Beneficial Effects of the Ketogenic Diet in Metabolic Syndrome: A Systematic Review
Abstract
1. Introduction
1.1. From Obesity to Metabolic Syndrome: Epidemiology and Physiopathology
1.2. Lifestyle Recommendations for the Prevention and Management of Metabolic Syndrome
1.3. Ketogenic Diet: Definition, History and Principle
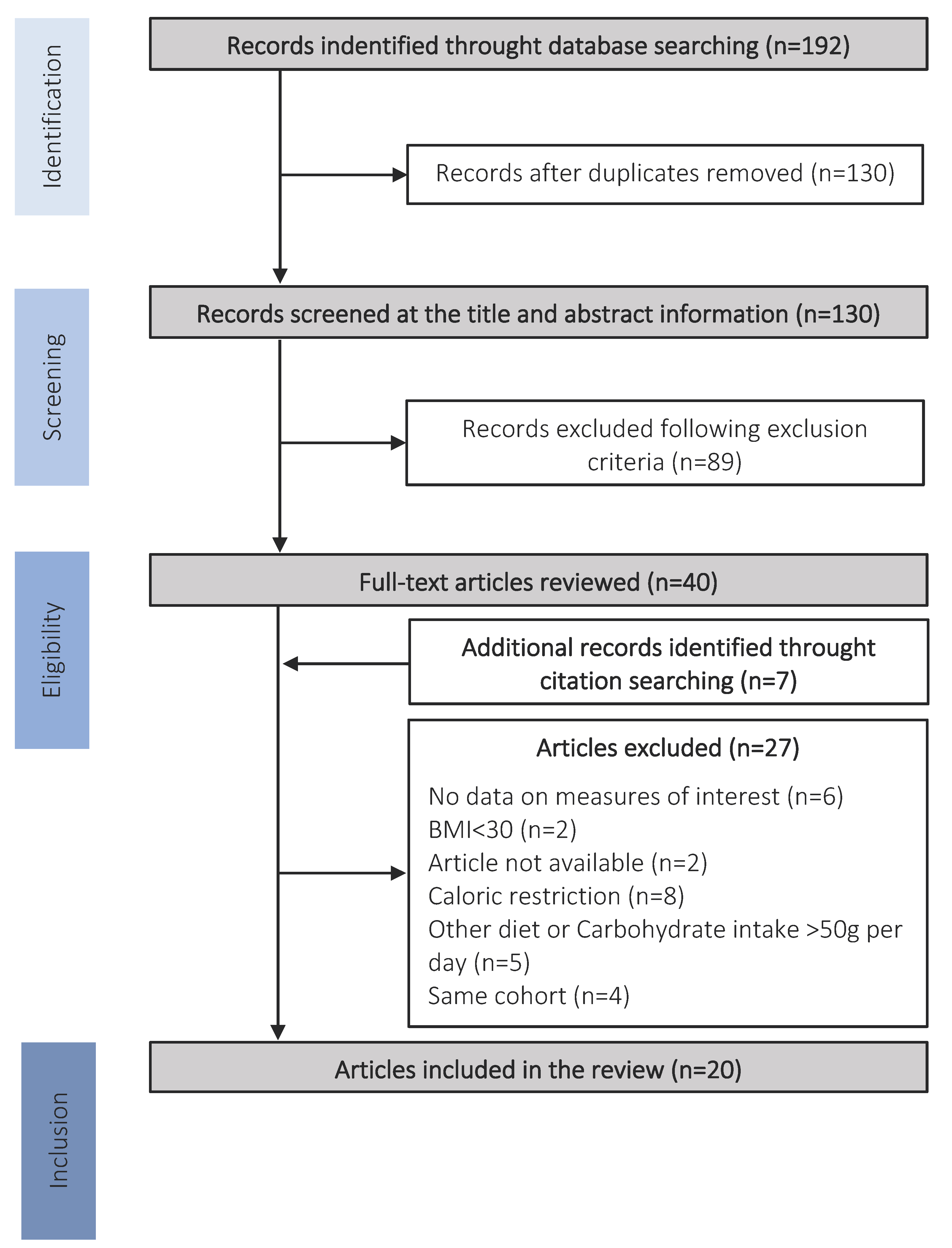
2. Methods
2.1. Eligibility Criteria
2.2. Search and Study Selection
2.3. Data Extraction
3. Results
3.1. Effects of KD on Obesity and Cardiovascular Risk
3.2. Effects of KD on Type 2 Diabetes
3.3. Effects of KD on Nonalcoholic Steatohepatitis
4. Discussion
4.1. Beneficial Effects of the Ketogenic Diet
4.2. Recommendations and Limitations of KD
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 26 November 2018).
- Odermatt, A. The Western-style diet: A major risk factor for impaired kidney function and chronic kidney disease. Am. J. Physiol. Physiol. 2011, 301, F919–F931. [Google Scholar] [CrossRef] [PubMed]
- Foufelle, F.; Ferré, P. Mechanism of Storage and Synthesis of Fatty Acids and Triglycerides in White Adipocytes. In Physiology and Physiopathology of Adipose Tissue; Bastard, J.-P., Fève, B., Eds.; Springer: Paris, France, 2013; pp. 101–121. ISBN 978-2-8178-0342-5. [Google Scholar]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 421–449. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipose Tissue, Obesity and Non-Alcoholic Fatty Liver Disease. Minerva Endocrinol. 2017, 42, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Milić, S.; Lulić, D.; Štimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Muller, F.L.; Liu, Y.; Chavez, A.O.; Balas, B.; Zuo, P.; Chang, Z.; Tripathy, D.; Jani, R.; Molina-Carrion, M.; et al. Deleterious action of FA metabolites on ATP synthesis: Possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am. J. Physiol. Metab. 2008, 295, E678–E685. [Google Scholar] [CrossRef] [PubMed]
- Algoblan, A.; Alalfi, M.; Khan, M. Mechanism linking diabetes mellitus and obesity. Diabetes, Metab. Syndr. Obesity: Targets Ther. 2014, 7, 587–591. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef]
- Ferré, P. Signalisation insulinique et résistance à l’insuline. Therapies 2007, 62, 277–284. [Google Scholar] [CrossRef]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef]
- Charlot, A.; Boumiza, R.; Roux, M.; Zoll, J. Obésité, inflammation et COVID-19: Intérêt préventif de l’alimentation Cétogène? Biol. Aujourd’hui 2021, 215, 63–72. [Google Scholar] [CrossRef]
- Das, S.R.; Drazner, M.H.; Dries, D.L.; Vega, G.L.; Stanek, H.G.; Abdullah, S.M.; Canham, R.M.; Chung, A.K.; Leonard, D.; WiansJr, F.H.; et al. Impact of Body Mass and Body Composition on Circulating Levels of Natriuretic Peptides: Results from the Dallas Heart Study. Circulation 2005, 112, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.; Levy, D.; Benjamin, E.; Leip, E.P.; Wilson, P.W.; Vasan, R.S. Impact of Obesity on Plasma Natriuretic Peptide Levels. Circulation 2004, 109, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Birkenfeld, A.L.; Melander, O.; Moro, C. Natriuretic Peptides in Cardiovascular and Metabolic Crosstalk. Hypertension 2018, 72, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.; Keyes, M.J.; Levy, D.; Benjamin, E.; Vasan, R.S. Association of Plasma Natriuretic Peptide Levels With Metabolic Risk Factors in Ambulatory Individuals. Circulation 2007, 115, 1345–1353. [Google Scholar] [CrossRef]
- Sarzani, R.; Spannella, F.; Giulietti, F.; Balietti, P.; Cocci, G.; Bordicchia, M. Cardiac Natriuretic Peptides, Hypertension and Cardiovascular Risk. High Blood Press. Cardiovasc. Prev. 2017, 24, 115–126. [Google Scholar] [CrossRef]
- Schlueter, N.; de Sterke, A.; Willmes, D.M.; Spranger, J.; Jordan, J.; Birkenfeld, A.L. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol. Ther. 2014, 144, 12–27. [Google Scholar] [CrossRef]
- Howard, B.V.; Ruotolo, G.; Robbins, D.C. Obesity and dyslipidemia. Endocrinol. Metab. Clin. N. Am. 2003, 32, 855–867. [Google Scholar] [CrossRef]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and Cardiovascular Disease: From Pathogenesis to Therapeutic Target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Soysal, P.; Arik, F.; Smith, L.; Jackson, S.E.; Isik, A.T. Inflammation, Frailty and Cardiovascular Disease. In Frailty and Cardiovascular Diseases; Veronese, N., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Volume 1216, pp. 55–64. ISBN 978-3-030-33329-4. [Google Scholar]
- Lee, M.-K.; Han, K.; Kim, M.K.; Koh, E.S.; Kim, E.S.; Nam, G.E.; Kwon, H.-S. Changes in metabolic syndrome and its components and the risk of type 2 diabetes: A nationwide cohort study. Sci. Rep. 2020, 10, 2313. [Google Scholar] [CrossRef]
- Lemieux, I.; Després, J.-P. Metabolic Syndrome: Past, Present and Future. Nutrients 2020, 12, 3501. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020. [Google Scholar]
- Ligue contre l’obésité. Enquête Épidémiologique Nationale Sur Le Surpoids et l’obésité; ObÉpi-Roche: Paris, France, 2021. [Google Scholar]
- Chan, J.C.; Malik, V.; Jia, W.; Kadowaki, T.; Yajnik, C.S.; Yoon, K.-H.; Hu, F.B. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. JAMA 2009, 301, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the Metabolic Syndrome in Children and Adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Standards of Medical Care in Diabetes—2009. Diabetes Care 2009, 32, S13–S61. [CrossRef] [PubMed]
- Babio, N.; Bulló, M.; Salas-Salvadó, J. Mediterranean diet and metabolic syndrome: The evidence. Public Health Nutr. 2009, 12, 1607–1617. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Poulsen, S.K.; Due, A.; Jordy, A.B.; Kiens, B.; Stark, K.D.; Stender, S.; Holst, C.; Astrup, A.; Larsen, T.M. Health effect of the New Nordic Diet in adults with increased waist circumference: A 6-mo randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 35–45. [Google Scholar] [CrossRef]
- Feldeisen, S.E.; Tucker, K.L. Nutritional strategies in the prevention and treatment of metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 46–60. [Google Scholar] [CrossRef]
- Pérez-Martínez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Díaz-López, A.; Drevon, C.A.; et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar] [CrossRef]
- Wilder, R.M.; Winter, M.D. The Threshold of Ketogenesis. J. Biol. Chem. 1922, 52, 393–401. [Google Scholar] [CrossRef]
- Sampaio, L.P.D.B. Ketogenic diet for epilepsy treatment. Arq. Neuro-Psiquiatr. 2016, 74, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Kerndt, P.R.; Naughton, J.L.; Driscoll, C.E.; Loxterkamp, D.A. Fasting: The history, pathophysiology and complications. West. J. Med. 1982, 137, 379–399. [Google Scholar] [PubMed]
- Schönfeld, P.; Reiser, G. Why does Brain Metabolism not Favor Burning of Fatty Acids to Provide Energy?—Reflections on Disadvantages of the Use of Free Fatty Acids as Fuel for Brain. J. Cereb. Blood Flow Metab. 2013, 33, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.J.; Wodschow, H.Z.; Nilsson, M.; Rungby, J. Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8767. [Google Scholar] [CrossRef]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 243–251. [Google Scholar] [CrossRef]
- Pierre, K.; Pellerin, L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J. Neurochem. 2005, 94, 1–14. [Google Scholar] [CrossRef]
- Meyer-Rogge, S.; Meyer-Rogge, K. Biochimie Métabolique: Ler Cycle, PACES; De Boeck: Bruxelles, Belgium, 2012; ISBN 978-2-8041-7147-6. [Google Scholar]
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes. Rev. 2020, 21, e13024. [Google Scholar] [CrossRef]
- Terzikhan, N.; Doets, E.; Noordegraaf-Schouten, M.V. Extensive literature search and review as preparatory work for the evaluation of the essential composition of total diet replacement products for weight control. EFSA Support. Publ. 2015, 12, 2–21. [Google Scholar] [CrossRef]
- Oh, R.; Gilani, B.; Uppaluri, K.R. Low Carbohydrate Diet. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional Ketosis for Weight Management and Reversal of Metabolic Syndrome. Curr. Nutr. Rep. 2018, 7, 97–106. [Google Scholar] [CrossRef]
- Kosinski, C.; Jornayvaz, F.R. Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 2017, 9, 517. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Ballard, K.D.; Quann, E.E.; Kupchak, B.R.; Volk, B.M.; Kawiecki, D.M.; Fernandez, M.L.; Seip, R.L.; Maresh, C.M.; Kraemer, W.J.; Volek, J.S. Dietary carbohydrate restriction improves insulin sensitivity, blood pressure, microvascular function, and cellular adhesion markers in individuals taking statins. Nutr. Res. 2013, 33, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.D.; Vasey, F.B.; Valeriano, J. The effect of a low-carbohydrate diet on bone turnover. Osteoporos. Int. 2006, 17, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.M.; Al-Zaid, N.S.; Mathew, T.C.; Al-Mousawi, M.; Talib, H.; Asfar, S.K.; Behbahani, A.I. Long Term Effects of Ketogenic Diet in Obese Subjects with High Cholesterol Level. Mol. Cell. Biochem. 2006, 286, 1. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.M.; Mathew, T.C.; Khadada, M.; Al-Mousawi, M.; Talib, H.; Asfar, S.K.; Behbahani, A.I.; Al-Zaid, N.S. Beneficial effects of ketogenic diet in obese diabetic subjects. Mol. Cell. Biochem. 2007, 302, 249–256. [Google Scholar] [CrossRef]
- Francois, M.E.; Myette-Cote, E.; Bammert, T.D.; Durrer, C.; Neudorf, H.; DeSouza, C.A.; Little, J.P. Carbohydrate restriction with postmeal walking effectively mitigates postprandial hyperglycemia and improves endothelial function in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H105–H113. [Google Scholar] [CrossRef]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.; Phinney, S.D.; et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef]
- Hernandez, T.L.; Sutherland, J.P.; Wolfe, P.; Allian-Sauer, M.; Capell, W.H.; Talley, N.D.; Wyatt, H.R.; Foster, G.D.; Hill, J.O.; Eckel, R.H. Lack of suppression of circulating free fatty acids and hypercholesterolemia during weight loss on a high-fat, low-carbohydrate diet. Am. J. Clin. Nutr. 2010, 91, 578–585. [Google Scholar] [CrossRef]
- Hussain, T.A.; Mathew, T.C.; Dashti, A.A.; Asfar, S.; Al-Zaid, N.; Dashti, H.M. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 2012, 28, 1016–1021. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef]
- McKenzie, A.L.; Hallberg, S.J.; Creighton, B.C.; Volk, B.M.; Link, T.M.; Abner, M.K.; Glon, R.M.; McCarter, J.P.; Volek, J.S.; Phinney, S.D. A Novel Intervention Including Individualized Nutritional Recommendations Reduces Hemoglobin A1c Level, Medication Use, and Weight in Type 2 Diabetes. JMIR Diabetes 2017, 2, e5. [Google Scholar] [CrossRef]
- Myette-Côté, É.; Durrer, C.; Neudorf, H.; Bammert, T.D.; Botezelli, J.D.; Johnson, J.D.; DeSouza, C.A.; Little, J.P. The effect of a short-term low-carbohydrate, high-fat diet with or without postmeal walks on glycemic control and inflammation in type 2 diabetes: A randomized trial. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R1210–R1219. [Google Scholar] [CrossRef] [PubMed]
- Partsalaki, I.; Karvela, A.; Spiliotis, B.E. Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2012, 25, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Saslow, L.R.; Daubenmier, J.J.; Moskowitz, J.T.; Kim, S.; Murphy, E.J.; Phinney, S.D.; Ploutz-Snyder, R.; Goldman, V.; Cox, R.M.; Mason, A.E.; et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr. Diabetes 2017, 7, 304. [Google Scholar] [CrossRef]
- Saslow, L.R.; Mason, A.E.; Kim, S.; Goldman, V.; Ploutz-Snyder, R.; Bayandorian, H.; Daubenmier, J.; Hecht, F.M.; Moskowitz, J.T. An Online Intervention Comparing a Very Low-Carbohydrate Ketogenic Diet and Lifestyle Recommendations Versus a Plate Method Diet in Overweight Individuals with Type 2 Diabetes: A Randomized Controlled Trial. J. Med. Internet Res. 2017, 19, e36. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Barbarisi, A.; Cesaretti, M.; Iannelli, A. A 4-Week Preoperative Ketogenic Micronutrient-Enriched Diet Is Effective in Reducing Body Weight, Left Hepatic Lobe Volume, and Micronutrient Deficiencies in Patients Undergoing Bariatric Surgery: A Prospective Pilot Study. Obes. Surg. 2018, 28, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Tendler, D.; Lin, S.; Yancy, W.S., Jr.; Mavropoulos, J.; Sylvestre, P.; Rockey, D.C.; Westman, E.C. The Effect of a Low-Carbohydrate, Ketogenic Diet on Nonalcoholic Fatty Liver Disease: A Pilot Study. Dig. Dis. Sci. 2007, 52, 589–593. [Google Scholar] [CrossRef]
- Walton, C.M.; Perry, K.; Hart, R.H.; Berry, S.L.; Bikman, B.T. Improvement in Glycemic and Lipid Profiles in Type 2 Diabetics with a 90-Day Ketogenic Diet. J. Diabetes Res. 2019, 2019, 8681959. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Olsen, M.K.; Guyton, J.R.; Bakst, R.P.; Westman, E.C. A Low-Carbohydrate, Ketogenic Diet versus a Low-Fat Diet to Treat Obesity and Hyperlipidemia: A randomized, controlled trial. Ann. Intern. Med. 2004, 140, 769–777. [Google Scholar] [CrossRef]
- Yancy, W.S.; Olsen, M.K.; Dudley, T.; Westman, E.C. Acid-base analysis of individuals following two weight loss diets. Eur. J. Clin. Nutr. 2007, 61, 1416–1422. [Google Scholar] [CrossRef]
- Yancy, W.S.; Westman, E.C.; McDuffie, J.R.; Grambow, S.; Jeffreys, A.S.; Bolton, J.; Chalecki, A.; Oddone, E.Z. A Randomized Trial of a Low-Carbohydrate Diet vs Orlistat Plus a Low-Fat Diet for Weight Loss. Arch. Intern. Med. 2010, 170, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.M. Obesity: Risk Factors, Complications, and Strategies for Sustainable Long-Term Weight Management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129 (Suppl. S2), S102–S138. [Google Scholar] [CrossRef] [PubMed]
- Jin, J. Risk Assessment for Cardiovascular Disease with Nontraditional Risk Factors. JAMA J. Am. Med. Assoc. 2018, 320, 316. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: A report of the american college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Kerner, W.; Brückel, J.; German Diabetes Association. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2014, 122, 384–386. [Google Scholar] [CrossRef]
- Indonesian Diabetes Association. Guidelines on the Management and Prevention of Prediabetes. Acta Med. Indones. 2014, 46, 348–359. [Google Scholar]
- Tang, Q.; Li, X.; Song, P.; Xu, L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug Discov. Ther. 2015, 9, 380–385. [Google Scholar] [CrossRef]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018, 24, 3361–3373. [Google Scholar] [CrossRef]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; De Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of Modest Weight Loss in Improving Cardiovascular Risk Factors in Overweight and Obese Individuals with Type 2 Diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy. Diabetes Care 2009, 32, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kurukulasuriya, L.R.; Sowers, J.R. Therapies for type 2 diabetes: Lowering HbA1c and associated cardiovascular risk factors. Cardiovasc. Diabetol. 2010, 9, 45. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Barr, E.L.; Zimmet, P.Z.; Welborn, T.A.; Jolley, D.; Magliano, D.J.; Dunstan, D.W.; Cameron, A.J.; Dwyer, T.; Taylor, H.R.; Tonkin, A.M.; et al. Risk of Cardiovascular and All-Cause Mortality in Individuals with Diabetes Mellitus, Impaired Fasting Glucose, and Impaired Glucose Tolerance: The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007, 116, 151–157. [Google Scholar] [CrossRef]
- Huang, D.; Refaat, M.; Mohammedi, K.; Jayyousi, A.; Al Suwaidi, J.; Khalil, C.A. Macrovascular Complications in Patients with Diabetes and Prediabetes. BioMed Res. Int. 2017, 2017, 7839101. [Google Scholar] [CrossRef]
- Liao, H.-W.; Saver, J.; Yeh, H.-C.; Chen, C.-H.S.; Wu, Y.-L.; Lee, M.; Ovbiagele, B. Low fasting glucose and future risks of major adverse outcomes in people without baseline diabetes or cardiovascular disease: A systematic review and meta-analysis. BMJ Open 2019, 9, e026010. [Google Scholar] [CrossRef]
- Park, C.; Guallar, E.; Linton, J.A.; Lee, D.-C.; Jang, Y.; Son, D.K.; Han, E.-J.; Baek, S.J.; Yun, Y.D.; Jee, S.H.; et al. Fasting Glucose Level and the Risk of Incident Atherosclerotic Cardiovascular Diseases. Diabetes Care 2013, 36, 1988–1993. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Yaghi, S.; Elkind, M.S. Lipids and Cerebrovascular Disease: Research and Practice. Stroke 2015, 46, 3322–3328. [Google Scholar] [CrossRef] [PubMed]
- Marston, N.A.; Giugliano, R.P.; Im, K.; Silverman, M.G.; O’Donoghue, M.L.; Wiviott, S.D.; Ference, B.A.; Sabatine, M.S. Association Between Triglyceride Lowering and Reduction of Cardiovascular Risk Across Multiple Lipid-Lowering Therapeutic Classes: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Circulation 2019, 140, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Choi, S.; Kim, K.; Kim, S.M.; Lee, G.; Park, S.Y.; Kim, Y.; Son, J.S.; Yun, J.; Park, S.M. Effect of Change in Total Cholesterol Levels on Cardiovascular Disease among Young Adults. J. Am. Hear. Assoc. 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.M.; Wonnerth, A.; Huber, K.; Wojta, J. Cardiovascular disease risk reduction by raising HDL cholesterol—current therapies and future opportunities. J. Cereb. Blood Flow Metab. 2012, 167, 1177–1194. [Google Scholar] [CrossRef]
- Antonakoudis, G.; Poulimenos, L.; Kifnidis, K.; Zouras, C.; Antonakoudis, H. Blood pressure control and cardiovascular risk reduction. Hippokratia 2007, 11, 114–119. [Google Scholar] [PubMed]
- Sanyal, A.J. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 1705–1725. [Google Scholar] [CrossRef]
- Zilberter, T.; Zilberter, Y. Ketogenic Ratio Determines Metabolic Effects of Macronutrients and Prevents Interpretive Bias. Front. Nutr. 2018, 5, 75. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, Y.M.; Lee, J.S.; Kang, H.C.; Kim, H.D. Efficacy and Tolerability of the Ketogenic Diet According to Lipid:Nonlipid Ratios? Comparison of 3:1 with 4:1 Diet. Epilepsia 2007, 48, 801–805. [Google Scholar] [CrossRef]
- El-Rashidy, O.F.; Nassar, M.F.; Abdel-Hamid, I.A.; Shatla, R.H.; Abdel-Hamid, M.H.; Gabr, S.S.; Mohamed, S.G.; El-Sayed, W.S.; Shaaban, S.Y. Modified Atkins diet vs classic ketogenic formula in intractable epilepsy. Acta Neurol. Scand. 2013, 128, 402–408. [Google Scholar] [CrossRef]
- Ye, F.; Li, X.-J.; Jiang, W.-L.; Sun, H.-B.; Liu, J. Efficacy of and Patient Compliance with a Ketogenic Diet in Adults with Intractable Epilepsy: A Meta-Analysis. J. Clin. Neurol. 2015, 11, 26–31. [Google Scholar] [CrossRef]
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Kempf, K.; Röhling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Narbonne, P.; Roy, R. La protéine kinase activée par l’AMP (AMPK) protège les réserves énergétiques. Med. Sci. 2009, 25, 565–566. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McNamara, N.; Carbone, L.A.; Shellhaas, R. Epilepsy Characteristics and Psychosocial Factors Associated with Ketogenic Diet Success. J. Child Neurol. 2013, 28, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Masood, W.; Annamaraju, P.; Uppaluri, K.R. Ketogenic Diet. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wirrell, E.C. Ketogenic ratio, calories, and fluids: Do they matter? Epilepsia 2008, 49, 17–19. [Google Scholar] [CrossRef]

| Clinical Measures | Glycemic Profile | Lipid Profile | Liver Profile | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Weight | BMI | Systolic BP | Diastolic BP | Fasting Glucose | Fasting Insulin | HOMA-IR | HbA1c | Total Cholesterol | HDL | LDL | TG | AST | ALT | Volume of Left Hepatic Lobe | Biopsy |
| [49] Ballard and et al., 2013 | x | x | x | x | x | x | x | x | x | |||||||
| [50] Carter and et al., 2006 | x | x | ||||||||||||||
| [51] Dashti and et al., 2006 | x | x | x | x | x | x | x | |||||||||
| [52] Dashti and et al., 2007 | x | x | x | x | x | x | ||||||||||
| [53] François and et al., 2018 | x | x | ||||||||||||||
| [54] Hallberg and et al., 2018 | x | x | x | x | x | x | x | x | x | x | ||||||
| [55] Hernandez and et al., 2010 | x | x | x | x | x | x | ||||||||||
| [56] Hussain and et al., 2012 | x | x | x | x | x | x | x | x | ||||||||
| [57] Johnstone et al., 2008 | x | x | x | x | x | x | x | x | ||||||||
| [58] McKenzie and et al., 2017 | x | x | x | x | x | x | x | x | x | x | x | x | ||||
| [59] Myette-Coté and et al., 2018 | x | x | x | x | ||||||||||||
| [60] Partsalaki and et al., 2012 | x | x | x | x | x | x | x | x | x | x | x | |||||
| [61] Saslow and et al., 2017 (1) | x | x | x | x | x | x | x | x | x | x | ||||||
| [62] Saslow and et al., 2017 (2) | x | x | x | x | x | |||||||||||
| [63] Schiavo and et al., 2018 | x | x | x | x | x | x | x | x | x | x | x | |||||
| [64] Tendler and et al., 2007 | x | x | x | x | x | x | ||||||||||
| [65] Walton and et al., 2019 | x | x | x | x | x | x | x | x | x | x | ||||||
| [66] Yancy and et al., 2004 | x | x | x | x | x | x | x | |||||||||
| [67] Yancy and et al., 2007 | x | |||||||||||||||
| [68] Yancy and et al., 2010 | x | x | x | x | x | x | x | x | x | x | ||||||
| Reference | Patients Characteristic | Maximum Carbohydrate Intake of | Duration | Sample Size |
|---|---|---|---|---|
| [49] Ballard and et al., 2013 |
Participants > 18 y.o BMI > 30 | 50 g per day | 6 weeks | n = 21 |
| [50] Carter and et al., 2006 |
Participants > 18 y.o BMI > 30 | 40 g per day | 3 months | n = 13 |
| [51] Dashti and et al., 2006 |
Normal cholesterol level and high cholesterol level adults (>18 y.o) BMI > 30 | 20 g per day | 56 weeks |
Normal cholesterol level adults: n = 23 High cholesterol level adults: n = 26 |
| [52] Dashti and et al., 2007 |
Diabetics and non-diabetics adults (>18 y.o) BMI > 30 | 20 g per day | 56 weeks | Diabetics: n = 31 Non-diabetics: n = 33 |
| [53] François and et al., 2018 |
48 to 72 y.o diabetics participants BMI > 30 | 50 g per day | 4 days | n = 11 |
| [54] Hallberg and et al., 2018 |
Diabetic participants (>18 y.o) BMI > 30 | 30 g per day | 1 year | n = 218 |
| [55] Hernandez and et al., 2010 | Obese participants > 18 y.o | 20 g per day | 6 weeks | n = 32 |
| [56] Hussain and et al., 2012 |
Diabetics and non-diabetics adults (>18 y.o) BMI > 30 | 30 g per day | 24 weeks | Diabetics: n = 102 Non-diabetics: n = 261 |
| [57] Johnstone et al., 2008 |
20 to 65 y.o men BMI > 30 | 5.5 g per day | 4 weeks | n = 17 |
| [58] McKenzie and et al., 2017 |
21 to 65 y.o diabetic participants BMI > 30 | 30 g per day | 10 weeks | n = 238 |
| [59] Myette-Coté and et al., 2018 | 48 to 72 y.o diabetic participants | 50 g per day | 4 days | n = 11 |
| [60] Partsalaki and et al., 2012 |
8 to 18 y.o children and adolescents BMI > 30 | 20 g per day | 6 months | n = 21 |
| [61] Saslow and et al., 2017 (1) |
Diabetic participants > 18 y.o BMI > 30 | 20 to 50 g per day | 6 months | n = 16 |
| [62] Saslow and et al., 2017 (2) | Diabetic obese participants > 18 y.o | 20 to 50 g per day | 32 weeks | n = 12 |
| [63] Schiavo and et al., 2018 |
Participants > 18 y.o BMI > 40 | 15 g per day | 4 weeks | n = 27 |
| [64] Tendler and et al., 2007 |
18 to 65 y.o participants with NAFLD BMI > 30 | 20 g per day | 6 months | n = 5 |
| [65] Walton and et al., 2019 |
18 to 45 y.o diabetic women BMI > 30 | 30 g per day | 90 days | n = 11 |
| [66] Yancy and et al., 2004 |
18 to 65 y.o participants BMI > 30 | 20 g per day | 24 weeks | n = 60 |
| [67] Yancy and et al., 2007 |
18 to 65 y.o participants BMI > 30 | 20 g per day | 24 weeks | n = 21 |
| [68] Yancy and et al., 2010 |
18 to 70 y.o participants BMI > 30 | 20 g per day | 48 weeks | n = 52 |
| Clinical Measures | Glycemic Profile | Lipid Profile | Liver Profile | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Weight | BMI | Systolic BP | Diastolic BP | Fasting Glucose | Fasting Insulin | HOMA-IR | HbA1c | Total Cholesterol | HDL | LDL | TG | AST | ALT | Volume of Left Hepatic Lobe | Biopsy |
| [49] Ballard and et al., 2013 | ns | ↓ | ↓ | ns | ↓ | ↓ | ns | ns | ↓ | |||||||
| p = 0.01 | p = 0.001 | p < 0.01 | p < 001 | p < 0.001 | ||||||||||||
| [50] Carter and et al., 2006 | ↓ | ↓ | ||||||||||||||
| p < 0.0008 | p < 0.0001 | |||||||||||||||
| [51] Dashti and et al., 2006 | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | |||||||||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | ||||||||||
| ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | ||||||||||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | ||||||||||
| [52] Dashti and et al., 2007 | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | ||||||||||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||||||||||
| ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | |||||||||||
| p < 0.0001 | p < 0.0069 | p < 0.002 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||||||||||
| [53] François and et al., 2018 | ns | ↓ | ||||||||||||||
| p < 0.05 | ||||||||||||||||
| [54] Hallberg and et al., 2018 | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | ||||||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||||||
| [55] Hernandez and et al., 2010 | ↓ | ns | ↓ | ns | ns | ↓ | ||||||||||
| p < 0.0001 | p = 0.03 | p = 0.01 | ||||||||||||||
| [56] Hussain and et al., 2012 | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | |||||||||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | ||||||||||
| ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | |||||||||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.05 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||||||||
| [57] Johnstone et al., 2008 | ↓ | ↓ | ↓ | ↓ | ns | ns | ns | ns | ||||||||
| p = 0.006 | p < 0.001 | p < 0.001 | p < 0.001 | |||||||||||||
| [58] McKenzie and et al., 2017 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ns | ns | ↓ | ↓ | ↓ | ||||
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.009 | p < 0.001 | p < 0.001 | p < 0.001 | |||||||
| [59] Myette-Coté and et al., 2018 | ↓ | ↓ | ns | ns | ||||||||||||
| p < 0.001 | p < 0.001 | |||||||||||||||
| [60] Partsalaki and et al., 2012 | ↓ | ↓ | ns | ns | ns | ↓ | ↓ | ns | ns | ns | ns | |||||
| p < 0.001 | p < 0.001 | p = 0.017 | p = 0.014 | |||||||||||||
| [61] Saslow and et al., 2017 (1) | ↓ | ↓ | ns | ns | ns | ns | ↓ | ns | ns | ↓ | ||||||
| p < 0.001 | p < 0.001 | p = 0.007 | p = 0.022 | |||||||||||||
| [62] Saslow and et al., 2017 (2) | ↓ | ↓ | ns | ns | ↓ | |||||||||||
| p < 0.001 | p < 0.002 | p < 0.01 | ||||||||||||||
| [63] Schiavo and et al., 2018 | ↓ | ↓ | ↓ | ↓ | ns | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||
| p < 0.001 | p < 0.001 | p = 0.0108 | p < 0.001 | p < 0.001 | p < 0.0001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||||
| [64] Tendler and et al., 2007 | ↓ | ↓ | ns | ns | ns | ↓ steatosis (p = 0.02) ↓ necro-inflammation (p = 0.02) ↓ fibrosis (p = 0.07) | ||||||||||
| p < 0.036 | p < 0.006 | |||||||||||||||
| [65] Walton and et al., 2019 | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ns | ↓ | ns | ns | ||||||
| p < 0.001 | p < 0.0001 | p < 0.0001 | p < 0.005 | p < 0.0001 | p < 0.005 | p < 0.005 | ||||||||||
| [66] Yancy and et al., 2004 | ↓ | ns | ns | ↓ | ↑ | ns | ↓ | |||||||||
| p < 0.001 | p = 0.08 | p < 0.001 | p < 0.001 | |||||||||||||
| [67] Yancy and et al., 2007 | ns | |||||||||||||||
| [68] Yancy and et al., 2010 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ns | ↑ | ns | ↓ | ||||||
| p-val not available | p-val not available | p-val not available | p-val not available | p-val not available | p-val not available | p-val not available | p-val not available | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charlot, A.; Zoll, J. Beneficial Effects of the Ketogenic Diet in Metabolic Syndrome: A Systematic Review. Diabetology 2022, 3, 292-309. https://doi.org/10.3390/diabetology3020020
Charlot A, Zoll J. Beneficial Effects of the Ketogenic Diet in Metabolic Syndrome: A Systematic Review. Diabetology. 2022; 3(2):292-309. https://doi.org/10.3390/diabetology3020020
Chicago/Turabian StyleCharlot, Anouk, and Joffrey Zoll. 2022. "Beneficial Effects of the Ketogenic Diet in Metabolic Syndrome: A Systematic Review" Diabetology 3, no. 2: 292-309. https://doi.org/10.3390/diabetology3020020
APA StyleCharlot, A., & Zoll, J. (2022). Beneficial Effects of the Ketogenic Diet in Metabolic Syndrome: A Systematic Review. Diabetology, 3(2), 292-309. https://doi.org/10.3390/diabetology3020020







