Abstract
The prevalence of diet-induced obesity and type-2 diabetes remains a growing concern in the United States. As best management practices still include improved diet and physical activity, bioactive food components, contained within functional foods, show promise in curbing the cardiometabolic complications associated with excess weight and diabetes. Quinoa is an emerging candidate crop for its versatility in wide-ranging growing conditions as one approach to address food security, but it also contains several components that may serve as a dietary tool for post-industrial countries struggling with the health complications of caloric excess. Preliminary rodent feeding studies demonstrate that components within quinoa, namely, phytosteroids, phenolics, polysaccharides, and peptides, can prevent adiposity, dyslipidemia, and hyperglycemia. Mechanistic activity may involve reduced lipid absorption and adipogenesis, increased energy expenditure and glucose oxidation and corrected gut microbiota. Other intestinal actions may include blocked carbohydrate digestion with enhanced incretin signaling. Evidence in clinical trials is lacking and future research spanning cells to the clinic is needed to further elucidate the interesting preliminary reports reviewed here. Quinoa offers several unique attributes that could be harnessed to improve the dietary management of obesity and diabetes.
1. Introduction
Obesity, defined as a body mass index (BMI) above 30 kg/m2, remains a pressing issue in the United States. According to the Centers for Disease Control and Prevention (CDC), the current prevalence of obesity is 42% [1,2]. Obesity prevalence plotted against time shows that it is in a linear growth phase with no signs of slowing; with current trends, a recent study estimates obesity prevalence will reach 50% by 2030 [3]. Further, the prevalence of severe adult obesity (BMI > 40) has doubled within the past 20 years [1,2]. Obesity disproportionately burdens minorities, females, and lower socioeconomic status populations [3].
Obesity is associated with several health complications, including insulin resistance and resultant type-2 diabetes [4]. In 2018, diabetes, defined as a fasting blood glucose concentration above 126 mg/dL, or hemoglobin A1c above 6.4%, affected approximately 11% of the US population (34.2 million people) and 90–95% of cases were type-2 (adult-onset/insulin-independent) diabetes [5]. These ailments are a costly burden to the healthcare industry, where the majority of their expense is attributed to the management of their comorbidities via prescription drugs [6]. With the exception of invasive surgical procedures, modern medicine does not offer a solution to obesity and type-2 diabetes as robust as proper nutrition and physical activity. In an effort to improve our food supply, scientists across a wide spectrum of agricultural and life sciences research are interested in developing food products that have the potential to augment human health [7]. Bioactive food components are a central feature in this paradigm, as constituents within foods have the potential to go beyond conventional nutritional needs and their chronic consumption may promote health and longevity and minimize age-related, noncommunicative diseases, including diet-induced obesity and type-2 diabetes.
One candidate food crop gaining considerable attention this past decade for its nutritional and bioactive potential is quinoa. Quinoa (Chenopodium quinoa Willd.) is a member of the Amaranthaceae family. Despite its underutilization by industrialized agriculture, quinoa has been cultivated in South America for thousands of years; part of its emerging appeal involves its broad adaptation and agronomic versatility: it can be grown at sea level or at altitude, in marginal soils with suboptimal pH and high salinity and across a wide range of precipitation zones [8,9]. Quinoa has a genetic diversity spanning thousands of unique varieties, landraces, and genotypes, which contain an array of chemical composition and physicochemical properties [9,10,11]. This genetic diversity is manifested in varieties with unique flavor, texture, aroma, and color profiles that are specifically adapted for different end uses, including grain bowls, bread, pancakes, cakes, drinks, noodles, and many other processed and extruded foods [10,11]. Combined with its myriad and varied end uses and potential for production on a global scale, it has a robust nutritional profile, including an attractive essential amino acid profile, and is a good source of dietary fiber, B vitamins, essential fatty acids, and polyphenols [12,13]. For these reasons, the Food and Agricultural Organization of the United Nations has brought this candidate crop to the world stage for its potential to address food security and even declared 2013 as the “International Year of Quinoa” [14].
With a unique nutritional profile, providing essential nutrients, fiber and phytochemicals, quinoa is considered a functional food, that is, a food that promotes human health and longevity beyond the classical understanding of physiological homeostasis offered by sufficient macro and micronutrient intake [15]. As quinoa varieties are developed, there is a novel opportunity to create varieties that are informed by their potential to maximize human health. As an example, Washington state quinoa varieties and breeding lines have been characterized for their potential to meet daily amino acid requirements for all age groups and this consideration can inform breeding line selection and development [16]. Further, the diverse class of plant metabolites contained within quinoa that may augment human health have been extensively summarized [17]. Consumption of functional foods such as quinoa may offer protection against cardiometabolic diseases related to poor diet and physical inactivity. Owing to the prevalence of obesity and type 2 diabetes in the United States, an examination of the current scientific evidence for quinoa consumption to augment dietary strategies for their management is warranted. The aim of this literature review is to highlight both the translational and clinical work, which was designed to investigate the utility of quinoa or its components to improve the metabolic ailments centered around obesity and diabetes. This review will describe both the findings from rodent and clinical studies, as well as select mechanistic work that could help explain quinoa’s action.
2. In Vivo Experiments with Quinoa
2.1. Rodent Studies
Quinoa is a rich source of one of the most common plant steroids, 20-hydroxyecdysone (20HE), which has been reported to significantly improve obesity and diabetes in a rodent model of diet-induced obesity [18]. Six-week-old C57BL/6 male mice were fed a 60% high-fat diet for 13 weeks. The experimental group was treated with 10 mg 20HE per kg body weight per day by oral gavage. Weight gain and ratio of adipose to lean mass in the 20HE group were 18% and 38% lower when compared to the high-fat diet group, respectively. There was no indication that 20HE induced higher body temperature or stimulated energy expenditure. The 20HE group expressed adiponectin 7.9-fold higher than the high-fat diet group in their visceral fat tissue. Although absolute plasma levels of adiponectin were not different by the end of the study, when normalized to fat mass or body weight, there was a respective 37% and 73% increase in the 20HE-treated mice. Furthermore, 20HE treatment also prevented the development of insulin resistance in mice fed the high-fat diet. It decreased plasma insulin levels by 4.5-fold compared to the high-fat group and reduced blood glucose levels within 60 min of a glucose tolerance test (glucose administered via oral gavage, data collected at 30, 60, and 120 min). The findings were supported by hepatocyte cultures where 20HE treatment reduced gene expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) via phosphoinositol-3-kinase (PI3K) and increased expression of adenosine monophosphate-activated protein kinase (AMPK).
Quinoa is up to 12-fold more concentrated with 20HE compared to spinach and one study confirmed the 20HE bioactivity described above, specifically from quinoa [19]. To induce obesity and hyperglycemia, C57Bl/6J mice were fed a 60% fat diet for 15 weeks. A polyphenol and 20HE-rich quinoa leachate (QL) was then studied for utility as an acute hypoglycemic. A dose-response effect showed that 500 mg/kg body weight (BW), but not 250 mg/kg, QL significantly reduced 4hr blood glucose (63.8% of baseline) to the same extent as the positive control, 300 mg/kg metformin (73.6% of baseline). The extraction method captured 60% of the 20HE contained within intact red quinoa seeds and it could be concentrated 17.5-fold compared to starting levels. In addition to 20HE, the QL also contained 2.59% flavonoids, as well as fatty and amino acids, which may have offered synergistic effects.
With the benefits of pure 20HE on glucose homeostasis described earlier, in addition to its positive influence on muscle synthesis [18,20], a separate group fed C57BL/6J mice one of four possible diets for 3 weeks: low fat (negative control), high fat (51% of energy from fat, positive control), high fat supplemented with quinoa extract (HFQ), or high fat supplemented with pure 20HE (HF20HE) [21]. Both experimental groups provided a similar dose of 20HE, 6 mg per day per kg BW. Despite no difference in body weight gain in all high-fat diet groups, HFQ and HF20HE reduced epididymal fat mass by 26% and 38%, respectively, compared to the high-fat control group, and similar trends were also true for subcutaneous fat depots. Commensurate with these findings were reductions in plasma leptin with both HFQ and HF20HE relative to high-fat control. Imaging of epididymal adipocytes showed reductions in cell size with HFQ and HF20HE, but not cell number compared to high-fat control. In gene expression studies of the adipose tissue, a two-fold increase in lipoprotein lipase (LPL) and peroxisome proliferator-activated receptor-γ (PPARγ) with the high-fat control diet compared to low fat was blocked with both HFQ and HF20HE and both groups also reduced PEPCK expression relative to high-fat control. Uncoupling proteins (UCP), present in the inner mitochondrial membrane to allow for futile proton cycling and heat dissipation in place of oxidative phosphorylation, were upregulated in both experimental groups by up to 2.5-fold compared to the high-fat control. There were no differences across all 4 treatment groups in measures of glucose homeostasis, suggesting that 3 weeks of feeding was insufficient to produce diet-induced hyperglycemia and insulin resistance. Despite this, low-grade inflammation was evident in the high-fat control animals, as indicated by increases in gene expression in several adipokines and this increase was also blocked by both experimental treatments, albeit there was no effect on increased plasma plasminogen activator inhibitor-1 (PAI-1) caused by high-fat feeding. While the acute study described above highlighted the potential synergistic effect of the many bioactives present in a quinoa extract, the HFQ vs. HF20HE experiment may argue that quinoa’s anti-obesity activity is explained simply by its 20HE content, but that the potency of 20HE in its pure form is not inhibited when delivered as part of a food extract. However, the study’s priority of extracting 20HE from quinoa could have been at the expense of other bioactives, as the quinoa extract was not characterized for other quinoa phytochemicals. Conversely, a more dramatic reduction in some of the measured adipokines with HFQ relative to HF20HE could be attributable to the antioxidant effects of the flavonoids present in quinoa, as such activity is described in other rodent experiments [22]. Foucault et al. note that they could prevent fat mass gain with a lower dose of 20HE compared to earlier studies [18,23], which may be due to their delivery in food form vs. the oral gavages of the other works. It is also worth noting that both HFQ and HF20HE groups had positive anti-obesity findings despite no differences in food intake relative to the high-fat control group. The major limitation of the positive findings of this work is the relatively short duration; longer high-fat feeding with more pronounced metabolic perturbations would inform the utility of quinoa and 20HE in more advanced stages of obesity and its accompanying inflammation, dyslipidemia, and glucose intolerance/type-2 diabetes. The authors speculate that one possible mechanism of action of 20HE could be similar to biological activities of active-form 1-α,25-dihydroxyvitamin D3 by binding to membrane (not nuclear) receptors to activate signal transduction pathways [24].
In a follow-up study, using the same exact experimental design, the mice in each of the four groups were tested with indirect calorimetry, including energy expenditure, respiratory quotient (RQ), and glucose and lipid oxidation [25]. In this study, the HF20HE treatment significantly increased energy expenditure by 10% compared to the high-fat control, where food intake and physical activity also trended higher, but were not significantly different. The HFQ group trended in the same direction but was not different from the high-fat or HF20HE groups. The HFQ group had a significant 2% increase in RQ compared to the high-fat control and both HFQ and HF20E oxidized significantly more glucose for energy compared to the high-fat control despite no differences in lipid oxidation across the three groups. In their assessment of fecal lipid content, HFQ and HF20HE treatments also increased fecal lipids by 68% and 104%, respectively, compared to the high-fat control. The combination of increased energy expenditure with reduced lipid absorption over time could help explain the decrease in fat depots of both the HFQ and HF20HE groups observed in the other work. A significant increase in glucose oxidation may help explain the hypoglycemic effect of 20HE described by others [18,26] and may also be due to lower availability of energy from fat evidenced by its higher excretion compared to the high-fat control. Taken together, this suggests dual action of hypoglycemia and less substrate for fatty acid synthesis in adipose tissue with quinoa or 20HE treatment. Finally, fecal lipid content was similar between low-fat and high-fat control groups, as the high-fat control undergoes adaptation in the small intestine to increase lipid absorption (more is coming from the diet, but the same is excreted in feces, and, therefore, more is absorbed). There was no increase in fecal weight between the high-fat control, HFQ, or HF20HE groups; independent of fecal mass, there was an increase in fecal lipids with HFQ and HF20HE treatments. Therefore, increased fecal lipid content is due to HFQ and HF20HE blocking the adaptation in the small intestine to increase lipid absorption, not due to an increased fecal bulk. Follow-up work designed around this question would be required to identify which aspect of intestinal adaptation is blocked by quinoa or 20HE. The authors speculate that modulation in bile acid synthesis or the fecal microbiome could explain their findings.
In a recent study, genetically obese db/db (type-2 diabetic) mice were fed a quinoa-supplemented AIN-93G or control diet for 8 weeks to assess improvements in the metabolic disturbances associated with this rodent model, including obesity, diabetes, dyslipidemia, liver disease, and inflammation [27]. As a negative control, lean rodents were fed the standard AIN-93G diet. The red quinoa flour-supplemented diet was matched for energy to the control diet. Major differences between diets included 39 g/kg more fiber and 35 g/kg less protein with the quinoa diet. The quinoa diet also provided 3456 mg total phenolics (gallic acid equivalents) per kg diet, which were identified as quercetin and flavonone glycosides, kaempferol, and phenolic acids. The db/db mice fed the quinoa-supplemented diet had delayed weight gain, where body weight was lower in the first 5 weeks of feeding, which was attributed to lower food intake, possibly due to differences in satiety hormones, but weight and body composition were not different by week 8. The quinoa group also had lower kidney weights and higher cecum weight, due to both greater caecal tissue and contents, which was attributed to changes in the gut microbiome described below. A surprising finding was a 1.7-fold increase in fasting insulin in db/db mice fed the quinoa diet compared to control. Plasma glucose was not reported and glucose tolerance testing was not conducted. Therefore, it is difficult to interpret the significance of differences in insulin and the authors mention it could be attributable to experimental error, or because the utility of the db/db model in the study of human diabetes is problematic [28]. Conversely, quinoa offered protection against dyslipidemia: db/db mice fed quinoa had a significant 24% reduction in total cholesterol and approximate 35% and 50% reductions in LDL cholesterol and oxidized LDL cholesterol (oxLDL), respectively, compared to the control diet. The reductions in these three measures were comparable to concentrations in lean rodents fed the control diet. Db/db mice fed quinoa also had a normalization in liver lipids and weight, in line with the reduction in serum cholesterol. The authors attribute the hypocholesterolemic and antioxidant effects of the quinoa treatment to a possible synergy between its phenolic, fiber, protein, phytosterol, and saponin components, which illustrates the manifold ways in which quinoa could offer protection against the cardiovascular complications of obesity.
The same mice were also studied for the influence of the quinoa diet on the fecal microbiome with the same 3 treatment groups at the end of 8 weeks of feeding [29]. Comparisons of the relative abundances of bacterial groups as well as the measurement of alpha and beta diversity demonstrated unique profiles across the 3 animal groups. A significant increase in operational taxonomic units was observed with the quinoa diet compared to both obese (34% increase) and lean (57% increase) groups. UniFrac analysis showed separate clustering between the three treatments. However, similar abundances between the quinoa and lean treatments included Enterococcus, Turicibacter, and Akkermansia bacterial groups, all of which may have an influence on metabolic health and, therefore, these similarities warrant further investigation. Quinoa’s polyphenol content could have influenced the changes in the microbiome identified in this study. Interestingly, compared to the quinoa-derived 20HE experiments described above [21,25], the quinoa diet in this study provided approximately 50 mg ecdysteroids per kg body weight per day, or about an 8-fold higher dose, which the authors suggest may explain the latency in body weight gain in the db/db mice fed the quinoa vs. AIN-93G diets; although food intake was not measured during that phase, the food intake was not different between groups at the end of 8 weeks. Follow-up rodent experiments of diet-induced obesity testing the bioactivity of this high 20HE quinoa flour are warranted.
Quinoa action against dyslipidemia by way of positive changes to the gut microbiota is corroborated by independent research [30]. Here, investigators were specifically interested in the bioactivity of a polysaccharide fraction of quinoa, which was comprised mainly of glucose and arabinose. Obesity and dyslipidemia were established in Wistar rats via high-fat feeding for 8 weeks. In addition to diet, two experimental groups received either 300 or 600 mg per kg BW per day quinoa polysaccharide via oral gavage. After 8 weeks, despite no changes in body weight, both polysaccharide levels attenuated the increase in triglycerides (TG) and LDL cholesterol attributable to high-fat feeding to concentrations not different from the administration of a statin positive control (0.9 mg per kg BW per day simvastatin). Both levels also attenuated serum lipid peroxides and circulating alpha-alanine aminotransferase (ALT). In addition, the lower-dose quinoa polysaccharide increased alpha diversity and corrected the Firmicutes-to-Bacteroidetes ratio (a common gut disturbance of obesity). Across all taxonomic levels measured, it normalized relative abundances to the low-fat diet. These findings support the positive effects of quinoa in the db/db model on dyslipidemia occurring in concert with reversal or protection to the fecal microbiome, here, with a background of high-fat feeding. These findings also indicate that protection can be partially explained by the polysaccharide fraction of quinoa.
Quinoa peptide fractions may also offer protective effects against cardiometabolic complications associated with obesity. To test potential improvements in blood pressure, 11-week-old spontaneously hypertensive rats were treated with increasing doses of a quinoa protein hydrolysate (QH) [31]. After quinoa proteins were passed through simulated gastrointestinal digestion with pepsin and pancreatin, the hydrolysate was administered orally in a dose response: 100, 200, or 400 mg per kg BW and blood pressure was measured out to 10 h from dosing. Captopril (10 mg per kg BW) was used as a positive control. A dose-response was observed, where high-dose QH was as or near as effective as Captopril in significantly reducing both systolic and diastolic blood pressure at the majority of time points out to six hours. At the 8 and 10-hour timepoints, medium and high-dose QH was more effective than Captopril at reducing both measures. Protein fractions of QH were characterized and angiotensin-converting enzyme (ACE) inhibitory activities were confirmed. Moreover, in silico molecular docking studies revealed 3 prospective peptides from the most bioactive protein fraction, which can be pursued with follow-up experimentation on the ability of quinoa to address hypertension. This group has also reported inhibitory effects of quinoa peptides against dipeptidyl peptidase-IV (DPP-IV) in vitro, suggesting that quinoa peptides also offer beneficial glucoregulatory effects, but the findings need confirmation in vivo [32].
An early experiment with rats and a rudimentary approach to a quinoa diet intervention reported some of the first effects on dyslipidemia with quinoa [33]. When fed a high-fructose diet, rats present with a three-fold increase in free radicals as well as hypertriglyceridemia, hypertension, obesity, and T2D [33]. Wistar rats were fed one of four possible diets for five weeks: a standard chow (C), a standard chow with half the corn starch replaced with fructose (F), a standard chow with half the corn starch replaced with quinoa seeds (Q), or a diet where half the corn starch was replaced with quinoa seeds and the other half replaced with fructose (QF). Although the energy density was comparable across the 4 diets, the quinoa diets had significantly more protein and fat than the cornstarch-based diets. Body weight and food intake measurements were not reported. The quinoa-based diets appeared to reduce both total and HDL cholesterol. The increase in serum TG expected with high-fructose feeding (F treatment) was dampened with quinoa (QF treatment), but significance at a standard alpha level is unclear. Quinoa did not prevent the rise in blood glucose due to the introduction of fructose into the diet.
In another study utilizing Wistar rats, animals were fed one of three possible diets for fifteen days using casein, amaranth flour, or quinoa flour as a protein source [34]. At study completion, compared to the casein diet, rats fed quinoa consumed 22% less diet and those fed amaranth consumed 32% less; this corresponded to a respective 13% and 20% reduction in energy intake. Significant postprandial differences in the satiety hormones ghrelin, leptin, and cholecystokinin between casein and the experimental diets may help explain differences in intake.
Sprouting and fermenting offer food processing approaches to reduce the glycemic index of foods. One group aimed to determine their utility or benefit with quinoa in diet-stressed animals [35]. Wistar rats were fed one of several diet treatments for 47 days: control, high glucose (HG), HG with control quinoa, HG with sprouted quinoa, HG with fermented quinoa, or HG glucose with sprouted and fermented quinoa [35]. Diet formulation was problematic, as the only manipulated ingredient was corn starch replaced with glucose and in the case of the quinoa treatments, corn starch replaced with glucose and respective quinoa flours; energy, macronutrient, and fiber profiles were not reported and were likely to be highly disparate. Moreover, the high glucose control diet did not result in increases in fasting or fed glucose homeostasis compared to the normal glucose control diet, albeit it did significantly increase glycated hemoglobin. Despite these pitfalls, the control quinoa, sprouted quinoa, and fermented quinoa diets did significantly reduce fat mass. Further, both the control and fermented quinoa diets significantly reduced total cholesterol, LDL and VLDL fractions, increased HDL, and reduced TG relative to the high glucose control diet. Although these are interesting findings, their utility is questionable due to concerns surrounding the validity of the diet formulation.
Taken together, these preliminary rodent experiments highlight several possible modes of quinoa action against obesity and diabetes. They illustrate the manifold ways in which quinoa may offer protection, namely its 20HE, phenolic, polysaccharide, and peptide components. Follow-up work in highly controlled feeding experiments with careful consideration for diet formulation and appropriate testing for anthropometrics, adiposity, energy metabolism, and blood lipid and glucose homeostasis are needed. Further, metabolomics, pharmacokinetics, and interactions with the fecal microbiome will aid in the characterization of quinoa’s bioactivity.
2.2. Human Studies
Quinoa bioactivity is poorly studied in clinical trials, but the few studies available offer encouraging insight. In a seminal clinical trial with quinoa, 22 generally healthy volunteers were fed two quinoa-containing cereal bars per day for 30 days to assess potential modulation of anthropometric and biochemical measures related to cardiovascular disease [36]. Half the subjects were healthy weight while the other half were overweight. Measures were performed at baseline and after 30 days of cereal bar consumption; there was no control group. Significant findings included a 10% reduction in total cholesterol, 12% reduction in TG, and 21% reduction in LDL cholesterol after quinoa treatment. When separated by gender, women, but not men, had a decrease in total cholesterol and TG and both genders had a reduction in LDL cholesterol. Conversely, men, but not women, experienced a significant 8% reduction in fasting blood glucose concentrations. This preliminary study shows promising findings, but major limitations including heterogeneity in biochemical and anthropometric measures in the subjects studied, as well as a lack of a control group, makes interpretation difficult. The findings do support the translational work described above and warrant follow-up experiments with more robust designs. The treatment provided 19.5 g of quinoa per day and as noted below, this dose may not reach the threshold to result in sustained changes in blood lipids, compared to 50 g per day.
A separate diet study fed 35 postmenopausal women 25 g per day of either quinoa or corn flakes for 4 weeks to determine the influence on anthropometrics, blood lipids, and fasting glucose using a parallel-arm design [37]. No significant differences were detected between treatments in the outcomes measured, which may in part be explained by the variability in the subjects’ background diets, which changed considerably between baseline and follow-up visits, as measured by food diaries. Despite this, there was a significant 5% and 6% reduction in serum total and LDL cholesterol, respectively, in the quinoa flakes group. Both corn flake and quinoa flake groups saw significant reductions in serum TG when comparing baseline to follow-up. While reductions in serum lipids are encouraging, stronger control of subjects’ background diets and a robust placebo may help identify the potential protective effect offered by quinoa.
Given the findings of potential health benefits of quinoa in postmenopausal women, another study determined the potential health benefits of quinoa consumption in a more general population [38]. The primary outcome was blood lipid profiles and secondary outcomes were body composition, dietary intake, and circulating hormones: adiponectin, leptin, insulin, and c-peptide. Subjects were randomized to one of three possible groups: control, 25 g per day quinoa, and 50 g per day quinoa. The intervention period was 12 weeks, with test measurements taken at baseline, 6, and 12 weeks. The major finding was a 36% reduction in serum TG, compared to baseline, in the high quinoa group after 12 weeks, which was also 42% lower than TG concentration in the control group at the end of the intervention. At the study mid-point, the high quinoa group had a non-significant 28% reduction in TG compared to baseline, demonstrating the dose-response of a significant reduction by 12 weeks. Further, due to improved TG and HDL, participants moved out of the metabolic syndrome (metS) classification (3 of 5 possible criteria [39]) in both the 25 g per day quinoa group (41% reduction compared to baseline) and 50 g per day quinoa group (70% reduction compared to baseline), whereas a 6.8% increase in metS occurred in the control group. No significant changes were observed in any other anthropometric or biomarkers measured and dietary intake, based on 3 d diet records at all three time points, was not different between treatments. The authors conclude that their findings support mechanistic changes related to bile acid activity and lipid absorption, but not due to changes in fiber intake, as their diet records showed no differences between groups. Teasing apart the mechanisms of action in human feeding studies would require more involved interventions, such as controlling all dietary intake across treatments, which, albeit the gold standard, requires significant financial and resource investment.
Rodent and human studies are summarized in Table 1.

Table 1.
Summary of rodent and human intervention studies with quinoa or its components.
3. Quinoa Bioactive Components Characterized by In Vitro Research
3.1. Effects on Adipogenesis in the 3T3-L1 Adipocyte Model
Adipocytes in culture have been extensively researched in nutrition for several reasons including the influence of visceral adipose on exacerbating insulin resistance, dyslipidemia, and the metabolic syndrome, but also because recent work describes its responsiveness to nutritional interventions, including remediation of inflammation and even modulation of its energetics via mitochondrial content and subcomponents [40]. Quinoa too is investigated for its influence on adipocyte biology. A recent study characterized and described the ability of quinoa-derived polysaccharides to prevent the differentiation of 3T3-L1 adipocytes via inhibition of the PPARγ, C/EBPα pathway, known to lead to maturity and lipid storage [41]. Despite the promising in vitro activity described, applicability is questionable, as quinoa polysaccharides would not be expected to be absorbed into systemic circulation [42]. Instead, they would be digested into monosaccharides, or reach the large intestine for colonic fermentation. Technologies, such as microencapsulation [43], would need to be elucidated before quinoa polysaccharides could offer systemic bioactivity beyond the already known benefits of non-digestible polysaccharides in the human digestive tract [44]. This same group also looked at the ability of a quinoa protein hydrolysate to inhibit lipid accumulation in 3T3-L1 adipocytes [45]. The quinoa protein hydrolysate was not toxic to growing cells when treated for 48 h with concentrations ranging from 50 to 1600 µg/mL. The protein hydrolysate prepared by digestion with pepsin for 120 min was described as the most inhibitive to adipogenesis, citing the same prevention of lipid accumulation and blockage of the same regulatory pathway mentioned in the previous study. Positive effects would need to be confirmed with in vivo work, as exposure to digestion again could eliminate potential bioactivity described here.
Saponins, common antinutritional compounds of quinoa, which are typically rinsed off during processing or in-home prior to cooking due to their bitter taste, have also been studied for their potential bioactivity in adipocyte cultures [46]. Both 12.5 and 25 µg/mL were not cytotoxic to differentiated 3T3-L1 cultures for 48 h. Both doses significantly reduced lipid content compared to control. Regulatory pathways related to lipid accumulation that were down-regulated by both levels of saponins included transcriptional and translational reductions in PPAR-γ, C/EBPα, and sterol regulatory element-binding protein 1c (SREBP-1c). Further, both doses also reduced gene and protein expression of glucose transporter 4 (GLUT4), LPL, and adipocyte protein-2 (aP2), all of which are involved in lipogenesis in adipocytes [46]. The saponin content of unwashed quinoa can range from <1 to 5 g per 100 g quinoa [13]. Despite their undesirable astringency, they are reported to possess serval possible bioactivities including antiglycemic and anti-dyslipidemic qualities that may be relevant to populations with diet-induced obesity and diabetes [13]. In addition to reports of possible bioavailability (in one study, as measured by incorporation into micelles [47]), the quantity that could be provided via quinoa is arguably safe, as saponin supplements are generally regarded as safe in the United States [13]. However, concerns or issues surrounding safety, nutrient and drug interactions, and bioavailability require future research to determine its utility to augment metabolic aberrations caused by poor diet and sedentary behavior. Because of their poor sensory characteristics, saponins from quinoa will likely continue to be removed before consumption. However, further elucidation of their potential health benefits may be cause for their recapture for ingredient or supplement preparation, instead of simple disposal.
3.2. Phytochemicals
In addition to the other possible bioactive nutrients reviewed earlier, quinoa is a rich and diverse source of phytochemicals, which have gained attention for their ability to positively modulate the metabolic complications of obesity and diabetes. Beyond their unique offering of 20HE, quinoa also contains polyphenols, phenolic acids, betacyanins, and saponins. Quinoa’s diverse phytochemical composition has been comprehensively reviewed elsewhere [17]. Here, we briefly discuss a few studies centering on quinoa’s polyphenol and betacyanin content and in vitro activities related to antioxidant and antiglycemic effects.
Seven colored quinoa varieties were investigated for antioxidant and α-glucosidase inhibitory activities [48]. Both free (dissolves into solution) and bound (not extractable, i.e., has the greatest chance of reaching the large intestine) forms of quinoa phenolics and their activities were assessed. Total phenolic content, using the Folin–Ciocalteu method, was highest in red quinoa varieties (200–300 mg gallic acid equivalents (GAE) per 100 g dry weight (DW)) compared to black and white (150–250 mg GAE per 100 g DW), where bound forms were also more prominent in red than the other colored varieties and represented 41–47% of the total phenolic content. The same trends were true when measuring total flavonoids, reported as catechin equivalents. Profiling of specific phenolic compounds using HPLC showed gallic acid (170–330 µg per g DW), ferulic acid (150–275 µg per g DW), p-coumaric acid (30–85 µg per g DW), and p-hydroxybenzoic acid (45–75 µg per g DW) were major phenolic constituents while lesser amounts of rutin (20–60 µg per g DW), quercetin (10–40 µg per g DW), vanillic acid (20–50 µg per g DW), and protocatechuic acid (15–70 µg per g DW) were also detected in the varieties tested. Additionally, others have reported quercetin trisaccharides as the major flavonoid constituent present in quinoa at concentrations ranging from 200–800 µg per g DW [19,49,50]. Antioxidant capacity of phenolic extracts, measured with both the oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays, showed that 60–80% of the activity were attributable to the free form extracts and bound forms were most active in the red varieties [48]. In both assays, red had the greatest antioxidant capacity compared to black and white varieties. Notably, red and white quinoa varieties had 28% and 40% lower IC50 concentrations for α-glucosidase, respectively, compared to acarbose, a commonly used hypoglycemic drug prescribed for the treatment of type 2 diabetes [48].
Another study assessed the antioxidant capacities of 29 different Peruvian quinoa varieties using FRAP and [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] (ABTS) assays, except to determine to what extent they could be explained by the betacyanin content of quinoa [51]. In addition to the phenolics listed above, quinoa contains betalains, which are nitrogen-containing plant pigments most associated with red beets. They are specific to the Caryophyllales plant order and replace anthocyanins. Of the varieties tested, the red and orange-yellow varieties contained betalains. In red varieties, the primary pigments were amaranthin (120–150 mg/kg) and to a lesser extent, dopaxanthin (18–85 mg/kg) and betanin (5–10 mg/kg), whereas the orange-yellow varieties predominantly contained dopaxanthin (2–55 mg/kg). The betalain extracts possessed antioxidant activities up to 25 mmol Trolox equivalent antioxidant capacity (TEAC), which are higher than TEAC values reported for blackberries and raspberries (20 and 17 mmol, respectively) [52].
3.3. Bioactive Peptides
Quinoa and other Central and South American grains have recently gained attention for their potential as a source of bioactive peptides in the control of type 2 diabetes [53]. Specific in vitro studies to test their effect include inhibitory assays of DPP-IV, α-glucosidase, and pancreatic amylase. Quinoa protein bioactivity was tested following the simulation of gastrointestinal digestion for potential inhibitory effects on the three enzymes [54]. Quinoa protein that underwent gastro-duodenal digestion (exposed to pepsin, pancreatic enzymes, and bile acids) for 60 min had the greatest inhibitory effect on DPP-IV with an IC50 of 0.23 mg protein/mL. Of this sample, its fraction below 5 kilodaltons had a greater inhibitory effect than the higher fraction (0.31 vs. 0.46 mg protein/mL, respectively). The 120-minute duodenal digest also exhibited inhibitory potency against the three enzymes tested and had a greater inhibitory effect against α-amylase compared to the 60-minute digest (0.19 vs. 0.53 mg protein/mL, respectively). Mass spectrometry analysis identified several bioactive peptides responsible for the inhibitory activity originating from a seed storage protein, 11S globulin B. The inhibitory activities of the isolated peptides were confirmed with the same assays, none of which reproduced the strength of the whole digestates, suggesting an additive effect of several peptides [54]. Independent work corroborates the DPP-IV inhibition of quinoa protein digestates [55]. Additionally, quinoa protein digestates also exhibit antioxidant properties [55,56]. Carefully controlled experiments in rodents and humans with quinoa proteins are needed to confirm these attractive characteristics in vivo.
Taken together, i.e., to take both the in vivo and in vitro experimental evidence reviewed here into consideration, the bioactive components of quinoa are summarized in Figure 1.
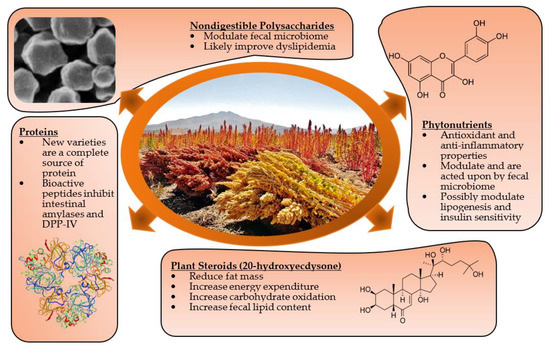
Figure 1.
Candidate bioactive components from quinoa.
4. Conclusions
In conclusion, greater agricultural production of quinoa provides a unique opportunity to both study and inform its development as a functional food in the United States and abroad. As reviewed here, several quinoa components offer potential protection against the metabolic complications of obesity and type-2 diabetes. Candidate components include its fiber, protein, and 20HE and polyphenol contents, which can be honed through both breeding and food ingredient processing research. Highly controlled trials in both rodents and humans are needed to confirm the preliminary findings described here. Primary endpoints of future work need to include indirect calorimetry, glucose tolerance, and body composition.
Author Contributions
Conceptualization, P.S.; writing—original draft preparation, P.S., A.L. and K.M.; writing—review and editing, P.S., A.L. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
CDC: Centers for Disease and Prevention, 20HE; 20-hydroxyecdystone, PEPCK; phosphoenolpyruvate carboxy kinase, G6Pase; glucose-6-phosphatase, PI3K; phosphoinositol-3-kinase, AMPK; adenosine monophosphate activated protein kinase, QL; quinoa leachate, HFQ; high fat supplemented with quinoa extract, HF20HE; high fat supplemented with pure 20HE, LPL; lipoprotein lipase, PPARγ; peroxisome proliferator-activated receptor-γ, UCP; uncoupling proteins, PAI-1; plasma plasminogen activator inhibitor-1, RQ; respiratory quotient, oxLDL; Oxidized LDL cholesterol, TG; triglycerides, ALT; alpha-alanine aminotransferase, QH; quinoa protein hydrolysate, ACE; angiotensin converting enzyme, DPP-IV; dipeptidyl peptidase-IV, C; chow, F; fructose, Q; quinoa, QF; quinoa with fructose, HG; high glucose, metS; metabolic syndrome, SREBP-1c; sterol regulatory element binding protein 1c, GLUT4; glucose transporter 4, aP2; adipocyte protein-2, GAE; gallic acid equivalents, DW; dry weight, ORAC; oxygen radical absorbance capacity, FRAP; ferric reducing antioxidant power, ABTS; [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)], TEAC; Trolox equivalent antioxidant capacity.
References
- Centers for Disease Control and Prevention. The Health Effects of Overweight and Obesity. Available online: https://www.cdc.gov/healthyweight/effects/index.html (accessed on 1 October 2020).
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8. [Google Scholar]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Insulin Resistance and Diabetes. Available online: https://www.cdc.gov/diabetes/basics/insulin-resistance.html (accessed on 14 February 2021).
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2020; pp. 12–15.
- Finkelstein, E.A.; Trogdon, J.G.; Cohen, J.W.; Dietz, W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff. 2009, 28, w822–w831. [Google Scholar] [CrossRef]
- Functional Food and Health: A Paradigm Shift in Agriculture. Available online: https://www.mdpi.com/journal/agriculture/special_issues/functional_food#info (accessed on 15 September 2020).
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef]
- Murphy, K.M.; Matanguihan, J.B.; Fuentes, F.F.; Gómez-Pando, L.R.; Jellen, E.N.; Maughan, P.J.; Jarvis, D.E. Quinoa breeding and genomics. Plant Breed. Rev. 2018, 42, 257–320. [Google Scholar]
- Aluwi, N.A.; Murphy, K.M.; Ganjyal, G.M. Physicochemical characterization of different varieties of quinoa. Cereal Chem. 2017, 94, 847–856. [Google Scholar] [CrossRef]
- Wu, G.; Ross, C.F.; Morris, C.F.; Murphy, K.M. Lexicon development, consumer acceptance, and drivers of liking of quinoa varieties. J. Food Sci. 2017, 82, 993–1005. [Google Scholar] [CrossRef]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kaniwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Tanwar, B.; Goyal, A.; Irshaan, S.; Kumar, V.; Sihag, M.K.; Patel, A.; Kaur, I. Quinoa. In Whole Grains and Their Bioactives: Composition and Health; Wiley: Hoboken, NJ, USA, 2019; pp. 269–305. [Google Scholar]
- Bazile, D.; Bertero, H.D.; Nieto, C. State of the Art Report on Quinoa around the World in 2013; FAO: Rome, Italy, 2015. [Google Scholar]
- Vega-Galvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martinez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Craine, E.B.; Murphy, K.M. Seed Composition and Amino Acid Profiles for Quinoa Grown in Washington State. Front. Nutr. 2020, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Han, P.; Li, Y.; Wang, W.; Lai, D.; Zhou, L. Quinoa Secondary Metabolites and Their Biological Activities or Functions. Molecules 2019, 24, 2512. [Google Scholar] [CrossRef] [PubMed]
- Kizelsztein, P.; Govorko, D.; Komarnytsky, S.; Evans, A.; Wang, Z.; Cefalu, W.T.; Raskin, I. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E433–E439. [Google Scholar] [CrossRef]
- Graf, B.L.; Poulev, A.; Kuhn, P.; Grace, M.H.; Lila, M.A.; Raskin, I. Quinoa seeds leach phytoecdysteroids and other compounds with anti-diabetic properties. Food Chem. 2014, 163, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Gorelick-Feldman, J.; Maclean, D.; Ilic, N.; Poulev, A.; Lila, M.A.; Cheng, D.; Raskin, I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J. Agric. Food Chem. 2008, 56, 3532–3537. [Google Scholar] [CrossRef] [PubMed]
- Foucault, A.S.; Mathe, V.; Lafont, R.; Even, P.; Dioh, W.; Veillet, S.; Tome, D.; Huneau, J.F.; Hermier, D.; Quignard-Boulange, A. Quinoa extract enriched in 20-hydroxyecdysone protects mice from diet-induced obesity and modulates adipokines expression. Obesity 2012, 20, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Pasko, P.; Barton, H.; Zagrodzki, P.; Izewska, A.; Krosniak, M.; Gawlik, M.; Gawlik, M.; Gorinstein, S. Effect of diet supplemented with quinoa seeds on oxidative status in plasma and selected tissues of high fructose-fed rats. Plant Foods Hum. Nutr. 2010, 65, 146–151. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Ehrhardt, C.; Wuttke, W. Metabolic effects of 20-OH-ecdysone in ovariectomized rats. J. Steroid Biochem. Mol. Biol. 2010, 119, 121–126. [Google Scholar] [CrossRef]
- Toth, N.; Hunyadi, A.; Bathori, M.; Zador, E. Phytoecdysteroids and vitamin D analogues--similarities in structure and mode of action. Curr. Med. Chem. 2010, 17, 1974–1994. [Google Scholar] [CrossRef]
- Foucault, A.S.; Even, P.; Lafont, R.; Dioh, W.; Veillet, S.; Tome, D.; Huneau, J.F.; Hermier, D.; Quignard-Boulange, A. Quinoa extract enriched in 20-hydroxyecdysone affects energy homeostasis and intestinal fat absorption in mice fed a high-fat diet. Physiol. Behav. 2014, 128, 226–231. [Google Scholar] [CrossRef]
- Chen, Q.; Xia, Y.; Qiu, Z. Effect of ecdysterone on glucose metabolism in vitro. Life Sci. 2006, 78, 1108–1113. [Google Scholar] [CrossRef]
- Noratto, G.D.; Murphy, K.; Chew, B.P. Quinoa intake reduces plasma and liver cholesterol, lessens obesity-associated inflammation, and helps to prevent hepatic steatosis in obese db/db mouse. Food Chem. 2019, 287, 107–114. [Google Scholar] [CrossRef]
- Wang, B.; Chandrasekera, P.C.; Pippin, J.J. Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Mills, D.; Noratto, G. Molecular exploration of fecal microbiome in quinoa-supplemented obese mice. FEMS Microbiol. Ecol. 2016, 92, fiw089. [Google Scholar] [CrossRef]
- Cao, Y.; Zou, L.; Li, W.; Song, Y.; Zhao, G.; Hu, Y. Dietary quinoa (Chenopodium quinoa Willd.) polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota. Int. J. Biol. Macromol. 2020, 163, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Hao, Y.; Richel, A.; Everaert, N.; Chen, Y.; Liu, M.; Yang, X.; Ren, G. Antihypertensive effect of quinoa protein under simulated gastrointestinal digestion and peptide characterization. J. Sci. Food Agric. 2020, 100, 5569–5576. [Google Scholar] [CrossRef]
- Guo, H.M.; Richel, A.; Hao, Y.Q.; Fan, X.; Everaert, N.; Yang, X.S.; Ren, G.X. Novel dipeptidyl peptidase-IV and angiotensin-I-converting enzyme inhibitory peptides released from quinoa protein by in silico proteolysis. Food Sci. Nutr. 2020, 8, 1415–1422. [Google Scholar] [CrossRef]
- Pasko, P.; Zagrodzki, P.; Barton, H.; Chlopicka, J.; Gorinstein, S. Effect of quinoa seeds (Chenopodium quinoa) in diet on some biochemical parameters and essential elements in blood of high fructose-fed rats. Plant Foods Hum. Nutr. 2010, 65, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Mithila, M.V.; Khanum, F. Effectual comparison of quinoa and amaranth supplemented diets in controlling appetite; a biochemical study in rats. J. Food Sci. Technol. 2015, 52, 6735–6741. [Google Scholar] [CrossRef]
- Lopes, C.O.; Barcelos, M.F.P.; Vieira, C.N.G.; de Abreu, W.C.; Ferreira, E.B.; Pereira, R.C.; de Angelis-Pereira, M.C. Effects of sprouted and fermented quinoa (Chenopodium quinoa) on glycemic index of diet and biochemical parameters of blood of Wistar rats fed high carbohydrate diet. J. Food Sci. Technol. 2019, 56, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Farinazzi-Machado, F.M.V.; Barbalho, S.M.; Oshiiwa, M.; Goulart, R.; Pessan, O. Use of cereal bars with quinoa (Chenopodium quinoa W) to reduce risk factors related to cardiovascular diseases. Food Sci. Technol. 2012, 32, 239–244. [Google Scholar] [CrossRef]
- De Carvalho, F.G.; Ovidio, P.P.; Padovan, G.J.; Jordao Junior, A.A.; Marchini, J.S.; Navarro, A.M. Metabolic parameters of postmenopausal women after quinoa or corn flakes intake--a prospective and double-blind study. Int. J. Food Sci. Nutr. 2014, 65, 380–385. [Google Scholar] [CrossRef]
- Navarro-Perez, D.; Radcliffe, J.; Tierney, A.; Jois, M. Quinoa Seed Lowers Serum Triglycerides in Overweight and Obese Subjects: A Dose-Response Randomized Controlled Clinical Trial. Curr. Dev. Nutr. 2017, 1, e001321. [Google Scholar] [CrossRef]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 2019, 1, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Shi, Z.; Yao, Y.; Ren, G. Structural Characterization of Quinoa Polysaccharide and Its Inhibitory Effects on 3T3-L1 Adipocyte Differentiation. Foods 2020, 9, 1511. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Cummings, J.H. Digestion of the polysaccharides of some cereal foods in the human small intestine. Am. J. Clin. Nutr. 1985, 42, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.K.; Rankin, S.A.; Lee, M.R.; Lee, W.J. Development and Characterization of Whey Protein-Based Nano-Delivery Systems: A Review. Molecules 2019, 24, 3254. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef]
- Shi, Z.X.; Hao, Y.Q.; Teng, C.; Yao, Y.; Ren, G.X. Functional properties and adipogenesis inhibitory activity of protein hydrolysates from quinoa (Chenopodium quinoa Willd.). Food Sci. Nutr. 2019, 7, 2103–2112. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.Y.; Gao, Y.; Shi, Z.X.; Hu, Y.B.; Ren, G.X. Suppressive effects of saponin-enriched extracts from quinoa on 3T3-L1 adipocyte differentiation. Food Funct. 2015, 6, 3282–3290. [Google Scholar] [CrossRef]
- del Hierro, J.N.; Reglero, G.; Martin, D. Chemical Characterization and Bioaccessibility of Bioactive Compounds from Saponin-Rich Extracts and Their Acid-Hydrolysates Obtained from Fenugreek and Quinoa. Foods 2020, 9, 1159. [Google Scholar] [CrossRef]
- Han, Y.; Chi, J.; Zhang, M.; Zhang, R.; Fan, S.; Huang, F.; Xue, K.; Liu, L. Characterization of saponins and phenolic compounds: Antioxidant activity and inhibitory effects on alpha-glucosidase in different varieties of colored quinoa (Chenopodium quinoa Willd). Biosci. Biotechnol. Biochem. 2019, 83, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Caravaca, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Simultaneous determination of phenolic compounds and saponins in quinoa (Chenopodium quinoa Willd) by a liquid chromatography-diode array detection-electrospray ionization-time-of-flight mass spectrometry methodology. J. Agric. Food Chem. 2011, 59, 10815–10825. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.L.; Rojo, L.E.; Delatorre-Herrera, J.; Poulev, A.; Calfio, C.; Raskin, I. Phytoecdysteroids and flavonoid glycosides among Chilean and commercial sources of Chenopodium quinoa: Variation and correlation to physico-chemical characteristics. J. Sci. Food Agric. 2016, 96, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; Cabanes, J.; Jimenez-Atienzar, M.; Ibanez-Tremolada, M.; Gomez-Pando, L.R.; Garcia-Carmona, F.; Gandia-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, F.V.; Campos, M.R.S. Amaranth, quinoa and chia bioactive peptides: A comprehensive review on three ancient grains and their potential role in management and prevention of Type 2 diabetes. Crit. Rev. Food Sci. 2020. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Martinez-Villaluenga, C.; Hernandez-Ledesma, B. Release of dipeptidyl peptidase IV, alpha-amylase and alpha-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Le Maux, S.; Dubrulle, C.; Barre, C.; FitzGerald, R.J. Quinoa (Chenopodium quinoa Willd.) protein hydrolysates with in vitro dipeptidyl peptidase IV (DPP-IV) inhibitory and antioxidant properties. J. Cereal Sci. 2015, 65, 112–118. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Miralles, B.; Carrillo, W.; Hernandez-Ledesma, B. In vitro chemopreventive properties of peptides released from quinoa (Chenopodium quinoa Willd.) protein under simulated gastrointestinal digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).