Abstract
Individuals with type 1 diabetes suffer from impaired angiogenesis, decreased capillarization, and higher fatigability that influence their muscular system beyond the detriments caused by decreased glycemic control. In order to combat exacerbations of these effects, the American Diabetes Association recommends that individuals with type 1 diabetes participate in regular resistance exercise. However, traditional resistance exercise only induces hypertrophy when loads of ≥65% of an individual’s one repetition maximum are used. Combining blood flow restriction with resistance exercise may serve as a more efficient means for stimulating anabolic pathways that result in increased protein synthesis and angiogenesis at lower loads, while also promoting better glycemic control. The purpose of this paper is to provide a review on the literature surrounding the benefits of resistance exercise, specifically for individuals with type 1 diabetes, and postulate potential effects of combining resistance exercise with blood flow restriction in this clinical population.
1. Introduction
Type 1 diabetes (T1D) is an autoimmune condition in which the insulin-producing beta cells of the pancreas are destroyed [1]. Unfortunately, being the less common type of diabetes mellitus (comprising only 5–10% of cases), T1D has been studied less extensively than type 2 diabetes [2]. Despite the small percentage of cases, this amounts to approximately 1.25 million Americans suffering from T1D [3]. Individuals with T1D are 22% weaker and have a higher fatigability (29.4%) than individuals without diabetes [4]. Additionally, T1D typically results in further complications than impaired glycemic control, including endothelial dysfunction [5] and impaired angiogenic capacity due to increased downregulation of the vascular endothelial growth factor (VEGF-A) [6]. Reduction in these factors impairs muscle perfusion and exacerbates metabolic dysfunction.
Individuals with T1D are recommended by the American Diabetes Association to maintain a stringent exercise routine that incorporates resistance exercise (RE) [7]. Further, RE is suggested to be as safe as aerobic exercise in regard to glycemic control and risk of cardiovascular events [8]. However, a 2006 study by Plotnikoff et al. revealed that 64% of surveyed Canadian citizens with T1D self-reported that they were not reaching the recommended physical activity levels [9]. This is even greater than the proportion of the general population not reaching physical activity recommendations in the United States, at approximately 46% [10]. The strongest reported barrier to physical activity among individuals with T1D is fear of hypoglycemia [2], though other barriers include work schedule and low fitness levels. Altogether, greater perception of these barriers is associated with higher HbA1c levels (r = 0.203; p = 0.042) and a lower self-appraisal of wellbeing (r = −0.45; p < 0.001)—statistics that may be useful in explaining the discrepancy in strength and fatiguability between individuals with and without diabetes.
Since the hypertrophic effects of RE are not observed unless loads of ≥65% of an individual’s one repetition maximum (1RM) are utilized [11], this intensity may not seem appealing for an individual only beginning to incorporate strength training. It may not only be risky for individuals with T1D to engage in RE of this intensity but may also seem daunting to someone who must carefully manage blood glucose during and post-exercise. However, a modern addition to exercise training, blood flow restriction (BFR), may serve as a feasible gateway to a hypertrophy-inducing RE program. Though the use of BFR in T1D has yet to be studied, results from studies in healthy samples suggest there may be some benefit to its application when introducing an RE program in this clinical population. The purpose of this paper is to provide a brief review on the literature regarding RE in T1D, current knowledge regarding BFR in healthy individuals, and finally postulate potential effects of combining RE with BFR (BFR + RE) in individuals with T1D.
2. Benefits of RE for Individuals with T1D
2.1. Glycemic Control
The benefits of RE for individuals with T1D include preventing muscle mass loss that occurs with age, decreasing fat mass, lowering blood pressure, and preventing several pathological states [12]. While evidence is limited and somewhat conflicting [13,14], a 2012 meta-analysis concluded there was a tendency for RE programs to improve long-term glycemic control [14], and a 2014 systematic review concluded that there is a promising role for exercise in reducing glycated hemoglobin (HbA1c) levels, increasing acute circulating interleukin-6 (IL-6) levels, and lowering an individual’s daily exogenous insulin requirement [15]. As little as three weeks of RE was shown to significantly increase time spent in a euglycemic range for individuals with T1D when compared to control participants [16].
2.2. Cardiovascular Health
Individuals with T1D are also significantly more likely to experience symptoms or diagnoses of cardiovascular disease, largely due to accompanying glycemic irregularities and inflammation [17]. RE decreases systemic inflammation by reducing high-sensitive C-reactive protein (hs-CRP) [18]. This has vast implications, as CRP is indicative of systemic inflammation and is a key risk factor for cardiovascular diseases, even in asymptomatic individuals [19]. Because adipocytes produce CRP [20], several studies have associated weight loss with CRP reduction [21,22,23]. Notably, the main stimulus upregulating expression of the CRP gene is IL-6 [24]. Chronically elevated levels of CRP and IL-6 are correlated with a lower capacity for general physical function (e.g., grip strength, four-minute walk, and sit–stand tests)—a trend that is independent of age, gender, race, and disease status [25].
2.3. Aging
The pathophysiology of aging also appears to be accelerated in individuals with T1D, in which individuals may experience declines in muscle mass and altered mitochondrial structure and function [26], thereby increasing risk of physical and functional disability as they age [27]. As such, the increase in physical function afforded by RE could have significant effects on independence and wellbeing. RE is considered the most effective means of maintaining or improving the ability to perform daily functional tasks [28]. In older adults, this muscular strength is also vital in improving quality of life by minimizing fear of falling [29]. Additionally, there is an overall association between T1D and decreased bone mineral density (BMD), though meta-analysis of this body of research concludes this association depends on site, age, gender, and lifestyle factors [30]. Although there are many proposed mechanisms through which RE improves BMD, research in animal models suggest RE enhances differentiation of bone marrow mesenchymal stromal cells into osteoblasts, promoting bone growth [31,32].
3. Current Knowledge Regarding BFR
3.1. Implementation
Blood flow restriction employs use of either an inflatable cuff or a tourniquet around a muscle group or joint to limit arterial blood flow to the muscles distal to occlusion points [33]. Though the protocols employed in research vary drastically, there are several reviews of BFR available to the interested reader [34,35,36,37], and it is generally recommended that protocols for implementation of a BFR + RE program are individualized based on some general guidelines [33]. For example, a systematic review and meta-analysis of the literature deems determination of an individual’s limb occlusion pressure (LOP) an important component of a safe and effective BFR training program [38]. This then allows researchers and clinicians to determine a percentage of LOP at which to occlude blood flow. Though research on BFR utilizes a multitude of different occlusion pressures, Counts et al. demonstrated that 8 weeks of BFR at 40% and 90% LOP caused similar increases in muscle size, strength, and endurance [39], concluding that high pressures may not be necessary to achieve these desired effects. To maximize physiological stimulus, Scott et al. recommend that BFR be used in conjunction with low exercise loads (20–40% 1RM) and high training volumes (50–80 repetitions per exercise). This can vary depending on the participant’s level of accustomization to exercise, though. Clinicians and researchers seeking to implement BFR are encouraged to use the progression model proposed in the review by Loenneke et al., in which participants start with BFR during bed rest, then progress to BFR during walking, low-load BFR + RE, and finally low-load BFR + RE as well as high-load RE [40]. The mechanisms discussed in the present review are related to these aforementioned guidelines. It is important to note that, while not discussed here, there is evidence that high-load BFR + RE does not increase markers of myotrauma or inflammation [41], though this area of research is still developing.
3.2. Research Regarding BFR in Healthy Individuals
3.2.1. Glycemic Control
Skeletal muscle mass is critical in the maintenance of not only functional ability, but also glycemic control, as evidenced in bed rest and step reduction studies [42]. Where BFR + RE differs from traditional RE is, traditionally, hypertrophy-inducing resistance loads primarily enhance muscular protein synthesis via mechanotransduction and exercise-induced muscular damage [43,44]. BFR, conversely, enhances RE adaptations by inducing hypoxia and, therefore, metabolic stress during exercise [34]. By this, BFR + RE induces mechanisms of muscular hypertrophy even at loads as low as 20% of 1RM, increasing protein synthesis while limiting myofibrillar damage [45,46]. Indeed, it has been shown that BFR during barbell squats at 75% of 1RM does not elicit significant increases in plasma markers of exercise-induced muscular damage to a greater extent than observed in load-matched controls, though BFR does induce fatigue more rapidly [41].
BFR has been established as a resource for increasing muscle size and mass at low-resistance loads in several studies and systematic reviews of the literature [47,48,49], via mechanisms such as hypoxia-induced nNOS and p38 MAPK signaling [50,51,52], which could elicit long-term benefits for glycemic control. As evidenced in cultured myotubes, acute tissue hypoxia enhances translocation of GLUT to the cell membrane to facilitate glucose uptake into the myofiber [53]. Christiansen et al. demonstrated the long-term effects of this after a six-week training study, determining that, when exercise was performed with unilateral BFR, localized glucose uptake was higher after training in only the BFR leg when compared to baseline, directly correlating with increased GLUT4 and neuronal nitric oxide synthase (nNOS) [51]. This is important for myogenesis, as nNOS is known to initiate production of hepatocyte growth factor (HGF) by binding to the c-Met receptor on satellite cells, ultimately inducing their activation and proliferation, as cited in a review of the literature [54]. Additionally, p38 is a critical initiator of myogenesis involved in activating quiescent satellite cells and stimulating differentiation and fusion of myoblasts [55] and is phosphorylated to a significantly greater extent during BFR than non-BFR conditions [42].
In the short term, low-load BFR + RE may stimulate gluconeogenesis that could prevent post-exercise hypoglycemia. Low-load BFR + RE causes acute increases in muscle-derived IL-6 as non-occlusion RE [56], which is believed to play a role in stimulation of cortisol production and release from the adrenal cortex [57]. It is important to note that, while acute secretion of muscle-derived IL-6 is beneficial in stimulating glucose uptake, chronically elevated levels of circulating IL-6 are detrimental, as discussed prior in this review.
3.2.2. Cardiovascular Health
Hypoxia from low-load BFR + RE stimulates also stimulates angiogenic mechanisms comparably [58] or to a greater extent (Ferguson 2018) than high-load RE without occlusion. Mechanistically, p38 MAPK activates transcription of PGC1-α [40], the dominant regulator of mitochondrial biogenesis [59]. Furthermore, a study in healthy adult human participants utilizing low-load (20% 1RM) BFR + RE demonstrated increased mRNA expression of multiple angiogenic markers including VEGF, VEGF-Receptor 2, hypoxia-inducible factor 1-alpha (HIF1-alpha), and endothelial nitric oxide synthase (eNOS) [50]. Further, the aforementioned study [50] and Patterson et al. report post-exercise VEGF levels that are significantly greater following low-load BFR + RE than low-load RE without occlusion [56]. Though not evidenced in RE, BFR walk training also increases carotid arterial compliance, suggesting that enhanced endothelial function may occur as a result of BFR-induced vascular remodeling [60].
Hypoxia also decreases inflammation by increasing antioxidant capacity. Hypoxic preconditioning, the endogenous adaptation to a brief period of hypoxia, activates the nuclear factor erythroid-derived 2-like-2 (NFE2L2) transcription factor to bind to the antioxidant response element (ARE), resulting in the transcription of genes for antioxidative enzymes such as heme oxygenase-1 (HO-1) and manganese superoxide dismutase (MnSOD) [61]. Similarly, hypoxia induced by low-load BFR + RE reduces systemic inflammation by increasing aerobic capacity and the abundance of antioxidant enzymes such as Cu/Zn-superoxide dismutase (SOD) and GPX-1 [51], which mitigate inflammatory damage caused by ROS [62,63]. For more information on the overarching effects of BFR on hemodynamics, we invite readers to see the systematic review by Neto et al. [64].
3.2.3. Aging
Both acute and chronic RE of either low- or high-intensity enhance circulating IGF-1 [65,66,67]. Further, IGF-1 increases over time with low-load BFR training programs [68]. For these reasons, BFR + RE may be effective in helping to prevent aging-related muscular atrophy [61] and neurodegeneration [69,70]. Additionally, low-load BFR + RE has demonstrated great efficacy in significantly elevating strength gain when compared to non-occlusion training [71,72]. Twelve weeks of low-load BFR + RE in older adults (mean of 75.6 years) produced strength gains comparable to high-load training, suggesting that BFR may be a suitable training alternative to delay or reverse muscle weakening or limitations in joint loading that present with age [73]. These increases in strength are supported by increases in myofibrillar protein synthesis. Six weeks of low-load BFR + RE increase muscle protein synthesis comparably to high-load RE without occlusion (1.34%/day compared to 1.12%/day) [74]. Despite its profound effect on improving strength at low loads, though, a review of the literature suggests that research on the direct impacts of BFR on bone metabolism is limited [75]. However, low-load BFR + RE causes a greater growth hormone response than high-load RE without occlusion [76]. Because growth hormone stimulates IGF-1 production and has a known anabolic effect on bone [77], consistent BFR + RE could provide benefits to BMD and should be researched.
4. Potential Benefits for BFR in Individuals with T1D
4.1. Glycemic Control
It has also been demonstrated that BFR + RE increases glucose uptake and glucose transporter (GLUT4) translocation to the membrane, while sometimes increasing and yet sometimes decreasing insulin [78]. This suggests the possibility that glucose uptake is being primarily driven via catecholamine (epinephrine and norepinephrine) stimulation under BFR + RE. Therefore, BFR training could serve as a safe mechanism in which individuals with T1D can strength train and utilize the glucose liberated during exercise without an additional insulin injection.
Mechanistically, muscle contractions stimulate glucose uptake via increases in intracellular calcium, leading to phosphorylation of calmodulin-dependent protein kinase II (CAMKII) [79]. Additionally, AMP-activated protein kinase (AMPK) is activated upon exercise, which phosphorylates histone deacetylase-5 (HDAC5) and protein kinase B (PKB/Akt), both promoting GLUT4 docking in the plasma membrane [80]. CAMKII and AMPK are proposed to act together to increase GLUT4 translocation independent of insulin [81]. Moreover, skeletal muscle contraction acutely produces reactive oxygen species (ROS) such as superoxide (O2-) and nitric oxide (NO) [79]. Superoxide forms peroxynitrite and hydrogen peroxide, which further stimulate AMPK signaling of GLUT, while NO activates PKG via cyclic GMP (cGMP). Since ROS-mediated mechanisms are potent stimulators of glucose uptake and act independently of insulin, this, in conjunction with the aforementioned acute increase in IL-6, makes it is very plausible that BFR + RE could enhance post-exercise glycemic control in an individual with T1D and should be investigated. Further, since low-load BFR + RE enhances muscle hypertrophy, a training program of low-load BFR + RE could improve long-term glycemic control and, therefore, HbA1c levels. The implications of increased muscle mass and therefore increased glucose utility are substantial, as even the slightest reduction in HbA1c can induce significant alleviation of observed T1D pathologies. Furthermore, a 1% reduction in HbA1c is associated with a 16% reduction for risk of myocardial infarction and an estimated 14% reduction for risk of all-cause mortality [82]. A summary of the impacts of BFR + RE on glycemic control can be found in Figure 1.
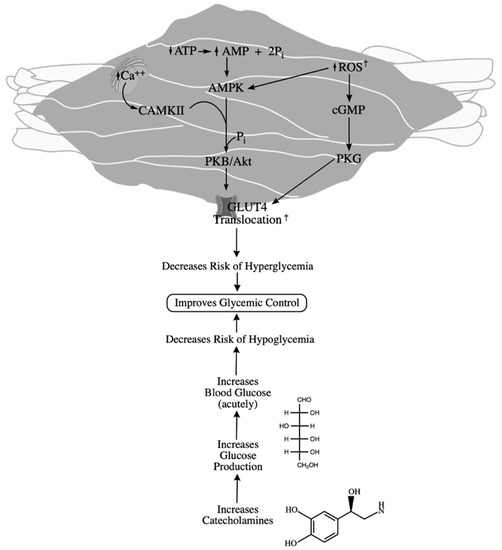
Figure 1.
Impacts of resistance exercise on glycemic stability. Contraction stimulates calcium release, activating CAMKII, and ATP utilization, activating AMPK. Both of these kinases stimulate PKB/Akt activity, upregulating GLUT translocation. Exercising the skeletal muscle also results in endogenous ROS production, which upregulates GLUT translocation both by AMPK and PKG. Increased glucose uptake by these mechanisms decreases the risk of hyperglycemia. Resistance exercise also decreases the risk of hypoglycemia by increasing catecholamine production, increasing glucose availability. † denotes outcomes exacerbated by BFR according to current literature.
4.2. Cardiovascular
Lowering risk of adverse cardiovascular events is especially important in individuals with T1D, who have a lessened capacity for endothelial regeneration and impaired angiogenesis [83,84]. This is thought to be caused by increased endothelial adhesion demonstrated by monocytes in individuals with T1D [85]. The chronic inflammation associated with T1D pathologies perturbs the function of systemic leukocytes, promoting a pro-inflammatory phenotype, thus promoting intravascular adhesion. The additional tissue hypoxia associated with BFR + RE could also promote increases in aerobic capacity through induction of mitochondrial biogenesis and angiogenesis, mitigating inflammation and damage to the endothelium common among individuals with T1D. The combined outcomes of RE and hypoxic stimulation of VEGF via BFR training could potentially elicit synergistic benefits in individuals with T1D. For these reasons, it has been proposed that BFR training may be an effective approach for individuals with T1D, especially with circulatory impairments or low fitness levels [86]. A summary of RE and BFR’s benefits for cardiovascular function is provided in Figure 2.

Figure 2.
The impacts resistance exercise has on cardiovascular-related outcomes. Aerobic capacity increases as a product of increased angiogenesis, through VEGF and other endogenous factors, as well as mitochondrial biogenesis via ROS and PGC1α signaling. The cardiovascular system also benefits from decreased adiposity as a moderator of systemic inflammation by decreasing chronic IL-6 and high-sensitive C-reactive protein. † denotes outcomes exacerbated by BFR according to current literature.
4.3. Aging
Individuals with T1D are at elevated risk of progressive motor dysfunction, which results in impaired ability to complete tasks of daily living. Though muscle atrophy in T1D is attributed to cellular damage from hyperglycemia and oxidative stress, these abnormalities in morphology of myofibers and mitochondria are also present in recently diagnosed individuals (duration from 1 to 28 weeks), even before neuropathy has occurred [87], asserting that some detriments of T1D can occur at disease onset. This risk of progressive motor dysfunction is due to lower-serum IGF-1, dysregulated hormone signaling, and increased cellular damage due to reduced oxidative capacity and increased chronic ROS production by the mitochondria [88,89,90,91], often manifesting as muscle weakness and exercise intolerance in individuals with type 1 diabetes [92]. RE prevents sarcopenia and gains in fat mass, and therefore oxidative stress, which may ensue as a result of insulin therapy in individuals with T1D [93]. However, it is important to note that, while chronic ROS can be detrimental to health, acute releases of ROS, such as during exercise, can promote mitochondrial biogenesis [94]. Decreased IGF-1 and dysregulated insulin also correlate with significantly lower BMD than observed in healthy peers, to the point that diabetes-related osteoporosis is a common comorbidity [88,95].
Pediatric research has shown that children with T1D who participated in weight-bearing activity for several months demonstrated increased BMD [96], though there are few, if any, studies that clearly demonstrate the impact RE has on IGF-1 production in individuals with T1D. However, increases in BMD due to RE are induced by osteogenic differentiation of mesenchymal stem cells, which appears to be at least partly regulated by NO production [32]. Because BFR increases inducible NOS (iNOS) [97], a prominent regulator of BMD [98], BFR + RE has the potential to enhance BMD in individuals with T1D. Additionally, hypoxia induced by low-intensity BFR + RE has been shown to increase circulating insulin-like growth factor (IGF-1) and muscle-bone cross sectional area [71], increase muscular strength, and provide cardiovascular benefits, all of which are fundamental in preventing age-related declines in physical function.
Finally, individuals with T1D who self-report less leisure-time physical activity are found to have a greater presence of diabetic complications, including nephropathy, retinopathy, and cardiovascular disease [99]. Thus, maintaining functional ability is critical for the T1D population. There are two prominent mechanisms responsible for the observed increase in strength stimulated by BFR + RE that is proposed to be common to individuals both with and without diabetes: metabolite accumulation and increased motor unit recruitment. BFR restricts venous return, and therefore, low-load BFR + RE results in post-exercise concentrations of metabolites and cell signaling molecules such as growth hormone, VEGF, IGF-1, norepinephrine, and lactate that are greater than or comparable to non-occluded training at the same load [56,71,86]. Additionally, BFR + RE results in greater post-activation potentiation than non-occlusion RE [100], which is greater in type II muscle fibers, suggesting that BFR accelerates fatigue and results in the need to recruit more, and likely larger, motor units. This increased recruitment is thought to contribute to the increases in strength associated with occlusion training. Thus, BFR + RE is proposed to be an effective tool for individuals with T1D to increase participation in non-exercise activities of daily life, simultaneously reducing sedentary behaviors that can exaggerate pathological conditions. Figure 3 provides a summary of the mechanisms by which BFR + RE contribute to increased muscle mass and strength.
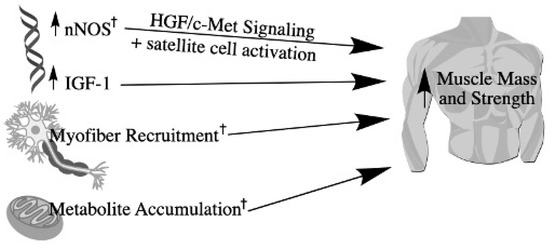
Figure 3.
Mechanisms by which resistance exercise and BFR increase muscle mass and strength. BFR-induced hypoxia stimulates nNOS signaling, leading to the production of hepatocyte growth factor (HGF), which binds to the c-Met receptor on satellite cells to activate their proliferation. Myofibrillar protein synthesis also increases following resistance exercise due to increased IGF-1. Muscle strength also increases as a product of hypoxia and venous occlusion from BFR, causing increased metabolite accumulation. This accelerates fatigue, requiring increased myofiber recruitment. † denotes outcomes exacerbated by BFR according to current literature.
5. Contraindications to BFR
To date, there has been no published research on the application of BFR + RE in individuals with T1D. This population faces vascular complications that could pose a health risk when using BFR, including lack of arterial compliance and increased ROS production. Therefore, it is pertinent to address some of the common concerns regarding the safety of BFR. While BFR + RE may amplify the pressor reflex, blood pressure has been found to return to baseline after cessation of the exercise, even resulting in greater post-exercise hypotension compared to high-load RE alone [101]. Further, training with BFR has been shown to increase large artery compliance similar to non-BFR training in adults aged 57–76 years [60]. Additionally, there is no published evidence that BFR during RE increases risk of venous thromboembolism. Therefore, it is likely that the use of BFR could be safely utilized in clinical populations, particularly if the individual is free of peripheral vascular disease.
6. Conclusions
The purpose of this review was to outline the mechanisms through which RE influences T1D-associated pathologies and BFR + RE may improve these outcomes, as summarized in Figure 4. Current literature suggests that RE successfully helps to manage glycemic control, increase muscle mass and strength, and decrease risk of cardiovascular disease—all of which decrease risk of mortality and improve quality of life. The addition of blood flow restriction augments some of the benefits of traditional resistance training while using significantly lighter loads than typically required during non-occluded training. Therefore, RE utilizing BFR could be an appropriate intervention to prevent the onset or further decline of T1D-associated pathologies. However, given the lack of research in this clinical population, it is clear that more research is needed to fully delineate the effects of BFR on key elements that would impact individuals with T1D.

Figure 4.
Benefits discussed in this review afforded by resistance exercise in combination with BFR from which individuals with type 1 diabetes could benefit, including increased glycemic control and muscle mass, both of which contribute to decreased HbA1c. Further, resistance exercise and BFR can increase strength and aerobic capacity while decreasing systemic inflammation, all of which contribute to decreased risk of diabetic complications, decreased risk of mortality, and improved quality of life.
Author Contributions
Conceptualization, M.T.J. and L.J.W.; writing—original draft preparation, M.T.J.; writing—review and editing, L.J.W. and E.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Brazeau, A.-S.; Rabasa-Lhoret, R.; Strychar, I.; Mircescu, H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 2008, 31, 2108–2109. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020; Department of Health and Human Services: Atlanta, GA, USA, 2020. [Google Scholar]
- Orlando, G.; Balducci, S.; Bazzucchi, I.; Pugliese, G.; Sacchetti, M. The impact of type 1 diabetes and diabetic polyneuropathy on muscle strength and fatigability. Acta Diabetol. 2017, 54, 543–550. [Google Scholar] [CrossRef]
- Začiragić, A.; Huskić, J.; Mulabegović, N.; Avdagić, N.; Valjevac, A.; Hasić, S.; Jadrić, R. An assessment of correlation between serum asymmetric dimethylarginine and glycated haemoglobin in patients with type 2 diabetes mellitus. Bosn. J. Basic Med. Sci. 2014, 14, 21–24. [Google Scholar] [CrossRef][Green Version]
- Jenkins, A.J.; Zhang, S.X.; Rowley, K.G.; Karschimkus, C.S.; Nelson, C.L.; Chung, J.S.; O’Neal, D.N.; Januszewski, A.S.; Croft, K.D.; Mori, T.A.; et al. Increased serum pigment epithelium-derived factor is associated with microvascular complications, vascular stiffness and inflammation in Type 1 diabetes. Diabet. Med. 2007, 24, 1345–1351. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the american diabetes association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Wróbel, M.; Rokicka, D.; Czuba, M.; Gołaś, A.; Pyka, Ł.; Greif, M.; Szymborska-Kajanek, A.; Strojek, K.; Gąsior, M. Aerobic as well as resistance exercises are good for patients with type 1 diabetes. Diabetes Res. Clin. Pr. 2018, 144, 93–101. [Google Scholar] [CrossRef]
- Plotnikoff, R.C.; Taylor, L.M.; Wilson, P.M.; Courneya, K.S.; Sigal, R.J.; Birkett, N.; Raine, K.; Svenson, L.W. Factors associated with physical activity in canadian adults with diabetes. Med. Sci. Sports Exerc. 2006, 38, 1526–1534. [Google Scholar] [CrossRef]
- Early Release of Selected Estimates Based on Data from the 2018 National Health Interview Survey CDC.gov: Centers for Disease Control and Prevention. 2018. Available online: https://www.cdc.gov/nchs/fastats/exercise.htm (accessed on 1 June 2021).
- Schoenfeld, B.J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef]
- Fiataraone Singh, M.; Hackett, D.; Schoenfeld, B.; Vincent, H.K.; Wescott, W. ACSM Guidelines for Strength Training: Resistance Training for Health. 2019. Available online: https://www.acsm.org/blog-detail/acsm-certified-blog/2019/07/31/acsm-guidelines-for-strength-training-featured-download (accessed on 1 June 2021).
- Kennedy, A.; Nirantharakumar, K.; Chimen, M.; Pang, T.T.; Hemming, K.; Andrews, R.; Narendran, P. Does Exercise Improve Glycaemic Control in Type 1 Diabetes? A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e58861. [Google Scholar] [CrossRef]
- Tonoli, C.; Heyman, E.; Roelands, B.; Buyse, L.; Cheung, S.S.; Berthoin, S.; Meeusen, R. Effects of different types of acute and chronic (training) exercise on glycaemic control in type 1 diabetes mellitus: A meta-analysis. Sports Med. 2012, 42, 1059–1080. [Google Scholar] [CrossRef] [PubMed]
- Yardley, J.E.; Hay, J.; Abou-Setta, A.M.; Marks, S.D.; McGavock, J. A systematic review and meta-analysis of exercise interventions in adults with type 1 diabetes. Diabetes Res. Clin. Pr. 2014, 106, 393–400. [Google Scholar] [CrossRef]
- Reddy, R.; Wittenberg, A.; Castle, J.R.; El Youssef, J.; Winters-Stone, K.; Gillingham, M.; Jacobs, P.G. Effect of aerobic and resistance exercise on glycemic control in adults with type 1 diabetes. Can. J. Diabetes 2018, 43, 406–414. [Google Scholar] [CrossRef] [PubMed]
- De Ferranti, S.D.; De Boer, I.H.; Fonseca, V.; Fox, C.S.; Golden, S.H.; Lavie, C.J.; Magge, S.N.; Marx, N.; McGuire, D.K.; Orchard, T.J. Type 1 diabetes mellitus and cardiovascular disease: A scientific statement from the American Heart Association and American Diabetes Association. Circulation 2014, 130, 1110–1130. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.A.; Neves, A.P.; Coelho-Silva, M.J.; Veríssimo, M.T.; Teixeira, A.M. The effect of aerobic versus strength-based training on high-sensitivity C-reactive protein in older adults. Graefe's Arch. Clin. Exp. Ophthalmol. 2010, 110, 161–169. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002, 347, 1557–1565. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131. [Google Scholar] [CrossRef]
- Hellbronn, L.K.; Noakes, M.; Clifton, P.M. Energy restriction and weight loss on very-low-fat diets reduce c-reactive protein concentrations in obese, healthy women. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 968–970. [Google Scholar] [CrossRef]
- Pannacciulli, N.; Cantatore, F.; Minenna, A.; Bellacicco, M.; Giorgino, R.; DE Pergola, G. C-reactive protein is independently associated with total body fat, central fat, and insulin resistance in adult women. Int. J. Obes. 2001, 25, 1416–1420. [Google Scholar] [CrossRef]
- Szalai, A.J.; Van Ginkel, F.W.; Dalrymple, S.A.; Murray, R.; McGhee, J.R.; Volanakis, J.E. Testosterone and IL-6 requirements for human C-reactive protein gene expression in transgenic mice. J. Immunol. 1998, 160, 5294–5299. [Google Scholar]
- Brinkley, T.E.; Leng, X.; Miller, M.E.; Kitzman, D.W.; Pahor, M.; Berry, M.J.; Marsh, A.P.; Kritchevsky, S.; Nicklas, B.J. Chronic Inflammation Is Associated with Low Physical Function in Older Adults Across Multiple Comorbidities. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2009, 64, 455–461. [Google Scholar] [CrossRef]
- Monaco, C.M.F.; Gingrich, M.A.; Hawke, T.J. Considering type 1 diabetes as a form of accelerated muscle aging. Exerc. Sport Sci. Rev. 2019, 47, 98–107. [Google Scholar] [CrossRef]
- Dhaliwal, R.; Weinstock, R.S. Management of type 1 diabetes in older adults. Diabetes Spectr. 2014, 27, 9–20. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance training for older adults: Position statement from the national strength and conditioning association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.; Donat, H.; Gelecek, N.; Ozdirenc, M.; Karadibak, D. The relationship between risk factors for falling and the quality of life in older adults. BMC Public Health 2005, 5, 90. [Google Scholar] [CrossRef]
- Pan, H.; Wu, N.; Yang, T.; He, W. Association between bone mineral density and type 1 diabetes mellitus: A meta-analysis of cross-sectional studies. Diabetes/Metab. Res. Rev. 2014, 30, 531–542. [Google Scholar] [CrossRef]
- Singulani, M.P.; Stringhetta-Garcia, C.T.; Santos, L.; Morais, S.; Louzada, M.; Oliveira, S.; Chaves-Neto, A.H.; Dornelles, R.C. Effects of strength training on osteogenic differentiation and bone strength in aging female Wistar rats. Sci. Rep. 2017, 7, 42878. [Google Scholar] [CrossRef]
- Ocarino, N.; Boeloni, J.; Goes, A.; Silva, J.; Marubayashi, U.; Serakides, R. Osteogenic differentiation of mesenchymal stem cells from osteopenic rats subjected to physical activity with and without nitric oxide synthase inhibition. Nitric Oxide 2008, 19, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Loenneke, J.P.; Slattery, K.M.; Dascombe, B.J. Exercise with blood flow restriction: An updated evidence-based approach for enhanced muscular development. Sports Med. 2014, 45, 313–325. [Google Scholar] [CrossRef]
- Pearson, S.J.; Hussain, S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2014, 45, 187–200. [Google Scholar] [CrossRef]
- Pope, Z.K.; Willardson, J.M.; Schoenfeld, B.J. Exercise and blood flow restriction. J. Strength Cond. Res. 2013, 27, 2914–2926. [Google Scholar] [CrossRef]
- Abe, T.; Loenneke, J.P.; Fahs, C.A.; Rossow, L.M.; Thiebaud, R.S.; Bemben, M.G. Exercise intensity and muscle hypertrophy in blood flow-restricted limbs and non-restricted muscles: A brief review. Clin. Physiol. Funct. Imaging 2012, 32, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Lixandrão, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceição, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: A systematic review and meta-analysis. Sports Med. 2018, 48, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef]
- Counts, B.R.; Dankel, S.; Barnett, B.E.; Kim, D.; Mouser, J.G.; Allen, K.M.; Thiebaud, R.S.; Abe, T.; Bemben, M.G.; Loenneke, J.P. Influence of relative blood flow restriction pressure on muscle activation and muscle adaptation. Muscle Nerve 2015, 53, 438–445. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Abe, T.; Wilson, J.M.; Thiebaud, R.S.; Fahs, C.A.; Rossow, L.M.; Bemben, M.G. Blood flow restriction: An evidence based progressive model (Review). Acta Physiol. Hung. 2012, 99, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Winchester, L.J.; Morris, C.E.; Badinger, J.; Wiczynski, T.L.; VanWye, W.R. Blood flow restriction at high resistance loads increases the rate of muscular fatigue, but does not increase plasma markers of myotrauma or inflammation. J. Strength Cond. Res. 2020, 34, 2419–2426. [Google Scholar] [CrossRef]
- Thyfault, J.P.; Krogh-Madsen, R. Metabolic disruptions induced by reduced ambulatory activity in free-living humans. J. Appl. Physiol. 2011, 111, 1218–1224. [Google Scholar] [CrossRef]
- Evans, W.J.; Cannon, J.G. The metabolic effects of exercise-induced muscle damage. Exerc. Sport Sci. Rev. 1991, 19, 99–125. [Google Scholar] [CrossRef]
- Hornberger, T.A. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int. J. Biochem. Cell Biol. 2011, 43, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Fales, J.T.; Heisey, S.R.; Zierler, K.L. Blood flow from and oxygen uptake by muscle, during and after partial venous occlusion. Am. J. Physiol. 1962, 203, 470–474. [Google Scholar] [CrossRef]
- Kawada, S. What phenomena do occur in blood flow-restricted muscle? Int. J. KAATSU Train. Res. 2005, 1, 37–44. [Google Scholar] [CrossRef]
- Fry, C.S.; Glynn, E.L.; Drummond, M.J.; Timmerman, K.L.; Fujita, S.; Abe, T.; Dhanani, S.; Volpi, E.; Rasmussen, B. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J. Appl. Physiol. 2010, 108, 1199–1209. [Google Scholar] [CrossRef]
- Lopes, K.G.; Bottino, D.A.; Farinatti, P.; de Souza, M.d.G.C.; Maranhão, P.A.; de Araujo, C.M.S.; Bouskela, E.; Lourenco, R.A.; de Oliviera, R.B. Strength training with blood flow restriction—A novel therapeutic approach for older adults with sarcopenia? A case report. Clin. Interv. Aging. 2019, 14, 1461. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.; Stannard, M.S.; Duren, D.L.; Cook, J.L.; Stannard, J.P. Does blood flow restriction therapy in patients older than age 50 result in muscle hypertrophy, increased strength, or greater physical function? A systematic review. Clin. Orthop. Relat. Res. 2019, 478, 593–606. [Google Scholar] [CrossRef]
- Ferguson, R.A.; Hunt, J.E.A.; Lewis, M.P.; Martin, N.R.W.; Player, D.J.; Stangier, C.; Taylor, C.W.; Turner, M.C. The acute angiogenic signalling response to low-load resistance exercise with blood flow restriction. Eur. J. Sport Sci. 2017, 18, 397–406. [Google Scholar] [CrossRef]
- Christiansen, D.; Eibye, K.; Hostrup, M.; Bangsbo, J. Blood flow-restricted training enhances thigh glucose uptake during exercise and muscle antioxidant function in humans. Metabolism 2019, 98, 1–15. [Google Scholar] [CrossRef]
- Ozaki, H.; Kakigi, R.; Kobayashi, H.; Loenneke, J.P.; Abe, T.; Naito, H. Effects of walking combined with restricted leg blood flow on mTOR and MAPK signalling in young men. Acta Physiol. 2014, 211, 97–106. [Google Scholar] [CrossRef]
- Sakagami, H.; Makino, Y.; Mizumoto, K.; Isoe, T.; Takeda, Y.; Watanabe, J.; Fujita, Y.; Takiyama, Y.; Abiko, A.; Haneda, M. Loss of HIF-1α impairs GLUT4 translocation and glucose uptake by the skeletal muscle cells. Am. J. Physiol. Metab. 2014, 306, E1065–E1076. [Google Scholar] [CrossRef]
- Anderson, J.E.; Wozniak, A.C. Satellite cell activation on fibers: Modeling events in vivo—An invited review. Can. J. Physiol. Pharmacol. 2004, 82, 300–310. [Google Scholar] [CrossRef]
- Segalés, J.; Perdiguero, E.; Muñoz-Cánoves, P. Regulation of muscle stem cell functions: A Focus on the p38 MAPK signaling pathway. Front. Cell Dev. Biol. 2016, 4, 91. [Google Scholar] [CrossRef]
- Patterson, S.D.; Leggate, M.; Nimmo, M.; Ferguson, R.A. Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Graefe's Arch. Clin. Exp. Ophthalmol. 2012, 113, 713–719. [Google Scholar] [CrossRef]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Constant, J.S.; Feng, J.J.; Zabel, D.D.; Yuan, H.; Suh, D.Y.; Scheuenstuhl, H.; Hunt, T.K.; Hussain, M.Z. Lactate elicits vascular endothelial growth factor from macrophages: A possible alternative to hypoxia. Wound Repair Regen. 2000, 8, 353–360. [Google Scholar] [CrossRef]
- Kelly, D.P.; Scarpulla, R.C. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004, 18, 357–368. [Google Scholar] [CrossRef]
- Ozaki, H.; Miyachi, M.; Nakajima, T.; Abe, T. Effects of 10 weeks walk training with leg blood flow reduction on carotid arterial compliance and muscle size in the elderly adults. Angiology 2010, 62, 81–86. [Google Scholar] [CrossRef]
- Huang, X.-S.; Chen, H.-P.; Yu, H.-H.; Yan, Y.-F.; Liao, Z.-P.; Huang, Q.-R. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for hypoxic preconditioning-mediated delayed cardioprotection. Mol. Cell. Biochem. 2013, 385, 33–41. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Włodarski, A.; Strycharz, J.; Wróblewski, A.; Kasznicki, J.; Drzewoski, J.; Śliwińska, A. The role of microRNAs in metabolic syndrome-related oxidative stress. Int. J. Mol. Sci. 2020, 21, 6902. [Google Scholar] [CrossRef]
- Neto, G.R.; Novaes, J.S.; Dias, I.B.F.; Brown, A.; Vianna, J.; Cirilo-Sousa, M.S. Effects of resistance training with blood flow restriction on haemodynamics: A systematic review. Clin. Physiol. Funct. Imaging 2016, 37, 567–574. [Google Scholar] [CrossRef]
- Borst, S.E.; De Hoyos, D.V.; Garzarella, L.; Vincent, K.; Pollock, B.H.; Lowenthal, D.T.; Pollock, M.L. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med. Sci. Sports Exerc. 2001, 33, 648–653. [Google Scholar] [CrossRef]
- Gregory, S.M.; Spiering, B.A.; Alemany, J.A.; Tuckow, A.P.; Rarick, K.R.; Staab, J.S.; Hatfield, D.L.; Kraemer, W.J.; Maresh, C.M.; Nindl, B.C. Exercise-induced insulin-like growth factor I system concentrations after training in women. Med. Sci. Sports Exerc. 2013, 45, 420–428. [Google Scholar] [CrossRef]
- Vega, S.R.; Knicker, A.; Hollmann, W.; Bloch, W.; Struder, H.K. Effect of resistance exercise on serum levels of growth factors in humans. Horm. Metab. Res. 2010, 42, 982–986. [Google Scholar] [CrossRef]
- Centner, C.; Lauber, B.; Seynnes, O.R.; Jerger, S.; Sohnius, T.; Gollhofer, A.; König, D. Low-load blood flow restriction training induces similar morphological and mechanical Achilles tendon adaptations compared with high-load resistance training. J. Appl. Physiol. 2019, 127, 1660–1667. [Google Scholar] [CrossRef]
- Barton-Davis, E.R.; Shoturma, D.I.; Musaro, A.; Rosenthal, N.; Sweeney, H.L. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc. Natl. Acad. Sci. USA 1998, 95, 15603–15607. [Google Scholar] [CrossRef]
- Törpel, A.; Herold, F.; Hamacher, D.; Müller, N.G.; Schega, L. Strengthening the brain—Is resistance training with blood flow restriction an effective strategy for cognitive improvement? J. Clin. Med. 2018, 7, 337. [Google Scholar] [CrossRef]
- Abe, T.; Yasuda, T.; Midorikawa, T.; Sato, Y.; Kearns, C.F.; Inoue, K.; Koizumi, K.; Ishii, N. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int. J. KAATSU Train. Res. 2005, 1, 6–12. [Google Scholar] [CrossRef]
- Takarada, Y.; Takazawa, H.; Sato, Y.; Takebayashi, S.; Tanaka, Y.; Ishii, N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J. Appl. Physiol. 2000, 88, 2097–2106. [Google Scholar] [CrossRef]
- Cook, S.B.; LaRoche, D.P.; Villa, M.R.; Barile, H.; Manini, T.M. Blood flow restricted resistance training in older adults at risk of mobility limitations. Exp. Gerontol. 2017, 99, 138–145. [Google Scholar] [CrossRef]
- Sieljacks, P.; Wang, J.; Groennebaek, T.; Rindom, E.; Jakobsgaard, J.E.; Herskind, J.; Gravholt, A.; Moller, A.B.; Musci, R.V.; de Paoli, F.V.; et al. Six weeks of low-load blood flow restricted and high-load resistance exercise training produce similar increases in cumulative myofibrillar protein synthesis and ribosomal biogenesis in healthy males. Front. Physiol. 2019, 10, 649. [Google Scholar] [CrossRef]
- Bittar, S.T.; Pfeiffer, P.S.; Santos, H.H.; Cirilo-Sousa, M.S. Effects of blood flow restriction exercises on bone metabolism: A systematic review. Clin. Physiol. Funct. Imaging 2018, 38, 930–935. [Google Scholar] [CrossRef]
- Manini, T.M.; Yarrow, J.F.; Buford, T.W.; Clark, B.C.; Conover, C.F.; Borst, S.E. Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Horm. IGF Res. 2012, 22, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Gillberg, P.; Mallmin, H.; Petrén-Mallmin, M.; Ljunghall, S.; Nilsson, A.G. Two years of treatment with recombinant human growth hormone increases bone mineral density in men with idiopathic osteoporosis. J. Clin. Endocrinol. Metab. 2002, 87, 4900–4906. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakajima, T.; Koide, S.; Yasuda, T.; Hasegawa, T.; Yamasoba, T.; Obi, S.; Toyoda, S.; Nakamura, F.; Inoue, T.; Poole, D.C.; et al. Muscle hypertrophy following blood flow-restricted, low-force isometric electrical stimulation in rat tibialis anterior: Role for muscle hypoxia. J. Appl. Physiol. 2018, 125, 134–145. [Google Scholar] [CrossRef]
- Merry, T.L.; McConell, G.K. Skeletal muscle glucose uptake during exercise: A focus on reactive oxygen species and nitric oxide signaling. IUBMB Life 2009, 61, 479–484. [Google Scholar] [CrossRef]
- McGee, S.L.; Van Denderen, B.J.; Howlett, K.; Mollica, J.; Schertzer, J.D.; Kemp, B.; Hargreaves, M. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes 2008, 57, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Saatmann, N.; Zaharia, O.P.; Loenneke, J.P.; Roden, M.; Pesta, D.H. Effects of blood flow restriction exercise and possible applications in type 2 diabetes. Trends Endocrinol. Metab. 2021, 32, 106–117. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.W.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. Bmj 2000, 321, 405. [Google Scholar] [CrossRef] [PubMed]
- Waclawovsky, G.; Umpierre, D.; Figueira, F.R.; Lima, E.D.S.D.; Alegretti, A.P.; Schneider, L.; Matte, U.S.; Rodrigues, T.C.; Schaan, B. Exercise on progenitor cells in healthy subjects and patients with type 1 diabetes. Med. Sci. Sports Exerc. 2016, 48, 190–199. [Google Scholar] [CrossRef]
- Kivelä, R.; Silvennoinen, M.; Touvra, A.-M.; Lehti, M.; Kainulainen, H.; Vihko, V. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. FASEB J. 2006, 20, 1570–1572. [Google Scholar] [CrossRef]
- Dosquet, C.; Weill, D.; Wautier, J.L. Molecular mechanism of blood monocyte adhesion to vascular endothelial cells. Nouv. Rev. Francaise D'hematologie 1992, 34, S55–S59. [Google Scholar]
- Takano, H.; Morita, T.; Iida, H.; Kato, M.; Uno, K.; Hirose, K.; Matsumoto, A.; Takenaka, K.; Hirata, Y.; Furuichi, T.; et al. Effects of low-intensity “KAATSU” resistance exercise on hemodynamic and growth hormone responses. IJKTR 2012, 8, 13–18. [Google Scholar] [CrossRef]
- Krause, M.P.; Riddell, M.C.; Hawke, T.J. Effects of type 1 diabetes mellitus on skeletal muscle: Clinical observations and physiological mechanisms. Pediatr. Diabetes 2010, 12, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Raisingani, M.; Preneet, B.; Kohn, B.; Yakar, S. Skeletal growth and bone mineral acquisition in type 1 diabetic children; abnormalities of the GH/IGF-1 axis. Growth Horm. IGF Res. 2017, 34, 13–21. [Google Scholar] [CrossRef]
- Crowther, G.J.; Milstein, J.M.; Jubrias, S.A.; Kushmerick, M.J.; Gronka, R.K.; Conley, K.E. Altered energetic properties in skeletal muscle of men with well-controlled insulin-dependent (type 1) diabetes. Am. J. Physiol. Metab. 2003, 284, E655–E662. [Google Scholar] [CrossRef]
- Kacerovsky, M.; Brehm, A.; Chmelik, M.; Schmid, A.I.; Szendroedi, J.; Kacerovsky-Bielesz, G.; Nowotny, P.; Lettner, A.; Wolzt, M.; Jones, J.; et al. Impaired insulin stimulation of muscular ATP production in patients with type 1 diabetes. J. Intern. Med. 2011, 269, 189–199. [Google Scholar] [CrossRef]
- Monaco, C.M.F.; Hughes, M.C.; Ramos, S.V.; Varah, N.E.; Lamberz, C.; Rahman, F.A.; McGlory, C.; Tarnopolski, M.A.; Krause, M.P.; Laham, R.; et al. Altered mitochondrial bioenergetics and ultrastructure in the skeletal muscle of young adults with type 1 diabetes. Diabetologia 2018, 61, 1411–1423. [Google Scholar] [CrossRef]
- Nishitani, M.; Shimada, K.; Sunayama, S.; Masaki, Y.; Kume, A.; Fukao, K.; Sai, E.; Yamashita, H.; Ohmura, H.; Onishi, T.; et al. Impact of diabetes on muscle mass, muscle strength, and exercise tolerance in patients after coronary artery bypass grafting. J. Cardiol. 2011, 58, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; Byrne, N.; Sirikul, B.; Fernández, J.R.; Zuckerman, P.A.; Darnell, B.E.; Gower, B.A. Resistance Training Conserves Fat-free Mass and Resting Energy Expenditure Following Weight Loss. Obesity 2008, 16, 1045–1051. [Google Scholar] [CrossRef]
- Bouchez, C.L.; Devin, A. Mitochondrial Biogenesis and Mitochondrial Reactive Oxygen Species (ROS): A Complex Relationship Regulated by the cAMP/PKA Signaling Pathway. Cells 2019, 8, 287. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Lumpkin, C.K.; Bunn, R.C.; Kemp, S.F.; Fowlkes, J.L. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am. J. Physiol. Metab. 2005, 289, E735–E745. [Google Scholar] [CrossRef]
- McCarthy, O.; Moser, O.; Eckstein, M.L.; Deere, R.; Bain, S.C.; Pitt, J.; Bracken, R.M. Resistance isn’t futile: The physiological basis of the health effects of resistance exercise in individuals with type 1 diabetes. Front. Endocrinol. 2019, 10, 507. [Google Scholar] [CrossRef]
- Larkin, K.A.; MacNeil, R.G.; Dirain, M.; Sandesara, B.; Manini, T.M.; Buford, T.W. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med. Sci. Sports Exerc. 2012, 44, 2077–2083. [Google Scholar] [CrossRef] [PubMed]
- Watanuki, M.; Sakai, A.; Sakata, T.; Tsurukami, H.; Miwa, M.; Uchida, Y.; Watanabe, K.; Ikeda, K.; Nakamura, T. Role of Inducible Nitric Oxide Synthase in Skeletal Adaptation to Acute Increases in Mechanical Loading. J. Bone Miner. Res. 2002, 17, 1015–1025. [Google Scholar] [CrossRef]
- Wadén, J.F.; Forsblom, C.; Thorn, L.M.; Saraheimo, M.; Rosengård-Bärlund, M.; Heikkilä, O.; Lakka, T.A.; Tikkanen, H.; Groop, P.-H. Physical activity and diabetes complications in patients with type 1 diabetes: The finnish diabetic nephropathy (FinnDiane) study. Diabetes Care 2008, 31, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Burgomaster, K.A.; Schofield, L.M.; Gibala, M.J.; Sale, D.G.; Phillips, S.M. Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. Eur. J. Appl. Physiol. 2004, 92, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood flow restriction exercise: Considerations of methodology, application, and safety. Front. Physiol. 2019, 10, 533. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).