Abstract
Edible mushrooms are macroscopic fungi that have been recognized as the “new superfoods” due to their high nutritional and medicinal values. The aim of this study was to develop and optimize a method for the wet digestion of edible mushrooms using a closed digestion block for the determination of macro- and micronutrients (Ca, Cr, Cu, Fe, K, Mg, Mn, Ni, and Zn) using microwave-induced plasma emission spectrometry (MIP OES). For the digestion of the samples, a 23 factorial design was used to evaluate the amount of HNO3 65% (m m−1), H2O2 30% (m m−1) and the digestion time, in 500 mg of the sample (dry and crushed) at 200° C. The method was applied to eleven species of edible or medicinal mushrooms (edible cultivated from wild strains, wild edible, and commercials medicinal). The average concentrations (in mg kg−1) showed higher levels of K (1442.85–17,534.97), Mg (1295.40–13,550.72), Fe (11.33–27.38), Zn (28.86–36.09), and Mn (10.22–10.97). This study contributed to the determination of the multi-element composition and nutritional potential of edible mushrooms from Brazil.
1. Introduction
Popularly known as mushrooms, macroscopic fungi are estimated to comprise approximately 470,000 species of ecological, economic, and medicinal importance, including many edible species. This estimate is based on the projected global fungal diversity of around 2.5 million species [1] and the proportion of mushroom-forming fungi, which is approximately 18.75% [2]. Notably, between 92.5% and 95% of all fungal species remain undescribed, highlighting the vast and largely unexplored diversity of the fungal kingdom [1]. Therefore, researching the chemical composition of edible mushrooms is relevant to considering them as functional foods, due to their potential nutritional values and benefits for human health [3].
Species of wild mushrooms consumed by the indigenous peoples of the Amazon, Brazil, have already been reported in the literature [4]. Many mushroom species are sources of essential amino acids, vitamins, minerals and also contain a variety of bioactive compounds such as polysaccharides, proteoglycans, terpenoids, phenolic compounds, and steroids, among others [5,6]. Although there is a great diversity of wild macrofungi, five genera account for about 85% of the world production of edible mushrooms: Lentinula (23% of world production, represented by the shiitake mushroom); Pleurotus (19%); Auricularia (17%); Agaricus (15%); and Flammulina (11%) [7,8]. A recent comprehensive study documented that more than 400 species of wild, edible mushrooms have been identified in Brazil. Of these, 86 have robust occurrence records in the country. Among the six Brazilian biomes recognized, the Atlantic Forest (one of the world’s biodiversity hotspots) is the phytogeographic area with the largest record of wild edible species in the country, with 317 species already mentioned [9]. However, there are few studies on the chemical composition and edibility of wild macrofungi from these areas of the Brazilian Atlantic Forest. This research highlights the rich diversity of edible fungi in Brazil and underscores the need for further investigation into their chemical composition, potential nutritional value, and benefits for human health.
To determine and quantify the total content of inorganic chemical species in biological samples, analytical strategies are used to pre-treat and decompose the matrix prior to quantification by sensitive analytical techniques. Methods include conductive heating in a closed digestion block and digestion in an oven with microwave-assisted heating [10]. Microwave-induced plasma optical emission spectrometry (MIP OES) has gained increasing interest in recent years due to its diverse applications, with sensitive and suitable analytical results. It is therefore a viable alternative to traditional inductively coupled plasma optical emission spectrometry (ICP OES) [11]. MIP OES is a very useful tool due to its low instrumentation and operating costs, which are comparable to those of ICP OES and also the Flame Atomic Absorption Spectrophotometer (F AAS), enabling rapid, multi-element analysis [12].
Considering the high diversity of naturally occurring species in areas of the Brazilian Atlantic Forest, research aimed at expanding knowledge of wild edible mushrooms is both relevant and necessary, contributing to the understanding of their distribution in the region and the assessment of their potential as sources of essential inorganic elements for human health. This study aims to develop and optimize a method for the wet digestion of edible mushrooms using a closed digestion block for the determination of macro- and micronutrients (Ca, Cr, Cu, Fe, K, Mg, Mn, Ni, and Zn) using MIP OES.
2. Materials and Methods
2.1. Mushroom Sampling and Preparation
A total of nine species of edible mushrooms (wild or cultivated from wild strains) from the Atlantic Forest were studied here. The cultivation from wild strains was conducted by the IFungiLab research group at the Federal Institute of Education, Science and Technology of São Paulo (IFSP), Campus São Paulo, Brazil [13,14], corresponding to the following species/strains: Auricularia cornea MPD594/CCIBt4755 [wild strain from Ilha do Cardoso, São Paulo]; Irpex rosettiformis MPD167 [wild strain from Guarapuava, Paraná]; Oudemansiella cubensis MPD319 [wild strain from Paraty, Rio de Janeiro]; Oudemansiella platensis MPD407 [wild strain from Guarapuava, Paraná]; and Pleurotus albidus MPD161 [wild strain from Guarapuava, Paraná]. Wild specimens of other four edible species were also studied from material available at the fungarium FIFUNGI (IFungiLab, IFSP, Brazil): Auricularia fuscosuccinea MPD576 (FIFUNGI005) [Campos do Jordão, São Paulo]; Favolus brasiliensis DAZ183 (FIFUNGI299) [São Paulo, São Paulo]; Lentinus berteroi MPCS95 (FIFUNGI152) [Caraguatatuba, São Paulo]; and Laetiporus gilbertsonii MPD466 (FIFUNGI007) [São Paulo, São Paulo]. The use of wild collections complies with Brazilian legislation on access to genetic biodiversity heritage and is registered in the ‘Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado’ (SisGen #A7E8308). Two species of medicinal mushrooms, Cordyceps militaris (Brazil, Santa Catarina, Guaramirim) and Cordyceps sinensis (Brazil, Paraná, Campos Lagos), were purchased from suppliers specialized in macrofungi through e-commerce platforms.
Wild-cultivated species were selected for their potential for imminent commercial production, while wild-collected species were chosen for their ease of identification and popularity among foragers. Cordyceps militaris was specifically selected for its widespread commercial demand. Whole mushroom samples (including both pileus and stipe) were dried at 40 °C using a fruit dehydrator. All samples were ground in a knife mill and stored in a desiccator until analysis. Cultivated mushrooms from wild strains of Auricularia cornea were used in the development of the acid digestion method, in a closed block digester.
2.2. Reagents, Solutions, and Decontamination of Materials
All reagents were of analytical grade and the solutions were prepared with ultrapure water with a specific resistivity of 18 MΩ cm−1 using the Milli-Q® purification system (Millipore®, Bedford, MA, USA). The reagents HNO3 suprapur grade 65% (m m−1) (Merck®, Darmstadt, DA, Germany), H2O2 30% (m m−1) (Vetec®, São Paulo, SP, Brazil), and ultrapure water were used for acid digestion of the samples. The multi-element solutions were prepared from SpecSol® 10,000 mg L−1 single-element solutions of Ca, K, Mg, Na, and 1000 mg L−1 of Cr, Cu, Fe, Mn, Ni, and Zn.
A multi-element stock solution (500 mg L−1) containing macroelements was prepared for the construction of the analytical curve, ranging from 20 to 90 mg L−1. For the microelements, the analytical curve ranged from 0.5 to 7 mg L−1, starting from a stock solution (50 mg L−1).
The glassware and materials were decontaminated in a 10% (v v−1) HNO3 solution in a bath for 24 h, and the modified polytetrafluoroethylene (TMF®) tubes used for digestion were decontaminated in the closed digestion block for 1 h and 15 min at 180 °C, using 10 mL of 65% m m−1 nitric acid (HNO3). After decontamination, all the glassware, tubes, and materials were washed thoroughly with ultrapure water.
2.3. Instrumentation and Operational Parameters
An analytical balance (Shimadzu®, model ATX224R, Kyoto, Japan) was used for weighing. For the total acid digestion of the samples, a closed digester block (Tecnal®, model 015-1, São Paulo, Brazil) equipped with 15 polytetrafluoroethylene (TMF®) tubes modified for digestion and coupled with temperature control (Tecnal®, model E-007 MOP, São Paulo, Brazil) was used. Analytical determinations were performed using a Microwave-Induced Plasma Optical Emission Spectrometer (MIP OES) (model 4210, Agilent Technologies®, Santa Clara, CA, USA). The nebulizer used has a double-pass glass cyclonic spray chamber system and the standard torch with axial viewing position. A nitrogen generator (model 4107, Agilent Technologies®, Santa Clara, CA, USA) was used for removing air from the environment through a Rotor Plus air compressor (Metalplan Airpower®, São Paulo, Brazil). The instrumental parameters used were: Peristaltic pump rotation (25 rpm); Stabilization time (15 s); Capture time (35 s), with 3 replicates and the Nebulization Flow (L min−1) variable for each element: Ca ( 0,60); Cr (0,90); Cu (0,70); Fe (0,65); K (0,75); Mg (0,9); Mn (0,90); Ni (0,70) e Zn (0,45). The spectral lines (nm) used were the most intense for each microelement: Ca (616.217 nm); Cr (425.433 nm); Cu (324.754 nm); Fe (371.993 nm); K (769.897 nm); Mg (285.213 nm); Mn (403.076 nm); Ni (352.450 nm); and Zn (213.857 nm).
2.4. Wet Digestion Optimization and Factorial Design
To develop the method, a complete factorial design of 23 (Table 1) with two levels was initially carried out to evaluate the influence of the independent variables (volume of nitric acid, volume of hydrogen peroxide, and digestion time) in the wet digestion process, using a closed digestion block. For this, approximately 500 mg of the Auricularia cornea sample was used and heating in a digestion block was carried out in three stages, with a final temperature of 200 °C. The residual acidity of the digested samples was assessed by acid-base titration with a standard solution of sodium hydroxide (0.0963 mol L−1) and phenolphthalein (1.0% m v−1, in ethanol). Multiple response (MR) was used to evaluate the factorial design, by generating a single response, since several analytes were studied. The experimental data were processed using the Statistica® 7.0 program (StatSoft®, Tulsa, OK, USA). Based on the MR results, a Pareto chart was generated to evaluate the effects and interactions of the studied variables.

Table 1.
Experimental conditions for full factorial design 23.
2.5. Analytical Validation Parameters
In the proposed method, the parameters of linearity, matrix effect, accuracy, precision, limits of detection (LOD), and quantification (LOQ) were evaluated following the recommendations of the International Union of Pure and Applied Chemistry (IUPAC) [15]. Accuracy was obtained through addition and recovery tests at three concentration levels (0.5; 3.0 and 6.0 µg L−1), since no certified mushroom reference material was available. The precision of the method was verified by the repeatability of the results observed intra- and inter-day, expressed as relative standard deviation (% RDS). For the limits of detection (LOD) and quantification (LOQ), the background equivalent concentration (BEC) and the signal-to-noise ratio (SBR) were considered. The LOD values were then calculated as (3 × RSD × BEC/100) and the LOQ values were calculated as (10 × RSD × BEC/100).
2.6. Recommended Daily Intake (RDI) and Adequate Intake (AI)
To assess the potential of these mushrooms as dietary supplements or sources of macro- and micronutrients, the concentrations of these elements in mushroom samples were compared with the recommended daily intake (RDI) and adequate intake (AI) levels established by the National Institutes of Health (NIH) [16,17].
The RDI represents the average level of daily intake sufficient to meet the nutritional needs of 97–98% of healthy individuals, which varies depending on gender and stage of life, such as age, pregnancy, or lactation. RDI calculations are based on the Estimated Average Requirement (EAR), as shown in Equation (1):
where SD corresponds to the standard deviation of the need (estimated from the variability between individuals). When SD is not known, it can be estimated as 10% of EAR. Some RDI values were approximated considering the variations in EAR values by age, according to Equation (2):
RDI = EAR + 2 × SD
RDI = EAR + 0.2 × EAR
Based on the EAR values (in mg/day), conversions were made to μg/day (1 mg = 1000 μg). Equation (3) was used to determine the average percentage by which each element present in the mushrooms meets the NIH-recommended values for the daily reference indices RDI and AI:
where VμD = Value in µg/day and VP (in%) = Value in percent.
VP = VμD/(RDI or AI) × 100
2.7. Statistical Analysis
The results obtained were expressed as a confidence interval at the 95% level. Statistica 10.0 software (StatSoft® Inc., Tulsa, OK, USA) was used to develop and optimize the proposed methodology for evaluating the total content of chemical species in the Auricularia cornea samples.
3. Results and Discussion
3.1. Wet Digestion Optimization and Factorial Design Based on Auricularia cornea
In the process of digesting biological samples, the influence of temperature, acidity, digestion time, and the use of oxidizing agents are commonly assessed. Based on the MR results (Table 2), a Pareto chart (Figure 1) was generated to evaluate the effects and interactions of the studied variables. At a 95% confidence level, the factors with the most significant influence on MR were the volume of HNO₃ and the reaction time. Table 3 presents the analysis of variance (ANOVA), and the parameters proved reliable with a coefficient of determination (R-square) of 0.9274 and a pure error of 0.0527999.

Table 2.
Multiple response (MR) of analytes.
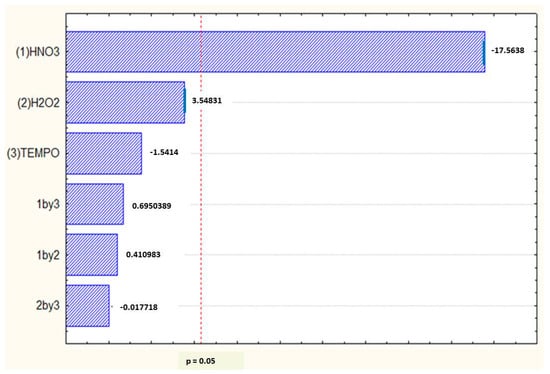
Figure 1.
Pareto chart to assess the significance of the digestion method variables.

Table 3.
ANOVA for model adjustment.
The results showed that the volume of (HNO3 65% m m−1) was the only significant factor, with better analytical signals for lower volumes of the acid. With no influence from the other variables, the volume of 30% H2O2 (m m−1) was set at 2 mL to ensure the presence of the oxidizing agent and better digestion of the samples. The digestion time was set using the center point (75 min).
After univariate optimization for the volume of (HNO3 65% m m−1), the optimum conditions for the acid digestion method were defined: 2 mL of HNO3 65% (m m−1) and 2 mL of H2O2 30% (m m−1) in 75 min at 200 °C, in a closed digestion block. These reagents were added to a mass of 500 mg of Auricularia cornea mushroom, previously dried and crushed. A volume of 4 mL of ultrapure water was added to the digestion tubes, giving a final volume of 8 mL of solution [18]. The amount of acid used was relatively lower when compared to studies in the literature for samples of the same mushrooms undergoing acid digestion [18,19,20]. Using a smaller volume of acid is important because it makes the proposed method more sustainable and environmentally friendly, reducing operating costs and the risk of deterioration of the device’s accessories.
Digestion consists of opening samples using chemical and physical agents that encourage the complete decomposition of all organic matter, thus releasing the inorganic chemical species (Ca, Cr, Cu, Fe, K, Mg, Mn, Ni, and Zn) present in the mushroom species under study. In order to avoid the risk associated with an abrupt increase in pressure and the internal temperature of the tubes, a heating ramp was carried out, varying as follows: (1) 0 to 75 °C; (2) 75 to 150 °C; and (3) 150 to 200 °C. A 5-min interval was maintained between the first two stages. Blanks were prepared and all experiments were carried out in triplicate. The digested samples were diluted with ultrapure water to a final volume of 10.0 mL. The concentrations of Cr, Cu, Fe, K, Mg, Mn, Ni, and Zn were determined by MIP OES.
3.2. Analytical Validation Parameters
The validation parameters applied to the multi-element determination method are described in Table 4. The linear equations and correlation coefficients indicated good linearity of the method, following IUPAC recommendations [15]. Table 5 shows the results of evaluating the matrix effect, through the ratio between the angular coefficient of the curve equation in acidified medium and the angular coefficient of the straight line of the curve equation using the digested sample (Auricularia cornea). The matrix effect results (except for potassium) were in the range of 0.9 to 1.1, in agreement with IUPAC, indicating that the chemical elements did not significantly influence the results obtained in the analyses.

Table 4.
Equations of the analytical curves, determination coefficients (R2), accuracy%, LOD and LOQ for macro- and microelements by MIP OES.

Table 5.
Linear equations (acidified and sample) of analytical curves for matrix effect.
The results obtained for LOD and LOQ indicated good sensitivity of the proposed method for the detection and quantification of Ca, Cr, Cu, Fe, K, Mg, Mn, Ni, and Zn. The accuracy tests consisted of fortifying the samples with the addition of a standard (0.5, 3.0, and 6 ppm) and the results showed an agreement of 90 to 120%, respecting the acceptable range for accuracy tests according to IUPAC [15]. Precision (intra- and inter-day), expressed as a percentage of the relative standard deviation (RSD%), was assessed by digesting certified reference material from tomato leaves (CRM-Agro FT_012016) supplied by the University of São Paulo/Brazilian Agricultural Research Corporation (Embrapa). The results indicated that the precision of the method was less than 10%.
3.3. Multi-Element Analysis and Nutritional Potential of Wild Edible and Medicinal Commercial Mushrooms
The nutritional value of edible and medicinal mushrooms has gained increasing attention due to their rich composition of bioactive compounds and essential nutrients [14,21,22]. The Brazilian Atlantic Forest, a hotspot of fungal biodiversity, is the phytogeographic area where we have the largest record of wild edible species in the country [9]. This section presents the multi-elemental composition of selected mushroom species, highlighting variations in macro- and microelement content and their potential nutritional relevance (Table 6).

Table 6.
Concentration of macro- and microelements (mg kg−1) in mushroom samples.
The highest and lowest levels (in mg kg−1) of macroelements were found for K (32,437.01 ± 2445.66) in the Oudemansiella platensis mushroom and Na (21.68 ± 7.47) in the Pleurotus albidus samples, respectively. Regarding microelements, the lowest levels (in mg kg−1) were found for Cu (0.17 ± 0.09) in the mushroom species Oudemansiella platensis. On the other hand, showing their potential as supplements, the highest levels were found for Fe (109.72 ± 2.59) and Zn (100.87 ± 2.72), in samples of Laetiporus gilbertsonii and Favolus brasiliensis, respectively.
Studies have employed analytical techniques such as inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled plasma optical emission spectroscopy (ICP OES) to quantify macro- and microelements in a variety of edible and medicinal mushroom species [19,20]. However, the use of microwave-induced plasma optical emission spectrometry (MIP OES) for the analysis of mushrooms is still relatively limited, despite the fact that this technique has advantages such as lower operating costs, high sensitivity for certain elements, and the possibility of simultaneous multi-element analysis [23]. In addition to this gap, there is a lack of data in the literature on the analysis of elements present in the mushrooms discussed in this study. This reinforces the relevance of this study, which seeks to contribute to expanding knowledge of the mineral content in these organisms, as well as assessing the feasibility and effectiveness of this analytical technique in this type of biological matrix.
Among the species studied, Oudemansiella cubensis and Oudemansiella platensis had the highest levels (in mg kg−1) of Na (618.83 ± 36.85) and K (32,437.01 ± 2445.66), respectively. On the other hand, the species Favolus brasiliensis and Lentinus berteroi showed the highest Ca (4028.26 ± 180.84 mg kg−1) and Mg (1722.79 ± 87.81 mg kg−1) concentrations, respectively. Among the mushrooms of the Cordyceps genus, Cordyceps sinensis showed the highest values (in mg kg−1) for Ca (1568.45 ± 4.13), Mg (1576.82 ± 8.22), and Na (819.53 ± 6.32), while the Cordyceps militaris was richer in K (2051.10 ± 46.85 mg kg−1). Therefore, these species could be potential supplementary daily sources of macronutrients.
Jasinska et al. (2022) [24] quantified macroelements by ICP OES in samples (mg kg−1) of mushrooms from the Pleurotus genus: Ca (880 to 1270), K (11,000 to 14,000), Mg (526 to 718), and Na (218 to 406). In this study, for Pleurotus albidus, the values for Ca, K, and Na were lower than those reported by the aforementioned authors, and higher for Mg. Mleczek et al. (2024) [25] determined macroelements in 14 mushroom species of the same order (Agaricales) by ICP-MS, with levels (in mg kg−1) of Ca (160 to 768), K (25,200 to 49,000), Mg (769 to 1800), and Na (35.1 to 558). When comparing across taxonomic orders, the macroelement levels found in the Agaricales species from this study were consistent with those reported by Mleczek et al. (2024) [25], particularly for potassium in O. cubensis and O. platensis, and for magnesium across all three species examined.
Macroelements help with body demands above 100 mg/day and are essential for ensuring adequate levels of physiological processes, such as cardiocirculatory function, bone quality, body fluid homeostasis, and nerve function [26,27]. The relevance of these elements for human nutrition and development is highlighted in the health literature, in mushrooms of the most varied species [28,29,30].
Calcium is the main macroelement related to bone integrity and metabolism, especially in the elderly community, reducing the risk of osteoporosis [30,31]. In addition to its important contribution to bone integrity, Ca is involved in the activation of many enzymes, is associated with the process of nerve impulse transmission and muscle contraction, and participates in the blood coagulation cascade [26,30]. The nutritional and physiological importance of this element is therefore clear, and its deficiency is linked to a series of physiological problems and irregularities. In the literature, it is possible to relate a reduction in body calcium content to diseases such as ostoporosis, hypertension, and stroke [32].
Potassium is an essential element for the body, being the largest and main intracellular cation, guaranteeing the normal functioning of cells, balancing fluid homeostasis, muscle contraction, neuronal impulses, and the secretion of bioactive molecules [33,34]. According to research, this element has been a worrying factor for health in view of low consumption, which has led to complications due to a deficiency of this element in the body. Inadequate potassium levels can increase the incidence of cardiovascular events, especially those related to the brain [34,35].
Magnesium is considered the second main intracellular cation and the fourth in body concentration. The content of magnesium in the body is approximately 1 moL, ranging from 20 to 28 mg, with the greatest deposit in bones and muscles, followed by 6% in other organs. Studies on the role of magnesium for nutrition and maintaining body health have been expanding due to its transport of potassium and calcium ions, as well as its ability to articulate cell multiplication signals [33,36]. Although sodium is recognized as an essential macroelement for physiological functions, the main health organizations recommend a low intake of this element due to its association with the risk of cardiovascular problems [37]. Like potassium, sodium is responsible for controlling water homeostasis, propagating neuronal impulses, and stimulating muscle contraction; in contrast to potassium, sodium is the most abundant cation in extracellular fluid [33].
Mushrooms have long been consumed by populations around the world due to their rich nutritional content and health-promoting properties [29]. In this study, the concentrations of macroelements present in the studied mushrooms were compared with the recommended daily intake (RDI) and the adequate intake (AI) of the National Institutes of Health (NIH) to assess the potential of these mushrooms as dietary supplements or supplements of these macroelements. According to the NIH, the RDI values for these macroelements are: Ca (1 × 106 µg/day) for both sexes; Mg (4.010 × 105 and 3.2 × 105 µg/day) for men and women, respectively; and Na (1.5 × 106 µg/day) for both sexes. For potassium, the NIH does not recommend RDI values, but uses AI values of 3.4 × 106 and 2.6 × 106 µg/day as a reference for men and women, respectively [16,17].
Mleczek et al. (2024) [25] considered RDI and AI for a dry mass intake (25 g, dry weight) per adult human organism. Considering an adult over 19 years of age, and a daily consumption of 25 g of mushrooms, the average macroelements of the mushrooms analyzed can meet the NIH dietary reference values for Na (0.67%) and Ca (1.76%) in both sexes; for K (8.38 and 10.47%) for men and women, respectively; and for Mg (8.38 and 10.47%) for men and women, respectively. These values show that the mushrooms analyzed in this study could be potential sources of macroelements, contributing to food supplementation and dietary enrichment. If consumed fresh, an increase of approximately 10% in RDI from dry weight is estimated for many edible mushrooms, as reported in the literature [38].
For the microelement contents, the same behavior was observed for all studied species (Table 6). Favolus brasiliensis had the highest contents (in mg kg−1) of Cu (13.50 ± 0.86), Cr (2.84 ± 0.45), Fe (51.92 ± 0.42), and Zn (100.87 ± 2.72). Cordyceps sinensis was the medicinal species most enriched (in mg kg−1) in Cr (2.12 ± 0.01), Fe (17.14 ± 0.03 1), Mn (15.67 ± 0.02), and Ni (3.27 ± 0.01). Cordyceps militaris was the most effective bioaccumulator for Cu (4.31 ± 0.32 mg kg−1) and Zn (42.12 ± 3.31 mg kg−1). All the edible mushrooms studied had relevant levels of Fe and Zn. On the other hand, despite the vital functions of Cu and Cr, these elements showed reduced concentrations in these samples.
Zakaria et al. (2022) [18] determined microelements in Auricularia cornea samples using ICP OES with average contents (in mg kg−1) of Fe (32,015), Zn (11,499), Mn (3790), Cu (2.10), and Cr (0.149). In this study, the results obtained using MIP OES were consistent with those reported by the aforementioned authors for Zn. The results were higher for the other microelements. In both studies, Mn was the microelement with the highest concentrations in the Auricularia cornea mushroom samples. Jasinska et al. (2022) [24] highlighted the levels (in mg kg−1) of microelements in mushrooms of the Pleurotus genus: Cr (0.34 to 0.42), Cu (11.3 to 15), Fe (110 to 191), Mn (5.96 to 7.76), Ni (0.01 to 0.1), and Zn (48.7 to 93.5). The results found in this study for the Pleurotus albidus show similar levels of Cr and Zn, lower concentrations of Cu, Fe, and Mn, and higher values for Ni.
Mleczek et al. (2024) [25] also determined microelements in Oudemansiella cubensis, Oudemansiella platensis, and Pleurotus albidus. The aforementioned authors found levels (in mg kg−1) of Cu (14.2 to 192), Fe (98.6 to 477), Mn (10.9 to 98.2), Ni (0.093 to 2.4), and Zn (46.5 to 212). In this study, similar values were obtained for Mn (only for the Oudemansiella cubensis species), Zn (only for the Pleurotus albidus species), and Ni for the 3 species studied.
Microelements are important nutrients for the human and animal organism, as some are essential for nutrition, such as Cu, Fe, Mn, and Zn. There are others whose functions are not fully understood, such as Cr and Ni [39]. Chromium is a metal that has many valences, such as Cr3+ and Cr6+, and, initially, studies indicated that Cr3+ is an essential element with benefits for lipoprotein metabolism, potentiating the action of insulin, important for gene expression, and the maintenance of nucleic acids. Currently, chromium falls into the category of a potentially toxic element for health, as according to the European Food Safety Authority (EFSA) there is no evidence of the benefits of chromium for nutritional health, and it is only used as a medicine or food supplement [39,40,41].
Brazil’s National Health Surveillance Agency (ANVISA) sets limits for some elements that are potentially toxic to health. For Cr, ANVISA and the majority of world organizations do not set a permitted daily intake limit. However, ANVISA recommends in its food legislation that a maximum of 10 mg kg−1 be allowed for gelatins and collagens, and 1 mg kg−1 for gelatine-based candies and similar products, as well as powders for preparing gelatine desserts [42]. Among the mushroom species analyzed in this study, the average Cr levels (in mg kg−1) in the samples of Favolus brasiliensis (2.84 ± 0.45), Cordyceps sinensis (2.12 ± 0.01), and Laetiporus gilbertsonii (1.45 ± 0.06) stand out. Looking at the limits permitted by ANVISA, the Cr levels indicate viable food use of these mushrooms for gelatines and collagens; however, their use in candies and similar gelatine-based products should be evaluated.
Nickel is also classified as a potentially toxic element. Consuming foods with significant levels of Ni can cause abdominal discomfort, nausea, emesis, headaches, and dizziness [43] Currently, there are no ANVISA or international health agency regulations establishing tolerance limits for this element in edible mushrooms. In this study, the mushroom samples showed Ni concentrations ranging from 0.27 to 3.27 mg kg−1.
Copper acts as a cofactor for a series of enzymes involved in energy production, iron metabolism, connective tissue synthesis, and deneuropeptides. In addition, this element is involved in regulating gene expression, brain development, and neurohormonal balance [44]. Studies on symptoms and diseases caused by Cu deficiency in the body indicate osteoporosis and anemia [45].
Iron is an essential microelement that acts as an enzyme cofactor for many plant and animal enzymes and many foods naturally contain Fe [46]. This element is a component of hemoglobin, which helps cell transportation and participates in the synthesis of some hormones as well as in physical and neurological growth [47]. Around 90% of the bioavailable Fe present in edible mushrooms is easily absorbed by the body [48]. Fe deficiency inhibits DNA synthesis in the liver, impairing its development. It also affects the mitochondria and microsomes of hepatocytes, reducing the production of proteins and energy, which can lead to anemia due to a lack of Fe intake [45].
Manganese participates as an enzymatic cofactor in the metabolism of cholesterol, carbohydrates, amino acids, and glucose [45,49]. Due to its oxidizing activity, it plays an important role in immunoregulation [50]. Likewise, Zn makes up the enzyme structure in physiological processes and in the synthesis of DNA and RNA. The main deposits of Zn are located in skeletal muscles and bones. As well as taking part in enzymatic activity, the literature also highlights the role of zinc in the development of immune functions [51].
Essential microelements are important for the development and maintenance of health. It is therefore necessary to analyze the levels of these elements in mushrooms, comparing them with the RDI and the maximum levels of AI. The RDI for Cu, Fe, and Zn according to the NIH is 900, 8000, and 11,000 µg/day, respectively, for men, and 8000 µg/day for women. No RDI values were found for Mn, but AI values exist for men (2300 µg/day) and women (1800 µg/day). As for maximum tolerable intake levels (UL), the NIH recommends a maximum intake of 10,000, 45,000, 11,000, and 40,000 µg/day of Cu, Fe, Mn, and Zn, respectively [52].
Considering the NIH reference values, based on a daily intake of 25 g of mushrooms and taking into account the average micronutrient content of the mushrooms studied, it is possible to supply an RDI for Cu (10.89%) and Fe (7.64%) for both sexes and Zn (7.43% and 10.22% for men and women, respectively) for adults > 19 years. For Mn, the IA values for mushrooms are approximately 11.25% and 14.38% for men and women, respectively. In this sense, the edible mushrooms studied here are better able to meet the intake requirements of Fe, Cu, Mn, and Zn than the daily intake requirement for macroelements. There is also a need to monitor some potentially toxic microelements, such as Cr and Ni, in the studied mushrooms. If consumed fresh, an increase of approximately 10% in RDI from dry weight is estimated for many edible mushrooms, as reported in the literature [38].
Substrate enrichment has emerged as a promising strategy to enhance the nutritional value of cultivated mushrooms by increasing the accumulation of essential macro- and micronutrients. The composition of the growth substrate significantly influences the elemental profile of fruiting bodies, offering a means to biofortify mushrooms with minerals beneficial to human health. As demonstrated by Zięba et al. (2020) [53], supplementing substrates with mineral sources such as zinc and selenium resulted in a notable increase in the concentration of these elements in Pleurotus eryngii, especially zinc, without compromising biomass production or quality. Similarly, Madaan et al. (2024) [54] showed that the targeted enrichment of substrates used for Pleurotus spp. cultivation successfully elevated levels of Zn and Se, highlighting the species’ capacity for selective mineral accumulation through mycelial uptake.
These findings underscore the potential of substrate manipulation as a sustainable approach to produce functional foods, contributing to dietary strategies aimed at preventing micronutrient deficiencies in human populations.
4. Conclusions
Edible mushrooms have long been recognized for their culinary attributes and have been appreciated by various civilizations, although there are few records of the consumption of edible wild mushrooms. In conclusion, this study contributes to the growing interest in mushrooms as superfoods, particularly in light of their nutritional and medicinal potential. The Brazilian Atlantic Forest, with its vast and underexplored diversity of wild mushroom species, presents a unique opportunity to uncover valuable bioactive compounds and essential nutrients. This study investigated the chemical composition of nine species of wild mushrooms from the Brazilian Atlantic Forest and two medicinal mushrooms, assessing the levels of macro (Ca, K, Mg and Na) and micro elements (Cr, Cu, Fe, Mn, Ni and Zn). The use of a 23 factorial design to optimize a method for acid digestion of mushrooms in a closed digestion block and MIP OES for quantification proved to be adequate, showing good sensitivity, precision (< 10%), and accuracy.
The samples of edible mushrooms (wild and medicinal) showed concentrations of macro- (Ca, K and Mg) and essential microelements (Cr, Fe and Zn) indicating that they are potential research objects for use in food supplementation.
Author Contributions
Conceptualization, A.d.F.S.J., A.R.L., N.M.J. and E.S.d.S.; methodology, E.S.d.S., J.M.F.J., J.P.C.B. and I.F.S.; validation, E.S.d.S., J.M.F.J., M.d.P.D., Á.C.M., N.M.J. and A.d.F.S.J.; formal analysis, E.S.d.S., J.M.F.J., J.P.C.B. and I.F.S.; investigation, E.S.d.S., J.M.F.J. and M.d.P.D.; resources, A.d.F.S.J. and N.M.J.; writing—original draft preparation, E.S.d.S., J.M.F.J. and A.d.F.S.J.; writing—review and editing, A.d.F.S.J., A.R.L. and N.M.J.; visualization, A.d.F.S.J.; supervision, A.d.F.S.J. and N.M.J.; project administration, A.d.F.S.J.; funding acquisition, A.d.F.S.J. and N.M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from the ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico’ (CNPq, Brasília, Brazil—grant 406331/2023-5) and ‘Fundação de Amparo à Pesquisa do Estado de São Paulo’ (FAPESP, São Paulo, Brazil—grant #2018/15677–0).
Data Availability Statement
The data sets generated and/or analyzed during this study are not publicly available, as the data are not public, but are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília, Brasil)” for its financial support (No. 406331/2023-5) and grants; “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília, Brasil)”—Finance Code 001, and “Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB, Bahia, Brazil) for grant no. 705/2023” for the financial support and grants awarded for this research. N.M.J. would like to thank the CNPq for Research Productivity grant no. 314236/2021–0. M.d.P.D.; and Á.C.M. wishes to thank the “Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp, São Paulo, Brasil) for grants no. #2017/25754–9 and #2021/00245-0.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ANVISA | National Health Surveillance Agency |
| BEC | Background Equivalent Concentration |
| EFSA | European Food Safety Authority |
| AI | Adequate Intake |
| ICP OES | Inductively Coupled Plasma Optical Emission Spectrometry |
| LOD | Limit of Detection |
| LOQ | Limit of Quaantification |
| IUPAC | International Union of Pure and Applied Chemistry |
| MIP OES | Microwave-Induced Plasma Optical Emission Spectrometry |
| NIH | National Institutes of Health |
| RDS | Relative Standard Deviation |
| RDI | Recommended Daily Intake |
| MR | Multiple Response |
| SBR | Signal-to-noise Ratio |
References
- Niskanen, T.; Lucking, R.; Dahlberg, A.; Gaya, E.; Suz, L.M.; Mikryukov, V.; Liimatainen, K.; Druzhinina, I.; Westrip, J.R.S.; Mueller, G.M.; et al. Pushing the frontiers of biodiversity research: Unveiling the global diversity, distribution, and conservation of fungi. Annu. Rev. Environ. Resour. 2023, 48, 149–176. [Google Scholar] [CrossRef]
- Hawksworth, D.L. Mushrooms: The extent of the unexplored potential. Int. J. Med. Mushr. 2001, 3, 333–337. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5, 4. [Google Scholar] [CrossRef]
- Cardoso, D.O.S.; Queiroz, L.P.; Bandeira, F.P.; Góes-Neto, A. Correlations between Indigenous Brazilian Folk Classifications of Fungi and Their Systematics. J. Ethnobiol. 2010, 30, 252–264. [Google Scholar] [CrossRef]
- Elsayed, E.A.; Enshasy, H.E.; Wadaan, M.A.M.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediators Inflamm. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Helm, C.V.; Coradin, J.H.; Rigoni, D. Evaluation of the Chemical Composition of the Edible Mushrooms Agaricus bisporus, Agaricus brasiliensis, Agaricus bisporus Portobello, Lentinula edodes and Pleurotus ostreatus; Embrapa: Colombo, Brazil, 2009; Technical communication 234. [Google Scholar]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; Wiley: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Brazil Flora Group; Gomes-da-Silva, J.; Filardi, F.L.; Barbosa, M.R.V.; Baumgratz, J.F.A.; Bicudo, C.E.; Cavalcanti, T.B.; Coelho, M.A.N.; Costa, A.F.; Costa, D.P.; et al. Brazilian Flora 2020: Leveraging the power of a collaborative scientific network. Taxon 2022, 71, 178–198. [Google Scholar]
- Drewinski, M.P.; Corrêa-Santos, M.P.; Lima, V.X.; Lima, F.T.; Palacio, M.; Borges, M.E.A.; Trierveiler-Pereira, L.; Magnago, A.C.; Furtado, A.N.M.; Lenz, A.R.; et al. Over 400 food resources from Brazil: Evidence-based records of wild edible mushrooms. IMA Fungus 2024, 15, 40. [Google Scholar] [CrossRef]
- Santos Júnior, A.F.; Matos, R.A.; Andrade, E.M.J.; Santos, W.N.L.; Magalhães, H.I.F.; Costa, F.N.; Korn, M.D.G.A. Multielement determination of macro and micro contents in medicinal plants and phytomedicines from Brazil by ICP OES. J. Braz. Chem. Soc. 2017, 28, 376–384. [Google Scholar] [CrossRef]
- Fontoura, B.M.; Jofré, F.C.; Williams, T.; Savio, M.; Donati, G.L.; Nóbrega, J.A. Is MIP-OES a suitable alternative to ICP-OES for trace element analysis? J. Anal. At. Spectrom. 2022, 37, 966–984. [Google Scholar] [CrossRef]
- Niedzielski, P.; Kozak, L.; Wachelka, M.; Jakubowski, K.; Wybieralska, J. The microwave induced plasma with optical emission spectrometry (MIP–OES) in 23 elements determination in geological samples. Talanta 2015, 132, 591–599. [Google Scholar] [CrossRef]
- de Paula Drewinski, M.; Zied, D.C.; Menolli, N. First successful cultivation of wild strains of Irpex rosettiformis from the Brazilian Atlantic Rainforest. Mycol. Progress 2025, 24, 17. [Google Scholar] [CrossRef]
- Drewinski, M.P.; Zied, D.C.; Gomes, E.P.C.; Menolli, N., Jr. Cultivation of a wild strain of wood ear Auricularia cornea from Brazil. Curr. Microbiol. 2024, 81, 390. [Google Scholar] [CrossRef] [PubMed]
- International Union of Pure and Applied Chemistry (IUPAC). Harmonized guidelines for validation of methods of analysis in a single laboratory (IUPAC Technical Report). Pure Appl. Chem. 1995, 74, 835–855. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium. Dietary Reference Intakes for Sodium and Potassium; Oria, M., Harrison, M., Stallings, V.A., Eds.; National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- National Institutes of Health. Dietary Supplement Fact Sheets: List of All Nutrients. Office of Dietary Supplements. 2025. Available online: https://ods.od.nih.gov/factsheets/list-all/ (accessed on 17 March 2025).
- Zakaria, M.K.; Matanjun, P.; George, R.; Pindi, W.; Mamat, H.; Surugau, N.; Seelan, J.S.S. Nutrient Composition, Antioxidant Activities and Glycaemic Response of Instant Noodles with Wood Ear Mushroom (Auricularia cornea) Powder. Appl. Sci. 2022, 12, 12671. [Google Scholar] [CrossRef]
- Scherdien, S.H. Development of Analytical Methods for Elemental Determination and Bioaccessible Fraction in Edible Mushrooms by MIP OES. Master’s Thesis, Federal University of Pelotas, Pelotas, Brazil, 2022. [Google Scholar]
- Altıntığ, E.; Hişir, M.E.; Altundağ, H. Türkiye, Sakarya, Mantar Örneklerinde ICP-OES ile Cr, Cu, Fe, Ni, Pb ve Zn’nun Belirlenmesi. Saü Fen Bilim. Enstit. Derg. 2017, 21, 1. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, R. A review on nutritional advantages of edible mushrooms and its industrialization development situation in protein meat analogues. J. Future Foods 2023, 3, 1–7. [Google Scholar] [CrossRef]
- Silva, C.G.; Teixeira-Silva, M.A.; Santos, I.N.P.; Silveira, M.; Oliveira, M.H. Richness of edible fungi in the Lago do Amapá Environmental Protection Area. Rev. Multidiscip. Educ. Meio Ambiente 2022, 3, 20–27. [Google Scholar] [CrossRef]
- Balaram, V. Microwave plasma atomic emission spectrometry (MP-AES) and its applications—A critical review. Microchem. J. 2020, 159, 105483. [Google Scholar] [CrossRef]
- Jasinska, A.; Prasad, R.; Lisiecka, J.; Roszak, M.; Stoknes, K.; Mleczek, M.; Niedzielski, P. Combined dairy manure-food waste digestate as a medium for Pleurotus djamor—Mineral composition in substrate and bioaccumulation of elements in fruiting bodies. Horticulturae 2022, 8, 934. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Budka, A.; Niedzielski, P.; Mleczek, P.; Kuczyńska-Kippen, N.; Budzyńska, S.; Karolewski, Z.; Kalač, P.; Jędryczka, M. Can the concentration of elements in wild-growing mushrooms be deduced from the taxonomic rank? Environ. Res. 2024, 252, 119079. [Google Scholar] [CrossRef]
- Olechno, E.; Puśćion-Jakubik, A.; Socha, K.; Zujko, M.E. Coffee brews: Are they a source of macroelements in human nutrition? Foods 2021, 10, 1328. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, J.; Kumar, S.; Singh, P. Metabolism of macro-elements (calcium, magnesium, sodium, potassium, chloride and phosphorus) and associated disorders. In Clinical Applications of Biomolecules in Disease Diagnosis, 1st ed.; Yadav, S., Kumar, S., Singh, P., Eds.; Springer: Singapore, 2024; pp. 177–203. [Google Scholar] [CrossRef]
- Golian, M.; Hegedűsová, A.; Mezeyová, I.; Chlebová, Z.; Hegedűs, O.; Urminská, D.; Vollmannová, A.; Chlebo, P. Accumulation of selected metal elements in fruiting bodies of oyster mushroom. Foods 2021, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Budka, A.; Siwulski, M.; Mleczek, P.; Budzyńska, S.; Proch, J.; Gąsecka, M.; Niedzielski, P.; Rzymski, P. A comparison of toxic and essential elements in edible wild and cultivated mushroom species. Eur. Food Res. Technol. 2021, 247, 1249–1262. [Google Scholar] [CrossRef]
- Tang, Z.X.; Shi, L.E.; Jiang, Z.B.; Bai, X.L.; Ying, R.F. Calcium enrichment in edible mushrooms: A review. J. Fungi 2023, 9, 338. [Google Scholar] [CrossRef]
- Arnold, M.; Rajagukguk, Y.V.; Gramza-Michałowska, A. Functional food for elderly high in antioxidant and chicken eggshell calcium to reduce the risk of osteoporosis—A narrative review. Foods 2021, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Anzolin, A.P.; Fagundes, S.C.; Fagundes, M.A.; Pizzol, G.D.; Spassim, M.R.; Bertol, C.D. Therapeutic management in calcium deficiency: A systematic review. Rev. Bras. Ciênc. Envelhec. Hum. 2020, 17, 114115612. [Google Scholar] [CrossRef]
- Cozzolino, S.M.F. Biodisponibilidade de Nutrientes, 6th ed.; Manole: Barueri, Brazil, 2020; E-book; p. 345. ISBN 9786555761115. Available online: https://integrada.minhabiblioteca.com.br/reader/books/9786555761115/ (accessed on 17 March 2025).
- McLean, R.M.; Wang, N.X. Potassium. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; pp. 89–121. [Google Scholar] [CrossRef]
- Kiani, A.K.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Connelly, S.T.; Bellinato, F.; Gisondi, P.; et al. Main nutritional deficiencies. J. Prev. Med. Hyg. 2022, 63, 93–101. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, nutrition, detection, and social impact of diseases linked to its deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; O’Donnell, M.; Yusuf, S. Sodium intake and health: What should we recommend based on the current evidence? Nutrients 2021, 13, 3232. [Google Scholar] [CrossRef]
- Šnirc, M.; Jančo, I.; Hauptvogl, M.; Jakabová, S.; Demková, L.; Árvay, J. Risk Assessment of the Wild Edible Leccinum Mushrooms Consumption According to the Total Mercury Content. J. Fungi 2023, 9, 287. [Google Scholar] [CrossRef]
- Marshall, W.J. Bioquímica Clínica—Aspectos Clínicos e Metabólicos, 3rd ed.; GEN Guanabara Koogan: Rio de Janeiro, Brazil, 2016; E-book; p. 192. ISBN 9788595151918. Available online: https://integrada.minhabiblioteca.com.br/reader/books/9788595151918/ (accessed on 17 March 2025).
- Vincent, J.B. New evidence against chromium as an essential trace element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.J.; He, S.X.; Li, X.Y.; Zeng, J.Y.; Li, M.Y.; Guan, D.X.; Ma, L.Q. Chromium contents, distribution and bioaccessibility in cultivated mushrooms from market: Health implications for human consumption. J. Hazard. Mater. 2024, 461, 132643. [Google Scholar] [CrossRef]
- Brazil National Health Surveillance Agency. Normative Instruction no. 160, of July 1, 2022. Establishes the Maximum Tolerated Limits (MRL) for Contaminants in Food. Diário Oficial da União: Seção 1. Brasília, DF, 2022. Available online: https://www.in.gov.br/en/web/dou/-/instrucao-normativa-in-n-160-de-1-de-julho-de-2022-413367081 (accessed on 17 March 2025).
- Ĺirić, I.; Rukavina, K.; Mioč, B.; Držaić, V.; Kumar, P.; Taher, M.A.; Eid, E.M. Bioaccumulation and health risk assessment of nickel uptake by five wild edible saprotrophic mushroom species collected from Croatia. Forests 2023, 14, 879. [Google Scholar] [CrossRef]
- National Institutes of Health. Chromium: Fact Sheet for Health Professionals. Office of Dietary Supplements. 2025. Available online: https://ods.od.nih.gov/factsheets/Chromium-HealthProfessional/ (accessed on 17 March 2025).
- Liu, S.; Liu, H.; Li, J.; Wang, Y. Research progress on elements of wild edible mushrooms. J. Fungi 2022, 8, 964. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Guerinot, M.L. All together now: Regulation of the iron deficiency response. J. Exp. Bot. 2021, 72, 2045–2055. [Google Scholar] [CrossRef]
- National Institutes of Health. Iron: Fact Sheet for Health Professionals. Office of Dietary Supplements. 2025. Available online: https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/ (accessed on 17 March 2025).
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and nutritional value of prominent Pleurotus spp.: An overview. Mycobiology 2020, 49, 1–14. [Google Scholar] [CrossRef]
- National Institutes of Health. Manganese: Fact Sheet for Health Professionals. Office of Dietary Supplements. 2025. Available online: https://ods.od.nih.gov/factsheets/Manganese-HealthProfessional/ (accessed on 17 March 2025).
- Kumar, P.; Kumar, M.; Bedi, O.; Gupta, M.; Kumar, S.; Jaiswal, G.; Rahi, V.; Yedke, N.G.; Bijalwan, A.; Sharma, S.; et al. Role of vitamins and minerals as immunity boosters in COVID-19. Inflammopharmacology 2021, 29, 1001–1016. [Google Scholar] [CrossRef]
- National Institutes of Health. Zinc: Fact Sheet for Health Professionals. Office of Dietary Supplements. 2025. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/ (accessed on 17 March 2025).
- National Center for Biotechnology Information. Zinc. In Drugs and Lactation Database (LactMed); Lentz, A., Ed.; National Library of Medicine: Bethesda, MD, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222310/ (accessed on 17 March 2025).
- Zięba, P.; Kała, K.; Włodarczyk, A.; Szewczyk, A.; Kunicki, E.; Sękara, A.; Muszyńska, B. Selenium and Zinc Biofortification of Pleurotus eryngii Mycelium and Fruiting Bodies as a Tool for Controlling Their Biological Activity. Molecules 2020, 25, 889. [Google Scholar] [CrossRef]
- Madaan, K.; Sharma, S.; Kalia, A. Effect of selenium and zinc biofortification on the biochemical parameters of Pleurotus spp. under submerged and solid-state fermentation. J. Trace Elem. Med. Biol. 2024, 82, 127365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).