Quinoa’s Potential to Enhance Dietary Management of Obesity and Type-2 Diabetes: A Review of the Current Evidence
Abstract
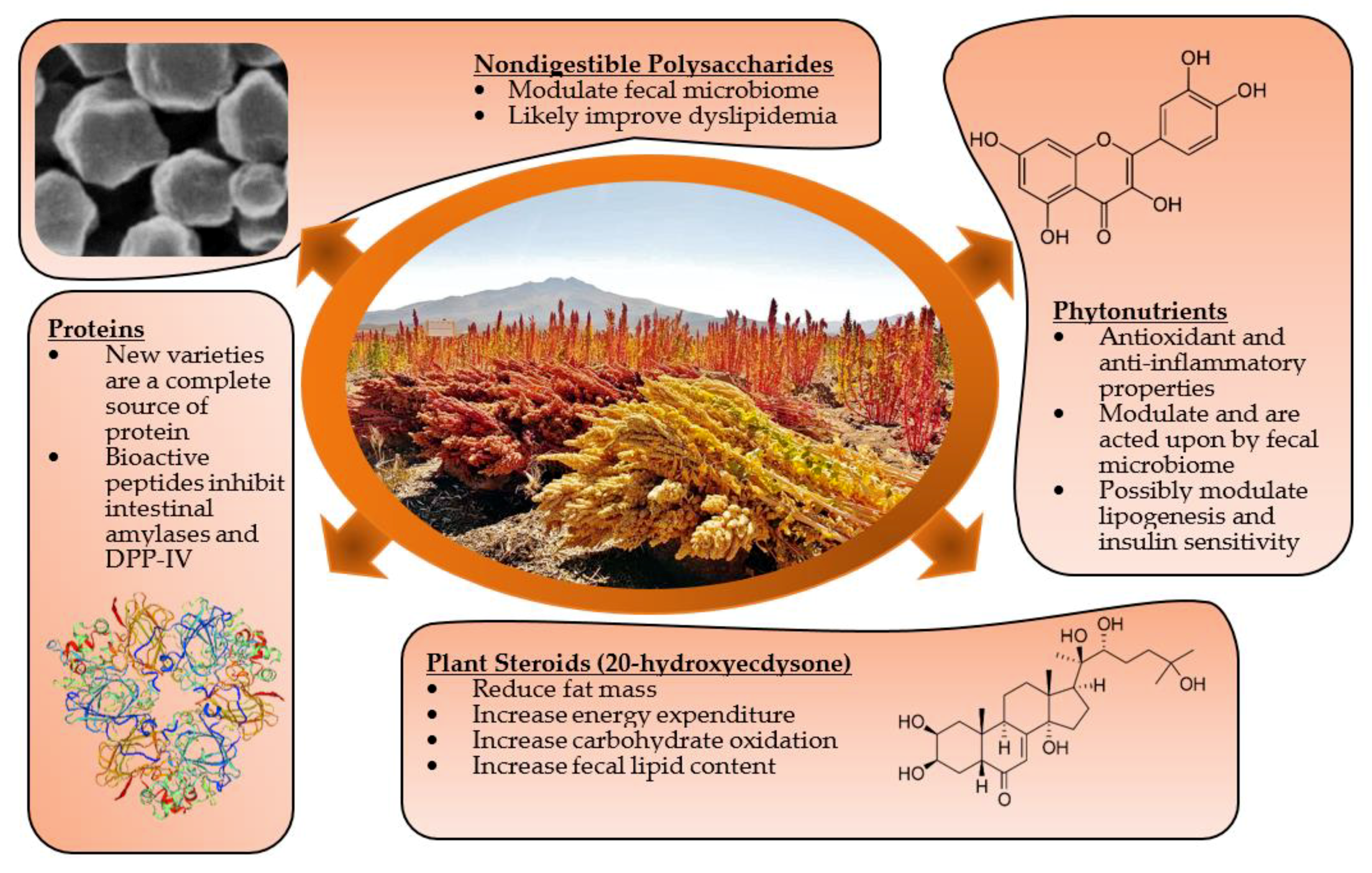
:1. Introduction
2. In Vivo Experiments with Quinoa
2.1. Rodent Studies
2.2. Human Studies
3. Quinoa Bioactive Components Characterized by In Vitro Research
3.1. Effects on Adipogenesis in the 3T3-L1 Adipocyte Model
3.2. Phytochemicals
3.3. Bioactive Peptides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Centers for Disease Control and Prevention. The Health Effects of Overweight and Obesity. Available online: https://www.cdc.gov/healthyweight/effects/index.html (accessed on 1 October 2020).
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8. [Google Scholar]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Insulin Resistance and Diabetes. Available online: https://www.cdc.gov/diabetes/basics/insulin-resistance.html (accessed on 14 February 2021).
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2020; pp. 12–15.
- Finkelstein, E.A.; Trogdon, J.G.; Cohen, J.W.; Dietz, W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff. 2009, 28, w822–w831. [Google Scholar] [CrossRef] [Green Version]
- Functional Food and Health: A Paradigm Shift in Agriculture. Available online: https://www.mdpi.com/journal/agriculture/special_issues/functional_food#info (accessed on 15 September 2020).
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.M.; Matanguihan, J.B.; Fuentes, F.F.; Gómez-Pando, L.R.; Jellen, E.N.; Maughan, P.J.; Jarvis, D.E. Quinoa breeding and genomics. Plant Breed. Rev. 2018, 42, 257–320. [Google Scholar]
- Aluwi, N.A.; Murphy, K.M.; Ganjyal, G.M. Physicochemical characterization of different varieties of quinoa. Cereal Chem. 2017, 94, 847–856. [Google Scholar] [CrossRef]
- Wu, G.; Ross, C.F.; Morris, C.F.; Murphy, K.M. Lexicon development, consumer acceptance, and drivers of liking of quinoa varieties. J. Food Sci. 2017, 82, 993–1005. [Google Scholar] [CrossRef]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kaniwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Tanwar, B.; Goyal, A.; Irshaan, S.; Kumar, V.; Sihag, M.K.; Patel, A.; Kaur, I. Quinoa. In Whole Grains and Their Bioactives: Composition and Health; Wiley: Hoboken, NJ, USA, 2019; pp. 269–305. [Google Scholar]
- Bazile, D.; Bertero, H.D.; Nieto, C. State of the Art Report on Quinoa around the World in 2013; FAO: Rome, Italy, 2015. [Google Scholar]
- Vega-Galvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martinez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Craine, E.B.; Murphy, K.M. Seed Composition and Amino Acid Profiles for Quinoa Grown in Washington State. Front. Nutr. 2020, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Han, P.; Li, Y.; Wang, W.; Lai, D.; Zhou, L. Quinoa Secondary Metabolites and Their Biological Activities or Functions. Molecules 2019, 24, 2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizelsztein, P.; Govorko, D.; Komarnytsky, S.; Evans, A.; Wang, Z.; Cefalu, W.T.; Raskin, I. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E433–E439. [Google Scholar] [CrossRef] [Green Version]
- Graf, B.L.; Poulev, A.; Kuhn, P.; Grace, M.H.; Lila, M.A.; Raskin, I. Quinoa seeds leach phytoecdysteroids and other compounds with anti-diabetic properties. Food Chem. 2014, 163, 178–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorelick-Feldman, J.; Maclean, D.; Ilic, N.; Poulev, A.; Lila, M.A.; Cheng, D.; Raskin, I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J. Agric. Food Chem. 2008, 56, 3532–3537. [Google Scholar] [CrossRef] [PubMed]
- Foucault, A.S.; Mathe, V.; Lafont, R.; Even, P.; Dioh, W.; Veillet, S.; Tome, D.; Huneau, J.F.; Hermier, D.; Quignard-Boulange, A. Quinoa extract enriched in 20-hydroxyecdysone protects mice from diet-induced obesity and modulates adipokines expression. Obesity 2012, 20, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Pasko, P.; Barton, H.; Zagrodzki, P.; Izewska, A.; Krosniak, M.; Gawlik, M.; Gawlik, M.; Gorinstein, S. Effect of diet supplemented with quinoa seeds on oxidative status in plasma and selected tissues of high fructose-fed rats. Plant Foods Hum. Nutr. 2010, 65, 146–151. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Ehrhardt, C.; Wuttke, W. Metabolic effects of 20-OH-ecdysone in ovariectomized rats. J. Steroid Biochem. Mol. Biol. 2010, 119, 121–126. [Google Scholar] [CrossRef]
- Toth, N.; Hunyadi, A.; Bathori, M.; Zador, E. Phytoecdysteroids and vitamin D analogues--similarities in structure and mode of action. Curr. Med. Chem. 2010, 17, 1974–1994. [Google Scholar] [CrossRef]
- Foucault, A.S.; Even, P.; Lafont, R.; Dioh, W.; Veillet, S.; Tome, D.; Huneau, J.F.; Hermier, D.; Quignard-Boulange, A. Quinoa extract enriched in 20-hydroxyecdysone affects energy homeostasis and intestinal fat absorption in mice fed a high-fat diet. Physiol. Behav. 2014, 128, 226–231. [Google Scholar] [CrossRef]
- Chen, Q.; Xia, Y.; Qiu, Z. Effect of ecdysterone on glucose metabolism in vitro. Life Sci. 2006, 78, 1108–1113. [Google Scholar] [CrossRef]
- Noratto, G.D.; Murphy, K.; Chew, B.P. Quinoa intake reduces plasma and liver cholesterol, lessens obesity-associated inflammation, and helps to prevent hepatic steatosis in obese db/db mouse. Food Chem. 2019, 287, 107–114. [Google Scholar] [CrossRef]
- Wang, B.; Chandrasekera, P.C.; Pippin, J.J. Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mazcorro, J.F.; Mills, D.; Noratto, G. Molecular exploration of fecal microbiome in quinoa-supplemented obese mice. FEMS Microbiol. Ecol. 2016, 92, fiw089. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Zou, L.; Li, W.; Song, Y.; Zhao, G.; Hu, Y. Dietary quinoa (Chenopodium quinoa Willd.) polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota. Int. J. Biol. Macromol. 2020, 163, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Hao, Y.; Richel, A.; Everaert, N.; Chen, Y.; Liu, M.; Yang, X.; Ren, G. Antihypertensive effect of quinoa protein under simulated gastrointestinal digestion and peptide characterization. J. Sci. Food Agric. 2020, 100, 5569–5576. [Google Scholar] [CrossRef]
- Guo, H.M.; Richel, A.; Hao, Y.Q.; Fan, X.; Everaert, N.; Yang, X.S.; Ren, G.X. Novel dipeptidyl peptidase-IV and angiotensin-I-converting enzyme inhibitory peptides released from quinoa protein by in silico proteolysis. Food Sci. Nutr. 2020, 8, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Pasko, P.; Zagrodzki, P.; Barton, H.; Chlopicka, J.; Gorinstein, S. Effect of quinoa seeds (Chenopodium quinoa) in diet on some biochemical parameters and essential elements in blood of high fructose-fed rats. Plant Foods Hum. Nutr. 2010, 65, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mithila, M.V.; Khanum, F. Effectual comparison of quinoa and amaranth supplemented diets in controlling appetite; a biochemical study in rats. J. Food Sci. Technol. 2015, 52, 6735–6741. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.O.; Barcelos, M.F.P.; Vieira, C.N.G.; de Abreu, W.C.; Ferreira, E.B.; Pereira, R.C.; de Angelis-Pereira, M.C. Effects of sprouted and fermented quinoa (Chenopodium quinoa) on glycemic index of diet and biochemical parameters of blood of Wistar rats fed high carbohydrate diet. J. Food Sci. Technol. 2019, 56, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Farinazzi-Machado, F.M.V.; Barbalho, S.M.; Oshiiwa, M.; Goulart, R.; Pessan, O. Use of cereal bars with quinoa (Chenopodium quinoa W) to reduce risk factors related to cardiovascular diseases. Food Sci. Technol. 2012, 32, 239–244. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, F.G.; Ovidio, P.P.; Padovan, G.J.; Jordao Junior, A.A.; Marchini, J.S.; Navarro, A.M. Metabolic parameters of postmenopausal women after quinoa or corn flakes intake--a prospective and double-blind study. Int. J. Food Sci. Nutr. 2014, 65, 380–385. [Google Scholar] [CrossRef]
- Navarro-Perez, D.; Radcliffe, J.; Tierney, A.; Jois, M. Quinoa Seed Lowers Serum Triglycerides in Overweight and Obese Subjects: A Dose-Response Randomized Controlled Clinical Trial. Curr. Dev. Nutr. 2017, 1, e001321. [Google Scholar] [CrossRef] [Green Version]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 2019, 1, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Shi, Z.; Yao, Y.; Ren, G. Structural Characterization of Quinoa Polysaccharide and Its Inhibitory Effects on 3T3-L1 Adipocyte Differentiation. Foods 2020, 9, 1511. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Cummings, J.H. Digestion of the polysaccharides of some cereal foods in the human small intestine. Am. J. Clin. Nutr. 1985, 42, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.K.; Rankin, S.A.; Lee, M.R.; Lee, W.J. Development and Characterization of Whey Protein-Based Nano-Delivery Systems: A Review. Molecules 2019, 24, 3254. [Google Scholar] [CrossRef] [Green Version]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.X.; Hao, Y.Q.; Teng, C.; Yao, Y.; Ren, G.X. Functional properties and adipogenesis inhibitory activity of protein hydrolysates from quinoa (Chenopodium quinoa Willd.). Food Sci. Nutr. 2019, 7, 2103–2112. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Zhu, Y.Y.; Gao, Y.; Shi, Z.X.; Hu, Y.B.; Ren, G.X. Suppressive effects of saponin-enriched extracts from quinoa on 3T3-L1 adipocyte differentiation. Food Funct. 2015, 6, 3282–3290. [Google Scholar] [CrossRef]
- del Hierro, J.N.; Reglero, G.; Martin, D. Chemical Characterization and Bioaccessibility of Bioactive Compounds from Saponin-Rich Extracts and Their Acid-Hydrolysates Obtained from Fenugreek and Quinoa. Foods 2020, 9, 1159. [Google Scholar] [CrossRef]
- Han, Y.; Chi, J.; Zhang, M.; Zhang, R.; Fan, S.; Huang, F.; Xue, K.; Liu, L. Characterization of saponins and phenolic compounds: Antioxidant activity and inhibitory effects on alpha-glucosidase in different varieties of colored quinoa (Chenopodium quinoa Willd). Biosci. Biotechnol. Biochem. 2019, 83, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Caravaca, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Simultaneous determination of phenolic compounds and saponins in quinoa (Chenopodium quinoa Willd) by a liquid chromatography-diode array detection-electrospray ionization-time-of-flight mass spectrometry methodology. J. Agric. Food Chem. 2011, 59, 10815–10825. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.L.; Rojo, L.E.; Delatorre-Herrera, J.; Poulev, A.; Calfio, C.; Raskin, I. Phytoecdysteroids and flavonoid glycosides among Chilean and commercial sources of Chenopodium quinoa: Variation and correlation to physico-chemical characteristics. J. Sci. Food Agric. 2016, 96, 633–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escribano, J.; Cabanes, J.; Jimenez-Atienzar, M.; Ibanez-Tremolada, M.; Gomez-Pando, L.R.; Garcia-Carmona, F.; Gandia-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamudio, F.V.; Campos, M.R.S. Amaranth, quinoa and chia bioactive peptides: A comprehensive review on three ancient grains and their potential role in management and prevention of Type 2 diabetes. Crit. Rev. Food Sci. 2020. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Martinez-Villaluenga, C.; Hernandez-Ledesma, B. Release of dipeptidyl peptidase IV, alpha-amylase and alpha-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; Le Maux, S.; Dubrulle, C.; Barre, C.; FitzGerald, R.J. Quinoa (Chenopodium quinoa Willd.) protein hydrolysates with in vitro dipeptidyl peptidase IV (DPP-IV) inhibitory and antioxidant properties. J. Cereal Sci. 2015, 65, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Vilcacundo, R.; Miralles, B.; Carrillo, W.; Hernandez-Ledesma, B. In vitro chemopreventive properties of peptides released from quinoa (Chenopodium quinoa Willd.) protein under simulated gastrointestinal digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef]
| Intervention | Method | Model | Parameters | Findings | Reference |
|---|---|---|---|---|---|
| Diet + Supplement | Duration: 13 weeks | Mouse—6 wk, male | Blood: Glucose, insulin, and adiponectin | Compared to the high-fat control, 20HE: | [18] |
| (1) Low fat (10% calories from fat) | C57BL6/J | Gluconeogenesis markers in hepatocytes | - prevented high-fat-induced hyperglycemia | ||
| (2) High fat (60% calories from fat) | n = 10 per group | - decreased insulin by 4.5-fold and increased adiponectin by 7.6-fold | |||
| (3) High fat with 20-Hydroxyecdysone (10 mg/kg day) via oral gavage | - decreased postprandial glucose and insulin resistance | ||||
| Ad libtum feeding | - decreased PEPCK and G6Pase RNA expression | ||||
| Diet + Supplement | Duration: 3 weeks | Mouse—6 wk, male | Blood: Glucose, insulin, lipids | Compared to HF, both HFQ and HF20HE: | [21] |
| (1) Low Fat (Control) | C57BL/6J | Fat: Cellularity, weight | - decreased fat mass in a high-fat diet | ||
| (2) High Fat (HF) | n = 12 per group | Fat and muscle RNA markers | - decreased subcutaneous fat weight | ||
| (3) High Fat + Quinoa (HFQ) | - reduced adipocyte weight | ||||
| (4) High Fat + 20-hydroxyecdystone (HF20HE) | - prevented increase of lipoprotein lipase and PPARG | ||||
| - increased CPT-1 mRNA expression | |||||
| HFQ and HF20HE had comparable 20-hydroxyecdysone levels | - upregulated UCP2 and UCP3 | ||||
| Ad libtum feeding | - prevented an increase in fat associated inflammatory markers | ||||
| Diet | Duration: 3 weeks | Mouse—6 wk, male | Blood: Glucose, insulin, lipids, leptin | 20HE increased food intake, physical activity, and energy expenditure | [25] |
| (1) Low Fat (9% calories from fat) | C57BL/6 | O2 and CO2 Volume | HFQ and 20HE increased glucose oxidation | ||
| (2) High Fat (51% calories from fat) | N value varied between 3 and 11 animals per group | Glucose oxidation | 20HE increased lipid excretion vs all diets | ||
| (3) High Fat + Quinoa Extract (HF + 2.8% QE) | Lipid oxidation | HFQ increased lipid excretion but less than 20HE | |||
| (4) High Fat + 20-hydroxyecdysone (HF + 0.0535% 20HE) | Food consumption | ||||
| Feces | |||||
| HFQ and HF20E had comparable 20E amounts | |||||
| Ad libtum feeding | |||||
| Supplement | Duration: 15 weeks | Mouse—5 wk, male | Blood: Glucose | Quinoa leachate decreased blood glucose in a dose-dependent manner—18.5% and 36.2%, respectively | [19] |
| High fat (60% calories from fat) | C56BL/6J | 500 mg/kg dose was significantly lower vs metformin | |||
| Oral gavage: 0.25 mL/50 g BW | n = 7 per group | ||||
| (1) Vehicle (70% Labrasol, Control) | |||||
| (2) 250 mg/kg quinoa leachate | |||||
| (3) 500 mg/kg quinoa leachate | |||||
| Ad libtum feeding | |||||
| Diet | Duration: 8 weeks | Mouse—5–6 wk, male | Feces | Lean diet group: increased Actinobacteria | [29] |
| (1) AIN-93G (Lean, control) | lean, obese (db/db) | Bacterial species (groups and families) | Obese diet group: decreased verrucomicrobia | ||
| (2) AIN-93G (Obese, control) | n = 10, 11, 11 | qPCR | Lean and Obese + Quinoa groups had similar amounts of Enterococcus, Turicibacter, and Akkermansia | ||
| (3) AIN-93G (Obese) + 838.98 g/kg quinoa | Weight | ||||
| Ad libtum feeding | |||||
| Diet | Duration: 8 weeks | Mouse | Blood: Insulin, lipids | Weight gain normalized for first 5 weeks in quinoa group but no difference compared to obese control at end of the study | [27] |
| (1) AIN-93G (Lean, Control)—679.5 g/kg carbs | lean, obese (db/db) | Body weight | Quinoa treatment: | ||
| (2) AIN-93G (db/db)—679.5 g/kg carbs | n ≥ 9 per group | Organ weight | - decreased kidney weight and increased fecal weight | ||
| (3) AIN-93G (db/db)—624 g/kg quinoa | Fat deposition | - increased blood insulin | |||
| Ad libtum feeding | - decreased total and LDL cholesterol by 24% and 35%, respectively | ||||
| - decreased oxidized-LDL by 50% | |||||
| - reduced fat deposition and total cholesterol in liver. | |||||
| Diet | Duration: 5 weeks | Rat—male | Blood: Glucose, Lipids, | Quinoa + Fructose decreased triglycerides (TG) | [33] |
| (1) Control—620 g/kg corn starch | Wistar | Total protein concentration, urea, creatine | Quinoa decreased total cholesterol, LDL and TG | ||
| (2) Fructose—310 g/kg cornstarch and 310 g/kg fructose | n = 6 per group | Quinoa prevented HDL reduction due to fructose | |||
| (3) Quinoa—310 g/kg corn starch and 310 g/kg quinoa | Quinoa meal replacement decreased total protein intake | ||||
| (4) Quinoa + Fructose—310 g/kg fructose and 310 g/kg quinoa | Quinoa decreased blood glucose | ||||
| Ad libtum feeding | |||||
| Diet | Duration: 15 days | Rat—Wistar | Blood: Glucose, ghrelin, | Amaranth caused less weight gain | [34] |
| (1) Casein (Control) | n = 8 per group | cholecystokinin | Quinoa and amaranth: | ||
| (2) Quinoa | Body weight | - decreased food consumption | |||
| (3) Amaranth | Food intake | - decreased plasma ghrelin, leptin, and CCK | |||
| All diets used variable as 20% of the formula | - increased plasma free fatty acids | ||||
| Ad libtum feeding | - increased blood glucose 3.6% and 15% vs. control, respectively | ||||
| Diet | Duration: 47 days | Rat—8 wk, male | Blood: Glucose | All quinoa groups: | [35] |
| (1) Corn starch (Control)—465.62 g/kg | Wistar | Body weight | - showed decreased epididymal adipose tissue | ||
| (2) Corn starch + glucose—315.62 and 150 g/kg | n = 6 per group | - reduced fasting and postprandial blood glucose | |||
| (3) Toasted Quinoa Flour—150 g/kg | Sprouted quinoa decreased food intake and increased average weight gain | ||||
| (4) Toasted Sprouted Quinoa Flour—150 g/kg | |||||
| (5) Fermented Quinoa Flour—150 g/kg | |||||
| (6) Toasted Sprouted Fermented Quinoa Flour—150 g/kg | |||||
| Ad libtum feeding | |||||
| Diet + Supplement | Duration: 8 weeks | Rat—6 wk, male | Blood: Lipids, Malondialdehyde, alanine transaminase (ALT) | HFHQ decreased liver weight | [30] |
| (1) Standard feed diet (Control) | Sprague-Dawley | Organ weights | HFHQ and HFLQ: | ||
| (2) High-fat diet (HF) | n = 6 per group | Gut microbiota | - prevented increase of plasma TG and LDL levels like Simvastatin | ||
| (3) HF + Simvastatin (0.9 mg/kg day) | - prevented increases of malondialdehyde and ALT | ||||
| (4) HF + High Quinoa polysaccharides (300 mg/kg day) (HFHQ) | - prevented alteration of gut microbiota constituent ratios and microbiota population ratios were comparable to control | ||||
| (5) HF + Low Quinoa polysaccharides (600 mg/kg day) (HFLQ) | |||||
| Quinoa polysaccharides delivered by oral gavage | |||||
| Ad libtum feeding | |||||
| Supplement | Duration: 10 h | Rat—9 wk, male | Blood pressure | Acute: high-dose QPH was equal to Captopril group | [31] |
| (1) Water (Control) | Spontaneously Hypertensive Rat | Chronic: lower QPH doses were equal to Captopril group | |||
| (2) Captopril | n = 6 per group | ||||
| (3) 100 mg/kg quinoa protein hydrolysate (QPH) | |||||
| (4) 200 mg/kg QPH | |||||
| (5) 400 mg/kg QPH | |||||
| Ad libtum feeding | |||||
| Supplement | Duration: 30 days | Human—18–45 y.o. | Blood: Glucose, lipids, aspartate transaminase, urea | Quinoa decreased total cholesterol, TG, and LDL | [36] |
| Quinoa cereal bar 2/day (19.5 g quinoa/day) | n = 22 (9 M, 13 F) | Blood pressure | Females show decreased cholesterol, TG and urea | ||
| Maintained a normal diet | Weight | Both sexes show decreased LDL and AST | |||
| 2 time points—0 days, 30 days | Height | ||||
| Supplement | Duration: 4 weeks | Human—Postmenopausal females: | Blood: Glucose, lipids, vitamin E, glutathione | QF: | [37] |
| (1) Corn flakes (CF) (Control)—25 g/day | (1) 2 yrs w/o hormone therapy | Weight | - increased daily fiber and protein intake | ||
| (2) Quinoa flakes (QF)—25 g/day | (2) estradiol 10–20 pg/mL | Height | - decreased serum END and increased urinary ENL | ||
| 2 Time points—0 wks, 4 wks | (3) follicle-stimulating hormone 35+ mIU/mL | BMI | - decreased cholesterol, LDL, and TG | ||
| n = 35 | Waist | - increased glutathione and decreased vitamin E | |||
| Urine: Enterodiol (END), enterolactone (ENL), lignans | CF: | ||||
| - decreased serum and urinary END and ENL | |||||
| - decreased TG | |||||
| Supplement | Duration: 12 weeks | Human—18 to 65 y.o. | Blood: Glucose, lipids, adiponectin, leptin, insulin, C-peptide | 50 g/d quinoa decreased TG after 12 weeks | [38] |
| (1) Control | BMI > 25 | Weight | Quinoa decreased TG in individuals with hypertriglyceridemia to normal range | ||
| (2) 25 g/d quinoa | n = 19, 22, 23 | Height | |||
| (3) 50 g/d quinoa | Waist to hip ratio | ||||
| Maintained normal diet | |||||
| 2 time points—6 wks, 12 wks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Little, A.; Murphy, K.; Solverson, P. Quinoa’s Potential to Enhance Dietary Management of Obesity and Type-2 Diabetes: A Review of the Current Evidence. Diabetology 2021, 2, 77-94. https://doi.org/10.3390/diabetology2020007
Little A, Murphy K, Solverson P. Quinoa’s Potential to Enhance Dietary Management of Obesity and Type-2 Diabetes: A Review of the Current Evidence. Diabetology. 2021; 2(2):77-94. https://doi.org/10.3390/diabetology2020007
Chicago/Turabian StyleLittle, Alexander, Kevin Murphy, and Patrick Solverson. 2021. "Quinoa’s Potential to Enhance Dietary Management of Obesity and Type-2 Diabetes: A Review of the Current Evidence" Diabetology 2, no. 2: 77-94. https://doi.org/10.3390/diabetology2020007
APA StyleLittle, A., Murphy, K., & Solverson, P. (2021). Quinoa’s Potential to Enhance Dietary Management of Obesity and Type-2 Diabetes: A Review of the Current Evidence. Diabetology, 2(2), 77-94. https://doi.org/10.3390/diabetology2020007








