Dietary Choline Deprivation Exacerbates Cardiomyopathy in Streptozotocin-Induced Diabetic Adult Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials
2.3. Experimental Procedure
2.4. Echocardiographic Assessment
2.5. Biochemical Assessment
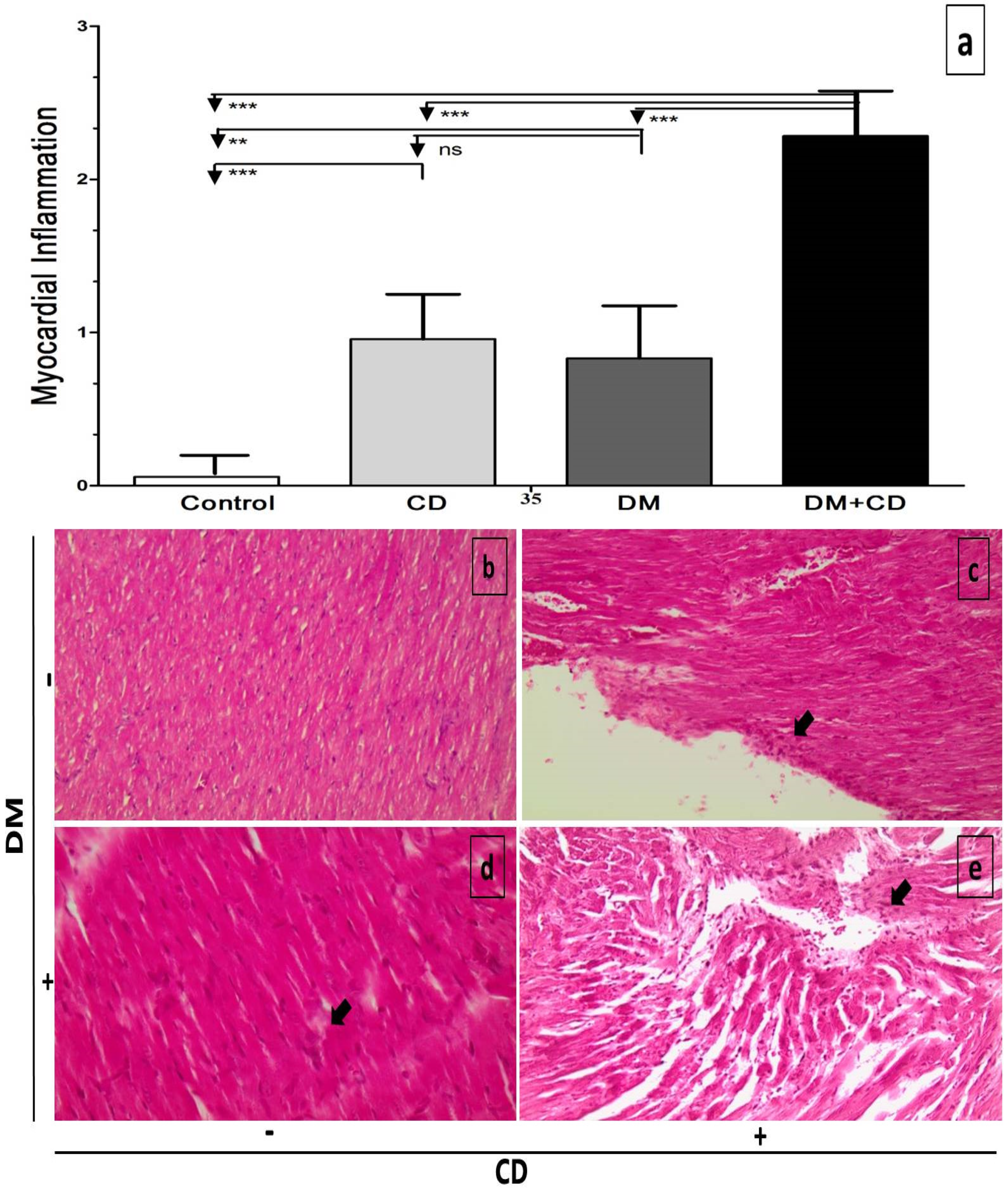
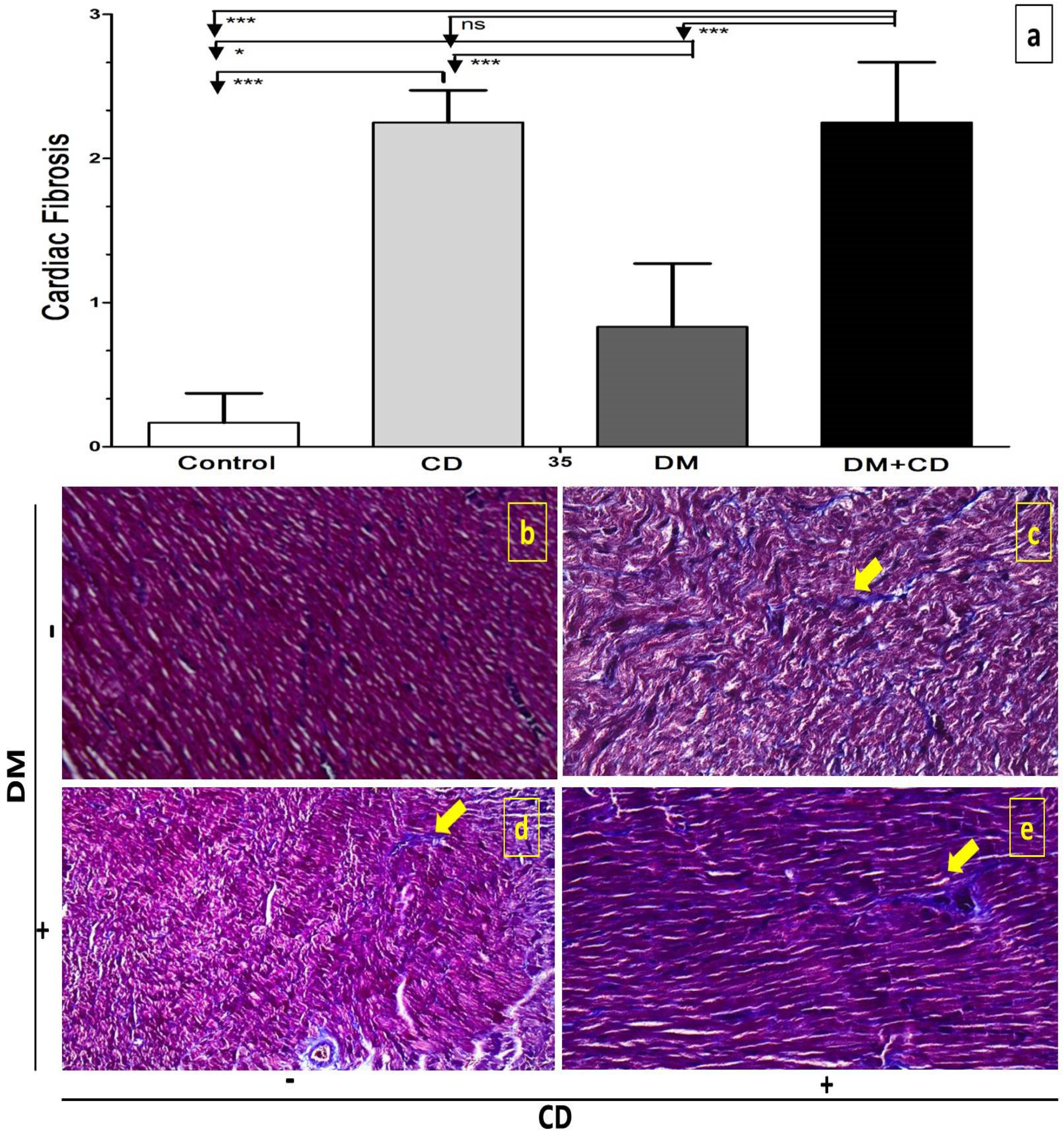
2.6. Histological Assessment
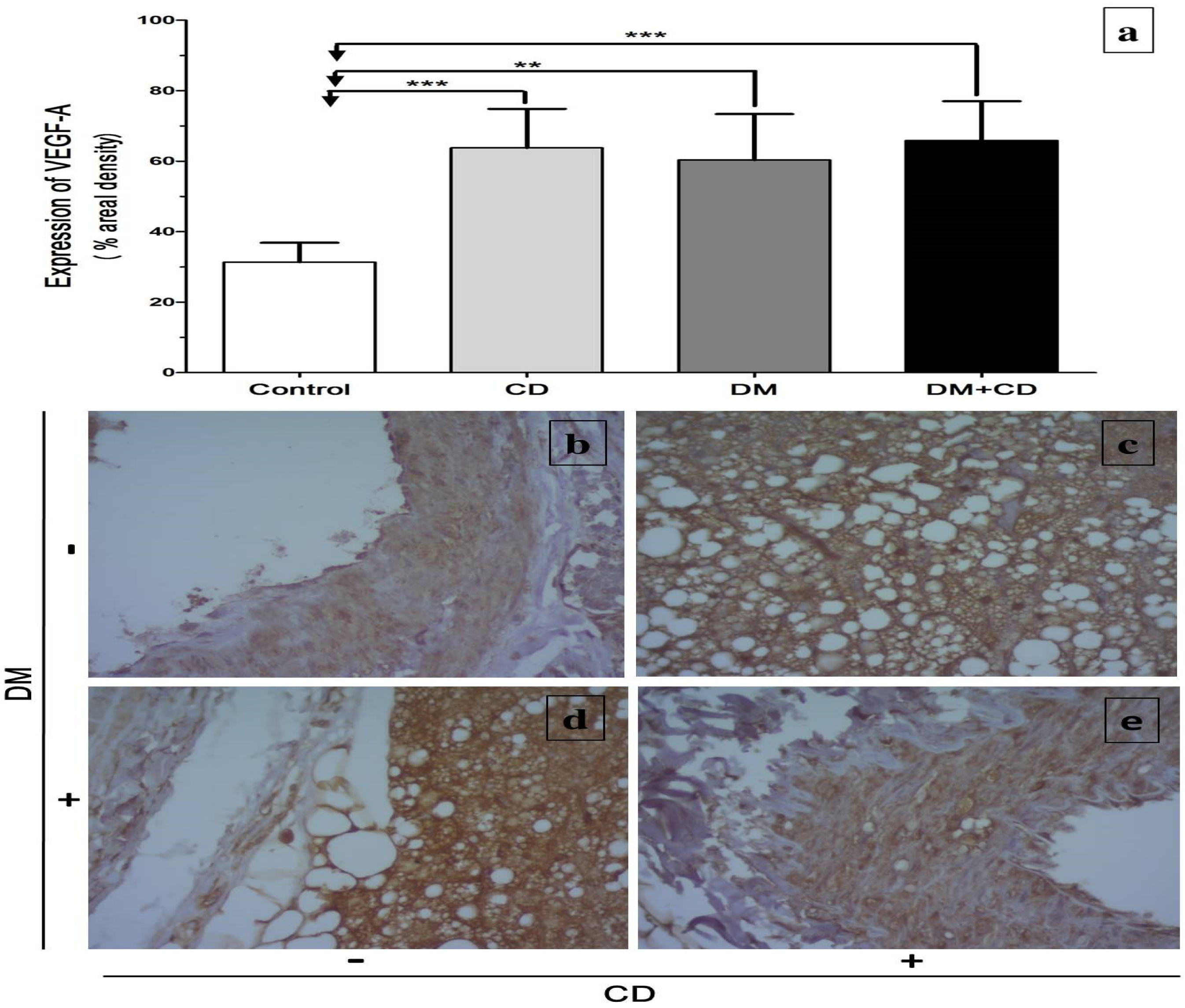
2.7. Immunohistochemical Assessment of Vascular Endothelial Factor-A165 (VEGF)
2.8. Statistical Analysis
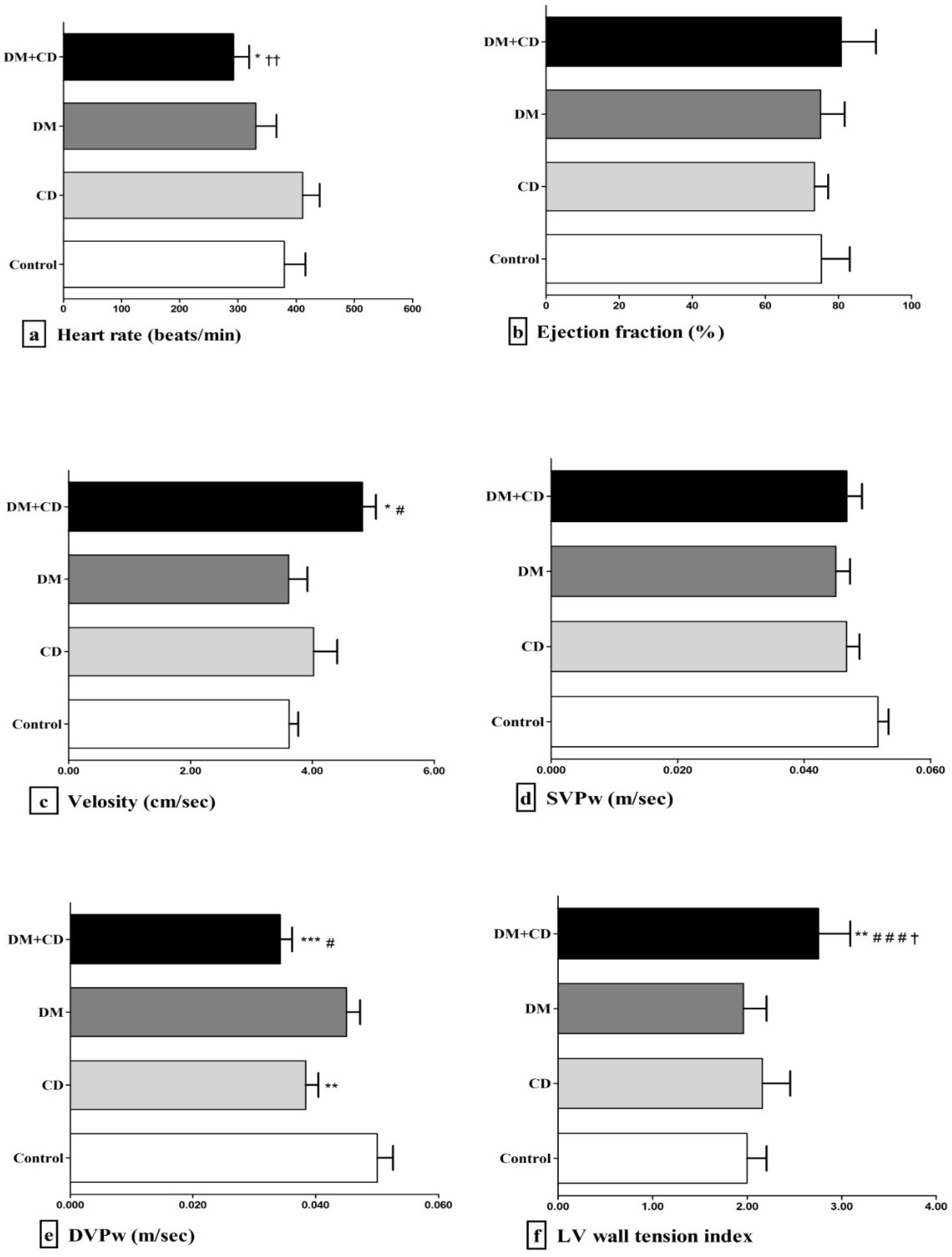
3. Results
4. Discussion
5. Conclusions
6. Clinical Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeisel, S.H.; Blusztajn, J.K. Choline and human nutrition. Annu. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Food and Nutrition Board. Choline. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998; pp. 390–422. [Google Scholar]
- European Food Safety Authority. Dietary reference values for choline. EFSA J. 2016, 14, e04484. [Google Scholar]
- Canty, D.J.; Zeisel, S.H. Lecithin and choline in human health and disease. Nutr. Rev. 1994, 52, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L.; Dubin, M.; Jenden, D.; Moukarzel, A.; Roch, M.H.; Rice, K.; Gornbein, J.; Ament, M.E.; Eckhert, C.D. Lecithin increases plasma free choline and decreases hepatic steatosis in long-term total parenteral nutrition patients. Gastroenterology 1992, 102, 1363–1370. [Google Scholar] [CrossRef]
- Konstandi, M.; Segos, D.; Galanopoulou, P.; Theocharis, S.; Zarros, A.; Lang, M.A. Effects of choline-deprivation on paracetamol- or phenobarbital-induced rat liver metabolic response. J. Appl. Toxicol. 2009, 29, 101–109. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: An essential nutrient for humans. Nutrition 2000, 16, 669–671. [Google Scholar] [CrossRef]
- Strilakou, A.A.; Lazaris, A.C.; Perelas, A.I.; Mourouzis, I.; Douzis, I.C.; Karkalousos, P.L.; Stylianaki, A.T.; Pantos, C.I.; Liapi, C.A. Heart dysfunction induced by choline-deficiency in adult rats: The protective role of l-carnitine. Eur. J. Pharmacol. 2013, 709, 20–27. [Google Scholar] [CrossRef]
- Padrón-Barthe, L.; Orero, M.V.; Gómez-Salinero, J.M.; Acin-Perez, R.; Cogliati, S.; López-Olañeta, M.; Ortiz-Sánchez, P.; Bonzon-Kulichenko, E.; Vázquez, J.; García-Pavía, P.; et al. Activation of Serine One-Carbon Metabolism by Calcineurin Aβ1 Reduces Myocardial Hypertrophy and Improves Ventricular Function. J. Am. Coll. Cardiol. 2018, 71, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, B.; Pani, P.; Schlunk, F.F. Choline-deficiency fatty liver: Impaired release of hepatic triglycerides. J. Lipid Res. 1968, 9, 437–446. [Google Scholar] [CrossRef]
- Veteläinen, R.; van Vliet, A.; van Gulik, T.M. Essential pathogenic and metabolic differences in steatosis induced by choline or methione-choline deficient diets in a rat model. J. Gastroenterol. Hepatol. 2007, 22, 1526–1533. [Google Scholar] [CrossRef]
- da Costa, K.A.; Badea, M.; Fischer, L.M.; Zeisel, S.H. Elevated serum creatine phosphokinase in choline-deficient humans: Mechanistic studies in C2C12 mouse myoblasts. Am. J. Clin. Nutr. 2004, 80, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L. The addition of choline to parenteral nutrition. Gastroenterology 2009, 137, S119–S128. [Google Scholar] [CrossRef]
- Al-Humadi, H.; Theocharis, S.; Dontas, I.; Stolakis, V.; Zarros, A.; Kyriakaki, A.; Al-Saigh, R.; Liapi, C. Hepatic injury due to combined choline-deprivation and thioacetamide administration: An experimental approach to liver diseases. Dig. Dis. Sci. 2012, 57, 3168–3177. [Google Scholar] [CrossRef]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef]
- Guerrerio, A.L.; Colvin, R.M.; Schwartz, A.K.; Molleston, J.P.; Murray, K.F.; Diehl, A.; Mohan, P.; Schwimmer, J.B.; Lavine, J.E.; Torbenson, M.S.; et al. Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2012, 95, 892–900. [Google Scholar] [CrossRef]
- Yu, D.; Shu, X.O.; Xiang, Y.B.; Li, H.; Yang, G.; Gao, Y.-T.; Zheng, W.; Zhang, X. Higher dietary choline intake is associated with lower risk of nonalcoholic fatty liver in normal-weight Chinese women. J. Nutr. 2014, 144, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhang, L.; Li, T.; Lopaschuk, G.; Vance, D.E.; Jacobs, R.L. Choline Deficiency Attenuates Body Weight Gain and Improves Glucose Tolerance in ob/ob Mice. J. Obes. 2012, 2012, 319172. [Google Scholar] [CrossRef] [PubMed]
- Strilakou, A.; Perelas, A.; Lazaris, A.; Papavdi, A.; Karkalousos, P.; Giannopoulou, I.; Kriebardis, A.; Panayiotides, I.; Liapi, C. Immunohistochemical determination of the extracellular matrix modulation in a rat model of choline-deprived myocardium: The effects of carnitine. Fundam. Clin. Pharmacol. 2016, 30, 47–57. [Google Scholar] [CrossRef]
- Liu, L.; Lu, Y.; Bi, X.; Xu, M.; Yu, X.; Xue, R.; He, X.; Zang, W. Choline ameliorates cardiovascular damage by improving vagal activity and inhibiting the inflammatory response in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 42553. [Google Scholar] [CrossRef]
- Factor, S.M.; Okun, E.M.; Minase, T. Capillary microaneurysms in the human diabetic heart. N. Engl. J. Med. 1980, 302, 384–388. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Techigawara, M.; Ishihata, T.; Asakura, T.; Saito, F.; Maehara, K.; Maruyama, Y. Comparison of ultrastructural changes on endomyocardial biopsy specimens obtained from patients with diabetes mellitus with and without hypertension. Heart Vessel. 1997, 12, 267–274. [Google Scholar] [CrossRef]
- De Boer, R.A.; Pinto, Y.M.; Van Veldhuisen, D.J. The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: The role of microvascular growth and abnormalities. Microcirculation 2003, 10, 113–126. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Touyz, R.M.; Herrmann, J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis. Oncol. 2018, 2, 13. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001, 106, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Fulgoni, V.L. 3rd Assessment of total choline intakes in the United States. J. Am. Coll. Nutr. 2016, 35, 108–112. [Google Scholar] [CrossRef]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Natker, E.; Zeisel, S.H.; Zelman, K.M. Choline: The Underconsumed and Underappreciated Essential Nutrient. Nutr Today 2018, 53, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Union. Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. J. Eur. Union 1986, 358, 1–28. [Google Scholar]
- Zarros, A.; Liapi, C.; Galanopoulou, P.; Marinou, K.; Mellios, Z.; Skandali, N.; Al-Humadi, H.; Anifantaki, F.; Gkrouzman, E.; Tsakiris, S. Effects of adult-onset streptozotocin-induced diabetes on the rat brain antioxidant status and the activities of acetylcholinesterase, (Na(+),K (+))-and Mg(2+)-ATPase: Modulation by l-cysteine. Metab. Brain Dis. 2009, 24, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Pantos, C.; Mourouzis, I.; Markakis, K.; Dimopoulos, A.; Xinaris, C.; Kokkinos, A.D.; Panagiotou, M.; Cokkinos, D.V. Thyroid hormone attenuates cardiac remodeling and improves hemodynamics early after acute myocardial infarction in rats. Eur. J. Cardiothorac. Surg. 2007, 32, 333–339. [Google Scholar] [CrossRef]
- Pantos, C.; Mourouzis, I.; Markakis, K.; Tsagoulis, N.; Panagiotou, M.; Cokkinos, D.V. Long-term thyroid hormone administration reshapes left ventricular chamber and improves cardiac function after myocardial infarction in rats. Basic Res. Cardiol. 2008, 103, 308–318. [Google Scholar] [CrossRef]
- Grossman, W.; Jones, D.; McLaurin, L.P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulou, I.; Mourouzis, I.; Lambrou, G.; Iliopoulou, D.; Koutsouris, D.; Pantos, C. Time-dependent and independent effects of thyroid hormone administration following myocardial infarction in rats. Mol. Med. Rep. 2018, 18, 864–876. [Google Scholar] [CrossRef]
- Chen, Y.X.; Nakashima, Y.; Tanaka, K.; Shiraishi, S.; Nakagawa, K.; Sueishi, K. Immunohistochemical expression of vascular endothelial growth factor/vascular permeability factor in atherosclerotic intimas of human coronary arteries. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.; Pellicori, P.; Zhang, J.; Weston, J.; Clark, A.L. Agreement and Classification Performance of Malnutrition Tools in Patients with Chronic Heart Failure. Curr. Dev. Nutr. 2020, 4, nzaa071. [Google Scholar] [CrossRef] [PubMed]
- Aurigemma, G.P.; Zile, M.R.; Gaasch, W.H. Response to Letter Regarding Article “Contractile Behavior of the Left Ventricle in Diastolic Heart Failure: With Emphasis on Regional Systolic Function”. Circulation 2006, 113, 3296–3304. [Google Scholar] [CrossRef]
- Rosenberg, M.A.; Manning, W.J. Diastolic dysfunction and risk of atrial fibrillation: A mechanistic appraisal. Circulation 2012, 126, 2353–2362. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Anderson, A.S. The sympathetic nervous system and heart failure. Cardiol. Clin. 2014, 32, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.J.; Park, M.H.; Lee, T.K.; Choi, S.H. Healing Effect of Platelet-rich Plasma on Decellularized Tracheal Allotransplantation in Rabbits. Vivo 2018, 32, 1433–1441. [Google Scholar] [CrossRef]
- Schaan, B.D.; Dall’Ago, P.; Maeda, C.Y.; Ferlin, E.; Fernandes, T.G.; Schmid, H.; Irigoyen, M.C. Relationship between cardiovascular dysfunction and hyperglycemia in streptozotocin-induced diabetes in rats. Braz. J. Med. Biol. Res. 2004, 37, 1895–1902. [Google Scholar] [CrossRef]
- Vinik, A.I.; Maser, R.E.; Mitchell, B.D.; Freeman, R. Diabetic autonomic neuropathy. Diabetes Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef]
- Roy, A.; Guatimosim, S.; Prado, V.F.; Gros, R.; Prado, M.A. Cholinergic activity as a new target in diseases of the heart. Mol. Med. 2015, 20, 527–537. [Google Scholar] [CrossRef]
- Das, A.V.; Padayatti, P.S.; Paulose, C.S. Effect of leaf extract of Aegle Marmelose (L.) Correa ex Roxb. On histological and ultrastructural changes in tissues of Streptozotocin induced diabetic rats. Indian J. Exp. Biol. 1996, 34, 341–345. [Google Scholar]
- Ohno, T.; Horio, F.; Tanaka, S.; Terada, M.; Namikawa, T.; Kitch, J. Fatty liver and hyperlipidemia in IDDM (insulin dependent diabetes mellitus) of Streptozotocin treated shrews. Life Sci. 2000, 66, 125–131. [Google Scholar] [CrossRef]
- Dobrin, J.S.; Lebeche, D. Diabetic cardiomyopathy: Signaling defects and therapeutic approaches. Expert Rev. Cardiovasc. Ther. 2010, 8, 373–391. [Google Scholar] [CrossRef][Green Version]
- Guha, M.; Bai, W.; Nadler, J.L.; Natarajan, R. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. J. Biol. Chem. 2000, 275, 17728–17739. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Qin, J.; Bu, P. Pathways involved in interleukin-1β-mediated murine cardiomyocyte apoptosis. Tex. Heart Inst. J. 2015, 42, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Gori, T.; Keaney, J.F., Jr.; Maack, C.; Daiber, A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart J. 2015, 36, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Niculescu, M.D. Modern Nutrition in Health and Disease, 10th ed.; Shils, M.E., Moshe, S., Shike, M., Ross, A.C., Caballero, B., Cousins, R.J., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; p. 531. [Google Scholar]
- Strunz, C.M.C.; Roggerio, A.; Cruz, P.L.; Pacanaro, A.P.; Salemi, V.M.C.; Benvenuti, L.A.; Mansur, A.D.P.; Irigoyen, M.C. Down-regulation of fibroblast growth factor 2 and its co-receptors heparan sulfate proteoglycans by resveratrol underlies the improvement of cardiac dysfunction in experimental diabetes. J. Nutr. Biochem. 2017, 40, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Dayanand, C.D.; Krishnamurthy, N.; Ashakiran, S.; Shashidhar, K.N. Carnitine: A novel health factor–an overview. Int. J. Pharm. Biomed. Res. 2011, 2, 79–89. [Google Scholar]
- Mingorance, C.; Rodriguez-Rodriguez, R.; Justo, M.L.; Herrera, M.D.; de Sotomayor, M.A. Pharmacological effects and clinical applications of propionyl-L-carnitine. Nutr. Rev. 2011, 69, 279–290. [Google Scholar] [CrossRef]
- Lin, Y.; Rajala, M.W.; Berger, J.P.; Moller, D.E.; Barzilai, N.; Scherer, P.E. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J. Biol. Chem. 2001, 276, 42077–42083. [Google Scholar] [CrossRef]
- Westermann, D.; Rutschow, S.; Jäger, S.; Linderer, A.; Anker, S.; Riad, A.; Unger, T.; Schultheiss, H.-P.; Pauschinger, M.; Tschöpe, C. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: The role of angiotensin type 1 receptor antagonism. Diabetes 2007, 56, 641–646. [Google Scholar] [CrossRef]
- Al-Humadi, H.; Zarros, A.; Kyriakaki, A.; Al-Saigh, R.; Liapi, C. Choline deprivation: An overview of the major hepatic metabolic response pathways. Scand. J. Gastroenterol. 2012, 47, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Japp, A.G.; Gulati, A.; Cook, S.A.; Cowie, M.R.; Prasad, S.K. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2996–3010. [Google Scholar] [CrossRef] [PubMed]
- Liapi, C.; Al-Humadi, H.; Zarros, A.; Galanopoulou, P.; Stolakis, V.; Gkrouzman, E.; Mellios, Z.; Skandali, N.; Anifantaki, F.; Tsakiris, S. Combined thirty-day exposure to thioacetamide and choline-deprivation alters serum antioxidant status and crucial brain enzyme activities in adult rats. Metab. Brain Dis. 2009, 24, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Al-Humadi, H.; Alhumadi, A.; Al-Saigh, R.; Strilakou, A.; Lazaris, A.C.; Gazouli, M.; Liapi, C. Extracellular matrix remodelling in the liver of rats subjected to dietary choline deprivation and/or thioacetamide administration. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1245–1256. [Google Scholar] [CrossRef]
- Battiprolu, P.K.; Gillette, T.G.; Wang, Z.V.; Lavandero, S.; Hill, J.A. Diabetic Cardiomyopathy: Mechanisms and Therapeutic Targets. Drug Discov. Today Dis. Mech. 2010, 7, e135–e143. [Google Scholar] [CrossRef] [PubMed]
- Tarquini, R.; Lazzeri, C.; Pala, L.; Rotella, C.M.; Gensini, G.F. The diabetic cardiomyopathy. Acta Diabetol. 2011, 48, 173–181. [Google Scholar] [CrossRef]
- Miki, T.; Yuda, S.; Kouzu, H.; Miura, T. Diabetic cardiomyopathy: Pathophysiology and clinical features. Heart Fail. Rev. 2013, 18, 149–166. [Google Scholar] [CrossRef]
- Liu, D.; Song, J.; Ji, X.; Liu, Z.; Cong, M.; Hu, B. Association of genetic polymorphisms on VEGFA and VEGFR2 with risk of coronary heart disease. Medicine 2016, 95, e3413. [Google Scholar] [CrossRef] [PubMed]
- Mehedint, M.G.; Craciunescu, C.N.; Zeisel, S.H. Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 12834–12839. [Google Scholar] [CrossRef] [PubMed]
- Kaji, K.; Yoshiji, H.; Kitade, M.; Ikenaka, Y.; Noguchi, R.; Shirai, Y.; Aihara, Y.; Namisaki, T.; Yoshii, J.; Yanase, K.; et al. Combination treatment of angiotensin II type I receptor blocker and new oral iron chelator attenuates progression of nonalcoholic steatohepatitis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1094–G1104. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi-Yorimoto, A.; Noto, T.; Yamada, A.; Miyamae, Y.; Oishi, Y.; Matsumoto, M. Persistent fibrosis in the liver of choline-deficient and iron-supplemented l-amino acid-defined diet-induced nonalcoholic steatohepatitis rat due to continuing oxidative stress after choline supplementation. Toxicol. Appl. Pharmacol. 2013, 268, 264–277. [Google Scholar] [CrossRef]
- Albright, C.D.; Siwek, D.F.; Craciunescu, C.N.; Mar, M.-H.; Kowall, N.W.; Williams, C.L.; Zeisel, S.H. Choline availability during embryonic development alters the localization of calretinin in developing and aging mouse hippocampus. Nutr. Neurosci. 2003, 6, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006, 20, 43–49. [Google Scholar] [CrossRef]
- Gogiraju, R.; Bochenek, M.L.; Schäfer, K. Angiogenic Endothelial Cell Signaling in Cardiac Hypertrophy and Heart Failure. Front. Cardiovasc. Med. 2019, 6, 20. [Google Scholar] [CrossRef]
- Givvimani, S.; Tyagi, N.; Sen, U.; Mishra, P.K.; Qipshidze, N.; Munjal, C.; Vacek, J.C.; Abe, O.A.; Tyagi, S.C. MMP-2/TIMP-2/TIMP-4 versus MMP-9/TIMP-3 in transition from compensatory hypertrophy and angiogenesis to decompensatory heart failure. Arch. Physiol. Biochem. 2010, 116, 63–72. [Google Scholar] [CrossRef][Green Version]
- Givvimani, S.; Kundu, S.; Narayanan, N.; Armaghan, F.; Qipshidze, N.; Pushpakumar, S.; Vacek, T.P.; Tyagi, S.C. TIMP-2 mutant decreases MMP-2 activity and augments pressure overload induced LV dysfunction and heart failure. Arch. Physiol. Biochem. 2013, 119, 65–74. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, W.; Chen, Y.; Ahokas, R.A.; Sun, Y. Vascular endothelial growth factor (VEGF)-A: Role on cardiac angiogenesis following myocardial infarction. Microvasc. Res. 2010, 80, 188–194. [Google Scholar] [CrossRef]
- Kim, P.; Chu, N.; Davis, J.; Kim, D.H. Mechanoregulation of Myofibroblast Fate and Cardiac Fibrosis. Adv. Biosyst. 2018, 2, 1700172. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.M.; da Costa, K.A.; Kwock, L.; Galanko, J.; Zeisel, S.H. Dietary choline requirements of women: Effects of estrogen and genetic variation. Am. J. Clin. Nutr. 2010, 92, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Krinsley, J.S. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin. Proc. 2004, 79, 992–1000. [Google Scholar] [CrossRef]
| Groups | TC (mg/dL) | TG (mg/dL) | HDL (mg/dL) | LDL (mg/dL) |
|---|---|---|---|---|
| Control | 75.17 ± 8.84 | 61.17 ± 36.55 | 52.83 ± 7.94 | 10.10 ± 15.18 |
| DM | 72.20 ± 12.38 | 137.00 ± 47.93 * | 35.80 ± 5.22 * | 9.00 ± 8.28 |
| CD | 59.50 ± 12.34 | 72.00 ± 22.67 | 38.33 ± 11.48 * | 6.77 ± 4.57 |
| DM + CD | 69.33 ± 12.04 | 86.00 ± 64.50 | 46.50 ± 9.81 | 5.63 ± 6.13 |
| Group | Heart Weight (g) | Long Axis (cm) | LVDd (cm) | LVDs (cm) | LVPw (cm) | FS (%) | LA (cm) |
|---|---|---|---|---|---|---|---|
| Control | 1.258 ± 0.203 | 1.67 ± 0.02 | 0.68 ± 0.06 | 0.35 ± 0.09 | 0.18 ± 0.01 | 49 ± 11.17 | 0.29 ± 0.02 |
| DM | 0.995 ± 0.093 | 1.66 ± 0.02 | 0.64 ± 0.03 | 0.38 ± 0.07 | 0.16 ± 0.02 | 44 ± 7.77 | 0.31 ± 0.02 |
| CD | 1.134 ± 0.141 | 1.70 ± 0.09 | 0.68 ± 0.06 | 0.38 ± 0.07 | 0.17 ± 0.02 | 39.83 ± 8.75 | 0.39 ± 0.04 **,## |
| DM + CD | 1.001 ± 0.099 | 1.63 ± 0.04 | 0.70 ± 0.03 # | 0.37 ± 0.08 | 0.13 ± 0.01 **,†,# | 44.66 ± 13.30 | 0.40 ± 0.03 **,## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Humadi, A.; Strilakou, A.; Al-Humadi, H.; Al-Saigh, R.; Agapitos, E.; Mourouzis, I.; Al-Najim, W.; Liapi, C. Dietary Choline Deprivation Exacerbates Cardiomyopathy in Streptozotocin-Induced Diabetic Adult Rats. Diabetology 2021, 2, 190-204. https://doi.org/10.3390/diabetology2040017
Al-Humadi A, Strilakou A, Al-Humadi H, Al-Saigh R, Agapitos E, Mourouzis I, Al-Najim W, Liapi C. Dietary Choline Deprivation Exacerbates Cardiomyopathy in Streptozotocin-Induced Diabetic Adult Rats. Diabetology. 2021; 2(4):190-204. https://doi.org/10.3390/diabetology2040017
Chicago/Turabian StyleAl-Humadi, Ahmed, Athina Strilakou, Hussam Al-Humadi, Rafal Al-Saigh, Emmanouel Agapitos, Iordanis Mourouzis, Werd Al-Najim, and Charis Liapi. 2021. "Dietary Choline Deprivation Exacerbates Cardiomyopathy in Streptozotocin-Induced Diabetic Adult Rats" Diabetology 2, no. 4: 190-204. https://doi.org/10.3390/diabetology2040017
APA StyleAl-Humadi, A., Strilakou, A., Al-Humadi, H., Al-Saigh, R., Agapitos, E., Mourouzis, I., Al-Najim, W., & Liapi, C. (2021). Dietary Choline Deprivation Exacerbates Cardiomyopathy in Streptozotocin-Induced Diabetic Adult Rats. Diabetology, 2(4), 190-204. https://doi.org/10.3390/diabetology2040017






