Boron Nitride Nanostructures (BNNs) Within Metal–Organic Frameworks (MOFs): Electrochemical Platform for Hydrogen Sensing and Storage
Abstract
1. Introduction
2. Boron Nitride Nanostructures (BNNs) Properties and Synthesis
3. Metal–Organic Frameworks (MOFs) Characteristics and Synthesis
| Material | Characteristics | Applications | References |
|---|---|---|---|
| ZIF-8 | High surface area and microporosity; stability under various conditions. | Hydrogen storage [67], adsorption [68], gas separation [69], flame retardancy [70], corrosion protection [71]. | [66,67,68,69,70,71] |
| MOF-5 | Zinc ions with terephthalic acid; high surface area; moderate stability. | Gas storage, adsorption, catalysis [76], polymer nanocomposites [77]. | [76,77,78,79,80] |
| MIL-101 | Chromium ions with terephthalic acid; exceptionally high surface area; large pore volume; high stability. | Gas storage, catalysis, photo-electrocatalysis [81], thermal management [82]. | [81,82] |
| UiO-66 | Zirconium ions with terephthalic acid; high surface area; chemical and thermal stability. | Electrochemical sensors [83], high energy storage dielectrics [C28]. | [83,84] |
| HKUST-1 | Copper ions with benzene-1,3,5-tricarboxylate; highly porous; moderate stability. | Gas storage, separation [85], photocatalysis [86]. | [85,86] |
4. Approach to Integrating BNNs Within MOFs and Characterization Through Electrochemical Techniques
5. Mechanisms of Hydrogen Sensing and Storage
6. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassan, Q.; Algburi, S.; Jaszczur, M.; Al-Jiboory, A.K.; Al Musawi, T.J.; Ali, B.M.; Viktor, P.; Fodor, M.; Ahsan, M.; Salman, H.M.; et al. Hydrogen role in energy transition: A comparative review. Process Saf. Environ. Prot. 2024, 184, 1069–1093. [Google Scholar] [CrossRef]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F.; Brandon, N.P. Hydrogen and fuel cells: Towards a sustainable energy future. Energy Policy 2008, 36, 4356–4362. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Lale, A.; Bernard, S.; Demirci, U.B. Boron Nitride for Hydrogen Storage. ChemPlusChem 2018, 83, 893–903. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, D.; Salameh, C.; Bechelany, M.; Miele, P. Nanostructured Boron Nitride–Based Materials: Synthesis and Applications. Mater. Today Adv. 2020, 8, 100107. [Google Scholar] [CrossRef]
- Revabhai, P.M.; Singhal, R.K.; Basu, H.; Kailasa, S.K. Progress on Boron Nitride Nanostructure Materials: Properties, Synthesis and Applications in Hydrogen Storage and Analytical Chemistry. J. Nanostruct. Chem. 2022, 13, 1–41. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Hamdy, M.S.; Taha, T.; AlSalem, H.S.; Alenad, A.M.; Amin, M.A.; Shah, R.; Palamanit, A.; Khan, J.; et al. Fabrication, Characteristics, and Applications of Boron Nitride and Their Composite Nanomaterials. Surf. Interfaces 2022, 29, 101725. [Google Scholar] [CrossRef]
- Moussa, G.; Salameh, C.; Bruma, A.; Malo, S.; Demirci, U.B.; Bernard, S.; Miele, P. Nanostructured Boron Nitride: From Molecular Design to Hydrogen Storage Application. Inorganics 2014, 2, 396–409. [Google Scholar] [CrossRef]
- Weng, Q.; Zeng, L.; Chen, Z.; Han, Y.; Jiang, K.; Bando, Y.; Golberg, D. Hydrogen Storage in Carbon and Oxygen Co-doped Porous Boron Nitrides. Adv. Funct. Mater. 2020, 31, 2007381. [Google Scholar] [CrossRef]
- Cao, L.; Dai, P.; Tang, J.; Li, D.; Chen, R.; Liu, D.; Gu, X.; Li, L.; Bando, Y.; Ok, Y.S.; et al. Spherical Superstructure of Boron Nitride Nanosheets Derived from Boron-Containing Metal–Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 8755–8762. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, A.; Gupta, V.; Sundramoorthy, A.K.; Arya, S. Sundramoorthy, and Sandeep Arya. Involvement of Metal Organic Frameworks in Wearable Electrochemical Sensor for Efficient Performance. Trends Environ. Anal. Chem. 2023, 38, e00200. [Google Scholar] [CrossRef]
- Fethi, A. Novel Materials for Electrochemical Sensing Platforms. Sens. Int. 2020, 1, 100035. [Google Scholar] [CrossRef]
- Tang, P.; Wang, Y.; He, F. Electrochemical Sensor Based on Super-Magnetic Metal–Organic Framework@molecularly Imprinted Polymer for Sarcosine Detection in Urine. J. Saudi Chem. Soc. 2020, 24, 620–630. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J.; Kumar, E.A.; Duraisamy, N.; Lee, A.-T. An Electrochemical Platform Based on Yttrium Oxide/Boron Nitride Nanocomposite for the Detection of Dopamine. Sens. Actuators B Chem. 2021, 349, 130787. [Google Scholar] [CrossRef]
- Sohrabi, H.; Arbabzadeh, O.; Falaki, M.; Vatanpour, V.; Majidi, M.R.; Kudaibergenov, N.; Joo, S.W.; Khataee, A. Advances in fabrication, physio-chemical properties, and sensing applications of non-metal boron nitride and boron carbon nitride-based nanomaterials. Surf. Interfaces 2023, 41, 103152. [Google Scholar] [CrossRef]
- Sohrabi, H.; Ghasemzadeh, S.; Ghoreishi, Z.; Majidi, M.R.; Yoon, Y.; Dizge, N.; Khataee, A. Metal-organic frameworks (MOF)-based sensors for detection of toxic gases: A review of current status and future prospects. Mater. Chem. Phys. 2023, 299, 127512. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Ghosh, S.; Jayababu, N.; Kang, C.-J.; Cho, H.D.; Kim, S.-G.; Kim, M.-D. Boron doped g-C3N4 quantum dots based highly sensitive surface acoustic wave NO2 sensor with faster gas kinetics under UV light illumination. Sens. Actuators B Chem. 2023, 378, 133140. [Google Scholar] [CrossRef]
- Sun, D.; Sun, Z.; Yang, D.; Jiang, X.; Tang, J.; Wang, X. Advances in boron nitride-based materials for electrochemical energy storage and conversion. Ecoenergy 2023, 1, 215–459. [Google Scholar] [CrossRef]
- Miele, P.; Bechelany, M. Boron Nitride Nanostructures; MDPI: Basel, Switzerland; Beijing, China; Wuhan, China; Barcelona, Spain; Belgrade, Serbia, 2018. [Google Scholar]
- Arenal, A.; Lopez-Bezanilla, A. Boron nitride materials: An overview from 0D to 3D (nano)structures. Wiley Interdiscip Rev. Comput. Mol. Sci. 2015, 5, 299–309. [Google Scholar] [CrossRef]
- Wong, T.L.; Vallés, C.; Nasser, A.; Abeykoon, C. Effects of boron-nitride-based nanomaterials on the thermal properties of composite organic phase change materials: A state-of-the-art review. Renew. Sustain. Energy Rev. 2023, 187, 113730. [Google Scholar] [CrossRef]
- Kostoglou, N.; Polychronopoulou, K.; Rebholz, C. Thermal and chemical stability of hexagonal boron nitride (h-BN) nanoplatelets. Vacuum 2015, 112, 42–45. [Google Scholar] [CrossRef]
- Rafiq, M.; Hu, X.; Ye, Z.; Qayum, A.; Xia, H.; Hu, L.; Lu, F.; Chu, P.K. Recent advances in structural engineering of 2D hexagonal boron nitride electrocatalysts. Nano Energy 2022, 91, 106661. [Google Scholar] [CrossRef]
- Wadhwa, G.K.; Late, D.J.; Charhate, S.; Sankhyan, S.B. 1D and 2D Boron Nitride Nano Structures: A Critical Analysis for Emerging Applications in the Field of Nanocomposites. ACS Omega 2024, 9, 26737–26761. [Google Scholar] [CrossRef] [PubMed]
- Simos, N.; Kotsina, Z.; Sprouster, D.; Zhong, Z.; Zhong, H.; Hurh, P. Hexagonal boron nitride (h-BN) irradiated with 140 MeV protons. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2020, 479, 110–119. [Google Scholar] [CrossRef]
- Maselugbo, A.O.; Harrison, H.B.; Alston, J.R. Boron nitride nanotubes: A review of recent progress on purification methods and techniques. J. Mater. Res. 2022, 37, 4438–4458. [Google Scholar] [CrossRef]
- Zeng, H.; Zhi, C.; Zhang, Z.; Wei, X.; Wang, X.; Guo, W.; Bando, Y.; Golberg, D. White Graphenes: Boron Nitride Nanoribbons via Boron Nitride Nanotube Unwrapping. Nano Lett. 2010, 10, 5049–5055. [Google Scholar] [CrossRef] [PubMed]
- Avasarala, S.; Bose, S. 2D nanochannels and huge specific surface area offer unique ways for water remediation and adsorption: Assessing the strengths of hexagonal boron nitride in separation technology. Funct. Compos. Mater. 2023, 4, 5. [Google Scholar] [CrossRef]
- Pan, D.; Su, F.; Liu, H.; Ma, Y.; Das, R.; Hu, Q.; Liu, C.; Guo, Z. The Properties and Preparation Methods of Different Boron Nitride Nanostructures and Applications of Related Nanocomposites. Chem. Rec. 2020, 20, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, J.; Hang, Y.; Yu, J.; Tai, G.; Li, X.; Zhang, Z.; Guo, W. Boron Nitride Nanostructures: Fabrication, Functionalization and Applications. Small 2016, 12, 2942–2968. [Google Scholar] [CrossRef] [PubMed]
- Shtansky, D.V.; Matveev, A.T.; Permyakova, E.S.; Leybo, D.V.; Konopatsky, A.S.; Sorokin, P.B. Recent Progress in Fabrication and Application of BN Nanostructures and BN-Based Nanohybrids. Nanomaterials. 2022, 12, 2810. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.S.; Padya, B.; Sarada, B.V.; Akhila, V.; Gowthami, C.; Krishna, P.V.; Joardar, J. Two-dimensional hexagonal boron nitride by cryo-milling: Microstructure and oxidation behavior at elevated temperature. J. Nanopart. Res. 2024, 26, 80. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y. Boron Nitride Nanotubes and Nanoribbons Produced by Ball Milling Method. In Nanotubes and Nanosheets; CRC Press: Boca Raton, FL, USA, 2015; pp. 33–58. [Google Scholar] [CrossRef]
- Leybo, D.V.; Konopatsky, A.S.; Fang, X.; Shtansky, D.V. Photocatalytic phenol oxidation over ball milled hexagonal boron nitride. J. Water Process Eng. 2023, 51, 103367. [Google Scholar] [CrossRef]
- Ranjithkumar, R.; Rosario, J.D.; Shanmugaraj, P.; Swaminathan, R.; Nandhakumar, R.; Bhojan, V.; Ayyasamy, S. Investigation on the influence of ball milling on the microstructural properties of 2D-hexaonal boron nitride towards electroadhesive applications. Micro Nanostruct. 2022, 173, 207453. [Google Scholar] [CrossRef]
- Ghosh, A.; Shukla, U.; Sahoo, N.; Ganguly, S.; Shrivastava, P.; Kumar, L.; Alam, S.N. Effect of ball milling on hexagonal boron nitride (hBN) and development of Al-hBN nanocomposites by powder metallurgy route. Mater. Sci.-Pol. 2023, 41, 68–93. [Google Scholar] [CrossRef]
- Bechelany, M.; Bernard, S.; Brioude, A.; Cornu, D.; Stadelmann, P.; Charcosset, C.; Fiaty, K.; Miele, P. Synthesis of Boron Nitride Nanotubes by a Template-Assisted Polymer Thermolysis Process. J. Phys. Chem. C 2007, 111, 13378–13384. [Google Scholar] [CrossRef]
- Wang, Y.; Yamamoto, Y.; Kiyono, H.; Shimada, S. Highly Ordered Boron Nitride Nanotube Arrays with Controllable Texture from Ammonia Borane by Template-Aided Vapor-Phase Pyrolysis. J. Nanomater. 2008, 2008, 1–7. [Google Scholar] [CrossRef]
- Yu, B.; Luo, Z.; Zhou, Y.; Zhang, Q.; He, J.; Fan, J. Highly Thermally Conductive Flexible Biomimetic APTES-BNNS/BC Nanocomposite Paper by Sol–Gel-Film Technology. ACS Appl. Mater. Interfaces 2024, 16, 21050–21060. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, F.; Cagil, E.M. Preparation of hexagonal boron nitride polymer systems with one-step sol–gel synthesis for pH-controlled drug delivery. Macromol. Res. 2023, 32, 337–348. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, P.; Wang, C.X.; Yang, G.W. External field-assisted laser ablation in liquid: An efficient strategy for nanocrystal synthesis and nanostructure assembly. Progress in Materials Science. 2017, 87, 140–220. [Google Scholar] [CrossRef]
- Kandadai, V.A.S.; Petersen, J.B.; Jasthi, B.K. Microstructural characterization of nanocrystalline hexagonal boron nitride thin films deposited by ion-beam assisted pulsed laser deposition. Surf. Coat. Technol. 2024, 487, 131035. [Google Scholar] [CrossRef]
- Haque, A.; Taqy, S.; Narayan, J. Recent Progress in Cubic Boron Nitride (c-BN) Fabrication by Pulsed Laser Annealing for Optoelectronic Applications. J. Electron. Mater. 2024, 53, 4308–4340. [Google Scholar] [CrossRef]
- Muneoka, H.; Koike, T.; Ito, T.; Terashima, K.; Miura, E. Size reduction and water dispersibility improvement of hexagonal boron nitride particles by femtosecond laser irradiation in water. J. Phys. D Appl. Phys. 2024, 57, 24520. [Google Scholar] [CrossRef]
- Ahmad, P.; Khandaker, M.U.; Khan, Z.R.; Amin, Y.M. Synthesis of boron nitride nanotubes via chemical vapour deposition: A comprehensive review. RSC Adv. 2015, 5, 35116–35137. [Google Scholar] [CrossRef]
- da Silva, W.M.; Ribeiro, H.; Ferreira, T.H.; Ladeira, L.O.; Sousa, E.M.B. Synthesis of boron nitride nanostructures from catalyst of iron compounds via thermal chemical vapor deposition technique. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 89, 177–182. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Peng, Y.; Zheng, Y.; Huang, D.; Hu, P.; Li, Y.; Fu, Z. Highly efficient synthesis of boron nitride nanotubes by catalytic chemical vapor deposition of boron/nickel containing precursors. J. Mater. 2022, 8, 1199–1204. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, K.; Wang, Y.; Zhou, Z.; He, Q.; Wang, N.; Xu, T.; Yao, Y. Experimental and Computational Research on the Effect of Flow Distribution on the Growth of Boron Nitride Nanotubes by Chemical Vapor Deposition. J. Phys. Chem. C 2023, 127, 12235–12242. [Google Scholar] [CrossRef]
- Shi, Y.; Hamsen, C.; Jia, X.; Kim, K.K.; Reina, A.; Hofmann, M.; Hsu, A.L.; Zhang, K.; Li, H.; Juang, Z.-Y.; et al. Synthesis of Few-Layer Hexagonal Boron Nitride Thin Film by Chemical Vapor Deposition. Nano Lett. 2010, 10, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Feng, L.; Liu, T.; Wang, H. Layer-selective growth of 2D hexagonal boron nitride using two-step chemical vapor deposition. Vacuum 2023, 213, 112126. [Google Scholar] [CrossRef]
- Nguyen, H.-U.-P.; Wani, S.-S.; Cyu, R.-H.; Rehman, B.; Chaudhary, M.; Yang, T.-Y.; Hsu, Y.-C.; Kimbulapitiya, K.M.M.; Kuo, Y.-R.; Aksu, K.H.; et al. Figure-of-Merit in 2D Insulators Pertaining to the Thermokinetics of Hexagonal Boron Nitride via Atmospheric-Pressure Chemical Vapor Deposition (APCVD) on Cu: Origin and Scenario. Chem. Mater. 2024, 36, 4518. [Google Scholar] [CrossRef]
- Yuan, Y.; Weber, J.; Li, J.; Tian, B.; Ma, Y.; Zhang, X.; Taniguchi, T.; Watanabe, K.; Lanza, M. On the quality of commercial chemical vapour deposited hexagonal boron nitride. Nat. Commun. 2024, 15, 1–12. [Google Scholar] [CrossRef]
- Yamamoto, M.; Murata, H.; Miyata, N.; Takashima, H.; Nagao, M.; Mimura, H.; Neo, Y.; Murakami, K. Low-Temperature Direct Synthesis of Multilayered h-BN without Catalysts by Inductively Coupled Plasma-Enhanced Chemical Vapor Deposition. ACS Omega 2023, 8, 5497–5505. [Google Scholar] [CrossRef]
- Brown, J.M.; Vishwakarma, S.; Smith, D.J.; Nemanich, R.J. Nucleation of cubic boron nitride on boron-doped diamond via plasma enhanced chemical vapor deposition. J. Appl. Phys. 2023, 133, 215303. [Google Scholar] [CrossRef]
- Torres-Castillo, C.S.; Tavares, J.R. Covalent functionalization of boron nitride nanotubes through photo-initiated chemical vapour deposition. Can. J. Chem. Eng. 2023, 101, 1410–1420. [Google Scholar] [CrossRef]
- Kuc, A.; Enyashin, A.; Seifert, G. Metal−Organic Frameworks: Structural, Energetic, Electronic, and Mechanical Properties. J. Phys. Chem. B 2007, 111, 8179–8186. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Düren, T.; Millange, F.; Férey, G.; Walton, K.S.; Snurr, R.Q. Calculating Geometric Surface Areas as a Characterization Tool for Metal−Organic Frameworks. J. Phys. Chem. C 2007, 111, 15350–15356. [Google Scholar] [CrossRef]
- Grünker, R.; Bon, V.; Müller, P.; Stoeck, U.; Krause, S.; Mueller, U.; Senkovska, I.; Kaskel, S. A new metal–organic framework with ultra-high surface area. Chem. Commun. 2014, 50, 3450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A historical overview of the activation and porosity of metal–organic frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Xu, Q. Porous metal–organic frameworks as platforms for functional applications. Chem. Commun. 2011, 47, 3351. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Zhang, M.; Zhou, H.-C. Increasing the Stability of Metal-Organic Frameworks. Adv. Chem. 2014, 2014, 182327. [Google Scholar] [CrossRef]
- He, T.; Kong, X.-J.; Li, J.-R. Chemically Stable Metal–Organic Frameworks: Rational Construction and Application Expansion. Acc. Chem. Res. 2021, 54, 3083–3094. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.; Patil, K.M.; Wilson, B.H.; Hermanspahn, L.; Harvey-Reid, N.C.; Howard, B.I.; Kleinjan, C.; Kolien, J.; Payet, F.; Telfer, S.G.; et al. The thermal stability of metal-organic frameworks. Coord. Chem. Rev. 2020, 419, 213388. [Google Scholar] [CrossRef]
- Saeed, M.; Marwani, H.M.; Shahzad, U.; Asiri, A.M.; Hussain, I.; Rahman, M.M. Utilizing Nanostructured Materials for Hydrogen Generation, Storage, and Diverse Applications. Chem.–Asian J. 2023, 19, e202300593. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.F.; Hong, Y.K.; Cui, Y.Q.; Guo, H.Y.; Long, Y.; Ye, J.S. Efficient Adsorption of Ofloxacin in a Novel Nanocomposite Formed by Nano-Hexagonal Boron Nitride Fused with Zeolite Imidazolite Skeleton-8: Experimental and Molecular Dynamics Simulation Studies. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4862385 (accessed on 12 August 2024).
- Guo, F.; Xiao, W.; Ma, C.; Ruan, X.; He, G.; Wang, H.; Yang, Z.; Jiang, X. Constructing Gas Transmission Pathways in Two-Dimensional Composite Material ZIF-8@BNNS Mixed-Matrix Membranes to Enhance CO2/N2 Separation Performance. Membranes 2023, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Gong, K.; Zhou, K.; Qian, X.; Shi, C.; Gui, Z.; Qian, L. Flame-retardant activity of ternary integrated modified boron nitride nanosheets to epoxy resin. J. Colloid Interface Sci. 2022, 608, 853–863. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, C.; Li, Y.; Wang, C.; Yuan, W.; Shen, T.; Liu, J.; Cheng, D.; Wu, C.; Shen, Q.; et al. A smart sol-gel coating incorporating pH-responsive BTA-ZIF-8 MOF assembled hexagonal boron nitride for active/passive corrosion protection of 1060 aluminum alloy. Surf. Coat. Technol. 2023, 474, 130072. [Google Scholar] [CrossRef]
- Tian, T.; Xu, J.; Xiong, Y.; Ramanan, N.; Ryan, M.; Xie, F.; Petit, C. Cu-functionalised porous boron nitride derived from a metal–organic framework. J. Mater. Chem. A 2022, 10, 20580–20592. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; An, X.; Nie, S.; Li, N.; Cao, H.; Cheng, Z.; Liu, H.; Ni, Y.; Liu, L. Design of B/N Co-Doped Micro/Meso Porous Carbon Electrodes from CNF/BNNS/ZIF-8 Nanocomposites for Advanced Supercapacitors. J. Bioresour. Bioprod. 2023, 8, 292–305. [Google Scholar] [CrossRef]
- Atta, M.R.; Shaharun, M.S.; Khan, M.M.R.; Abdullah, B.; Al-Mahmodi, A.F.; Ridzuan, N.D.M.; Wei, L.J. Enhancing the Photo-Electrocatalytic Properties of g-C3N4 by Boron Doping and ZIF-8 Hybridization. Inorg. Chem. Commun. 2022, 148, 110235. [Google Scholar] [CrossRef]
- Habibi, B.; Pashazadeh, A.; Pashazadeh, S.; Saghatforoush, L.A. Copper/zeolitic imidazolate Framework-8 integrated by boron nitride as an electrocatalyst at the glassy carbon electrode to sensing of the clopidogrel. J. Solid State Chem. 2023, 323, 123982. [Google Scholar] [CrossRef]
- Liu, W.; Yin, R.; Xu, X.; Zhang, L.; Shi, W.; Cao, X. Structural Engineering of Low-Dimensional Metal–Organic Frameworks: Synthesis, Properties, and Applications. Adv. Sci. 2019, 6, 1802373. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, V.; Zabihi, O.; Ahmadi, M.; Li, Q.; Blanchard, P.; Kiziltas, A.; Naebe, M. Metal–organic framework structure–property relationships for high-performance multifunctional polymer nanocomposite applications. J. Mater. Chem. A 2021, 9, 4348–4378. [Google Scholar] [CrossRef]
- Lotfi, R.; Saboohi, Y. Effect of metal doping, boron substitution and functional groups on hydrogen adsorption of MOF-5: A DFT-D study. Comput. Theor. Chem. 2014, 1044, 36–43. [Google Scholar] [CrossRef]
- Tan, X.; Wang, S.; Han, N. Metal organic frameworks derived functional materials for energy and environment related sustainable applications. Chemosphere 2022, 313, 137330. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Xu, L.; Chen, M.; Wu, C.-E.; Li, W.; Huang, B.; Cui, Y. Carbon Dioxide Captured by Metal Organic Frameworks and Its Subsequent Resource Utilization Strategy: A Review and Prospect. J. Nanosci. Nanotechnol. 2019, 19, 3059–3078. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.R.; Shima, S.M.; Al-Mahmodi, A.F.; Juma, A.K.; Merican, Z.M.A.; Khan, M. Maksudur Rahman, Nh2-Mil-101(Fe) Hybridization on Boron-Doped-G-C3n4 for Photo-Electrocatalytic Co2 Reduction. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4704955 (accessed on 10 September 2024).
- An, D.; Chen, H.; He, R.; Chen, J.; Liu, C.; Sun, Z.; Yu, H.; Liu, Y.; Wong, C.; Feng, W. MOF decorated boron nitride/natural rubber composites with heterostructure for thermal management application through dual passive cooling modes base on the improved thermal conductivity and water sorption-desorption process. Compos. Sci. Technol. 2024, 248, 110469. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Hashemzadeh, A.; Darroudi, M. A novel molecularly imprinted polymer decorated by CQDs@HBNNS nanocomposite and UiO-66-NH2 for ultra-selective electrochemical sensing of Oxaliplatin in biological samples. Sens. Actuators B Chem. 2020, 307, 127614. [Google Scholar] [CrossRef]
- Long, Y.; Qu, C.; Kang, Y.; Huang, X.; Zan, P.; Chen, J.; Sun, X.; Yu, Q.; Dai, Y.; Wang, W.; et al. Janus composite film comprising metal–organic framework/polyimide and BNNSs/polyimide as high energy storage dielectrics. Polym. Compos. 2024, 45, 4605–4617. [Google Scholar] [CrossRef]
- Samui, A.B.; Begum, S.S. 2D nanomaterials for removal of gas molecules. In Nanomaterials in Environmental Analysis; Elsevier: Amsterdam, The Netherlands, 2024; pp. 393–417. [Google Scholar] [CrossRef]
- Haroon, H.; Wahid, M.; Majid, K. Metal–Organic Framework-Derived p-Type Cu3P/Hexagonal Boron Nitride Nanostructures for Photocatalytic Oxidative Coupling of Aryl Halides to Biphenyl Derivatives. ACS Appl. Nano Mater. 2022, 5, 2006–2017. [Google Scholar] [CrossRef]
- Rasul, M.G.; Kiziltas, A.; Arfaei, B.; Shahbazian-Yassar, R. 2D boron nitride nanosheets for polymer composite materials. Npj 2D Mater. Appl. 2021, 5, 56. [Google Scholar] [CrossRef]
- Ma, P.; Spencer, J.T. Non-covalent stabilization and functionalization of boron nitride nanosheets (BNNSs) by organic polymers: Formation of complex BNNSs-containing structures. J. Mater. Sci. 2014, 50, 313–323. [Google Scholar] [CrossRef]
- Raman, A.; Aparna, A.; Aathira, U.; Sethulekshmi, A.S.; Saritha, A. Two-dimensional nanomaterial-based polymer nanocomposites for supercapacitor applications. Two-Dimens. Nanomater.-Based Polym. Nanocompos. 2024, 343–376. [Google Scholar] [CrossRef]
- Sharma, K.; Puri, N.K.; Singh, B. An efficient electrochemical nano-biosensor based on hydrothermally engineered ultrathin nanostructures of hexagonal boron nitride nanosheets for label-free detection of carcinoembryonic antigen. Appl. Nanosci. 2023, 14, 217–230. [Google Scholar] [CrossRef]
- Jia, D.; Tong, R.; Ning, L.; Yang, Z.; Zhang, Y.; Gu, W.; Liu, X. BN nanosheets in-situ mosaic on MOF-5 derived porous carbon skeleton for high-performance lithium-ion batteries. J. Alloys Compd. 2020, 857, 157571. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, P.; Zhang, C.; Wu, L.; Yang, J.; Zhang, M.; Ren, Z.; Zhang, J.; Luo, H. MOF(ZM)/Potassium Citrate-Derived Composite Porous Carbon and Its Electrochemical Properties. J. Miner. Mater. Charact. Eng. 2021, 9, 462–479. [Google Scholar] [CrossRef]
- Ren, J.; Stagi, L.; Innocenzi, P. Hydroxylated boron nitride materials: From structures to functional applications. J. Mater. Sci. 2020, 56, 4053–4079. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Ghosh, M.; Samantaray, P.K.; Kurungot, S.; Winter, M.; Nair, J.R. Chapter 5. 2D Nanomaterial-based Polymer Composite Electrolytes for Lithium-based Batteries. In Two-Dimensional Inorganic Nanomaterials for Conductive Polymer Nanocomposites; Royal Society of Chemistry: London, UK, 2021; pp. 204–274. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, K.; Subramanian, P.; Xu, M.; Luo, J.; Fransaer, J. Electrochemical deposition of metal–organic framework films and their applications. J. Mater. Chem. A 2020, 8, 7569–7587. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Glavin, N.R.; Robinson, J.A. Chapter Three—2D Boron Nitride: Synthesis and Applications. In Semiconductors and Semimetals; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Yan, Y.; Cao, Y.; Wang, Z.; Wang, K.; Ren, H.; Zhang, S.; Wang, Y.; Chen, J.; Zhou, Y.; Liu, L.; et al. Asymmetric orbital hybridization in Zn-doped antiperovskite Cu1−xZnxNMn3 enables highly efficient electrocatalytic hydrogen production. J. Energy Chem. 2023, 89, 304–312. [Google Scholar] [CrossRef]
- Yu, L.; Li, S.; Liu, B.; Liu, S.; Sheng, J.; Ao, Y. Determination of hydrogen gas by 1,4-bis(phenylethynyl)benzene hydrogenation coupled with gas chromatography-mass spectrometry. Talanta 2023, 266, 125071. [Google Scholar] [CrossRef]
- Kofahl, C.; Dörrer, L.; Wulfmeier, H.; Fritze, H.; Ganschow, S.; Schmidt, H. Hydrogen Diffusion in Li(Nb,Ta)O3 Single Crystals Probed by Infrared Spectroscopy and Secondary Ion Mass Spectrometry. Chem. Mater. 2024, 36, 1639–1647. [Google Scholar] [CrossRef]
- Day, M.C.; Jollands, M.C.; Novella, D.; Nestola, F.; Dovesi, R.; Pamato, M.G. Hydrogen-related defects in diamond: A comparison between observed and calculated FTIR spectra. Diam. Relat. Mater. 2024, 143, 110866. [Google Scholar] [CrossRef]
- Dorokhov, V.V.; Nyashina, G.S.; Strizhak, P.A. Thermogravimetric, kinetic study and gas emissions analysis of the thermal decomposition of waste-derived fuels. J. Environ. Sci. 2024, 137, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yong, H.; Gu, B.; Mukamel, S. Chemical bond reorganization in intramolecular proton transfer revealed by ultrafast X-ray photoelectron spectroscopy. Proc. Natl. Acad. Sci. USA 2024, 121, e2321343121. [Google Scholar] [CrossRef]
- You, X.; Zhang, D.; Zhang, X.-G.; Li, X.; Tian, J.-H.; Wang, Y.-H.; Li, J.-F. Exploring the Cation Regulation Mechanism for Interfacial Water Involved in the Hydrogen Evolution Reaction by In Situ Raman Spectroscopy. Nano-Micro Lett. 2023, 16, 53. [Google Scholar] [CrossRef]
- Zhang, R.; Ai, S.; Long, M.; Wan, L.; Li, Y.; Jia, D.; Duan, H.; Chen, D. Quantitative Study on Hydrogen Concentration–Hydrogen Embrittlement Sensitivity of X80 Pipeline Steel Based on Hydrogen Permeation Kinetics. Metals 2024, 14, 763. [Google Scholar] [CrossRef]
- Tang, D.; Tan, G.-L.; Li, G.-W.; Liang, J.-G.; Ahmad, S.M.; Bahadur, A.; Habib Ullah, M.H.; Khan, A.; Bououdina, M. State-of-the-art hydrogen generation techniques and storage methods: A critical review. J. Energy Storage 2023, 64, 107196. [Google Scholar] [CrossRef]
- Cheng, T.; Bets, K.V.; Yakobson, B.I. Synthesis Landscapes for Ammonia Borane Chemical Vapor Deposition of h-BN and BNNT: Unraveling Reactions and Intermediates from First-Principles. J. Am. Chem. Soc. 2024, 146, 9318–9325. [Google Scholar] [CrossRef]
- Burtch, N.C.; Heinen, J.; Bennett, T.D.; Dubbeldam, D.; Allendorf, M.D. Mechanical Properties in Metal-Organic Frameworks: Emerging Opportunities and Challenges for Device Functionality and Technological Applications. Adv. Mater. 2017, 30, 1704124. [Google Scholar] [CrossRef]
- Paul, A.; Banga, I.K.; Muthukumar, S.; Prasad, S. Engineering the ZIF-8 Pore for Electrochemical Sensor Applications–A Mini Review. ACS Omega 2022, 7, 26993–27003. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, S.; Dhamodharan, D.; Ghoderao, P.N.P.; Byun, H.-S. A systematic review on recent advances of metal–organic frameworks-based nanomaterials for electrochemical energy storage and conversion. Coord. Chem. Rev. 2022, 471, 214741. [Google Scholar] [CrossRef]
- Shet, S.P.; Shanmuga Priya, S.; Sudhakar, K.; Tahir, M. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, Y.; Xu, H.; He, X. Hydrogen storage mechanism of metal–organic framework materials based on metal centers and organic ligands. Carbon Neutralization 2023, 2, 632–645. [Google Scholar] [CrossRef]
- Lee, M.-H.; Vikrant, K.; Younis, S.A.; Szulejko, J.E.; Kim, K.-H. Chemisorption of hydrogen sulfide by metal-organic frameworks and covalent-organic polymers based on experimental/theoretical evaluation. J. Clean. Prod. 2020, 250, 119486. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Xin, X.; Yang, Z.; Gao, Y.; Shi, Y.; Zhao, Z.; An, K.; Wang, W.; Tan, J.; et al. Multi-stepwise charge transfer via MOF@MOF/TiO2 dual-heterojunction photocatalysts towards hydrogen evolution. J. Mater. Chem. A 2022, 10, 9717–9725. [Google Scholar] [CrossRef]
- Liu, X.-T.; Li, B.-H.; Wang, X.-J.; Li, Y.-L.; Zhao, J.; Li, Y.-P.; Li, F.-T. Enhanced Schottky Effect in the Ni2P Cocatalyst via Work Function Up-Shift Induced by MoO2 for Boosting Photocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2022, 10, 10627–10640. [Google Scholar] [CrossRef]
- Zhu, C.; Gerald, R.E.; Huang, J. Metal-Organic Framework Materials Coupled to Optical Fibers for Chemical Sensing: A Review. IEEE Sens. J. 2021, 21, 19647–19661. [Google Scholar] [CrossRef] [PubMed]
- Fortea-Pérez, F.R.; Mon, M.; Ferrando-Soria, J.; Boronat, M.; Leyva-Pérez, A.; Corma, A.; Herrera, J.M.; Osadchii, D.; Gascon, J.; Armentano, D.; et al. The MOF-driven synthesis of supported palladium clusters with catalytic activity for carbene-mediated chemistry. Nat. Mater. 2017, 16, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Psofogiannakis, G.M.; Froudakis, G.E. Fundamental studies and perceptions on the spillover mechanism for hydrogen storage. Chem. Commun. 2011, 47, 7933. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y. Research on Improved MOF Materials Modified by Functional Groups for Purification of Water. Molecules 2023, 28, 2141. [Google Scholar] [CrossRef] [PubMed]

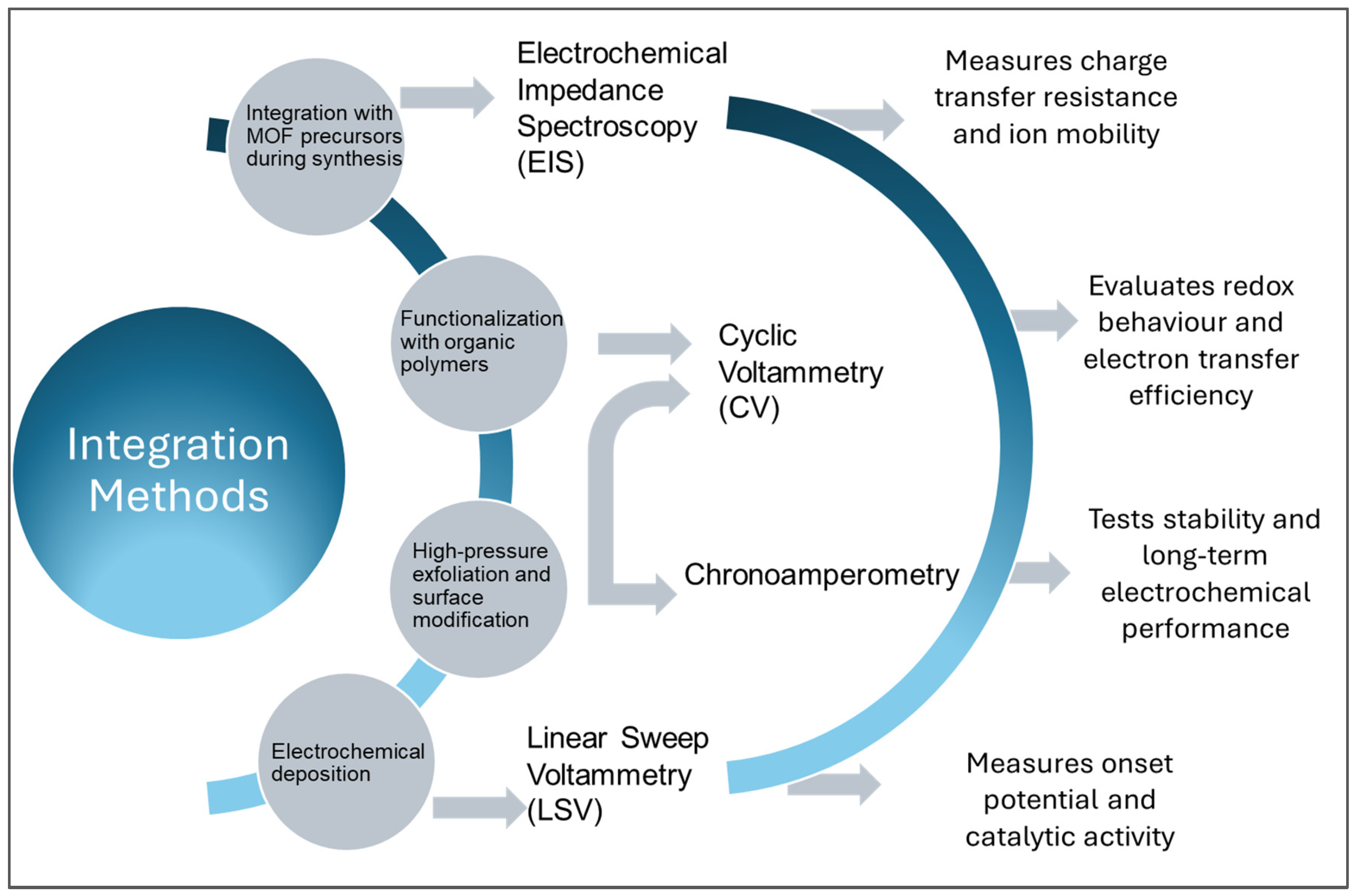
| Synthesis Method | Advantages | Limitations | Typical BNNs Produced | Applications |
|---|---|---|---|---|
| Ball Milling | Simple, accessible | Reduced crystallinity, potential particle re-agglomeration | Nanosheets, nanoparticles | Composite materials, photocatalysis |
| Template-Assisted Growth | High control over structure | Complex, requires precise conditions | Nanotubes | Ordered arrays, high-quality nanotubes |
| Sol–Gel | Flexible, tailored properties | Time-consuming, complex handling | Nanosheets, polysaccharide nanosystems | Electronic films, biomedical applications |
| Laser Ablation | Precise material control | High cost, requires sophisticated equipment | Films, reduced-size particles | Film fabrication, particle size reduction |
| Chemical Vapor Deposition (CVD) | High-quality materials, versatility | Requires high temperatures, complex equipment | BNNTs, h-BN films | High-quality films, nanotubes |
| Method | Characteristics | Applications | References |
|---|---|---|---|
| Solvothermal Synthesis | Dissolves reactants in solvent, heated in sealed vessel; effective for hydrogen storage [67], flame retardancy [70]. | Development of nanostructured materials. | [67,70] |
| Microwave-Assisted Synthesis | Rapid heating with microwave radiation; improves crystallinity and reduces reaction times. | Enhances material properties [C16]. | [72] |
| Electrochemical Synthesis | Anodic dissolution of metal in presence of organic ligand; straightforward route to MOFs. | Electrocatalytic properties [75]. | [75] |
| Sonochemical Synthesis | Uses ultrasound waves to accelerate mixing and reaction rates; improves dispersion of reactants. | Self-assembly approaches for material creation [70,71]. | [70,71] |
| Mechanochemical Synthesis | Physical grinding of reactants without solvents; used for creating micro/mesoporous carbon electrodes. | Fabrication of heterostructural carbon electrodes [73]. | [73] |
| In situ Growth | Direct formation of MOFs on substrates; precise control over composition and structure. | Development of ZIF-8@BN composites [68,69,71]. | [68,69,71] |
| Self-Assembly | Spontaneous organization of molecules; effective for creating hybrids. | Development of BN-OH/ZIF-8 hybrids [70]. | [70] |
| MOF-Derived Synthesis | MOFs used as precursors; tailored material properties. | Cu-functionalized porous boron nitride [72]. | [72] |
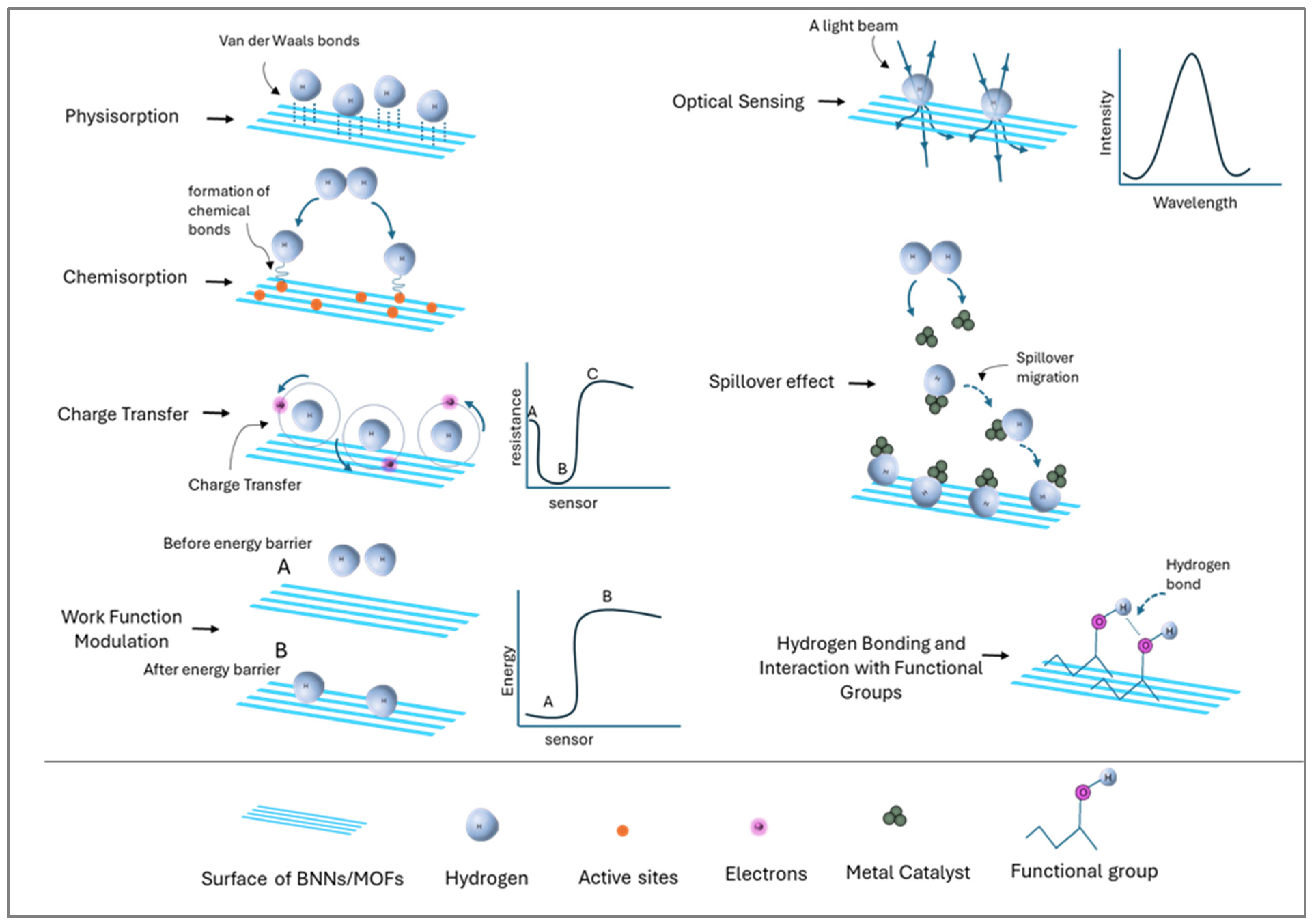
| Mechanism/Method | Applications | Key Features | References |
|---|---|---|---|
| Gas Chromatography (GC) | Hydrogen sensing | High sensitivity, quantitative analysis of hydrogen absorption or release. | [98] |
| Mass Spectrometry (MS) | Hydrogen sensing | Effective in detecting trace amounts of hydrogen and analyzing desorption characteristics. | [99] |
| Fourier-Transform Infrared Spectroscopy (FTIR) | Hydrogen interaction analysis | Provides molecular-level insights into hydrogen interaction and storage. | [100] |
| Thermogravimetric Analysis (TGA) | Hydrogen sensing and storage | Evaluates thermal stability and quantifies hydrogen release across temperatures. | [101] |
| X-ray Photoelectron Spectroscopy (XPS) | Hydrogen interaction analysis | Reveals surface interactions, chemical bonding, and oxidation state changes. | [102] |
| Raman Spectroscopy | Hydrogen sensing | Identifies adsorption sites and examines hydrogen interaction within composite materials. | [103] |
| Hydrogen Permeation Tests | Hydrogen diffusion analysis | Focuses on the kinetic behavior of hydrogen diffusion for storage and sensing applications. | [104] |
| Physisorption Mechanism | Reversible hydrogen storage | Involves weak van der Waals interactions, suitable for low-temperature, reversible adsorption. | [111] |
| Chemisorption Mechanism | Stable hydrogen storage | Forms strong covalent bonds with hydrogen; typically irreversible. | [112] |
| Charge Transfer Mechanism | Hydrogen sensing | Redistribution of electrons changes electrical properties for detection. | [113] |
| Work Function Modulation | Hydrogen sensing | Monitors adsorption-induced changes in work function for precise hydrogen detection. | [114] |
| Optical Sensing Mechanism | Non-invasive, real-time hydrogen detection | Detects changes in absorbance or fluorescence upon hydrogen adsorption. | [115,116] |
| Spillover Effect | Enhanced hydrogen storage | Hydrogen dissociates on metal catalysts and migrates to material surfaces, increasing storage capacity. | [117] |
| Hydrogen Bonding with Functional Groups | Improved hydrogen storage capacity | Functional groups (e.g., -OH, -NH2) facilitate adsorption and retention, enhancing storage performance. | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamro, A.; Balbaied, T. Boron Nitride Nanostructures (BNNs) Within Metal–Organic Frameworks (MOFs): Electrochemical Platform for Hydrogen Sensing and Storage. Analytica 2024, 5, 599-618. https://doi.org/10.3390/analytica5040040
Alamro A, Balbaied T. Boron Nitride Nanostructures (BNNs) Within Metal–Organic Frameworks (MOFs): Electrochemical Platform for Hydrogen Sensing and Storage. Analytica. 2024; 5(4):599-618. https://doi.org/10.3390/analytica5040040
Chicago/Turabian StyleAlamro, Azizah, and Thanih Balbaied. 2024. "Boron Nitride Nanostructures (BNNs) Within Metal–Organic Frameworks (MOFs): Electrochemical Platform for Hydrogen Sensing and Storage" Analytica 5, no. 4: 599-618. https://doi.org/10.3390/analytica5040040
APA StyleAlamro, A., & Balbaied, T. (2024). Boron Nitride Nanostructures (BNNs) Within Metal–Organic Frameworks (MOFs): Electrochemical Platform for Hydrogen Sensing and Storage. Analytica, 5(4), 599-618. https://doi.org/10.3390/analytica5040040






