Determination of Inorganic Elements in Paper Food Packaging Using Conventional Techniques and in Various Matrices Using Microwave Plasma Atomic Emission Spectrometry (MP-AES): A Review
Abstract
1. Introduction
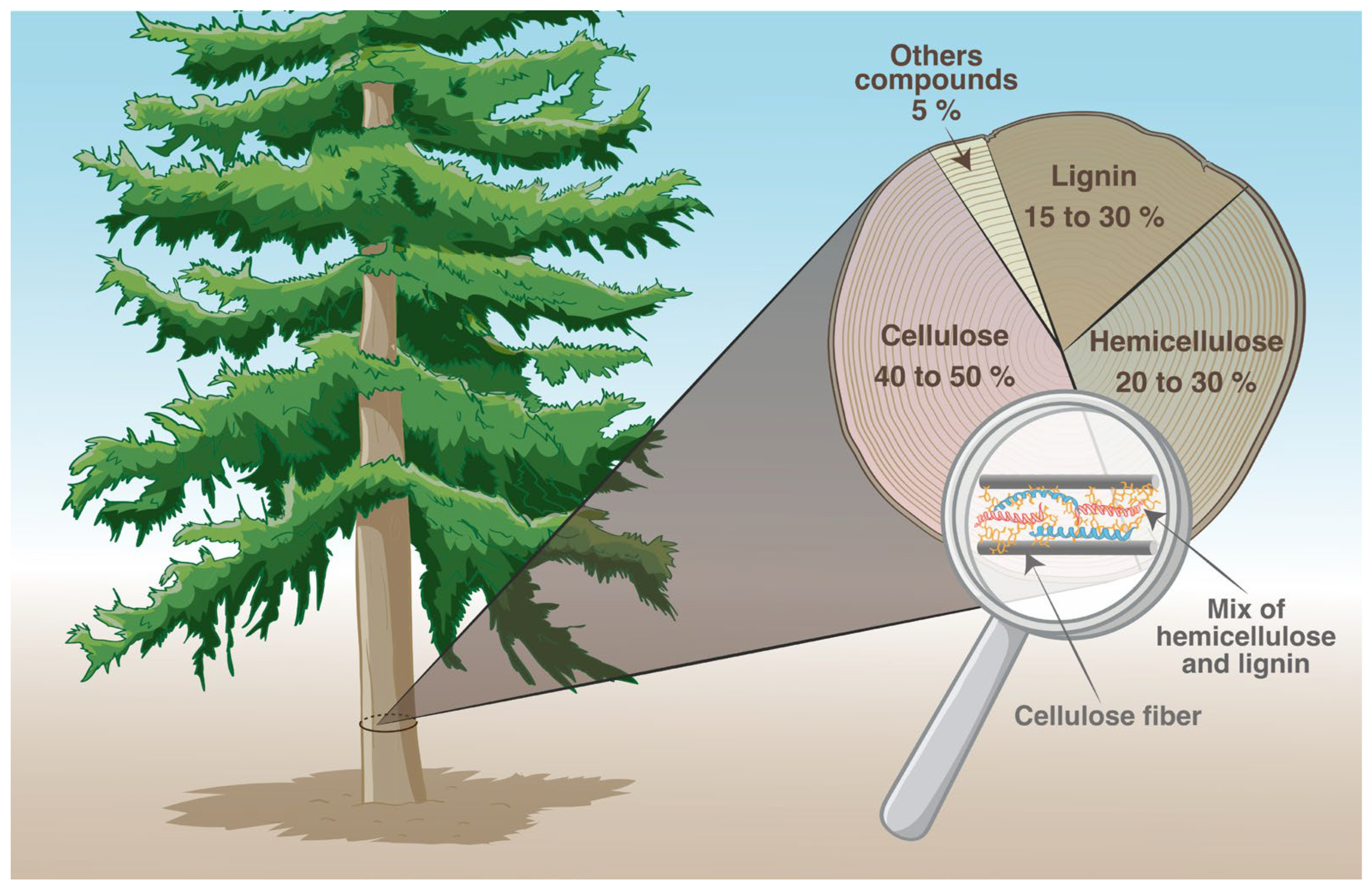
1.1. From Wood to Kraft Pulp Production
- Chemical composition of wood
- Kraft Process
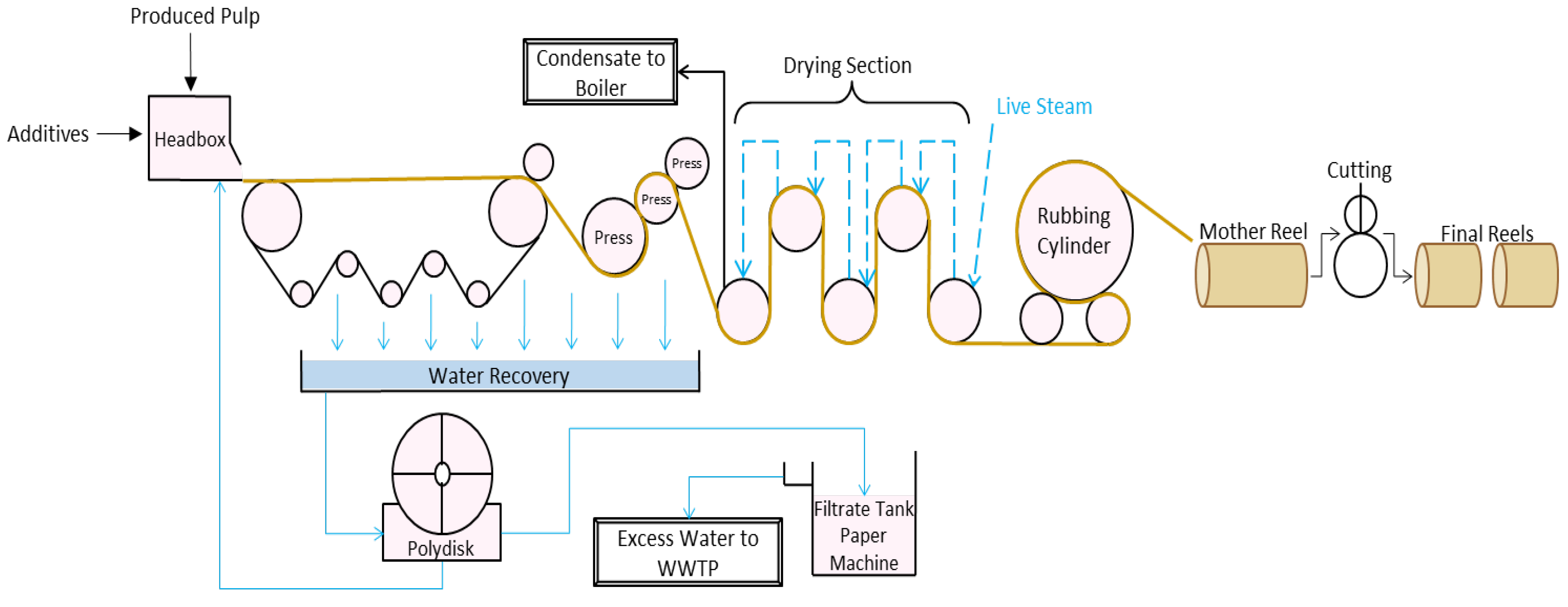
1.2. Paper-Manufacturing Process
2. Inorganic Elements in Paper Packaging Regulations and Analytical Techniques
2.1. Inorganic Elements in the Various Regulations Governing the Paper Industry
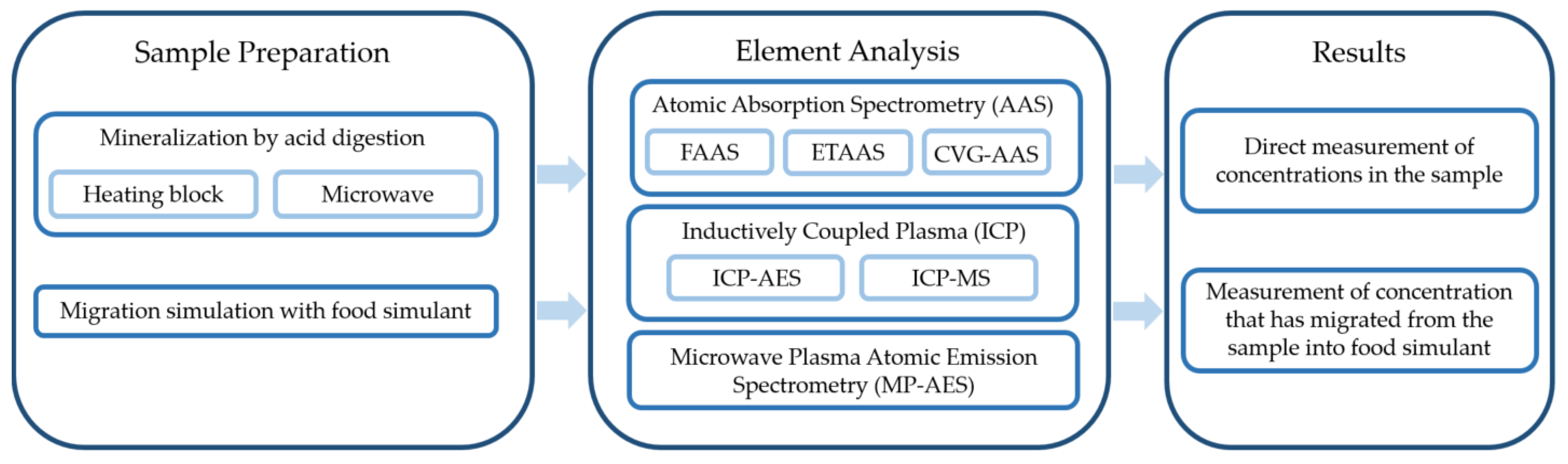
2.2. Commonly Used Techniques for the Analysis of Inorganic Elements
2.2.1. Sample Preparation Step
2.2.2. Element Analysis
- Atomic Absorption Spectrometry
- Inductively Coupled Plasma Atomic Emission Spectrometry
- Inductively Coupled Plasma Mass Spectrometry
- Microwave Plasma Atomic Emission Spectrometry
3. Analysis of Inorganic Elements in Food Packaging: State of the Art
4. MP-AES Elemental Analysis in Different Research Fields: State of the Art
5. Challenges and Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sjöström, E.; Westermark, U. Chemical Composition of Wood and Pulps: Basic Constituents and Their Distribution Chapter. In Analytical Methods in Wood Chemistry, Pulping, and Papermaking; Sjöström, E., Alén, R., Eds.; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–19. ISBN 978-3-662-03898-7. [Google Scholar]
- Blechschmidt, J.; Heinemann, S.; Hans-Joachim, P.; Duffy, G.G. Fibrous Materials for Paper and Board Manufacture chapter. In Handbook of Paper and Board, 2nd ed.; Holik, H., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 33–108. ISBN 978-3-527-65249-5. [Google Scholar]
- Chevalier, R.; Catapano, A.; Pommier, R.; Montemurro, M. A Review on Properties and Variability of Pinus pinaster Ait. Ssp. Atlantica Existing in the Landes of Gascogne. J. Wood. Sci. 2024, 70, 14. [Google Scholar] [CrossRef]
- Pu, Y.; Matyas, K.; Kalluri, U.C.; Tuskan, G.A.; Ragauskas, A.J. Challenges of the Utilization of Wood Polymers: How Can They Be Overcome? Appl. Microbiol. Biotechnol. 2011, 91, 1525–1536. [Google Scholar] [CrossRef]
- Rousseau, V.M. Preparation and Evaluation of New Recyclable Catalysts for Paper Baking. Ph.D. Thesis, Bordeaux 1 University, Bordeaux, France, 2012. [Google Scholar]
- Iglesias, M.C. Lignin-containing cellulose nanofibrils (LCNF): Processing and characterization. Master’s Thesis, Auburn University, Auburn, AL, USA, 2018. [Google Scholar]
- Tribot, A.; Ghenima, A.; Maarouf, A.A.; De Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.D.; Callois, J.M.; Vial, C.; Michaud, P.; et al. Wood-Lignin: Supply, Extraction Processes and Use as Bio-Based Material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Roberts, J.C. The Chemistry of Paper; Royal Society of Chemistry: Tokyo, Japan, 1998; ISBN 978-0-85404-518-1. [Google Scholar]
- Zborowska, M.; Niedzielski, P.; Budka, A.; Enenche, J.; Mleczek, M. Content of elements in contempory and archaeological wood as a marker in physico-chemical parameters. J. Cult. Herit. 2023, 63, 90–100. [Google Scholar] [CrossRef]
- Biermann, C.J. Pulping Fundamentals chapter. In Handbook of Pulping and Papermaking, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 55–100. ISBN 978-0-12-097362-0. [Google Scholar]
- Conte, F.; Casalini, G.; Prati, L.; Ramis, G.; Rossetti, I. Photoreforming of Model Carbohydrate Mixtures from Pulping Industry Wastewaters. Int. J. Hydrogen Energy 2022, 47, 41236–41248. [Google Scholar] [CrossRef]
- Polowski, N.V.; Vasco De Toledo, E.C.; Filho, R.M. A kinetic mathematical model of kraft pulping process for control and optimization applications. IFAC Proc. 2006, 39, 291–295. [Google Scholar] [CrossRef]
- Biermann, C.J. Paper Manufacture chapter. In Handbook of Pulping and Papermaking, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 209–262. ISBN 978-0-12-097362-0. [Google Scholar]
- Hubbe, M.A.; Venditti, R.A.; Rojas, O.J. What happens to cellulosic fibers during papermaking and recycling? A review. BioResources 2007, 2, 739–788. [Google Scholar] [CrossRef]
- Alamri, M.S.; Qasem, A.A.; Abdellatif, A.M.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food Packaging’s Materials: A Food Safety Perspective. Saudi. J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, V.I.; Akrida-Demertzi, K.; Demertzis, P.G. A Study on the Migration of Organic Pollutants from Recycled Paperboard Packaging Materials to Solid Food Matrices. J. Chromatogr. A 2007, 1077, 74–79. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Bosnea, L. Migration of Substances from Food Packaging Materials to Foods. Crit. Rev. Food Sci. Nutr. 2004, 44, 63–76. [Google Scholar] [CrossRef]
- Sood, S.; Sharma, C. Levels of Selected Heavy Metals in Food Packaging Papers and Paperboards Used in India. JEP 2019, 10, 360–368. [Google Scholar] [CrossRef]
- Senila, M. Metal and metalloid monitoring in water by passive sampling—A review. Rev. Anal. Chem. 2023, 42, 20230065. [Google Scholar] [CrossRef]
- Khan, R.; Srivastava, R.; Abdin, M.Z.; Manzoor, N.; Mahmooduzzafar. Effect of Soil Contamination with Heavy Metals on Soybean Seed Oil Quality. Eur. Food. Res. Technol. 2013, 236, 707–714. [Google Scholar] [CrossRef]
- Senila, M. Recent Advances in the Determination of Major and Trace Elements in Plants Using Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2024, 29, 3169. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh-Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 939161, 1–31. [Google Scholar] [CrossRef]
- Nouri, J.; Mahvi, A.H.; Bazrafshan, E. Application of Electrocoagulation Process in Removal of Zinc and Copper From Aqueous Solutions by Aluminum Electrodes. Int. J. Environ. Res. 2010, 4, 201–208. [Google Scholar] [CrossRef]
- Stahl, T.; Falk, S.; Rohrbeck, A.; Georgii, S.; Herzog, C.; Wiegand, A.; Hotz, S.; Boschek, B.; Zorn, H.; Brunn, H. Migration of Aluminum from Food Contact Materials to Food—A Health Risk for Consumers? Part I of III: Exposure to Aluminum, Release of Aluminum, Tolerable Weekly Intake (TWI), Toxicological Effects of Aluminum, Study Design, and Methods. Environ. Sci. Eur. 2017, 29, 19. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I. Iron toxicity and antioxidant nutrients. Toxicology 2002, 180, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Selin, E.; Svensson, K.; Gravenfors, E.; Giovanoulis, G.; Mitsura, L.; Oskarsson, A.; Lundqvist, J. Food Contact Materials: An Effect-Based Evaluation of the Presence of Hazardous Chemicals in Paper and Cardboard Packaging. Food Addit. Contam. Part A 2021, 38, 1594–1607. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to come into Contact with Food; European Commission, Office of the European Union: Luxembourg, 2011.
- Regulation (EC) No 1935/2004 of the European Parliament on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC; European Commission, Office of the European Union: Luxembourg, 2004.
- European Parliament and Council Directive 94/62/EC on Packaging and Packaging Waste; European Communities, Office of the European Union: Luxembourg, 1994.
- Food Contact Suitability of Organic Materials Based on Plant Fibers Intended to come into Contact with Foodstuffs; General Directorate for Competition, Consumer Affairs and Fraud Control: Paris, France, 2019.
- BfR XXXVI. Paper and Board for Food Contact; BfR German Federal Institute for Risk Assessment: Berlin, Germany, 2023. [Google Scholar]
- Conti, M.E. Heavy Metals in Food Packagings. In Mineral Components in Foods; Nriagu, J., Szefer, P., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 339–362. ISBN 978-1-4200-0398-7. [Google Scholar]
- Ibourki, M.; Hallouch, O.; Devkota, K.; Guillaume, D.; Hirich, A.; Gharby, S. Elemental Analysis in Food: An Overview. J. Food. Comp. Anal. 2023, 120, 105330. [Google Scholar] [CrossRef]
- Soares, S.; Moraes, B.L.; Rocha, F.R.P.; Virgilio, A. Sample Preparation and Spectrometric Methods for Elemental Analysis of Milk and Dairy Products—A Review. J. Food. Comp. Anal. 2023, 115, 104942. [Google Scholar] [CrossRef]
- Sapkota, A.; Krachler, M.; Scholz, C.; Cheburkin, A.K.; Shotyk, W. Analytical Procedures for the Determination of Selected Major (Al, Ca, Fe, K, Mg, Na, and Ti) and Trace (Li, Mn, Sr, and Zn) Elements in Peat and Plant Samples Using Inductively Coupled Plasma-Optical Emission Spectrometry. Anal. Chim. Acta 2005, 540, 247–256. [Google Scholar] [CrossRef]
- Krachler, M.; Mohl, C.; Emons, H.; Shotyk, W. Analytical Procedures for the Determination of Selected Trace Elements in Peat and Plant Samples by Inductively Coupled Plasma Mass Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 1277–1289. [Google Scholar] [CrossRef]
- Pastorino, P.; Pizzul, E.; Barceló, D.; Abete, M.C.; Magara, G.; Brizio, P.; Avolio, R.; Bertoli, M.; Dondo, A.; Prearo, M.; et al. Ecology of Oxidative Stress in the Danube Barbel (Barbus balcanicus) from a Winegrowing District: Effects of Water Parameters, Trace and Rare Earth Elements on Biochemical Biomarkers. Sci. Total Environ. 2021, 772, 145034. [Google Scholar] [CrossRef]
- Douvris, C.; Vaughan, T.; Bussan, D.; Bartzas, G.; Thomas, R. How ICP-OES Changed the Face of Trace Element Analysis: Review of the Global Application Landscape. Sci. Total Environ. 2023, 905, 167242. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Bezerra, M.A.; Santos, A.S.; Dos Santos, W.N.L.; Novaes, C.G.; De Oliveira, O.M.C.; Oliveira, M.L.; Garcia, R.L. Atomic Absorption Spectrometry—A Multi Element Technique. TrAC Trends Anal. Chem. 2018, 100, 1–6. [Google Scholar] [CrossRef]
- Mukhopadhyay, G. Atomic Spectroscopy Analysis of Heavy Metals in Plants. J. Pharm. Biol. Arch. 2018, 9, 175–179. [Google Scholar]
- Tyler, G.; Yvon, J. ICP-OES, ICP-MS and AAS Techniques Compared. Available online: https://www.semanticscholar.org/paper/ICP-OES-%2C-ICP-MS-and-AAS-Techniques-Compared-Tyler-Yvon/3a997ca1a20b00e68003ca04937b2035d0d76a5d (accessed on 17 April 2024).
- Sneddon, J.; Vincent, M.D. ICP-OES and ICP-MS for the Determination of Metals: Application to Oysters. Anal. Lett. 2008, 41, 1291–1303. [Google Scholar] [CrossRef]
- Yeung, V.; Miller, D.D.; Rutzke, M.A. References. In Atomic Absorption Spectroscopy, Atomic Emission Spectroscopy, and Inductively Coupled Plasma-Mass Spectrometry; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2017; pp. 129–150. ISBN 978-3-319-45776-5. [Google Scholar]
- Nageswara, R.; Kumar Talluri, M.V.N. An Overview of Recent Applications of Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) in Determination of Inorganic Impurities in Drugs and Pharmaceuticals. J. Pharm. Biomed. Anal. 2007, 43, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Poupon, J. Inductively coupled plasma mass spectrometry: Principle, equipment and benefits in clinical biology. Rev. Francoph. Lab. 2021, 553, 55–63. [Google Scholar] [CrossRef]
- Balaram, V. Microwave Plasma Atomic Emission Spectrometry (MP-AES) and Its Applications—A Critical Review. Microchem. J. 2020, 159, 105483. [Google Scholar] [CrossRef]
- Puppe, D.; Buhtz, C.; Kaczorek, D.; Schaller, J.; Stein, M. Microwave plasma atomic emission spectroscopy (MP-AES)—A useful tool for the determination of silicon contents in plant samples? Front. Environ. Sci. 2024, 12, 1378922. [Google Scholar] [CrossRef]
- Jung, M.Y.; Kang, J.H.; Choi, Y.S.; Lee, D.Y.; Lee, J.Y.; Park, J.S. Analytical Features of Microwave Plasma-Atomic Emission Spectrometry (MP-AES) for the Quantitation of Manganese (Mn) in Wild Grape (Vitis Coignetiae) Red Wines: Comparison with Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES). Food Chem. 2019, 274, 20–25. [Google Scholar] [CrossRef]
- Han, Y.; Cheng, J.; An, D.; He, Y.; Tang, Z. Occurrence, Potential Release and Health Risks of Heavy Metals in Popular Take-out Food Containers from China. Environ. Res. 2022, 206, 112265. [Google Scholar] [CrossRef]
- Skrzydlewska, E.; Balcerzak, M.; Vanhaecke, F. Determination of Chromium, Cadmium and Lead in Food-Packaging Materials by Axial Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Anal. Chim. Acta 2003, 479, 191–202. [Google Scholar] [CrossRef]
- Mertoglu-Elmas, G.; Çınar, G. Toxic Metals in Paper and Paperboard Food Packagings. BioResources 2018, 13, 7560–7580. [Google Scholar] [CrossRef]
- Bandara, R.; Indunil, G.M. Food Packaging from Recycled Papers: Chemical, Physical, Optical Properties and Heavy Metal Migration. Heliyon 2022, 8, e10959. [Google Scholar] [CrossRef]
- Khan, S.; Khan, A.R. Migrating Levels of Toxic Heavy Metals in Locally Made Food Packaging Containers. Egypt. J. Chem. 2022, 65, 521–527. [Google Scholar] [CrossRef]
- Whitt, M.; Vorst, K.; Brown, W.; Baker, S.; Gorman, L. Survey of Heavy Metal Contamination in Recycled Polyethylene Terephthalate Used for Food Packaging. J. Plast. Film Sheet. 2013, 29, 163–173. [Google Scholar] [CrossRef]
- Duran, A.; Mustafa, S. Evaluation of Metal Concentrations in Food Packaging Materials: Relation to Human Health. At. Spectrosc. 2013, 34, 99–103. [Google Scholar] [CrossRef]
- Oliveira, L.B.; De Melo, J.C.; Da Boa Morte, E.S.; De Jesus, R.M.; Teixeira, L.S.G.; Korn, M.G.A. Multi-Element Determination in Chocolate Bars by Microwave-Induced Plasma Optical Emission Spectrometry. Food. Chem. 2021, 351, 129285. [Google Scholar] [CrossRef] [PubMed]
- Sajtos, Z.; Herman, P.; Harangi, S.; Baranyai, E. Elemental Analysis of Hungarian Honey Samples and Bee Products by MP-AES Method. Microchem. J. 2019, 149, 103968. [Google Scholar] [CrossRef]
- São Bernardo Carvalho, L.; Santos Silva, C.; Araújo Nóbrega, J.; Santos Boa Morte, E.; Muniz Batista Santos, D.S.; Andrade Korn, M.G. Microwave Induced Plasma Optical Emission Spectrometry for Multielement Determination in Instant Soups. J. Food Compos. Anal. 2020, 86, 103376. [Google Scholar] [CrossRef]
- Ozbek, N.; Akman, S. Method Development for the Determination of Calcium, Copper, Magnesium, Manganese, Iron, Potassium, Phosphorus and Zinc in Different Types of Breads by Microwave Induced Plasma-Atomic Emission Spectrometry. Food. Chem. 2016, 200, 245–248. [Google Scholar] [CrossRef]
- Ozbek, N.; Akman, S. Microwave Plasma Atomic Emission Spectrometric Determination of Ca, K and Mg in Various Cheese Varieties. Food. Chem. 2016, 192, 295–298. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Carlier, J.D.; Costa, M.C.; Romano, A. Multi-Element Characterisation of Carob, Fig and Almond Liqueurs by MP-AES: Multi-Element Characterisation of Carob, Fig and Almond Liqueurs by MP-AES. J. Inst. Brew. 2018, 124, 300–309. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Ross, A.; Huang, Z.; Chang, W.; Ou-yang, K.; Chen, Y.; Wu, C. Determination of Heavy Metals in Leather and Fur by Microwave Plasma-Atomic Emission Spectrometry. Spectrochim. Acta Part B 2015, 112, 6–9. [Google Scholar] [CrossRef]
- Sungur, Ş.; Gülmez, F. Determination of Metal Contents of Various Fibers Used in Textile Industry by MP-AES. J. Spectrosc. 2015, 2015, 1–5. [Google Scholar] [CrossRef]
- Geisenblosen, M.C.; Oyhantçabal, P.; Pistón, M. Determination of Major Elements in Igneous Rocks Using Microwave Plasma Atomic Emission Spectrometry (MP-AES). MethodsX 2022, 9, 101793. [Google Scholar] [CrossRef]
- Vella, A.; Attard, E. Analysis of Heavy Metal Content in Conventional and Herbal Toothpastes Available at Maltese Pharmacies. Cosmetics 2019, 6, 28. [Google Scholar] [CrossRef]
| Reference | Sample Type | Targeted Elements | Sample Preparation | Digestion Conditions | Analysis | Measured Values |
|---|---|---|---|---|---|---|
| Bandara and Indunil [52] | Recycled papers for food packaging | Cd, Cr, Cu, Mn, Pb, and Zn | Hot-plate digestion with conc. HNO3 (12 mL), conc.H2O2 (4 mL) and conc. HCl (2 mL) | 60 min/50 °C 24 h/Room Temperature (RT) | AAS | Cd [0.0095; 1.94] mg·kg−1 Cr [0.0264; 0.1963] mg·kg−1 Cu [0.0095; 1.940] mg·kg−1 Mn [0.0134; 0.0156] mg·kg−1 Pb [0.1255; 0.2618] mg·kg−1 Zn [0.4520; 0.4573] mg·kg−1 |
| Elmas and Cinar [51] | Paper, paperboard, and corrugated board packaging | Al, Cd, Cr, Cu, Hg, Ni, Pb, and Zn | Microwave digestion with 5% HNO3 (5 mL) and 30% H2O2 (2 mL) | 2 min/120 °C 5 min/140 °C 15 min/170 °C 1 min/50 °C 1 min/50 °C | ICP-AES ICP-MS | Al [1.268; 3909] mg·kg−1 Cd [0.02; 0.18] mg·kg−1 Cr [0.51; 6.16] mg·kg−1 Cu [0.52; 166.6] mg·kg−1 Hg [0.01; 3.80] mg·kg−1 Ni [0.92; 4.93] mg·kg−1 Pb [1.39; 12.94] mg·kg−1 Zn [1.36; 61.30] mg·kg−1 |
| Han et al. [49] | Take-out food paper containers | Cd, Co, Cr, Mn, Ni, Pb, and Sb | Microwave digestion with analytical grade HNO3 (8 mL) | 5 min/up to 175 °C 4.5 min/175 °C | ICP-MS | Cd [0.004; 0.18] mg·kg−1 Co [0.02; 5.63] mg·kg−1 Cr [0.65; 6.58] mg·kg−1 Mn [1.61; 89.8] mg·kg−1 Ni [0.37; 3.76] mg·kg−1 Pb [0.004; 5.58] mg·kg−1 Sb [0.002; 1.19] mg·kg−1 |
| Skrzydlewska et al. [50] | Papers and paperboards for food packaging | Cd, Cr, and Pb | Microwave digestion with 65% HNO3 (6 mL) and 30% H2O2 (2 mL) | 15 min/up to 100 °C 10 min/up to 180 °C 15 min/180 °C 30 min/up to RT | ICP-TOF-MS | Cd [0.09; 0.12] µg·kg−1 Cr [0.25; 0.64] µg·kg−1 Pb [0.28; 0.99] µg·kg−1 |
| Sood and Sharma [18] | Papers and paperboards for food packaging | Al, As, B, Ba, Co, Cr, Cu, Fe, Mn, Ni, Pb, Te, Ti, and V | Microwave digestion with conc. HNO3 (10 mL) and conc. HCl (3 mL) | 5.5 min/up to 175 °C 4.5 min/175 °C | ICP-AES | Al [11.763; 102.722] mg·kg−1 As [0.008; 0.300] mg·kg−1 B [0.008; 0.035] mg·kg−1 Ba [0.265; 1.118] mg·kg−1 Co [0.024; 0.053] mg·kg−1 Cr [0.026; 2.174] mg·kg−1 Cu [0.045; 0.832] mg·kg−1 Fe [0.418; 37.209] mg·kg−1 Mn [0.208; 547] mg·kg−1 Ni [0.008; 0.196] mg·kg−1 Pb [0.051; 388] mg·kg−1 Te [0.004; 0.012] mg·kg−1 Ti [0.007; 0.153] mg·kg−1 V [0.032; 0.547] mg·kg−1 |
| Whitt et al. [54] | Recycled plastic food packaging | Cd, Cr, Ni, Pb, and Sb | Hot-plate digestion in two steps: (1) 67% HNO3 (20 mL) and trace metal gradeHClO4 (3 mL) (2) 67% HNO3 (1 mL) and 37% HCl (1 mL) | reduction up to 1 mL/500 °C cooled 5 min until boiling after add. of new reagent/500 °C cooled 5 min | ICP-AES | Cd [2.02; 22.61] mg·kg−1 Cr [1.71; 16.67] mg·kg−1 Ni [2.20; 23.59] mg·kg−1 Pb [0.02; 0.36] mg·kg−1 Sb [0.05; 11.38] mg·kg−1 |
| Bandara and Indunil [52] | Recycled papers for food packaging | Cd, Cr, Cu, Mn, Pb, and Zn | Migration of sample in 3% CH3COOH food simulant | 24 h/40 °C | AAS | Cd [0.0012; 0.011] µg·L−1 Cr [0.0069; 0.206] µg·L−1 Cu [0.262; 0.98] µg·L−1 Mn [0.001; 0.056] µg·L−1 Pb [0.0072; 0.126] µg·L−1 Zn [0.024; 0.45] µg·L−1 |
| Duran and Soylak [55] | Different food packaging materials | Co, Cd, Cr, Cu, Fe, Mn, Ni, and Pb | Migration of sample in 3% CH3COOH food simulant | 24 h/40 °C | FAAS | Cd [0.03; 0.64] µg·dm2 Co [0.40; 5.03] µg·dm2 Cr [0.30; 2.45] µg·dm2 Cu [0.04; 29.8] µg·dm2 Fe [0.15; 67.8] µg·dm2 Mn [0.006; 36.8] µg·dm2 Ni [0.02; 9.43] µg·dm2 Pb [0.41; 11.8] µg·dm2 |
| Khan and Khan [53] | Plastic food containers | Cu, Mn, Ni, Pb, and Zn | Migration of sample in aqueous food simulants (water, 3% CH3COOH, 8% EtOH, 0.9% NaCl + 5% Na2CO3) and then digestion of solution with conc. HNO3 (1 mL) | 3 different conditions: 72 h/4 °C 2 h/60 °C 24 h/25 °C | AAS | Cu [1.003; 1.61] mg·L−1 Mn [1.002; 1.01] mg·L−1 Ni [1.01; 1.31] mg·L−1 Pb [1.002; 1.9] mg·L−1 Zn [1.001; 1.02] mg·L−1 |
| Reference | Sample Type | Targeted Elements | Sample Digestion Protocol | Digestion Conditions | Detection Limits | Quantification Limits |
|---|---|---|---|---|---|---|
| Geisenblosen et al. [64] | Rock | Al, Ba, Ca, Fe, K, Mg, Mn, Na, Sr, and Ti | Microwave digestion with (1) 65% HNO3 (3 mL), 37% HCl (1 mL), and 48% HF (3 mL) (2) 5% H3BO3 (18 mL) | 5 min ramp + 60 min hold time at 1400 W 5 min ramp + 15 min hold time at 1400 W | 100 µg·g−1 (Al) 1.0 µg·g−1 (Ba) 4.0 µg·g−1 (Ca) 70 µg·g−1 (Fe) 140 µg·g−1 (K) 1.0 µg·g−1 (Mg) 1.0 µg·g−1 (Mn) 110 µg·g−1 (Na) 0.3 µg·g−1 (Sr) 6.0 µg·g−1 (Ti) | 300 µg·g−1 (Al) 4.0 µg·g−1 (Ba) 13 µg·g−1 (Ca) 210 µg·g−1 (Fe) 400 µg·g−1 (K) 2.0 µg·g−1 (Mg) 4.0 µg·g−1 (Mn) 320 µg·g−1 (Na) 0.8 µg·g−1 (Sr) 19 µg·g−1 (Ti) |
| Jung et al. [48] | Wine | Mn | Hot-plate digestion with 30–32% H2O2 (15 mL) 70% HNO3 (1 mL) | reduction up to 20 mL/80 °C reduction up to 5 mL/80 °C | 0.67 µg·L−1 | 2.22 µg·L−1 |
| Oliveira et al. [56] | Chocolate bar | Ba, Ca, Cu, Cr, Fe, K, Mg, Mn, Na, Ni, P, and Zn | Microwave digestion with analytical grade HNO3 (2.3 mL) and analytical grade H2O2 (1 mL) | 4 min/up to 120 °C 2 min/120 °C 4 min/up to 190 °C 20 min/190 °C 10 min/ventilation | 0.002 mg·kg−1 (Ba) 21 mg·kg−1 (Ca) 0.05 mg·kg−1 (Cu) 0.2 mg·kg−1 (Cr) 0.5 mg·kg−1 (Fe) 73 mg·kg−1 (K) 1.4 mg·kg−1 (Mg) 0.01 mg·kg−1 (Mn) 3.8 mg·kg−1 (Na) 0.1 mg·kg−1 (Ni) 11 mg·kg−1 (P) 0.2 mg·kg−1 (Zn) | 0.007 mg·kg−1 (Ba) 69 mg·kg−1 (Ca) 0.16 mg·kg−1 (Cu) 0.6 mg·kg−1 (Cr) 1.6 mg·kg−1 (Fe) 241 mg·kg−1 (K) 4.6 mg·kg−1 (Mg) 0.03 mg·kg−1 (Mn) 12.5 mg·kg−1 (Na) 0.3 mg·kg−1 (Ni) 36 mg·kg−1 (P) 0.6 mg·kg−1 (Zn) |
| Ozbek and Akman [59] | Bread | Ca, Cu, Fe, K, Mg, Mn, and Zn | Hot-plate digestion with 65% HNO3 (3 mL) and 35% H2O2 (1 mL) | 2 h/100 °C | 13.1 mg·kg−1 (Ca) 0.28 mg·kg−1 (Cu) 4.47 mg·kg−1 (Fe) 118 mg·kg−1 (K) 1.10 mg·kg−1 (Mg) 0.41 mg·kg−1 (Mn) 3.00 mg·kg−1 (Zn) | 43.8 mg·kg−1 (Ca) 0.93 mg·kg−1 (Cu) 14.9 mg·kg−1 (Fe) 393 mg·kg−1 (K) 3.66 mg·kg−1 (Mg) 1.38 mg·kg−1 (Mn) 10.1 mg·kg−1 (Zn) |
| Ozbek and Akman [60] | Cheese | Ca, K, and Mg | Hot-plate digestion with 65% HNO3 (3 mL) and 35% H2O2 (1 mL) | 4–5 h/100 °C | 0.036 mg·kg−1 (Ca) 0.19 mg·kg−1 (K) 0.012 mg·kg−1 (Mg) | 0.118 mg·kg−1 (Ca) 0.63 mg·kg−1 (K) 0.038 mg·kg−1 (Mg) |
| Rodriguez-Solana et al. [61] | Beverage | Ca, Cu, Cd, Fe, K, Mg, Mn, Na, P, Pb, and Zn | Hot-plate digestion with: Method 1: 65% HNO3 (6 mL) and 60% HClO4 (4 mL) Method 2: 65% HNO3 (2 mL) and 60% HClO4 (8 mL) Method 3: 65% HNO3 (4 mL) and 36% HCl (1 mL) Method 4: 65% HNO3 (1 mL) and 36% HCl (1 mL) Muffle furnace digestion with 65% HNO3 (10 mL) | Method 1: 20 min/60 °C and 45 min/90 °C Method 2: 20 min/60 °C and 45 min/90 °C Method 3: 20 min/RT, 30 min/80 °C, 30 min/100 °C and 30 min/110 °C Method 4: 20 min/60 °C and 45 min/90 °C Muffle furnace method: 30 min/80 °C, 120 min/150 °C and 240 min/450 °C | 0.52 mg·L−1 (Ca) 0.05 mg·L−1 (Cu) 0.11 mg·L−1 (Cd) 0.10 mg·L−1 (Fe) 0.07 mg·L−1 (K) 0.05 mg·L−1 (Mg) 0.08 mg·L−1 (Mn) 0.15 mg·L−1 (Na) 0.14 mg·L−1 (P) 0.13 mg·L−1 (Pb) 0.10 mg·L−1 (Zn) | 1.74 mg·L−1 (Ca) 0.17 mg·L−1 (Cu) 0.38 mg·L−1 (Cd) 0.33 mg·L−1 (Fe) 0.07 mg·L−1 (K) 0.17 mg·L−1 (Mg) 0.25 mg·L−1 (Mn) 0.49 mg·L−1 (Na) 0.46 mg·L−1 (P) 0.42 mg·L−1 (Pb) 0.32 mg·L−1 (Zn) |
| Sajtos et al. [57] | Honey | Al, B, Ba, Bi, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Na, Ni, Pb, Sr, and Zn | Hot-plate digestion with 65% HNO3 (4 mL) and 30% H2O2 (1 mL) | - | 0.2315 mg·kg−1 (Al) 0.2372 mg·kg−1 (B) 0.0777 mg·kg−1 (Ba) 1.2260 mg·kg−1 (Bi) 2.0610 mg·kg−1 (Ca) 0.3325 mg·kg−1 (Cd) 0.0720 mg·kg−1 (Co) 0.0044 mg·kg−1 (Cr) 0.0442 mg·kg−1 (Cu) 0.3694 mg·kg−1 (Fe) 0.1852 mg·kg−1 (K) 0.0138 mg·kg−1 (Li) 0.0802 mg·kg−1 (Mg) 0.0080 mg·kg−1 (Mn) 0.2126 mg·kg−1 (Na) 0.0162 mg·kg−1 (Ni) 0.0165 mg·kg−1 (Pb) 0.2878 mg·kg−1 (Sr) 0.1899 mg·kg−1 (Zn) | 0.7718 mg·kg−1 (Al) 0.7905 mg·kg−1 (B) 0.2590 mg·kg−1 (Ba) 4.0865 mg·kg−1 (Bi) 6.8701 mg·kg−1 (Ca) 1.1084 mg·kg−1 (Cd) 0.2399 mg·kg−1 (Co) 0.0148 mg·kg−1 (Cr) 0.1473 mg·kg−1 (Cu) 1.2312 mg·kg−1 (Fe) 0.6172 mg·kg−1 (K) 0.0459 mg·kg−1 (Li) 0.2673 mg·kg−1 (Mg) 0.0266 mg·kg−1 (Mn) 0.7085 mg·kg−1 (Na) 0.0540 mg·kg−1 (Ni) 0.0549 mg·kg−1 (Pb) 0.9594 mg·kg−1 (Sr) 0.6331 mg·kg−1 (Zn) |
| Sao Bernardo Carvalho et al. [58] | Instant soup | Cu, K, Mg, Mn, P, and Zn | Microwave digestion with 65% HNO3 (1 mL) and 30% H2O2 (1 mL) | 10 min/up to 120 °C 3 min/120 °C 13 min/up to 200 °C 14 min/200 °C | 0.09 mg·kg−1 (Cu) 4.9 mg·kg−1 (K) 1.0 mg·kg−1 (Mg) 0.04 mg·kg−1 (Mn) 5.4 mg·kg−1 (P) 0.88 mg·kg−1 (Zn) | 0.31 mg·kg−1 (Cu) 16 mg·kg−1 (K) 3.4 mg·kg−1 (Mg) 0.12 mg·kg−1 (Mn) 18 mg·kg−1 (P) 2.9 mg·kg−1 (Zn) |
| Sungur and Gülmez [63] | Textile | Al, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Tl, and Zn | Hot-plate digestion with 1:5 30% H2O2/70% HNO3 (10 mL) | 55 min/110 °C | 0.123 mg·L−1 (Al) 0.415 mg·L−1 (Cd) 0.202 mg·L−1 (Co) 0.101 mg·L−1 (Cr) 0.256 mg·L−1 (Cu) 0.343 mg·L−1 (Fe) 0.059 mg·L−1 (Mn) 0.118 mg·L−1 (Ni) 0.088 mg·L−1 (Pb) 0.285 mg·L−1 (Tl) 0.705 mg·L−1 (Zn) | 0.409 mg·L−1 (Al) 1.382 mg·L−1 (Cd) 0.673 mg·L−1 (Co) 0.336 mg·L−1 (Cr) 0.852 mg·L−1 (Cu) 1.142 mg·L−1 (Fe) 0.196 mg·L−1 (Mn) 0.393 mg·L−1 (Ni) 0.293 mg·L−1 (Pb) 0.949 mg·L−1 (Tl) 2.348 mg·L−1 (Zn) |
| Vella and Attard [65] | Toothpaste | Ag, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Sn, and Zn | Hot-plate digestion with 5% HNO3 (5 mL) and 34.5% H2O2 (2 mL) Then calcination in a muffle furnace with 5% HNO3 (5 mL) | 80–90 °C after each addition of reagent 6 h/500 °C in muffle furnace | 0.0549 mg·kg−1 (Ag) 0.0067 mg·kg−1 (Cd) 0.0005 mg·kg−1 (Cr) 0.0007 mg·kg−1 (Cu) 0.0037 mg·kg−1 (Fe) 0.0789 mg·kg−1 (Hg) 0.0042 mg·kg−1 (Mn) 0.0056 mg·kg−1 (Ni) 0.0169 mg·kg−1 (Pb) 0.0375 mg·kg−1 (Sn) 0.0301 mg·kg−1 (Zn) | 0.1665 mg·kg−1 (Ag) 0.0204 mg·kg−1 (Cd) 0.0014 mg·kg−1 (Cr) 0.0022 mg·kg−1 (Cu) 0.0113 mg·kg−1 (Fe) 0.2391 mg·kg−1 (Hg) 0.0127 mg·kg−1 (Mn) 0.0169 mg·kg−1 (Ni) 0.0511 mg·kg−1 (Pb) 0.1137 mg·kg−1 (Sn) 0.0912 mg·kg−1 (Zn) |
| Zhao et al. [62] | Leather and fur | Cd, Co, Cr, Cu, Hg, Ni, and Pb | Microwave digestion with 65% HNO3 (4 mL) and 30% H2O2 (1 mL) | up to 130 °C and hold for 5 min up to 180 °C and hold for 10 min up to 220 °C and hold for 20 min | 1.3 mg·kg−1 (Cd) 1.9 mg·kg−1 (Co) 0.9 mg·kg−1 (Cr) 1.5 mg·kg−1 (Cu) 2.0 mg·kg−1 (Hg) 0.9 mg·kg−1 (Ni) 1.2 mg·kg−1 (Pb) | Not determined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chivaley, M.; Bassim, S.; Vargas, V.; Lartigue, D.; Bouyssiere, B.; Pannier, F. Determination of Inorganic Elements in Paper Food Packaging Using Conventional Techniques and in Various Matrices Using Microwave Plasma Atomic Emission Spectrometry (MP-AES): A Review. Analytica 2025, 6, 41. https://doi.org/10.3390/analytica6040041
Chivaley M, Bassim S, Vargas V, Lartigue D, Bouyssiere B, Pannier F. Determination of Inorganic Elements in Paper Food Packaging Using Conventional Techniques and in Various Matrices Using Microwave Plasma Atomic Emission Spectrometry (MP-AES): A Review. Analytica. 2025; 6(4):41. https://doi.org/10.3390/analytica6040041
Chicago/Turabian StyleChivaley, Maxime, Samia Bassim, Vicmary Vargas, Didier Lartigue, Brice Bouyssiere, and Florence Pannier. 2025. "Determination of Inorganic Elements in Paper Food Packaging Using Conventional Techniques and in Various Matrices Using Microwave Plasma Atomic Emission Spectrometry (MP-AES): A Review" Analytica 6, no. 4: 41. https://doi.org/10.3390/analytica6040041
APA StyleChivaley, M., Bassim, S., Vargas, V., Lartigue, D., Bouyssiere, B., & Pannier, F. (2025). Determination of Inorganic Elements in Paper Food Packaging Using Conventional Techniques and in Various Matrices Using Microwave Plasma Atomic Emission Spectrometry (MP-AES): A Review. Analytica, 6(4), 41. https://doi.org/10.3390/analytica6040041







