Abstract
The objective of this research is to develop environmentally friendly, risk-free and effective adsorbent composite beads that remove Cu(II) ions from aqueous solutions using cost-effective biopolymers (Carboxymethylcellulose (CMC) and sodium alginate (AL)). The synthesized hydrogel beads (AL@CMC) were dried using two drying modes, namely air-drying and freeze-drying, and characterized using scanning electron microscopy (SEM), Fourier Transform Infrared Spectroscopy (FT-IR), and Brunauer–Emmett–Teller (BET) analysis. The study investigated factors such as pH, adsorbent dosage, reaction time, Cu(II) ions concentration, and temperature to elucidate the adsorption mechanisms involved in removing copper ions. The results indicated that the hydrogel exhibited a maximum adsorption capacity of 99.05 mg·g−1, which is highly competitive compared to previous studies; the AL@CMC beads prepared in this work show a significantly higher adsorption capacity, improved stability due to the interpenetrated biopolymer network, and a clear enhancement from freeze-drying, which greatly increases porosity and active surface area. In addition, the pseudo-second-order nonlinear kinetic model best described the experimental data, implying the chemical nature of the adsorption process. Furthermore, the thermodynamic studies revealed that the adsorption process was endothermic, spontaneous, and homogenous. A Monte Carlo simulation model was utilized to ensure compatibility with the adsorption mechanism, in order to delve deeper into the intricacies of the adsorption process and gain a more comprehensive understanding of its underlying mechanisms and behavior. In conclusion, the prepared hydrogel beads proved to be an effective adsorbent for efficiently removing copper ions, making them a promising solution for addressing Cu(II) ion pollution.
1. Introduction
Copper is considered one of the first known toxic elements for its widespread presence in various industrial applications such as electroplating, metal finishing, and paint. In excess, copper is highly toxic to aquatic life and can occur in three major forms: particulate, colloidal and soluble. According to drinking water guidelines, the maximum permissible concentration of Cu(II) is 2 mg·L−1 (WHO and EU) and 1.3 mg·L−1 (US EPA), above which chronic toxicity and organ damage may occur [1,2]. Thus, treating wastewater containing copper before being discharged into water streams is vital. For this purpose, substantial attention has been given to developing conventional, cost-effective, and environmentally friendly remediation technologies to reduce copper contamination [3]. Among these, adsorption is one of the most widely adopted methods to remove toxic Cu ions from aqueous solutions, due to its simplicity, low cost, and high efficiency in removing toxic Cu ions from aqueous solutions [4]. Particular attention has been given to natural biopolymers due to their biocompatibility, biodegradability, economic feasibility, and abundance [5,6]. In addition, the composition of these natural materials makes them an appealing host matrix for incorporating multivalent metal ions compared to synthetic polymers [7,8,9].
Many recent studies have focused on the adsorption of copper ions using different adsorbents under different experimental conditions. For instance, Sahebjamee et al. developed chitosan-based porous membranes for copper removal and reported an adsorption capacity of approximately 140 mg/g [10]. Additionally, they investigated the hybridization of bentonite with graphene oxide for the removal of copper from industrial wastewater, achieving adsorption capacities up to 558.4 mg/g with the bentonite/GO composite [11]. Moreover, Cunha et al. synthesized chitosan beads functionalized with ionic liquids and obtained a copper adsorption capacity of 1.214 mmol/g (≈77 mg/g) [12]. Among the adsorbents reported in the literature, Carboxymethyl cellulose (CMC) is one of the most important anionic polysaccharides prepared via cellulose modification with acetic acid in the presence of an alkali catalyst [13]. Due to the presence of carboxylate groups, CMC has been effectively used in wastewater treatment for removing copper ions through electrostatic interactions with positively charged Cu(II) ions [14]. However, CMC beads have low mechanical stability, and physical or chemical modification is usually applied to enhance their applicability, including grafting, crosslinking, and co-polymerization [15]. Consequently, forming an interpenetrating network with another polymer is often required.
Sodium alginate (AL), a biopolymer obtained cost-effectively from brown seaweed, has been widely studied due to its strong affinity for divalent cations and its ability to form crosslinked networks in the presence of metal ions [16,17]. Together, AL and CMC can form stable biopolymeric matrices with improved structural and adsorption properties.
Several studies have explored the applications of CMC–alginate beads in water treatment. Ren et al. synthesized gel beads using a combination of sodium alginate and CMC [18], while Dewangan et al. investigated their use for removing Pb(II) and Cr(VI) ions [19]. Although these works demonstrate the potential of CMC–alginate beads for heavy metal removal, their specific application in Cu(II) adsorption remains limited. Although alginate-CMC composites have been previously reported, two scientific aspects remain insufficiently addressed. First, the specific influence of drying methods on the structural, textural, and adsorption behavior of these biopolymeric matrices has not been systematically examined. Second, the use of molecular-level Monte Carlo modeling to provide energetic consistency with experimentally observed Cu(II) adsorption behavior in AL@CMC systems remains limited.
The present study reports the synthesis of hydrogel beads using AL and CMC via calcium chloride crosslinking and applying two different drying modes: air drying and freeze-drying. The obtained beads were characterized by SEM, FTIR and BET to be examined for their potential as adsorbents to remove Cu(II) from an aqueous solution. The effects of pH, temperature, contact time, adsorbent content, and initial concentration of copper ions on the adsorption process were investigated. Moreover, kinetics, equilibrium modeling and thermodynamic studies were evaluated to elucidate nature and mechanism of Cu(II) adsorption onto the adsorbents.
In addition to Monte Carlo (MC) calculations, an additional computational method known as molecular modeling was also utilized to provide a comprehensive explanation of the adsorption mechanism based on the molecular structure of Cu(II) and AL@CMC polymers substrate. These computational techniques were primarily focused on determining the most optimal binding mode between the Cu(II) cation (target) and AL@CMC substrate [20,21], with the ultimate goal of minimizing the interaction energies between them. This approach aimed to gain a deeper understanding of the Cu(II) uptake process on cellulose polymer by employing the Accelrys Inc.’s Materials Studio software package, specifically version 20.1. The simulations were conducted using the COMPASS and Universal force field, a widely recognized methods for accurately predicting molecular interactions and behaviors in various systems.
Moreover, the results obtained from the simulations were carefully compared and contrasted with experimental data to evaluate the accuracy and reliability of the current computational model. This comparative analysis served to provide a more precise insight into the adsorption mechanisms of Cu(II) on the surfaces of AL@CMC, which are mainly governed by electrostatic attraction between Cu2+ ions and the carboxylate groups (-COO−) of both polymers, as well as possible coordination with hydroxyl groups present in the biopolymeric matrix. This enhanced understanding clarifies the dominant interactions responsible for metal uptake. The insights gleaned from this study have significant implications in the development of alternative biomaterials designed for the efficient removal of Cu(II) from liquid industrial effluents. By leveraging these computational and experimental findings, it is possible to explore more advanced applications of these adsorbents in diverse industrial settings, potentially leading to groundbreaking innovations in wastewater treatment technologies and environmental remediation strategies.
2. Materials and Methods
2.1. Reagents
Sodium alginate (≥99%), sodium carboxymethylcellulose (MW ≈ 90,000; ≥98%), and calcium chloride (≥70%) were purchased from Sigma-Aldrich (Burlington, MA, USA). in the form of fine powders. Sodium hydroxide (NaOH) and chlorohydric acid (HCl) solutions were used to adjust the pH of the prepared aqueous solution.
2.2. Preparation of Adsorbents
The adsorbent materials were prepared according to the protocol illustrated in Figure 1. Sodium alginate solution 2.5% (w/v) was prepared in distilled water with slow agitation for one hour. Next, 2.5 g of carboxymethyl cellulose (CMC) was added to the first solution and mixed for another hour (Solution A). Afterward, approximately 20 mL of the mixture was dripped through a needle (0.20 mm diameter) into CaCl2 crosslinking solution 1.0% (w/v) was also prepared in distilled water at neutral conditions where the calcium ions, CMC, and sodium alginate start forming hydrogel beads by crosslinking under slow stirring to keep the droplets from agglomerating. This crosslinking was ensured by the well-known “egg-box” interaction between Ca2+ and the guluronic units of alginate, which induces immediate gelation. This network also stabilizes the embedded CMC chains, confirming successful hydrogel formation. After 3 h of maturation in the CaCl2 solution, the formed beads were separated and washed three times with deionized water to remove unreacted polymer chains before drying and split into two groups. The first group (D_AL@CMC) was air-dried at room temperature for nearly 72 h. The second group (F_AL@CMC) was frozen using liquid nitrogen and then lyophilized in Cosmos Freeze-dryer (Cryotec, Lunel-Viel, France). The beads were then stored in a closed glass container.
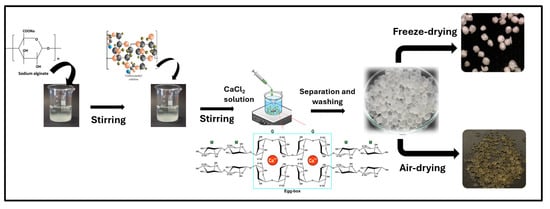
Figure 1.
Schematic representation of AL@CMC beads formation.
Beads Formation Mechanism
Sodium alginate is a polymer chain containing numerous negatively charged CO2− carboxyl groups. These negative charges are counterbalanced by the positive charge of sodium ions (Na+) to ensure a neutral molecule. When sodium alginate is brought into contact with calcium, calcium ions (Ca2+) replace sodium ions. As calcium ions have a valence of 2, each ion can interact with two CO2− groups, creating a network structure known as the egg-box crosslinking model. This crosslinking process generates a gel with a spherical structure [22].
2.3. Adsorption Experiments
Adsorption studies for Cu(II) removal by sodium alginate beads and AL@CMC hydrogel beads were conducted in batch mode. The effect of adsorbent dosage was studied in a mass range of (0.01 to 0.08 g). The influence of pH on Cu(II) was limited to 2–6 to avoid Cu(OH)2 precipitation, which begins above pH = 5.5 and would interfere with true adsorption measurements. The pH of the solutions was adjusted using 1 M NaOH and 1 M HCl. The effect of contact time on Cu(II) adsorption was performed at different time intervals [10 to 720 min]. Adsorption equilibrium studies were conducted by dispersing 20 mg of adsorbent in 40 mL of Cu(II) solution (initial concentrations of 10, 20, 30, 40, 50, 60, 80, and 100 mg·L−1) under agitation speed of 180 rpm. The temperature varied between 25 °C and 60 °C. Upon reaching equilibrium, samples were filtered using a Whatman no.42 filter paper. Equilibrium Cu(II) concentrations were measured on Atomic Absorption Spectroscopy. The amount of Cu(II) adsorbed per unit mass was calculated using Equation (1).
where qe (mg·g−1) is the amount of Cu(II) adsorbed per gram of adsorbent at equilibrium, Co (mg·L−1) is the initial concentration, Ce (m.L−1) is the equilibrium concentration of the adsorbate, V (mL) is the volume of the solution and m (mg) is the adsorbent weight.
2.4. Modeling of Adsorption Kinetics, Isotherms, and Thermodynamics
To determine the kinetic mechanism of the adsorption process, Cu(II) adsorption kinetics were fitted to the nonlinear pseudo-first-order and pseudo-second-order rate models (Equations (2) and (3)).
where qe and qt are adsorption capacities at equilibrium (mg·g−1) and t time, respectively, and k1 is the pseudo-first-order rate constant (min−1). The linear plot of log [(qe − qt) vs. t] provided the values of k1 and qe from the slope and intercept, respectively. The validity of the adsorption mechanism was obtained based on the values of the regression coefficient, R2, and the predicted qe values.
The chi-squared (χ2) error function was calculated using the expression
where and represent the experimental and model-predicted adsorption capacities, respectively.
Adsorption isotherm models provide information about the adsorption mechanisms and adsorbate-adsorbent interactions. Cu(II) equilibrium data were fitted to the Langmuir, Freundlich, and Sips equations. The Langmuir isotherm assumes that adsorption occurs on monolayer adsorbent surfaces with uniform energy. In contrast, Freundlich isotherm assumes adsorption occurs on multilayer surfaces with non-uniform energy distribution. Sips isotherm model combines Langmuir and Freundlich isotherm models and describes heterogeneous surfaces much better [23].
The nonlinear form of Langmuir, Freundlich and Sips isotherm models are given by Equations (4), (5) and (6), respectively:
where Ce is the equilibrium concentration of Cu(II) in solution, qe is the amount of Cu(II) adsorbed at equilibrium, qmax is Langmuir saturation adsorption capacity, and b is adsorption equilibrium constant. In addition, kL (L·mg−1) is the Langmuir adsorption equilibrium constant related to the affinity of Cu(II) for the adsorbent surface. For the Freundlich model, KF is the Freundlich constant associated with adsorption capacity, and nF is the heterogeneity factor describing the intensity of adsorption. For the Sips model, kS (L·mg−1) is the Sips adsorption constant related to the adsorption energy, and nS is the Sips heterogeneity index that accounts for the deviation from ideal homogeneous adsorption.
The values of thermodynamic factors, including Gibbs free energy change (ΔG°), enthalpy change (ΔH°), and entropy change (ΔS°), play a critical role in the feasibility and direction of the physicochemical sorption process of Cu(II) ions adsorption onto the adsorbents. The thermodynamic parameters can be written as Equations (6)–(9):
where Kd is the distribution coefficient, T is the temperature (K) and R is the gas constant (8.314 J mol−1 K−1). ΔH° and ΔS° values are determined from the slope and intercept of ln Kd versus 1/T plot.
Validity of Adsorption Kinetic and Isotherm Models
Apart from the correlation coefficient (R2), several error analysis methods were useful to validate the suitability of the models to the experimental equilibrium data. In this study, The applicability of the models is verified through Marquardt’s percent standard deviation (MPSD) error function for the validity of the isotherm models and normalized standard deviation (NSD) to validate the kinetics model. The equations are expressed as follows:
where qexp is observed from the batch experiment, qcal is estimated from the model, n is the number of observations in the experiment, and p is the number of parameters in the regression model [23].
2.5. Materials Characterization
For the FTIR analyses, a Spectrum 2000 spectrophotometer (PerkinElmer, Shelton, CT, USA) equipped with the attenuated total reflectance (ATR) accessory was used. The measurements were carried out over the 4000–400 cm−1 range in the transmittance mode. The morphology and surface elements of the adsorbents were investigated using a scanning electron microscope HIROX SH 4000M (Hirox Co., Tokyo, Japan). The specific surface area (BET) was determined using a Micromeritics instrument GEMINI VII 2390 (Micromeritics Instr. Corp., Norcross, GA, USA). The measurement involved the adsorption of N2 at the temperature of liquid nitrogen and a relative pressure (P/P0) range of 0.05 to 0.3. Prior to the measurement, the samples were subjected to degassing at 373 K for 24 h.
2.6. Monte Carlo Simulations
The utilization of the “3D atomistic” and “Build Polymer” modules was employed in the construction of the Alginate (AL) and carboxymethyl cellulose (CMC) substrate structure. In Figure 2 the 3D configuration of the AL@CMC, consisting of three monomers and the completed box, is visually depicted. To create the appropriate lattice at 298 K, a cubic form was utilized through the “amorphous cell” module. This lattice contained three Alginate and three carboxymethyl cellulose monomers with interactions among them, allowing for the investigation of their mutual adsorption behavior. The COMPASIII force field was specifically chosen for constructing the AL@CMC polymer [24], while the Universal Force Field (UFF) was applied to assess the binding and non-binding interactions (adsorption) between Cu and the AL@CMC polymer [25].
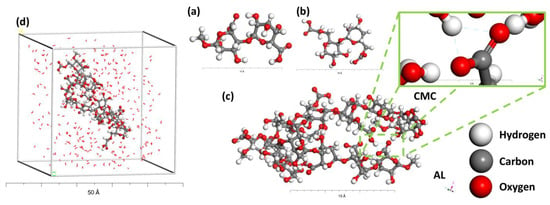
Figure 2.
(a) Schematic representation of Alginate monomer and (b) carboxymethyl cellulose monomer (c) AL@CMC polymer chain and (d) Simulation box containing water molecules and AL@CMC polymer.
For the geometry optimization calculations, a Van der Waals (vdWs) cut-off distance of 12 was established, along with a convergence threshold of 0.001 kcal/mol. Following cell construction, all cells exhibiting the lowest energy levels were selected and utilized. The interaction energy parameter [26,27], denoted as Eads(Cu(II)/AL@CMC-Water), was computed using Equation (3), which is directly associated with the relative affinities between Cu and the system comprising AL@CMC and water molecules.
Eads(Cu(II)/AL@CMC-Water) = E(Cu(II)/AL@CMC-Water) − [E(Cu(II)+ E(AL@CMC-Water)]
In the equation, E(Cu(II)/AL@CMC-Water), E(AL@CMC-Water), and E(Cu(II) represent the total energies of three distinct systems: one involving Cu adsorption on the surface of cellulose covered in water, another lacking AL@CMC, and a system solely composed of AL@CMC. As indicated by various research reports, the interaction energy demonstrates a greater magnitude in absolute terms due to the intermolecular forces and adsorption capacity [28,29].
3. Results and Discussion
Figure 3 presents the FTIR spectra of the pure biopolymers (alginate and CMC) alongside the composite beads (D_AL@CMC and F_AL@CMC). The spectra of the composites display all the characteristic functional groups of both alginate and CMC, but with noticeable shifts in band position and intensity that confirm intermolecular interactions between the polymers. A broad band between 3000 and 3500 cm−1 appears in all spectra and corresponds to O–H stretching vibrations. In the composites, this band becomes broader and more intense compared to the pure polymers, indicating the formation of stronger hydrogen bonding between CMC and alginate chains [30]. The peak at 2922 cm−1, attributed to asymmetric C–H stretching, is present in both pure polymers and the composite beads, with slightly reduced intensity in the composites due to chain interactions. The characteristic carboxylate bands of alginate and CMC appear at ~1609 cm−1 (C=O stretching) and ~1428 cm−1 (asymmetric/symmetric COO− vibrations). In the composite spectra, these bands exhibit slight shifts relative to the pure biopolymers, reflecting electrostatic interactions and partial reticulation between the COO− groups of alginate and the hydroxyl-rich backbone of CMC [31]. The shoulder observed around 1300 cm−1, more pronounced in CMC, is assigned to C–O stretching of the saccharide structure; its presence in the composites confirms the incorporation of CMC into the network. A well-defined band at 1037 cm−1 corresponds to C–O and C–O–C stretching vibrations in both polymers. The increased intensity in the composites suggests overlapping contributions from both alginate and CMC, consistent with a blended matrix. Finally, the band near 820 cm−1, associated with C–O stretching of alcohol groups, appears in all materials but is slightly attenuated in the composites due to structural interactions between the polymers [32]. Overall, the comparison with the pure alginate and CMC spectra clearly evidences molecular interactions, mainly hydrogen bonding and carboxylate-hydroxyl associations, supporting the formation of a reticulated AL@CMC network and confirming the absence of unreacted biopolymer species.
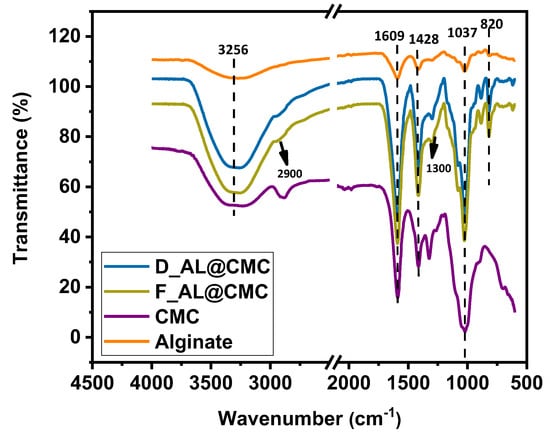
Figure 3.
FTIR spectra of Alginate, CMC, D_AL@CMC and F_AL@CMC.
Figure 4 illustrates the SEM images of the hydrogel beads in two different drying modes. The SEM images reveal that the surface of F_AL@CMC exhibits a sponge-like structure and shows fewer cracks and wrinkles compared to D_AL@CMC. This can be attributed to the freeze-drying process, which results in a smoother surface for F_AL@CMC [33]. On the other hand, D_AL@CMC showed a rougher, wrinkled, and crusty surface, with a more spherical and realistic shape compared to the freeze-dried beads. Furthermore, it was observed that the air-dried beads shrank gradually and uniformly due to slower water evaporation, resulting in reduced bead size [34]. It should be noted that the difference in available –COOH groups between D_AL@CMC and F_AL@CMC is inferred qualitatively from the relative FTIR carboxylate band intensities, the difference in BET surface area, and the adsorption behavior, which indicates enhanced diffusion into the porous structure of the freeze-dried beads.
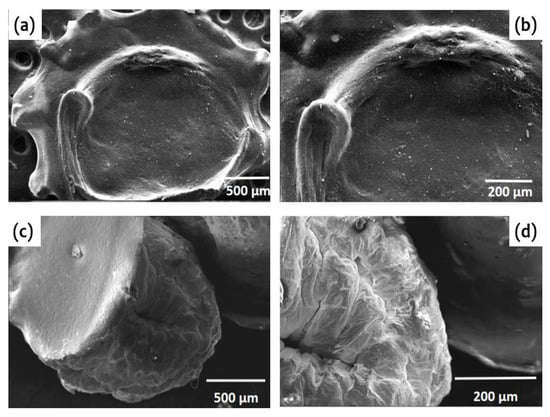
Figure 4.
SEM images of F_AL@CMC (a,b) and D_AL@CMC (c,d).
The BET specific surface area was determined from the linear region (P/P0 = 0.05–0.30) of the N2 adsorption data after degassing the samples at 373 K for 24 h. The obtained surface areas were 0.104 m2·g−1 for D_AL@CMC and 54.371 m2·g−1 for F_AL@CMC. This substantial difference is attributed to the drying method. Air-dried beads undergo structural collapse as water evaporates, resulting in a dense, non-porous material with minimal nitrogen uptake. In contrast, freeze-drying preserves the hydrogel architecture: the formation and sublimation of ice crystals generate interconnected voids within the polymer network, producing a porous structure with a markedly higher surface area. These results confirm that F_AL@CMC exhibits a porous morphology, while D_AL@CMC behaves as a non-porous or minimally porous material [3].
3.1. pH Effect
pH value affects the surface charge and the degree of ionization of the adsorbent surface, thereby affecting the adsorption capacity [35]. Therefore, the pH effect was examined at values under pH 6 since a higher pH could lead to increased precipitation of Cu(II) ions. In Figure 5a, the adsorption of copper ions appears to be very weak in low-pH, acidic medium because AL@CMC contains carboxylate COO− that gets protonated to R-COOH at low pH; therefore, coordination of copper ions from the solution with the protonated carboxylate groups is unlikely to occur, which directly and negatively affects the adsorption [36]. Thereafter, as the medium becomes less acidic and binding sites are more available, the optimal pH for Cu(II) ion adsorption was 5. As the pH increased to 6, the adsorption subsequently decreased, which can be attributed to the precipitation of copper and the formation of hydroxide compounds beyond a pH value of 5. This phenomenon confirms the slight reduction in the adsorption capacities of both materials at pH 6 [37]. The Cu ion adsorption in F_AL@CMC was marginally higher than that of D_AL@CMC. The observed difference can be attributed to the surface characteristics of the adsorbents. The adsorption in the case of D_AL@CMC is likely driven solely by electrostatic interactions, considering its relatively low surface area of 0.104 m2/g. On the other hand, in the case of F_AL@CMC, the adsorption is not only governed by electrostatic interactions but also facilitated by diffusion into the pores of the adsorbent surface, as indicated by its higher specific surface area of 54.371 m2/g [3].
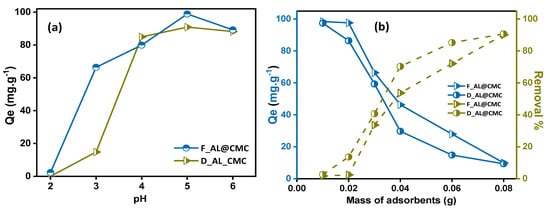
Figure 5.
(a) The effects of initial pH and (b) absorbent dosage on Cu(II) adsorption.
3.2. Effect of the Adsorbent Dosage
Figure 5b shows the importance of the dosage of adsorbent beads on the adsorption of Cu(II) ions. Dosage directly affects available active adsorption sites on the adsorbents [38]. The results demonstrate that the percentage of removal rate of Cu(II) increased with increasing the dose of both adsorbents [39]. The maximum removal rate of Cu(II) outreached 95.05% and 95.35% for D_AL@CMC and F_AL@CMC, respectively, when the mass of the beads was 0.08 g. However, for D_AL@CMC, the adsorption amount of Cu(II) decreased rapidly with increasing the mass of adsorbents in the range between 0.01 and 0.04 g; after that, the rate got slower from 0.04 to 0.08 g. This behavior is due to the fact that on increasing the adsorbent dose, more adsorption sites are available for interaction with the metal ions. No relevant increase in adsorption efficiency was noticed at higher dosages which may be due to the saturation of the surface binding sites and/or unavailability of adsorbed ions [15]. As for the F_AL@CMC, the rate of adsorption amount got slightly slower after 0.06 g. On the other hand, the particulate interaction, such as aggregation resulting from high adsorbent dosage, contributes to these findings [40]. The equilibrium point of the percentage removal rate and adsorption amount was selected as the optimal mass of the beads. Therefore, the most appropriate amount of adsorbents was 0.03 g.
3.3. Effect of Contact Time
The effect of time on the adsorption properties of copper ions using the prepared beads is shown in Figure 6a. The time range studied was from zero to 720 min for a concentration of 100 mg·L−1 and an adsorbent dosage of 0.03 g. As it is noticed, both adsorbents exhibited close adsorption kinetics of Cu(II). The rate of the adsorption amount kept increasing up to 170 min for D_AL@CMC and 150 min for F_AL@CMC until gradually reaching the equilibrium; This behavior could be explained by the presence of many available sites responsible for the adsorption process groups in the chemical structure of the adsorbents [41]. Furthermore, F_AL@CMC reaches equilibrium earlier than D_AL@CMC, confirming that freeze-drying enhances mass transfer kinetics. The similar equilibrium capacities reflect comparable densities of functional groups, consistent with a chemisorption-controlled process.
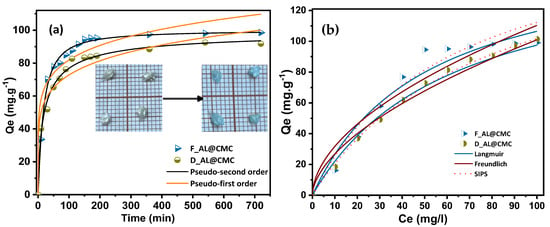
Figure 6.
Effect of (a) contact time and (b) initial concentration on the adsorption of Cu by D_AL@CMC and F_AL@CMC.
The impact of further increase in the contact time on the adsorption amount was minor as all the sites became occupied, the reason wherefore the surface of the adsorbents is saturated [42]. The adsorption capacities were improved as more adsorption sites were added, such as carboxylate groups, hydroxyl groups and oxygen atoms.
The kinetic parameters determined by the nonlinear method are listed in Table 1.

Table 1.
Kinetic parameters obtained from the nonlinear method.
Between the adsorption kinetic models, both adsorbents show a higher degree of correlation coefficient (0.992 and 0.999 for D_AL@CMC and F_AL@CMC, respectively) and a lower normalized standard deviation, NSD (3.14 and 1.252 in the same order), in the pseudo-second-order kinetic model compared to the values of pseudo-first-order kinetic model [43]. In addition, the calculated qe values through the second-order model are close to the experimental value. The goodness of fit of the kinetic models was further evaluated using the chi-squared (χ2) statistic. For both adsorbents, the pseudo-second-order (PSO) model exhibited markedly lower χ2 values (1.21 for D_AL@CMC and 0.89 for F_AL@CMC) compared to the pseudo-first-order (PFO) model (6.10 and 15.84, respectively). These results, together with the higher R2 values obtained for the PSO model, confirm that Cu(II) adsorption onto both AL@CMC beads follows pseudo-second-order kinetics, indicating that the rate-limiting step is predominantly chemisorption involving valence forces through electron sharing or exchange between Cu(II) and the functional groups on the biopolymeric matrix [44,45,46].
3.4. Effect of Initial Concentration
Figure 6b illustrates the effect of initial ion concentration on the adsorption of Cu(II). As the initial Cu(II) concentration increases, the equilibrium adsorption amount keeps increasing as well until reaching an equilibrium at an initial ion concentration of 50 mg·L−1 for F_AL@CMC, 60 mg·L−1 and 70 mg·L−1 for D_AL@CMC. After these values of initial ion concentrations, no increase in the adsorption amount was detected because the adsorption reached saturation, and there were no more active sorption sites for binding with Cu(II) [47]. According to the results of the isotherms analysis, the obtained nonlinear method and the MPSD error values are shown in Table 2. Evidently, the Langmuir isotherm model, with a high correlation ratio, explains the behavior of adsorbed Cu(II) behavior better than Freundlich and SIPS isotherm models. This proves that Cu(II) adsorption onto the adsorbents is monolayer and homogeneous with an adsorption capacity of 157.52 mg·g−1 and 159.8 mg·g−1 for D_AL@CMC and F_AL@CMC, respectively. However, MPSD values also suggest that Langmuir isotherm provides a better model of the sorption system (Table 2). The values of qm of the adsorbents studied were compared with other reported results (Table 3). It is shown that the adsorbents have greater potential and are comparable with many adsorbents for removing Cu(II). As a natural, low-cost, and easily synthesized material, AL@CMC beads represent a promising candidate for copper removal, particularly as a sustainable alternative to conventional adsorbents, pending further validation under real wastewater conditions. Nevertheless, the adsorption capacities reported in Table 3 should be interpreted in the context of the concentration ranges employed, as qm values are known to increase with higher initial metal concentrations.

Table 2.
Isotherm parameters for the adsorption of Cu(II).
Moreover, the values of parameter kl from the Langmuir model and 1/n from the Freundlich model lie between 0 and 1, indicating the favorable condition for the adsorption [48].
3.5. Effect of Temperature
The adsorption capacity of adsorbents at different temperatures is given in Figure 7. with respect to the initial copper ions concentration (100 mgL−1). The amount of copper ions adsorbed on the surface of both adsorbents slightly increased with the temperature from 25 to 60 °C. With increasing temperature, the number of active sorption sites increases because of the bond rupture of functional groups on the composite surface, enhancing the adsorption capacity towards the copper ions. Moreover, the adsorbents’ internal structure may enlarge due to increased temperature, allowing copper ions to penetrate further. The collision frequency is also important between the composites and copper ions [49].
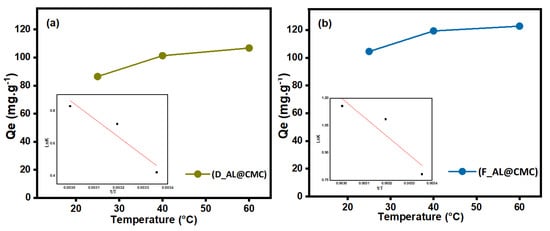
Figure 7.
Effect of temperature on Cu(II) adsorption by (a) D_AL@CMC, (b) F_AL@CMC.
From Table 4 the ∆H° values were 9.43 kJ·mol−1 and 8.16 kJ·mol−1 for D_AL@CMC and F_AL@CMC, respectively, which verifies the endothermic nature of Cu(II) adsorption process, indicating that as the adsorption process progresses, the system’s solid/solution interface becomes more unpredictable through copper ions uptake. The negative values of ∆G° at various temperatures indicate the spontaneous nature of the adsorption process. The increase in ∆G° with the increased temperature affirms that the adsorption is more favorable at high temperatures [50]. In addition, the structural changes in the adsorbate/adsorbent system are implied by the positive values of ∆S, indicating an increase in the randomness degree of the solid/liquid interface [51].

Table 3.
Comparison of adsorption capacity for Cu(II) using different adsorbents.
Table 3.
Comparison of adsorption capacity for Cu(II) using different adsorbents.
| Adsorbents | qmax (mg·g−1) | Ref |
|---|---|---|
| Chitosan-poly(vinyl alcohol) beads | 39.83 | [52] |
| Chitosan cellulose hydrogel beads | 53.20 | [53] |
| Chitosan beads | 64.62 | [39] |
| Chitosane@alginate beads | 67.66 | [39] |
| Clay/alginate beads | 27.10 | [54] |
| Alginate-graphene beads | 60.24 | [55] |
| C-phenylcalix[4]pyrogallolarene | 8.14 | [56] |
| Dopamine-functionalized tannic acid-templated mesoporous Silica nanoparticles MC/Alginate beads | 58.70 | [57] |
| Clay and sodium alginate beads | 92.44 | [1] |
| D_AL@CMC | 157.52 | This work |
| F_AL@CMC | 159.81 | This work |

Table 4.
Thermodynamic parameters.
Table 4.
Thermodynamic parameters.
| ΔG°(kJ·mol−1) | ΔH° | ΔS° | |||
|---|---|---|---|---|---|
| 298 K | 313 K | 333 K | kJ·mol−1 | J·mol−1 | |
| D_AL@CMC | −1.044 | −1.873 | −2.293 | 9.439 | 39.242 |
| F_AL@CMC | −1.940 | −2.823 | −3.201 | 8.616 | 29.680 |
3.6. Adsorption Mechanism
Figure 8 illustrates the final state of equilibrium adsorption of Cu(II) onto the surface of AL@CMC. It is visually apparent from the depiction that Cu(II) was in close proximity to the polymer, being entirely encompassed by it. This optimal encapsulation was successfully attained through electrostatic attraction between positively charged Cu2+ ions and electron-rich oxygen atoms of carboxylate and hydroxyl groups within the AL@CMC polymer, along with coordination interactions. Moreover, apart from the dipole–dipole interaction (Hydrogen bonding) and the interaction among Cu(II) atoms, there was a definite negative surface interaction between (AL@CMC-Water) that determined the interaction energy or adsorption energy.
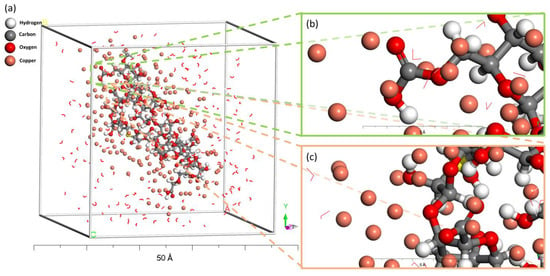
Figure 8.
Equilibrium adsorption configurations of Cu(II) on AL@CMC biosorbents using Monte Carlo simulation in liquid phase, (a) simulation box containing Cu(II) in AL@CMC polymer and water molecules, with (b,c) different views following zooming.
The observed behavior implies that the process of adsorption occurred spontaneously. Furthermore, the relatively elevated degree of interaction existing between the AL@CMC polymer within the adsorbent surfaces and Cu(II) is also substantiated by the relatively higher value of −34.7932 kcal/mol. The alignment between computational and experimental findings is notably strong, as the investigated metal exhibits a robust adsorption affinity and undergoes reactions with the AL@CMC polymer.
4. Conclusions
In this study, carboxymethyl cellulose alginate (AL@CMC) beads were prepared by a simple crosslinking process using a calcium chloride solution. Two different drying methods, air-drying and freeze-drying, were used to study their influence on the adsorption efficiency of AL@CMC on copper ions in aqueous solutions. FTIR analysis confirmed the successful synthesis of the material, which contained both carboxymethylcellulose and alginate. Nitrogen adsorption results indicated that D_AL@CMC had a negligible surface area (0.104 m2/g), suggesting that copper adsorption occurred primarily via electrostatic interactions. In contrast, the surface area of F_AL@CMC was higher (54.371 m2/g), indicating that copper adsorption occurred through both the pores of the material and electrostatic interactions. The optimum adsorbent dosage for all beads was determined to be 0.03 g. Adsorption experiments were carried out at a pH of 5 and a contact time of 170 min, using an initial Cu(II) concentration of 100 mg·g−1. F_AL@CMC showed the highest adsorption capacity (99.05 mg·g−1). The nonlinear pseudo-second-order model described adsorption kinetics well, while adsorption isotherms followed the Langmuir equation. Thermodynamic analysis revealed that the adsorption processes for both materials were spontaneous and endothermic.
Quantum chemistry simulation was utilized to analyze the interactions involving Cu(II) and AL@CMC, shedding light on the nature of the bonding between these entities. The results obtained from this simulation provided concrete evidence of the stability and advantageous characteristics of the adsorption process occurring between the aforementioned materials. Moreover, it was elucidated through the simulation data that the Copper (II) cation exhibited a higher susceptibility towards nucleophilic attacks, particularly those involving Hydrogen bonding, as opposed to electrophilic assaults such as π − π Interactions.
Overall, the results indicate that freeze-drying did indeed enhance the adsorption efficiency of the synthesized material. This finding presents a promising approach for the preparation of an adsorbent with enhanced heavy metal removal capabilities. In this study, model Cu(II) solutions were used to examine the effect of processing conditions under controlled experimental settings. The simplicity of the synthesis, mechanical integrity of the beads, and ease of solid–liquid separation suggest that further investigations under real wastewater conditions would be a logical extension of this work. This comprehensive analysis deepens our understanding of the intricate interplay between Cu(II) and AL@CMC, offering valuable insights for further research in this domain.
Author Contributions
Conceptualization, I.B., I.A., Z.K. and K.D.; methodology, I.B., I.A., Z.K., J.R. and K.D.; validation, I.B., I.A., Z.K., Y.A., J.R. and K.D.; formal analysis, I.B., I.A., J.R., S.S., M.E.A. and K.D.; investigation, I.B., I.A., M.E.A. and K.D.; data curation, I.B., I.A.,. J.R., S.S., Y.A. and K.D.; writing—original draft preparation, I.B., I.A., Z.K., J.R. and K.D.; writing—review and editing, I.A. and K.D.; visualization, I.B., I.A., Z.K., M.E.A. and K.D.; supervision, K.D.; project administration, K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Office Chérifien des Phosphates (OCP) Foundation (Research Project N°: ACD-DRA-01/2017.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no competing interests.
References
- Barrak, I.; Ayouch, I.; Kassab, Z.; El Achaby, M.; Barhoun, A.; Draoui, K. Sodium alginate encapsulated Moroccan clay as eco-friendly and efficient adsorbent for copper ions from aqueous medium. Mater. Today Proc. 2021, 51, 2040–2046. [Google Scholar] [CrossRef]
- He, W.; Yu, Q.; Wang, N.; Ouyang, X.-K. Efficient adsorption of Cu(II) from aqueous solutions by acid-resistant and recyclable ethylenediamine tetraacetic acid-grafted polyvinyl alcohol/chitosan beads. J. Mol. Liq. 2020, 316, 113856. [Google Scholar] [CrossRef]
- Ayouch, I.; Barrak, I.; Kassab, Z.; El Achaby, M.; Barhoun, A.; Draoui, K. Impact of the drying process on the efficiency of alginate beads for cadmium removal from water: Kinetic, isotherm and thermodynamic study. Environ. Technol. Innov. 2020, 20, 101157. [Google Scholar] [CrossRef]
- Kuczajowska-Zadrożna, M.; Filipkowska, U.; Jóźwiak, T. Adsorption of Cu (II) and Cd (II) from aqueous solutions by chitosan immobilized in alginate beads. J. Environ. Chem. Eng. 2020, 8, 103878. [Google Scholar] [CrossRef]
- Oualid, H.A.; Abdellaoui, Y.; Laabd, M.; El Ouardi, M.; Brahmi, Y.; Iazza, M.; Oualid, J.A. Eco-Efficient Green Seaweed Codium decorticatum Biosorbent for Textile Dyes: Characterization, Mechanism, Recyclability, and RSM Optimization. ACS Omega 2020, 5, 22192–22207. [Google Scholar] [CrossRef] [PubMed]
- Cimá-Mukul, C.A.; Abdellaoui, Y.; Abatal, M.; Vargas, J.; Santiago, A.A.; Barrón-Zambrano, J.A. Eco-Efficient Biosorbent Based on Leucaena leucocephala Residues for the Simultaneous Removal of Pb(II) and Cd(II) Ions from Water System: Sorption and Mechanism. Bioinorg. Chem. Appl. 2019, 2019, 2814047. [Google Scholar] [CrossRef]
- Jabli, M.; Almalki, S.G.; Agougui, H. An insight into methylene blue adsorption characteristics onto functionalized alginate bio-polymer gel beads with λ-carrageenan-calcium phosphate, carboxymethyl cellulose, and celite 545. Int. J. Biol. Macromol. 2020, 156, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Billah, R.E.K.; Islam, A.; Lgaz, H.; Lima, E.C.; Abdellaoui, Y.; Rakhila, Y.; Goudali, O.; Majdoubi, H.; Alrashdi, A.A.; Agunaou, M.; et al. Shellfish waste-derived mesoporous chitosan for impressive removal of arsenic(V) from aqueous solutions: A combined experimental and computational approach. Arab. J. Chem. 2022, 15, 104123. [Google Scholar] [CrossRef]
- Elhoudi, M.; Oukhrib, R.; Celaya, C.A.; Araiza, D.G.; Abdellaoui, Y.; Barra, I.; Brahmi, Y.; Bourzi, H.; Reina, M.; Albourine, A.; et al. Comparison of green bio-based cerium/alginate vs. copper/alginate beads: A study of vibrational and thermal properties using experimental and theoretical methods. J. Mol. Model. 2022, 28, 37. [Google Scholar] [CrossRef]
- Sahebjamee, N.; Soltanieh, M.; Mousavi, S.M.; Heydarinasab, A. Preparation and characterization of porous chitosan–based membrane with enhanced copper ion adsorption performance. React. Funct. Polym. 2020, 154, 104681. [Google Scholar] [CrossRef]
- Chang, Y.S.; Au, P.I.; Mubarak, N.M.; Khalid, M.; Jagadish, P.; Walvekar, R.; Abdullah, E.C. Adsorption of Cu(II) and Ni(II) ions from wastewater onto bentonite and bentonite/GO composite. Environ. Sci. Pollut. Res. 2020, 27, 33270–33296. [Google Scholar] [CrossRef]
- Cunha, B.S.; Bataglioli, R.A.; Taketa, T.B.; Lopes, L.M.; Beppu, M.M. Ionic liquid functionalization of chitosan beads for improving thermal stability and copper ions uptake from aqueous solution. J. Environ. Chem. Eng. 2019, 7, 103181. [Google Scholar] [CrossRef]
- Darbasizadeh, B.; Fatahi, Y.; Feyzi-Barnaji, B.; Arabi, M.; Motasadizadeh, H.; Farhadnejad, H.; Moraffah, F.; Rabiee, N. Crosslinked-polyvinyl alcohol-carboxymethyl cellulose/ZnO nanocomposite fibrous mats containing erythromycin (PVA-CMC/ZnO-EM): Fabrication, characterization and in-vitro release and anti-bacterial properties. Int. J. Biol. Macromol. 2019, 141, 1137–1146. [Google Scholar] [CrossRef]
- Chen, S.; Zou, Y.; Yan, Z.; Shen, W.; Shi, S.; Zhang, X.; Wang, H. Carboxymethylated-bacterial cellulose for copper and lead ion removal. J. Hazard. Mater. 2009, 161, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Ayouch, I.; Kassem, I.; Kassab, Z.; Barrak, I.; Barhoun, A.; Jacquemin, J.; Draoui, K.; El Achaby, M. Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surf. Interfaces 2021, 24, 101124. [Google Scholar] [CrossRef]
- Veglio’, F.; Esposito, A.; Reverberi, A. Copper adsorption on calcium alginate beads: Equilibrium pH-related models. Hydrometallurgy 2002, 65, 43–57. [Google Scholar] [CrossRef]
- Zhang, H.; Omer, A.; Hu, Z.; Yang, L.-Y.; Ji, C.; Ouyang, X.-K. Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu (II) adsorption. Int. J. Biol. Macromol. 2019, 135, 490–500. [Google Scholar] [CrossRef]
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb(II) removal using sodium alginate-carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr. Polym. 2016, 137, 402–409. [Google Scholar] [CrossRef]
- Dewangan, T.; Tiwari, A.; Bajpai, A.K. Removal of chromium(VI) ions by adsorption onto binary biopolymeric beads of sodium alginate and carboxymethyl cellulose. J. Dispers. Sci. Technol. 2011, 32, 1075–1082. [Google Scholar] [CrossRef]
- Samuel, Y.; Garg, A.; Mulugeta, E. Synthesis, DFT Analysis, and Evaluation of Antibacterial and Antioxidant Activities of Sulfathiazole Derivatives Combined with in Silico Molecular Docking and ADMET Predictions. Biochem. Res. Int. 2021, 2021, 7534561. [Google Scholar] [CrossRef]
- Vennila, P.; Venkatesh, G.; Sixto-López, Y.; Kamal, C.; Kaya, S.; Serdaroğlu, G.; Landeros-Rivera, B. Synthesis, spectroscopic characterization, molecular docking studies and DFT calculation of novel Mannich base 1-((4-ethylpiperazin-1-yl)(2-hydroxyphenyl)methyl)naphthalen-2-ol. J. Mol. Struct. 2021, 1246, 131164. [Google Scholar] [CrossRef]
- Voo, W.-P.; Lee, B.-B.; Idris, A.; Islam, A.; Tey, B.-T.; Chan, E.-S. Production of ultra-high concentration calcium alginate beads with prolonged dissolution profile. RSC Adv. 2015, 5, 36687–36695. [Google Scholar] [CrossRef]
- Hidalgo, J.; Hidalgo, L.; Aguiar, C.D.S.; Madroñero, D.B.G.; Galambos, I.; Vilasó-Cadre, J.E.; Reyes-Domínguez, I.A.; Brânzanic, A.M.V.; Ignat, N.; Turdean, G.L. Study of Caesalpinia spinosa Extracts as Green Corrosion Inhibitor for Mild Steel. Langmuir 2025, 41, 9406–9421. [Google Scholar] [CrossRef]
- Akkermans, R.L.C.; Spenley, N.A.; Robertson, S.H. COMPASS III: Automated fitting workflows and extension to ionic liquids. Mol. Simul. 2021, 47, 540–551. [Google Scholar] [CrossRef]
- Jaillet, L.; Artemova, S.; Redon, S. IM-UFF: Extending the universal force field for interactive molecular modeling. J. Mol. Graph. Model. 2017, 77, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Ouakki, M.; Dahmani, K.; Aribou, Z.; Ech-Chihbi, E.; Galai, M.; AlZeqri, N.; Warad, I.; Benzekri, Z.; Guo, L.; AlObaid, A.A.; et al. Adsorption of novel heterocyclic compounds of the purine derivatives as corrosion inhibitors over mild steel surface in acidic medium: Electrochemical, surface characterization and theoretical investigations. Inorg. Chem. Commun. 2023, 157, 111342. [Google Scholar] [CrossRef]
- You, X.; He, M.; Zhang, W.; Wei, H.; Lyu, X.; He, Q.; Li, L. Molecular dynamics simulations of nonylphenol ethoxylate on the Hatcher model of subbituminous coal surface. Powder Technol. 2018, 332, 323–330. [Google Scholar] [CrossRef]
- Dao, D.Q. Corrosion Inhibition Perfomance of Four Natural Thiazole Derivatives: Quantum Chemical and Monte Carlo Simulation Studie. Vietnam J. Sci. Technol. 2018, 55, 35. [Google Scholar] [CrossRef]
- Obot, I.; Kaya, S.; Kaya, C.; Tüzün, B. Density Functional Theory (DFT) modeling and Monte Carlo simulation assessment of inhibition performance of some carbohydrazide Schiff bases for steel corrosion. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 80, 82–90. [Google Scholar] [CrossRef]
- Swamy, B.Y.; Yun, Y.-S. In vitro release of metformin from iron (III) cross-linked alginate—Carboxymethyl cellulose hydrogel beads. Int. J. Biol. Macromol. 2015, 77, 114–119. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; El Salmawi, K.M. Preparation and Properties of Carboxymethyl Cellulose (CMC)/Sodium alginate (SA) Blends Induced by Gamma Irradiation. J. Polym. Environ. 2013, 21, 520–527. [Google Scholar] [CrossRef]
- Riyajan, S.-A.; Nuim, J. Interaction of Green Polymer Blend of Modified Sodium Alginate and Carboxylmethyl Cellulose Encapsulation of Turmeric Extract. Int. J. Polym. Sci. 2013, 2013, 364253. [Google Scholar] [CrossRef]
- Smrdel, P.; Bogataj, M.; Zega, A.; Planinšek, O.; Mrhar, A. Shape optimization and characterization of polysaccharide beads prepared by ionotropic gelation. J. Microencapsul. 2008, 25, 90–105. [Google Scholar] [CrossRef]
- Smrdel, P.; Bogataj, M.; Mrhar, A. The Influence of Selected Parameters on the Size and Shape of Alginate Beads Prepared by Ionotropic Gelation. Sci. Pharm. 2008, 76, 77–89. [Google Scholar] [CrossRef]
- Horsfall, M.; Spiff, A.I. Studies on the effect of pH on the sorption of Pb2+ and Cd2+ ions from aqueous solutions by Caladium bicolor (Wild Cocoyam) biomass. Electron. J. Biotechnol. 2004, 7, 310–320. [Google Scholar] [CrossRef]
- Luk, C.H.J.; Yip, J.; Yuen, C.W.M.; Pang, S.K.; Lam, K.H.; Kan, C.W. Biosorption performance of encapsulated Candida krusei for the removal of copper(II). Sci. Rep. 2017, 7, 2159. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, H.; Shao, T.; Zhao, X.; Peng, H.; Gong, Y.; Wan, H. Enhanced copper adsorption by DTPA-chitosan/alginate composite beads: Mechanism and application in simulated electroplating wastewater. Chem. Eng. J. 2018, 339, 322–333. [Google Scholar] [CrossRef]
- Billah, R.E.K.; El Bachraoui, F.; El Ibrahimi, B.; Oualid, H.A.; Kassab, Z.; Giácoman-Vallejos, G.; Sillanpää, M.; Agunaou, M.; Soufiane, A.; Abdellaoui, Y. Mechanistic understanding of Nickel(II) adsorption onto fluorapatite-based natural phosphate via Rietveld refinement combined with Monte Carlo simulations. J. Solid State Chem. 2022, 310, 123023. [Google Scholar] [CrossRef]
- Ngah, W.W.; Fatinathan, S. Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Özer, A.; Özer, D.; Özer, A. The adsorption of copper(II) ions on to dehydrated wheat bran (DWB): Determination of the equilibrium and thermodynamic parameters. Process Biochem. 2004, 39, 2183–2191. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; Gamero-Melo, P.; Díaz-Jiménez, L.; Ponce-Caballero, C.; Giácoman-Vallejos, G. Synthesis and Surface Modification of Small Pore Size Zeolite W for Improving Removal Efficiency of Anionic Contaminants from Water. Bull. Environ. Contam. Toxicol. 2020, 105, 934–940. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; Olguín, M.T.; Abatal, M.; Ali, B.; Méndez, S.E.D.; Santiago, A.A. Comparison of the divalent heavy metals (Pb, Cu and Cd) adsorption behavior by montmorillonite-KSF and their calcium- and sodium-forms. Superlattices Microstruct. 2019, 127, 165–175. [Google Scholar] [CrossRef]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Bazzi, L.; Ayouch, I.; Tachallait, H.; EL Hankari, S. Ultrasound and microwave assisted-synthesis of ZIF-8 from zinc oxide for the adsorption of phosphate. Results Eng. 2022, 13, 100378. [Google Scholar] [CrossRef]
- Han, R.; Zou, W.; Zhang, Z.; Shi, J.; Yang, J. Removal of copper(II) and lead(II) from aqueous solution by manganese oxide coated sand: I. Characterization and kinetic study. J. Hazard. Mater. 2006, 137, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Billah, R.E.K.; Ayouch, I.; Abdellaoui, Y.; Kassab, Z.; Khan, M.A.; Agunaou, M.; Soufiane, A.; Otero, M.; Jeon, B.-H. A Novel Chitosan/Nano-Hydroxyapatite Composite for the Adsorptive Removal of Cd(II) from Aqueous Solution. Polymers 2023, 15, 1524. [Google Scholar] [CrossRef]
- Ayouch, I.; Barrak, I.; Kassab, Z.; El Achaby, M.; Barhoun, A.; Draoui, K. Improved recovery of cadmium from aqueous medium by alginate composite beads filled by bentonite and phosphate washing sludge. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125305. [Google Scholar] [CrossRef]
- Kumar, K.V.; Sivanesan, S. Prediction of optimum sorption isotherm: Comparison of linear and non-linear method. J. Hazard. Mater. 2005, 126, 198–201. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Sorption of dyes and copper ions onto biosorbents. Process Biochem. 2003, 38, 1047–1061. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; El Ibrahimi, B.; Abou Oualid, H.; Kassab, Z.; Quintal-Franco, C.; Giácoman-Vallejos, G.; Gamero-Melo, P. Iron-zirconium microwave-assisted modification of small-pore zeolite W and its alginate composites for enhanced aqueous removal of As(V) ions: Experimental and theoretical studies. Chem. Eng. J. 2021, 421, 129909. [Google Scholar] [CrossRef]
- Vilela, P.B.; Matias, C.A.; Dalalibera, A.; Becegato, V.A.; Paulino, A.T. Polyacrylic acid-based and chitosan-based hydrogels for adsorption of cadmium: Equilibrium isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2019, 7, 103327. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption of copper ions onto chitosan/poly(vinyl alcohol) beads functionalized with poly(ethylene glycol). Carbohydr. Polym. 2020, 234, 115890. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bai, R. Copper adsorption on chitosan-cellulose hydrogel beads: Behaviors and mechanisms. Sep. Purif. Technol. 2005, 42, 237–247. [Google Scholar] [CrossRef]
- Ely, A.; Baudu, M.; Kankou, M.O.S.O.; Basly, J.-P. Copper and nitrophenol removal by low cost alginate/Mauritanian clay composite beads. Chem. Eng. J. 2011, 178, 168–174. [Google Scholar] [CrossRef]
- Algothmi, W.M.; Bandaru, N.M.; Yu, Y.; Shapter, J.G.; Ellis, A.V. Alginate-graphene oxide hybrid gel beads: An efficient copper adsorbent material. J. Colloid. Interface Sci. 2013, 397, 32–38. [Google Scholar] [CrossRef]
- Priastomo, Y.; Setiawan, H.R.; Kurniawan, Y.S.; Ohto, K. Simultaneous removal of lead(II), chromium(III), and copper(II) heavy metal ions through an adsorption process using C-phenylcalix[4]pyrogallolarene material. J. Environ. Chem. Eng. 2020, 8, 103971. [Google Scholar] [CrossRef]
- Gao, J.; Lei, H.; Han, Z.; Shi, Q.; Chen, Y.; Jiang, Y. Dopamine functionalized tannic-acid-templated mesoporous silica nanoparticles as a new sorbent for the efficient removal of Cu2+ from aqueous solution. Sci. Rep. 2017, 7, 45215. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.