Systematic Review of Different Methods for the Quantification of Vitamin C in Human Plasma Samples by HPLC and UV Detector
Abstract
1. Introduction
1.1. Vitamin C—Structure, Function, and Occurrence
1.2. Sampling, Sample Preparation and Analysis
1.2.1. Measurand
1.2.2. Sample
1.2.3. Blood Collection
1.2.4. Sample Preparation
1.2.5. Analysis
1.3. Clinical Relevance and Aim of This Work
2. Methods
Database Search Strategy
- Selection of publications
- Data collection, extraction, and analysis
3. Results
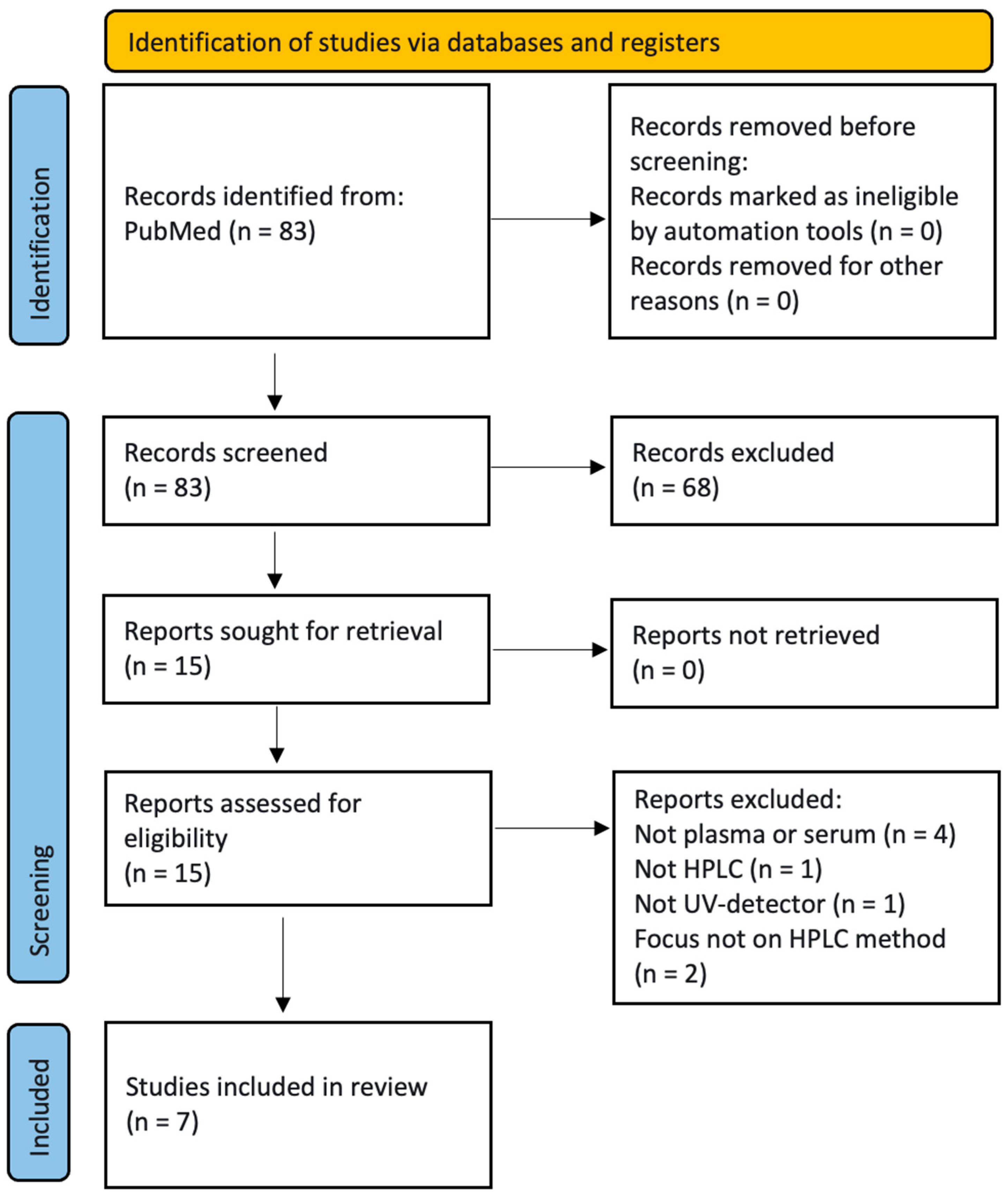
3.1. Search
3.2. Reporting on Sample Preparation, HPLC Parameters and Quality Criteria
3.3. Process Characteristics
3.3.1. Extraction and Measurement
3.3.2. Method Validation
4. Discussion
4.1. Method Selection
4.2. Sample Preparation and Analytical Consideration
4.2.1. Sample Preparation
4.2.2. Analysis
Internal Standard
RP-HPLC
Mobile Phase and Elution
Detector Types
Mass Spectrometry
UHPLC
HPLC Kits
HILIC
Precision
4.2.3. Summary
4.3. Limitations and Strengths
4.4. Outlook
4.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | ascorbic acid |
| DHA | dehydroascorbic acid |
| EC | electrochemical |
| HILIC | hydrophilic interaction chromatography |
| HPLC | high-pressure liquid chromatography |
| LLE | liquid–liquid extraction |
| LOD | limit of detection |
| LOQ | limit of quantification |
| MPA | metaphosphoric acid |
| RP-HPLC | reversed-phase high-performance liquid chromatography |
| SPE | solid phase extraction |
| TCA | trichloroacetic acid |
| TCEP | tris(2-carboxyethyl)phosphine |
| UHPLC | ultrahigh-pressure liquid chromatography |
| UV | ultraviolet |
References
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.; Van Montagu, M.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.; Strain, J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Karlsen, A.; Blomhoff, R.; Gundersen, T.E. High-throughput analysis of vitamin C in human plasma with the use of HPLC with monolithic column and UV-detection. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2005, 824, 132–138. [Google Scholar] [CrossRef]
- Nováková, L.; Solich, P.; Solichová, D. HPLC methods for simultaneous determination of ascorbic and dehydroascorbic acids. TrAC Trends Anal. Chem. 2008, 27, 942–958. [Google Scholar] [CrossRef]
- van Gorkom, G.; Gijsbers, B.; Ververs, E.J.; El Molla, A.; Sarodnik, C.; Riess, C.; Wodzig, W.; Bos, G.; Van Elssen, C. Easy-to-Use HPLC Method to Measure Intracellular Ascorbic Acid Levels in Human Peripheral Blood Mononuclear Cells and in Plasma. Antioxidants 2022, 11, 134. [Google Scholar] [CrossRef]
- Rozemeijer, S.; van der Horst, F.A.L.; de Man, A.M.E. Measuring vitamin C in critically ill patients: Clinical importance and practical difficulties-Is it time for a surrogate marker? Crit. Care 2021, 25, 310. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.T.; Niculescu, A.G.; Grumezescu, A.M. Vitamin C: A Comprehensive Review of Its Role in Health, Disease Prevention, and Therapeutic Potential. Molecules 2025, 30, 748. [Google Scholar] [CrossRef]
- Böttger, F.; Vallés-Martí, A.; Cahn, L.; Jimenez, C.R. High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J. Exp. Clin. Cancer Res. 2021, 40, 343. [Google Scholar] [CrossRef]
- Granger, M.; Eck, P. Dietary Vitamin C in Human Health. Adv. Food Nutr. Res. 2018, 83, 281–310. [Google Scholar] [CrossRef]
- Fitzpatrick, M.; Bonnitcha, P.; Nguyen, V.L. Streamlined three step total vitamin C analysis by HILIC-UV for laboratory testing. Clin. Chem. Lab. Med. 2021, 59, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. 2014, 5, 16–18. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian. J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Collie, J.T.B.; Greaves, R.F.; Jones, O.A.H.; Eastwood, G.; Bellomo, R. Vitamin C measurement in critical illness: Challenges, methodologies and quality improvements. Clin. Chem. Lab. Med. 2020, 58, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Emadi-Konjin, P.; Verjee, Z.; Levin, A.V.; Adeli, K. Measurement of intracellular vitamin C levels in human lymphocytes by reverse phase high performance liquid chromatography (HPLC). Clin. Biochem. 2005, 38, 450–456. [Google Scholar] [CrossRef]
- Kand’ár, R.; Záková, P. Determination of ascorbic acid in human plasma with a view to stability using HPLC with UV detection. J. Sep. Sci. 2008, 31, 3503–3508. [Google Scholar] [CrossRef]
- Clark, Z.D.; Frank, E.L. Development and implementation of an HPLC-ECD method for analysis of vitamin C in plasma using single column and automatic alternating dual column regeneration. Pract. Lab. Med. 2016, 6, 25–37. [Google Scholar] [CrossRef]
- Ross, M.A. Determination of ascorbic acid and uric acid in plasma by high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 1994, 657, 197–200. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for vitamin C. EFSA J. 2013, 11, 3418. [CrossRef]
- Robitaille, L.; Hoffer, L.J. A simple method for plasma total vitamin C analysis suitable for routine clinical laboratory use. Nutr. J. 2016, 15, 40. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tavazzi, B.; Lazzarino, G.; Leone, P.; Amorini, A.M.; Bellia, F.; Janson, C.G.; Di Pietro, V.; Ceccarelli, L.; Donzelli, S.; Francis, J.S.; et al. Simultaneous high performance liquid chromatographic separation of purines, pyrimidines, N-acetylated amino acids, and dicarboxylic acids for the chemical diagnosis of inborn errors of metabolism. Clin. Biochem. 2005, 38, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Auclair, J.; Rathore, A.S.; Bhattacharya, S. The Role of Ion Pairing Agents in Liquid Chromatography (LC) Separations. LCGC N. Am. 2023, 41, 268–273. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.E.; Yan, J.Q.; Liu, M.; Zhou, Y.; Shen, X.; Ma, Y.L.; Feng, X.S.; Yang, J.; Li, G.H. A Review of the Extraction and Determination Methods of Thirteen Essential Vitamins to the Human Body: An Update from 2010. Molecules 2018, 23, 1484. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.H.; Osman, R.; Abidin, N.; Kassim, N.S.A. Recent trends in the quantification of vitamin b. Malays. J. Anal. Sci. 2021, 25, 466–482. [Google Scholar]
- Handini, A.; Husni, P.; Agustin, I. Comparative Study of HPLC Detectors in PT. FIP: A Review. Calory J. Med. Lab. J. 2025, 3, 33–44. [Google Scholar] [CrossRef]
- Bhati, C.; Minocha, N.; Purohit, D.; Kumar, S.; Makhija, M.; Saini, S.; Kaushik, D.; Pandey, P. High Performance Liquid Chromatography: Recent Patents and Advancement. Biomed. Pharmacol. J. 2022, 15, 729–746. [Google Scholar] [CrossRef]
- Topkoska, M.; Miloshevska, M.; Piponski, M.; Lazarevska Todevska, E.; Slaveska Spirevska, I.; Acevska, J. Development of RP HPLC method with gradient elution for simultaneous Resveratrol and Vitamin E determination in solid dosage forms. Maced. Pharm. Bull. 2022, 68, 163–164. [Google Scholar] [CrossRef]
- Swartz, M. HPLC detectors: A brief review. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1130–1150. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. Determination of ascorbic acid and dehydroascorbic acid in biological samples by high-performance liquid chromatography using subtraction methods: Reliable reduction with tris[2-carboxyethyl]phosphine hydrochloride. Anal. Biochem. 2000, 282, 89–93. [Google Scholar] [CrossRef]
- Klimczak, I.; Gliszczyńska-Świgło, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015, 175, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.F.; Franke, A.A. Analysis of circulating lipid-phase micronutrients in humans by HPLC: Review and overview of new developments. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2013, 931, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Paliakov, E.M.; Crow, B.S.; Bishop, M.J.; Norton, D.; George, J.; Bralley, J.A. Rapid quantitative determination of fat-soluble vitamins and coenzyme Q-10 in human serum by reversed phase ultra-high pressure liquid chromatography with UV detection. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2009, 877, 89–94. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Plasma, serum, blood | Other body fluids than blood, plasma, serum (e.g., blood cells, urine, vitreous body, organs, tissue) |
| Human | Animal, food |
| UV detector | Other than UV detectors (e.g., electrochemical or fluorescence detectors), mass spectrometry |
| Publication and description of method | Application only (without the development of new method) |
| Author, Year | Mobile Phase | Elution | Flow Rate | Injection Volume | HPLC Oven Temperature | Detector Wavelength | LOQ | LOD | Reference Values | Calibration Curve | Linearity | Precision Intra-Assay | Precision Inter-Assay | Example of Chromatogram |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| van Gorkom et al., 2022 [5] | ||||||||||||||
| Fitzpatrick et al., 2021 [10] | ||||||||||||||
| Robitaille et al., 2016 [20] | ||||||||||||||
| Kand’ár et al., 2008 [16] | ||||||||||||||
| Tavazzi et al., 2005 [22] | ||||||||||||||
| Karlsen et al., 2005 [3] | ||||||||||||||
| Ross et al., 1994 [18] |
| Author | Precipitation Agent | Incubation | Centrifugation | Extra Step (Form Stabilization) | Phase System | Elution | Injection Volume (μL) | Column Temperature (°C) | Detector Wavelength (nm) | Mobile Phase | Flowrate (mL/min) | Specific Features |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Van Gorkom et al.a [5] | ACN in MPA | Yes | Yes | - | HILIC | Isocratic | 20 | 25 | 255 | 85% ACN, 15% water with 10 mM NH4Ac (pH 7) | 1 | HILIC, application also in other leukocyte subsets, determination of stability |
| Fitzpatrick et al. [10] | ACN | N/A | Yes | TCEP | UHPLC, HILIC | Isocratic | 10 | 40 | 254 | 80% ACN in 0.005 M potassium dihydrogen phosphate (pH 4,6) | 0.5 | HILIC, UHPLC, determination of stability |
| Robitaille et al. [20] | MPA in Na2EDTA | N/A | Yes | TCEP added (for total AA) | RP-HPLC | Isocratic | 20 | 25 | 245 | 1.8 mmol/L sulfuric acid (pH 2.7) | 0.8 | Comparison of UV-HPLC method with EC-HPLC method (result: equivalent) |
| Kand’ár et al. [16] | Mixture b | Yes | Yes | Filtering | RP-HPLC | Isocratic | 10 | 25 to 30 | 265 | 5% methanol in 25 mmol/L sodium dihydrogenphosphate (pH 4.8) | 0.5 | Investigation of protein precipitation procedures with regard to stability |
| Tavazzi et al. [22] | No | N/A | Yes | Filtering | RP-HPLC, ion-pairing reagent | Step gradient | N/A | 10 | 260 | A and B eluent c | 1.2 | Ion-pairing reagent (tetrabutylammonium hydroxide), synchronous separation of several compounds with a single analysis |
| Karlsen et al. [3] | MPA | - | Yes | Reduction of DHA (at pH 9.0) | RP-HPLC | Isocratic | 5 | N/A | 264 | 2.5 mmol/L NaH2PO4, 2.5 mmol/L dodecyltrimethyl ammonium chloride and 1.25 mmol/L Na2EDTA in water, 2% acetonitrile | 6 | Determination of ascorbic acid and determination of total ascorbic acid (by reduction of DHA with TCEP in trizmabuffer) |
| Ross et al. [18] | MPA | N/A | Yes | - | RP-HPLC | Isocratic | 20 | N/A | 262 | 25 mM myristyltrimethylammonium bromide, 0.05 M sodium hydroxide, 0.06 M acetic acid, 7.5% ACN (pH 5.5), adding homocysteine 100 mg/I and EDTA 200 mg/L before use | 0.55 | Simultaneous determination of ascorbic acid and uric acid, ion-pairing reagent (myristyltrimethylammonium bromide) |
| Author | Filling | Grain Size (µm) | Column Length (mm) |
|---|---|---|---|
| Van Gorkom et al. a [5] | HPLC column: XBridge Amide guard column: XBridge Amide | 3.5 | 150 |
| Fitzpatrick et al. [10] | HILIC column: Waters BEH Amide Column precolumn: Waters BEH amide Vanguard (Waters, Rydalmere, Australia) | 1.7 | 100 |
| Robitaille et al. [20] | reverse phase Agilent Zorbax Eclipse XDB-C18 guard column: Agilent Eclipse XDB-C18 | 3.5 | 150 |
| Kand’ár et al. [16] | Analytical column: Discovery C18 guard column: Discovery C18 (Supelco, Bellefonte, PA, USA) | 5 | 250 |
| Tavazzi et al. [22] | analytical column: Hypersil C-18 guard column (ThermoElectron Italia, Rodano, Milan, Italy) | 5 | 250 |
| Karlsen et al. [3] | analytical column: Chromolith Performance RP18-e guard column: Chromolith Performance RP18-e | Not mentioned | 100 |
| Ross et al. [18] | analytical column: Nucleosil ODS (Jones, Henygoed, Mid Glamorgan, UK) guard column: Perisorb RP18 (Anachem) | 5 | 250 |
| Author, Year | LOD | LOQ | Reference Values |
|---|---|---|---|
| van Gorkom et al., 2022 [5] | 1.93 μmol/L | - | Only shown as figure in publication, range ca. 28–113 μmol/L (n = 40) |
| Fitzpatrick et al., 2021 [10] | - | 1 mg/L (5.7 μmol/L #) | - |
| Robitaille et al., 2016 [20] | - | <4.0 μmol/L | Range: 4–133 μmol/L (n = 80, total) |
| Kand’ár R et al., 2008 [16] | 3 μmol/L | - | 50.8 ± 22.4 μmol/L (n = 70) |
| Tavazzi B et al., 2005 [22] | 0.05 μmol/L | 0.08 μmol/L * | 62.57 ± 4.71 μmol/L (n = 15) |
| Karlsen et al., 2005 [3] | 1.5 μmol/L | 4.95 μmol/L | 63.7 ± 18.9 μmol/L (n = 41 healthy) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Demtschuk, M.; Heinz, P. Systematic Review of Different Methods for the Quantification of Vitamin C in Human Plasma Samples by HPLC and UV Detector. Analytica 2026, 7, 2. https://doi.org/10.3390/analytica7010002
Demtschuk M, Heinz P. Systematic Review of Different Methods for the Quantification of Vitamin C in Human Plasma Samples by HPLC and UV Detector. Analytica. 2026; 7(1):2. https://doi.org/10.3390/analytica7010002
Chicago/Turabian StyleDemtschuk, Miriam, and Priska Heinz. 2026. "Systematic Review of Different Methods for the Quantification of Vitamin C in Human Plasma Samples by HPLC and UV Detector" Analytica 7, no. 1: 2. https://doi.org/10.3390/analytica7010002
APA StyleDemtschuk, M., & Heinz, P. (2026). Systematic Review of Different Methods for the Quantification of Vitamin C in Human Plasma Samples by HPLC and UV Detector. Analytica, 7(1), 2. https://doi.org/10.3390/analytica7010002





