Microcrystal Delivery Using a Syringe and Syringe Pump Method for Serial Crystallography
Abstract
1. Introduction
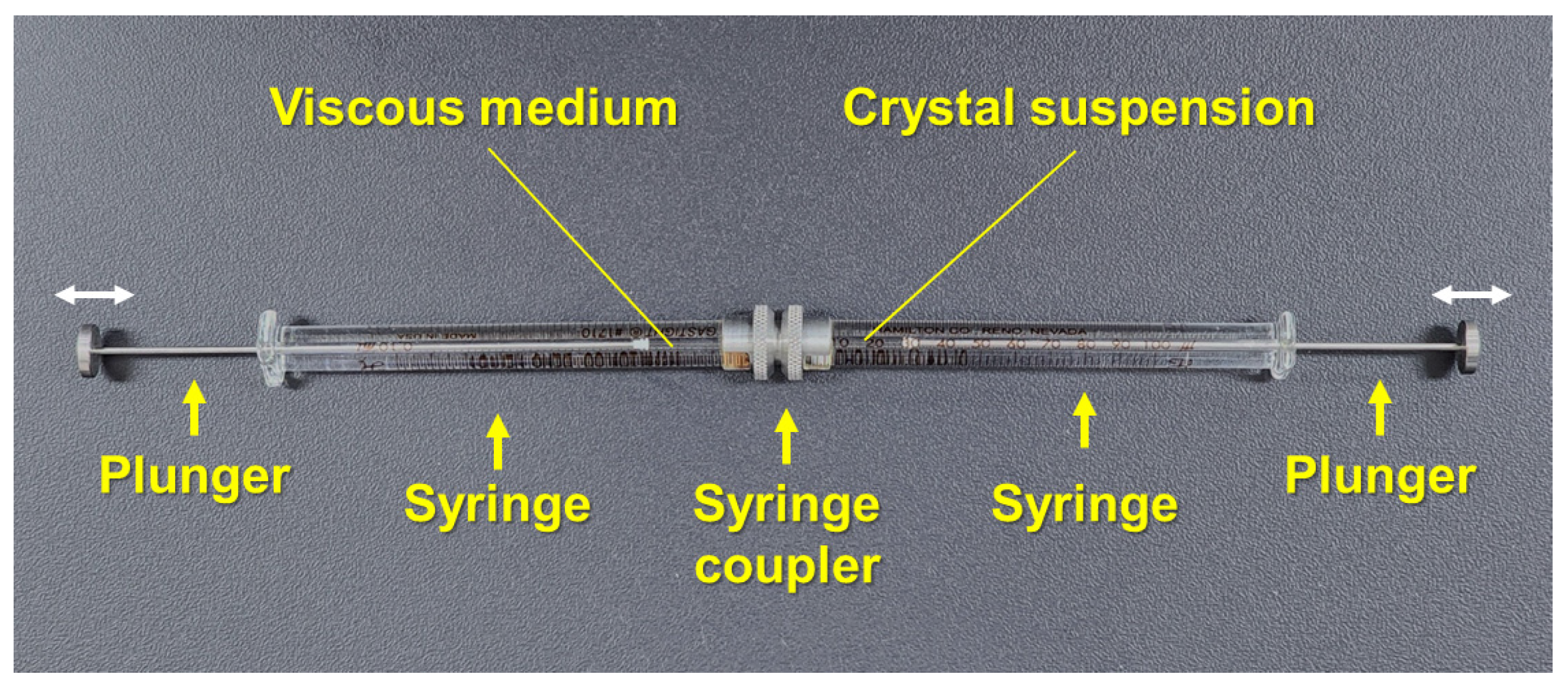
2. Syringe-Based Microcrystal Delivery Method
3. Sample Preparation
4. Syringe Plunger Pushing for Sample Extrusion
5. Advantages of Microcrystal Delivery Using Syringes and Syringe Pumps
5.1. Commercial Availability
5.2. Preliminary Injection Tests
5.3. Portability
5.4. Independent Operation
6. Applications of Syringe and Syringe Pump-Based Sample Delivery
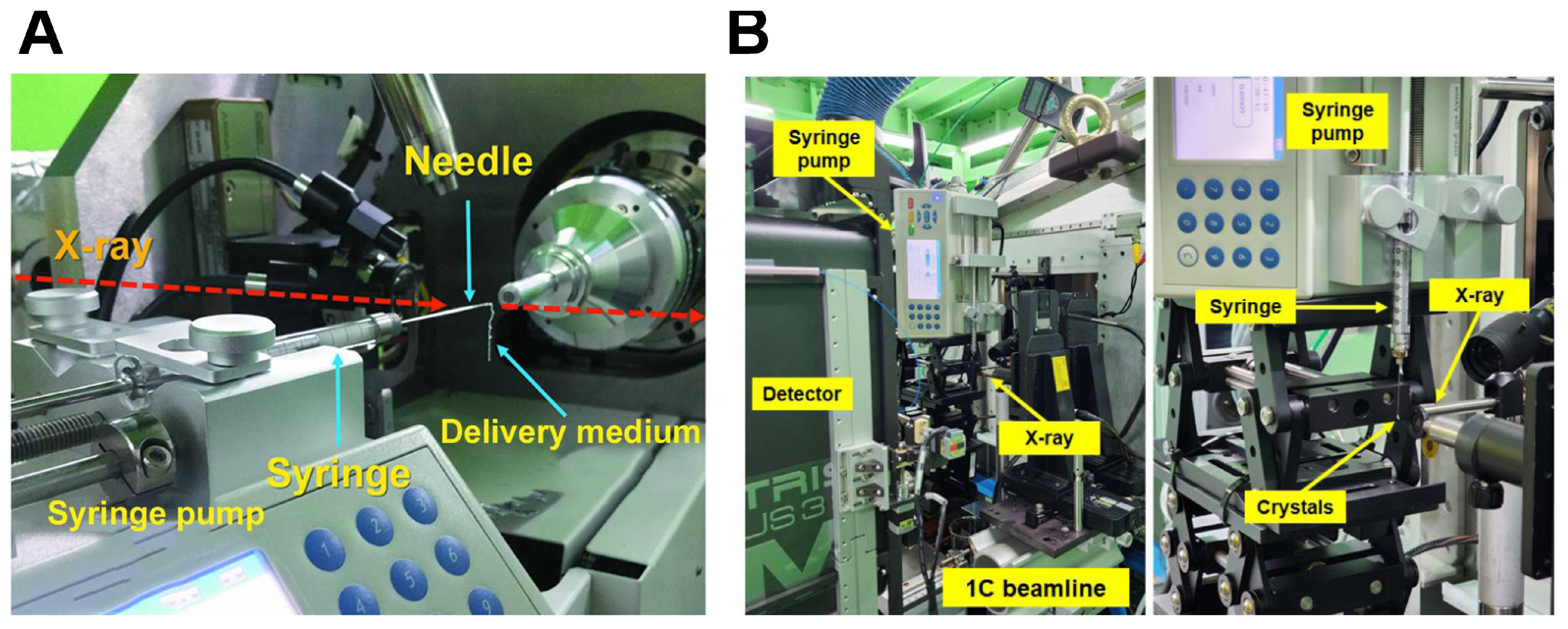
6.1. Demonstration of the SSX Experiment
6.2. Development of Viscous Media
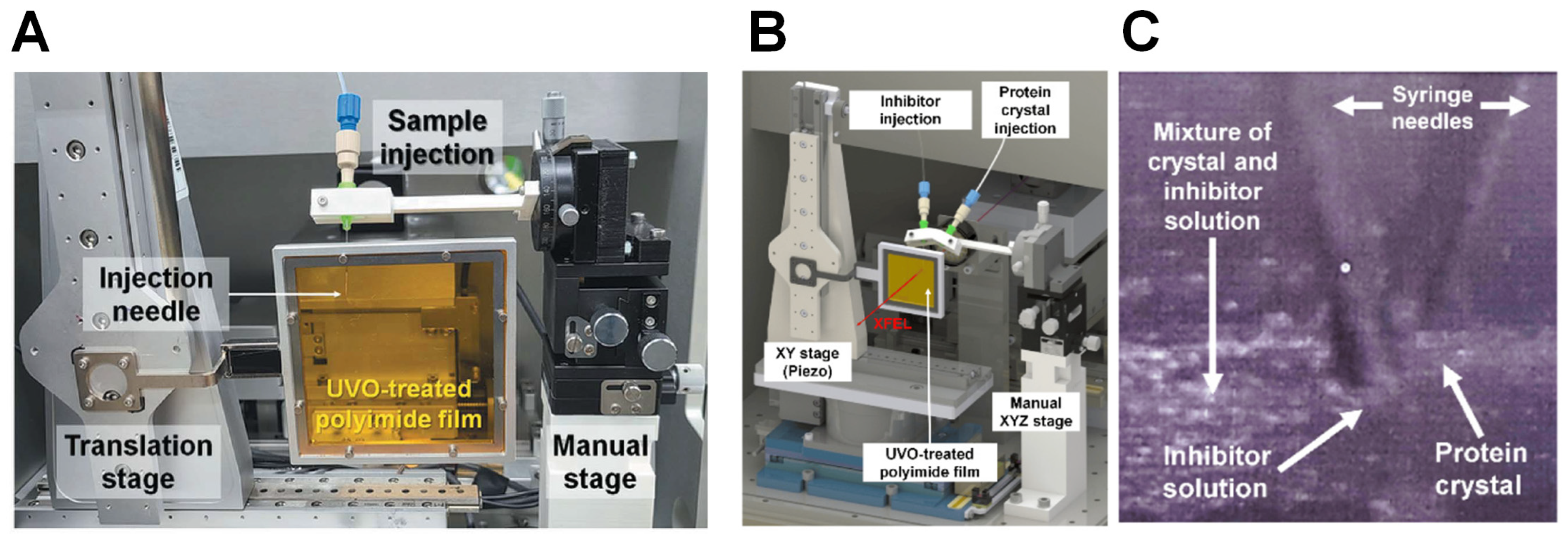
6.3. Hybrid Sample Delivery Methods
| Application | Comment | Reference |
|---|---|---|
| Demonstration of the SSX experiment | Demonstration of SSX with monochromatic X-ray beam using sample delivery using syringe and syringe pump | [35] |
| Pink-beam serial synchrotron crystallography at the Pohang Light Source II | [47] | |
| Development of the viscous medium | Shortening the injection matrix for serial crystallography. | [48] |
| Polysaccharide-based injection matrix for serial crystallography | [49] | |
| Lard injection matrix | [37] | |
| Beef tallow matrix | [50] | |
| Hybrid sample delivery method | Stable sample delivery in viscous media via a capillary for serial crystallography. | [51] |
| Stable sample delivery in a viscous medium via a polyimide-based single-channel microfluidics chip for serial crystallography. | [52] | |
| Combination of an inject-and-transfer system for serial femtosecond crystallography | [53] | |
| Upgraded combined inject-and-transfer system for serial femtosecond crystallography | [42] | |
| Data processing | Application of Serial Crystallography for Merging Incomplete Macromolecular Crystallography Datasets | [65] |
| Structure determination | Room-temperature structure analysis | [25] |
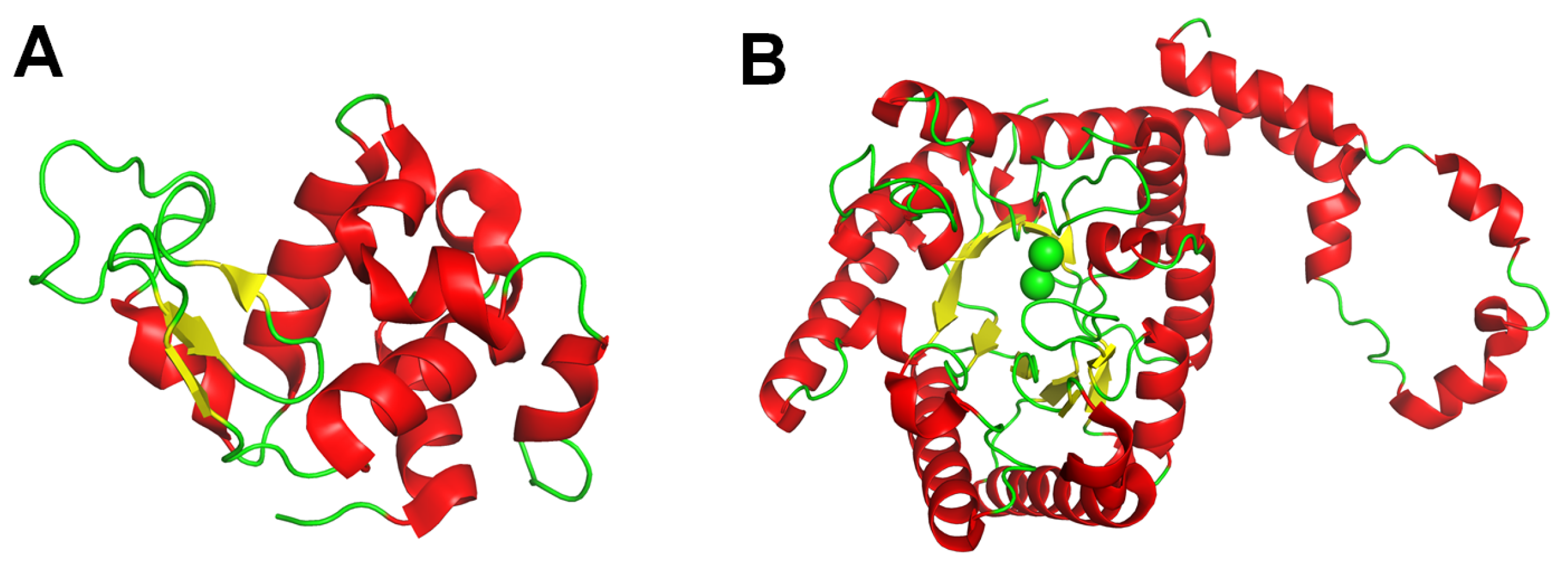
6.4. Room-Temperature Structure Determination
7. Discussion
8. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SX | Serial crystallography |
| SFX | Serial femtosecond crystallography |
| SSX | Serial synchrotron crystallography |
| XFEL | X-ray free electron laser |
| LCP | Lipidic Cubic Phase |
| PAL | Pohang Accelerator Laboratory |
| PLS-II | Pohang Light Source II |
| BITS | Combination of an inject-and-transfer system |
References
- Duke, E.M.H.; Johnson, L.N. Macromolecular crystallography at synchrotron radiation sources: Current status and future developments. Proc. R. Soc. A Math. Phys. Eng. Sci. 2010, 466, 3421–3452. [Google Scholar] [CrossRef]
- Walter, R.L.; Thiel, D.J.; Barna, S.L.; Tate, M.W.; Wall, M.E.; Eikenberry, E.F.; Gruner, S.M.; Ealick, S.E. High-resolution macromolecular structure determination using CCD detectors and synchrotron radiation. Structure 1995, 3, 835–844. [Google Scholar] [CrossRef]
- Mulholland, A.J. Modelling enzyme reaction mechanisms, specificity and catalysis. Drug Discov. Today 2005, 10, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, C.; Rudhra, O.; Alothaim, A.S.; Alkhanani, M.; Singh, S.K. Structure and chemistry of enzymatic active sites that play a role in the switch and conformation mechanism. In Protein Design and Structure; Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 59–83. [Google Scholar]
- Schwalbe, H.; Audergon, P.; Haley, N.; Amaro, C.A.; Agirre, J.; Baldus, M.; Banci, L.; Baumeister, W.; Blackledge, M.; Carazo, J.M.; et al. The future of integrated structural biology. Structure 2024, 32, 1563–1580. [Google Scholar] [CrossRef]
- Blundell, T.L.; Jhoti, H.; Abell, C. High-throughput crystallography for lead discovery in drug design. Nat. Rev. Drug Discov. 2002, 1, 45–54. [Google Scholar] [CrossRef]
- Zheng, H.; Hou, J.; Zimmerman, M.D.; Wlodawer, A.; Minor, W. The future of crystallography in drug discovery. Expert Opin. Drug Discov. 2013, 9, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Bajad, N.G.; Rayala, S.; Gutti, G.; Sharma, A.; Singh, M.; Kumar, A.; Singh, S.K. Systematic review on role of structure based drug design (SBDD) in the identification of anti-viral leads against SARS-Cov-2. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100026. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Shao, J.; Zhu, Z.; Zhang, L. Structure-driven protein engineering for production of valuable natural products. Trends Plant Sci. 2023, 28, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, W.-t.; Gong, Y.-m.; Zhang, Y.-k.; Fan, X.-x.; Wang, G.-c.; Lu, Z.-h.; Liu, F.; Liu, X.-h.; Zhu, Y.-s. Molecular engineering of PETase for efficient PET biodegradation. Ecotoxicol. Environ. Saf. 2024, 280, 116540. [Google Scholar] [CrossRef]
- Ndochinwa, G.O.; Wang, Q.-Y.; Okoro, N.O.; Amadi, O.C.; Nwagu, T.N.; Nnamchi, C.I.; Moneke, A.N.; Odiba, A.S. New advances in protein engineering for industrial applications: Key takeaways. Open Life Sci. 2024, 19, 20220856. [Google Scholar] [CrossRef] [PubMed]
- Nishimasu, H.; Shi, X.; Ishiguro, S.; Gao, L.; Hirano, S.; Okazaki, S.; Noda, T.; Abudayyeh, O.O.; Gootenberg, J.S.; Mori, H.; et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 2018, 361, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Nishimasu, H.; Ishitani, R.; Nureki, O. Structural Basis for the Altered PAM Specificities of Engineered CRISPR-Cas9. Mol. Cell 2016, 61, 886–894. [Google Scholar] [CrossRef]
- de la Mora, E.; Coquelle, N.; Bury, C.S.; Rosenthal, M.; Holton, J.M.; Carmichael, I.; Garman, E.F.; Burghammer, M.; Colletier, J.-P.; Weik, M. Radiation damage and dose limits in serial synchrotron crystallography at cryo- and room temperatures. Proc. Natl. Acad. Sci. USA 2020, 117, 4142–4151. [Google Scholar] [CrossRef] [PubMed]
- Shelley, K.L.; Garman, E.F. Quantifying and comparing radiation damage in the Protein Data Bank. Nat. Commun. 2022, 13, 1314. [Google Scholar] [CrossRef]
- Hattne, J.; Shi, D.; Glynn, C.; Zee, C.-T.; Gallagher-Jones, M.; Martynowycz, M.W.; Rodriguez, J.A.; Gonen, T. Analysis of Global and Site-Specific Radiation Damage in Cryo-EM. Structure 2018, 26, 759–766.e4. [Google Scholar] [CrossRef]
- Ravelli, R.B.G.; McSweeney, S.M. The ‘fingerprint’ that X-rays can leave on structures. Structure 2000, 8, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Garman, E. Investigation of possible free-radical scavengers and metrics for radiation damage in protein cryocrystallography. J. Synchrotron Radiat. 2002, 9, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Banumathi, S.; Zwart, P.H.; Ramagopal, U.A.; Dauter, M.; Dauter, Z. Structural effects of radiation damage and its potential for phasing. Acta Crystallogr. D Biol. 2004, 60, 1085–1093. [Google Scholar] [CrossRef]
- Nam, K.H. Radiation Damage on Thaumatin: A Case Study of Crystals That Are Larger Than the Microfocusing X-ray Beam. Appl. Sci. 2023, 13, 1876. [Google Scholar] [CrossRef]
- Taberman, H. Radiation Damage in Macromolecular Crystallography—An Experimentalist’s View. Crystals 2018, 8, 157. [Google Scholar] [CrossRef]
- Nam, K.H. Temperature-dependent structural changes in xylanase II from Trichoderma longibrachiatum. Carbohydr. Res. 2024, 541, 109173. [Google Scholar] [CrossRef] [PubMed]
- Bradford, S.Y.C.; El Khoury, L.; Ge, Y.; Osato, M.; Mobley, D.L.; Fischer, M. Temperature artifacts in protein structures bias ligand-binding predictions. Chem. Sci. 2021, 12, 11275–11293. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.S.; van den Bedem, H.; Samelson, A.J.; Lang, P.T.; Holton, J.M.; Echols, N.; Alber, T. Accessing protein conformational ensembles using room-temperature X-ray crystallography. Proc. Natl. Acad. Sci. USA 2011, 108, 16247–16252. [Google Scholar] [CrossRef]
- Nam, K.H. Temperature-Dependent Structural Changes of the Active Site and Substrate-Binding Cleft in Hen Egg White Lysozyme. Crystals 2025, 15, 111. [Google Scholar] [CrossRef]
- Chapman, H.N.; Barty, A.; Bogan, M.J.; Boutet, S.; Frank, M.; Hau-Riege, S.P.; Marchesini, S.; Woods, B.W.; Bajt, S.; Benner, W.H.; et al. Femtosecond diffractive imaging with a soft-X-ray free-electron laser. Nat. Phys. 2006, 2, 839–843. [Google Scholar] [CrossRef]
- Chapman, H.N.; Caleman, C.; Timneanu, N. Diffraction before destruction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130313. [Google Scholar] [CrossRef] [PubMed]
- Boutet, S.; Lomb, L.; Williams, G.J.; Barends, T.R.M.; Aquila, A.; Doak, R.B.; Weierstall, U.; DePonte, D.P.; Steinbrener, J.; Shoeman, R.L.; et al. High-Resolution Protein Structure Determination by Serial Femtosecond Crystallography. Science 2012, 337, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H. Guide to serial synchrotron crystallography. Curr. Res. Struct. Biol. 2024, 7, 100131. [Google Scholar] [CrossRef]
- Kupitz, C.; Basu, S.; Grotjohann, I.; Fromme, R.; Zatsepin, N.A.; Rendek, K.N.; Hunter, M.S.; Shoeman, R.L.; White, T.A.; Wang, D.; et al. Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser. Nature 2014, 513, 261–265. [Google Scholar] [CrossRef]
- Poddar, H.; Heyes, D.J.; Schirò, G.; Weik, M.; Leys, D.; Scrutton, N.S. A guide to time-resolved structural analysis of light-activated proteins. FEBS J. 2021, 289, 576–595. [Google Scholar] [CrossRef] [PubMed]
- Brändén, G.; Neutze, R. Advances and challenges in time-resolved macromolecular crystallography. Science 2021, 373, eaba0954. [Google Scholar] [CrossRef] [PubMed]
- Kupitz, C.; Olmos, J.L.; Holl, M.; Tremblay, L.; Pande, K.; Pandey, S.; Oberthür, D.; Hunter, M.; Liang, M.; Aquila, A.; et al. Structural enzymology using X-ray free electron lasers. Struct. Dyn. 2017, 4, 044003. [Google Scholar] [CrossRef] [PubMed]
- Worrall, J.A.R.; Hough, M.A. Serial femtosecond crystallography approaches to understanding catalysis in iron enzymes. Curr. Opin. Struc. Biol. 2022, 77, 102486. [Google Scholar] [CrossRef]
- Stagno, J.R.; Knoska, J.; Chapman, H.N.; Wang, Y.-X. Mix-and-Inject Serial Femtosecond Crystallography to Capture RNA Riboswitch Intermediates. In RNA Structure and Dynamics; Methods in Molecular Biology; Humana: New York, NY, USA, 2023; pp. 243–249. [Google Scholar]
- Kim, J.; Kim, Y.; Park, J.; Nam, K.H.; Cho, Y. Structural mechanism of Escherichia coli cyanase. Acta Crystallogr. D Biol. Crystallogr. 2023, 79, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.; Lee, K.; Chung, W.K.; Nam, K.H.; Cho, Y. Exploring the reaction dynamics of alanine racemase using serial femtosecond crystallography. Sci. Rep. 2024, 14, 31442. [Google Scholar] [CrossRef]
- White, T.A.; Kirian, R.A.; Martin, A.V.; Aquila, A.; Nass, K.; Barty, A.; Chapman, H.N. CrystFEL: A software suite for snapshot serial crystallography. J. Appl. Crystallogr. 2012, 45, 335–341. [Google Scholar] [CrossRef]
- White, T.A. Processing serial crystallography data with CrystFEL: A step-by-step guide. Acta Crystallogr. D Struct. Biol. 2019, 75, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Grünbein, M.L.; Nass Kovacs, G. Sample delivery for serial crystallography at free-electron lasers and synchrotrons. Acta Crystallogr. D Biol. Crystallogr. 2019, 75, 178–191. [Google Scholar] [CrossRef]
- Martiel, I.; Muller-Werkmeister, H.M.; Cohen, A.E. Strategies for sample delivery for femtosecond crystallography. Acta Crystallogr. D Struct. Biol. 2019, 75, 160–177. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Zhang, B.; Yan, E.K.; Sun, B.; Wang, Z.J.; He, J.H.; Yin, D.C. A guide to sample delivery systems for serial crystallography. FEBS J. 2019, 286, 4402–4417. [Google Scholar] [CrossRef]
- Aller, P.; Orville, A.M. Dynamic Structural Biology Experiments at XFEL or Synchrotron Sources. In Structural Proteomics; Methods in Molecular Biology; Humana: New York, NY, USA, 2021; pp. 203–228. [Google Scholar]
- Sugahara, M.; Mizohata, E.; Nango, E.; Suzuki, M.; Tanaka, T.; Masuda, T.; Tanaka, R.; Shimamura, T.; Tanaka, Y.; Suno, C.; et al. Grease matrix as a versatile carrier of proteins for serial crystallography. Nat. Methods 2015, 12, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, P.; Hadian Jazi, M.; Kusel, M.; Martin, A.V.; Ericsson, T.; Call, M.J.; Trenker, R.; Roque, F.G.; Darmanin, C.; Abbey, B. The serial millisecond crystallography instrument at the Australian Synchrotron incorporating the “Lipidico” injector. Rev. Sci. Instrum. 2019, 90, 085110. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Nam, K.H. Sample delivery using viscous media, a syringe and a syringe pump for serial crystallography. J. Synchrotron Radiat. 2019, 26, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Eom, I.; Chun, S.H.; Lee, J.H.; Nam, D.; Ma, R.; Park, J.; Park, S.; Park, S.H.; Yang, H.; Nam, I.; et al. Recent Progress of the PAL-XFEL. Appl. Sci. 2022, 12, 1010. [Google Scholar] [CrossRef]
- Nam, K.H. Lard injection matrix for serial crystallography. Int. J. Mol. Sci. 2020, 21, 5977. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H. Sample delivery media for serial crystallography. Int. J. Mol. Sci. 2019, 20, 1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ishchenko, A.; Cherezov, V. Preparation of microcrystals in lipidic cubic phase for serial femtosecond crystallography. Nat. Protoc. 2014, 9, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.E.; Basu, S.; James, D.; Wang, D.; Schaffer, A.; Roy-Chowdhury, S.; Zatsepin, N.A.; Aquila, A.; Coe, J.; Gati, C.; et al. A novel inert crystal delivery medium for serial femtosecond crystallography. IUCrJ 2015, 2, 421–430. [Google Scholar] [CrossRef]
- Stellato, F.; Oberthür, D.; Liang, M.; Bean, R.; Gati, C.; Yefanov, O.; Barty, A.; Burkhardt, A.; Fischer, P.; Galli, L.; et al. Room-temperature macromolecular serial crystallography using synchrotron radiation. IUCrJ 2014, 1, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, D.; Park, J.; Lee, J.-L.; Chung, W.K.; Cho, Y.; Nam, K.H. Upgraded Combined Inject-and-Transfer System for Serial Femtosecond Crystallography. Appl. Sci. 2022, 12, 9125. [Google Scholar] [CrossRef]
- DePonte, D.P.; Weierstall, U.; Schmidt, K.; Warner, J.; Starodub, D.; Spence, J.C.H.; Doak, R.B. Gas dynamic virtual nozzle for generation of microscopic droplet streams. J. Phys. D 2008, 41, 195505. [Google Scholar] [CrossRef]
- Weierstall, U.; James, D.; Wang, C.; White, T.A.; Wang, D.; Liu, W.; Spence, J.C.; Bruce Doak, R.; Nelson, G.; Fromme, P.; et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat. Commun. 2014, 5, 3309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, K.; Ji, A. A portable plug-and-play syringe pump using passive valves for microfluidic applications. Sens. Actuators B Chem. 2020, 304, 127331. [Google Scholar] [CrossRef]
- Bae, S.J.; Lee, S.J.; Heo, J.U.; Im, D.J. Syringe pump-embedded, on-demand sample supply and collection system for droplet 3D cell culture in digital microfluidics. Sens. Actuators B Chem. 2024, 419, 136439. [Google Scholar] [CrossRef]
- Kim, Y.; Nam, K.H. Pink-Beam Serial Synchrotron Crystallography at Pohang Light Source II. Crystals 2022, 12, 1637. [Google Scholar] [CrossRef]
- Nam, K.H. Shortening injection matrix for serial crystallography. Sci. Rep. 2020, 10, 107. [Google Scholar] [CrossRef]
- Nam, K.H. Polysaccharide-based injection matrix for serial crystallography. Int. J. Mol. Sci. 2020, 21, 3332. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H. Beef tallow injection matrix for serial crystallography. Sci. Rep. 2022, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H. Stable sample delivery in viscous media via a capillary for serial crystallography. J. Appl. Crystallogr. 2020, 53, 45–50. [Google Scholar] [CrossRef]
- Nam, K.H.; Cho, Y. Stable sample delivery in a viscous medium via a polyimide-based single-channel microfluidic chip for serial crystallography. J. Appl. Crystallogr. 2021, 54, 1081–1087. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.; Baek, S.; Park, J.; Park, S.; Lee, J.-L.; Chung, W.K.; Cho, Y.; Nam, K.H. Combination of an inject-and-transfer system for serial femtosecond crystallography. J. Appl. Crystallogr. 2022, 55, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H. Application of Serial Crystallography for Merging Incomplete Macromolecular Crystallography Datasets. Crystals 2024, 14, 1012. [Google Scholar] [CrossRef]
- Fromme, R.; Ishchenko, A.; Metz, M.; Chowdhury, S.R.; Basu, S.; Boutet, S.; Fromme, P.; White, T.A.; Barty, A.; Spence, J.C.; et al. Serial femtosecond crystallography of soluble proteins in lipidic cubic phase. IUCrJ 2015, 2, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, M.; Song, C.; Suzuki, M.; Masuda, T.; Inoue, S.; Nakane, T.; Yumoto, F.; Nango, E.; Tanaka, R.; Tono, K.; et al. Oil-free hyaluronic acid matrix for serial femtosecond crystallography. Sci. Rep. 2016, 6, 24484. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, M.; Nakane, T.; Masuda, T.; Suzuki, M.; Inoue, S.; Song, C.; Tanaka, R.; Nakatsu, T.; Mizohata, E.; Yumoto, F.; et al. Hydroxyethyl cellulose matrix applied to serial crystallography. Sci. Rep. 2017, 7, 703. [Google Scholar] [CrossRef] [PubMed]
- Kovacsova, G.; Grunbein, M.L.; Kloos, M.; Barends, T.R.M.; Schlesinger, R.; Heberle, J.; Kabsch, W.; Shoeman, R.L.; Doak, R.B.; Schlichting, I. Viscous hydrophilic injection matrices for serial crystallography. IUCrJ 2017, 4, 400–410. [Google Scholar] [CrossRef]
- Martin-Garcia, J.M.; Conrad, C.E.; Nelson, G.; Stander, N.; Zatsepin, N.A.; Zook, J.; Zhu, L.; Geiger, J.; Chun, E.; Kissick, D.; et al. Serial millisecond crystallography of membrane and soluble protein microcrystals using synchrotron radiation. IUCrJ 2017, 4, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, S.; Kim, J.; Park, G.; Cho, Y.; Nam, K.H. Polyacrylamide injection matrix for serial femtosecond crystallography. Sci. Rep. 2019, 9, 2525. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, M.; Motomura, K.; Suzuki, M.; Masuda, T.; Joti, Y.; Numata, K.; Tono, K.; Yabashi, M.; Ishikawa, T. Viscosity-adjustable grease matrices for serial nanocrystallography. Sci. Rep. 2020, 10, 1371. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, K.H. Microcrystal Delivery Using a Syringe and Syringe Pump Method for Serial Crystallography. Analytica 2025, 6, 5. https://doi.org/10.3390/analytica6010005
Nam KH. Microcrystal Delivery Using a Syringe and Syringe Pump Method for Serial Crystallography. Analytica. 2025; 6(1):5. https://doi.org/10.3390/analytica6010005
Chicago/Turabian StyleNam, Ki Hyun. 2025. "Microcrystal Delivery Using a Syringe and Syringe Pump Method for Serial Crystallography" Analytica 6, no. 1: 5. https://doi.org/10.3390/analytica6010005
APA StyleNam, K. H. (2025). Microcrystal Delivery Using a Syringe and Syringe Pump Method for Serial Crystallography. Analytica, 6(1), 5. https://doi.org/10.3390/analytica6010005







