rifai, N.; Cook, N.R. Development and Validation of Improved Algorithms for the Assessment of Global Cardiovascular Risk in Women: The Reynolds Risk Score. JAMA 2007, 297, 611–619, Erratum in JAMA 2007, 297, 1433. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Paynter, N.P.; Rifai, N.; Gaziano, J.M.; Cook, N.R. C-Reactive Protein and Parental History Improve Global Cardiovascular Risk Prediction. Circulation 2008, 118, 2243–2251. [Google Scholar] [CrossRef] [Green Version]
- Artigao-Rodenas, L.M.; Carbayo-Herencia, J.A.; Divisón-Garrote, J.A.; Gil-Guillén, V.F.; Massó-Orozco, J.; Simarro-Rueda, M.; Molina-Escribano, F.; Sanchis, C.; Carrión-Valero, L.; de Coca, E.L.; et al. Framingham Risk Score for Prediction of Cardiovascular Diseases: A Population-Based Study from Southern Europe. PLoS ONE 2013, 8, e73529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Istituto Superiore di Sanità. Carta del Rischio Cardiovascolare. Progetto CUORE 2016. Web Site. Available online: http://www.cuore.iss.it/valutazione/carte-pdf.pdf (accessed on 22 December 2021).
- Nesti, L.; Natali, A. Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323, Erratum in Eur. Heart J. 2020, 41, 4317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briones, R.; Sarmah, A.K.; Padhye, L. A global perspective on the use, occurrence, fate and effects of anti-diabetic drug metformin in natural and engineered ecosystems. Environ. Pollut. 2016, 219, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).