Chemical, Diagnostic, and Instrumental Analysis of an Ancient Roman Cippus funebris from the First Century AD
Abstract
1. Introduction
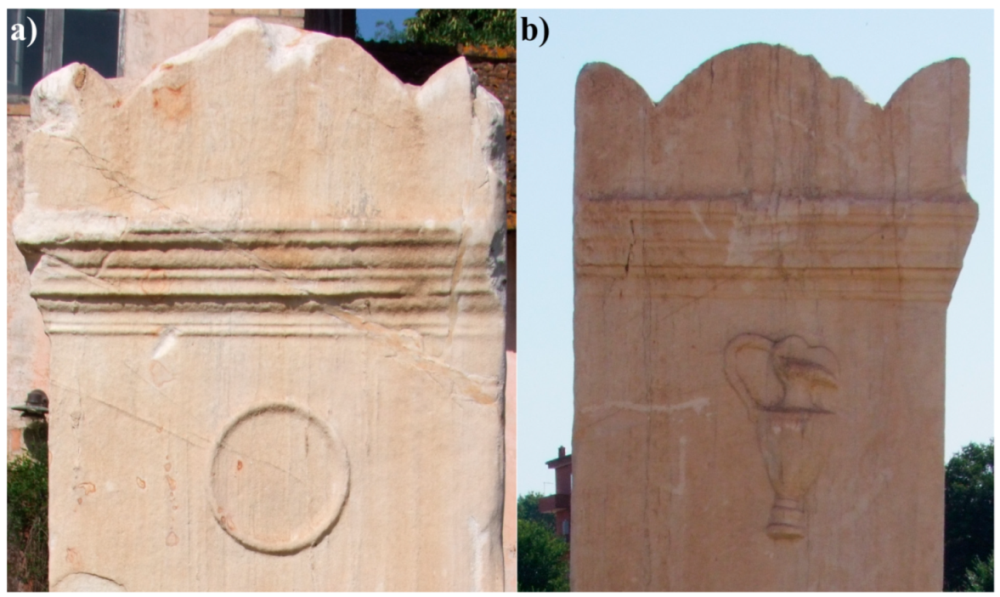
1.1. Roman Funebris Cippus
1.2. Historical–Artistic Information
1.3. Conservation State
2. Materials and Methods
2.1. Optical Microscopy
2.2. Colorimetric Measurements
2.3. Spectrophotometric Measurements
2.4. Electrochemical Measurements
2.5. Ion Chromatography Measurements
2.6. SEM/EDS
3. Results
3.1. Determination of the Soluble Salts Content
- The pH value of a solution coming from the solubilization of marble (calcium carbonate) would have been expected to be higher; the lowering of the pH value is probably due to biological contamination.
- The conductivity values are quite low, indicating low concentrations of soluble salts. This is confirmed by ion chromatography data (see paragraph 3.3).
- As expected, the solution obtained from sample 4 has higher pH and conductivity values (the latter almost double) than those of the three previous samples; in fact, samples scratched from the surface of the Cippus contain a high amount of biodeteriogens, while sample 4, being a fragment spontaneously fallen from the Cippus, has a smaller part of contaminated surface and a higher amount of marble.
- The ORP values are also lower than expected. In fact, a marble exposed outdoors, in good conservation conditions, should have values around 300 mV. It is, therefore, very likely that the degradation due to biological contamination, in contact with the underlying marble, has created a very reducing environment.
- The ORP measured for sample 4, which, as mentioned above, shows very little biological contamination, is more in line with what is expected from the dissolution of a carbonate rock.
3.2. Spectrophotometry UV-Vis
- In the visible range, between 400 and 750 nm (Table 2), a diffuse brown color component can be seen, perceptible to the eye; it is almost absent in sample 4 (obtained from the “original” marble, that is, almost without patina), of low intensity in sample 1, but higher than in sample 1 and in samples 2 and 3, whose spectra almost overlap in this range. These data are congruent with the color measures explained later in the text
- One of the most common indices of the presence of organic substances is an absorption in the UV, observable as a peak or as a shoulder, at about 254 nm (Table 2) [28,29]. Samples 2 and 3 show a similar and well-detectable absorption at this wavelength, while a lower intensity can be seen in the solution derived from sample 1. Unexpectedly, sample 4 also absorbs in this region but with an intensity about one-fifth of that of samples 2 and 3, which is certainly due to the fact that it does not contain a completely uncontaminated region (see Figure 4).
- Considering the location of the Cippus, it is reasonable to assume the presence of humic acids produced by the degradation of decomposing plant species. In Supplementary Materials Figure S2, in order to facilitate the identification of such organic substances, spectra reported in the literature by other authors [28,29] have been collected where the relative peaks are better highlighted.
- Finally, the peaks between 190 and 200 nm could be due to the presence of nitrates at very low concentrations [30], as confirmed by the ion chromatography results (see Section 3.3.1).
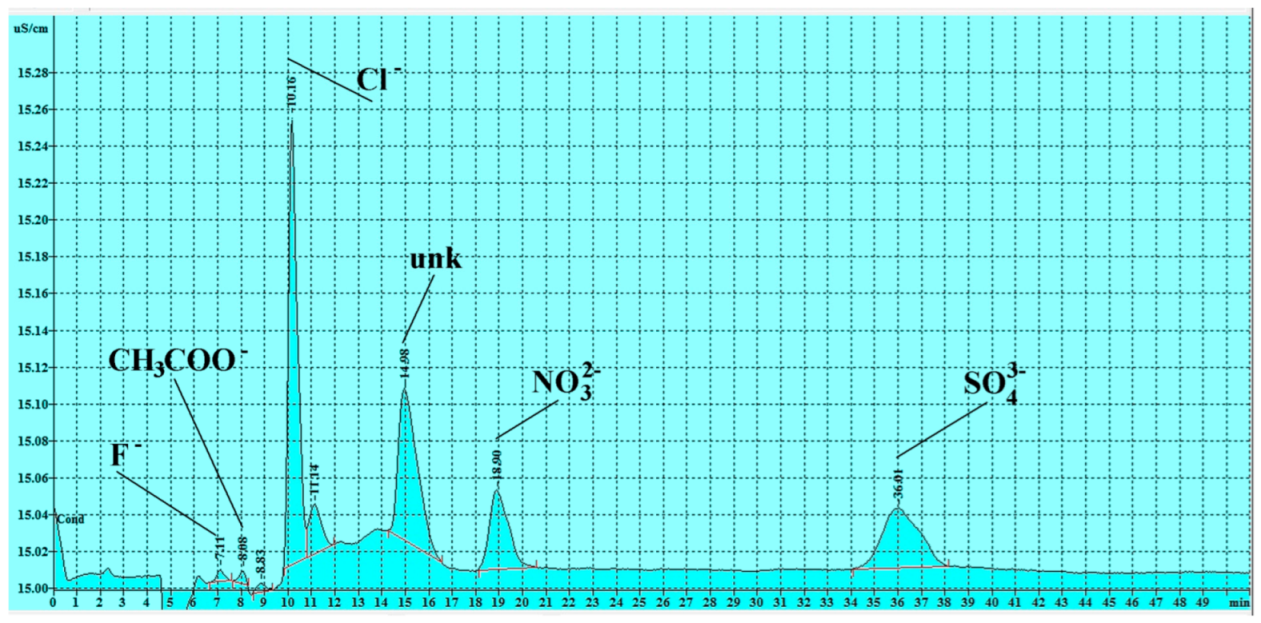
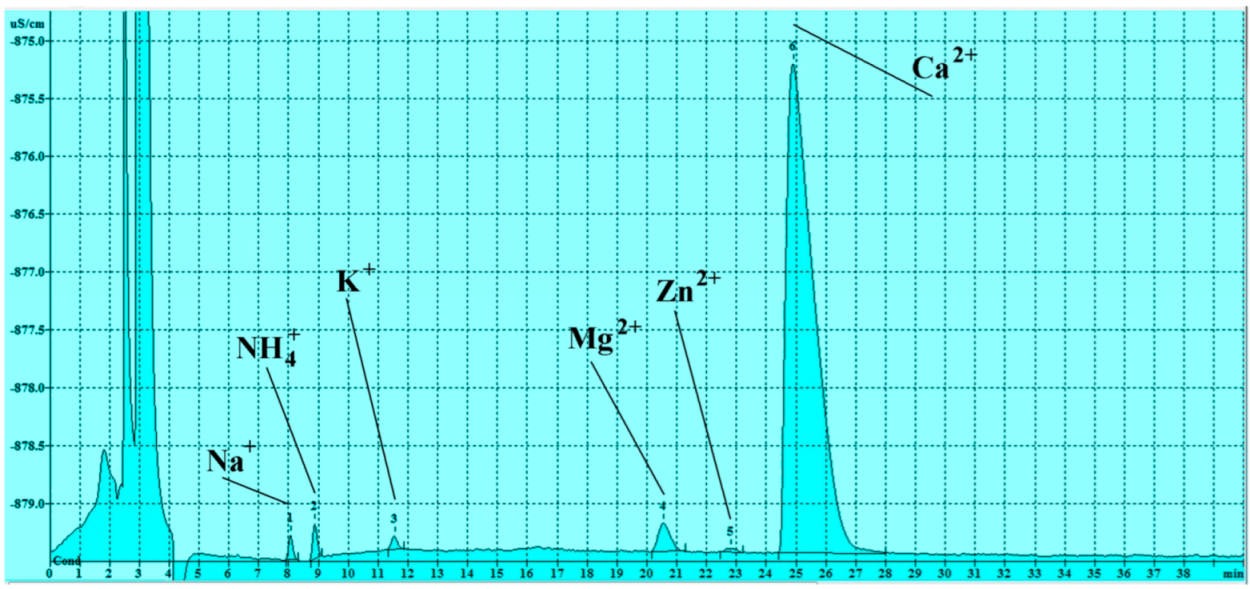
3.3. Soluble Salts Detected by Ion Chromatography
3.3.1. Anions
3.3.2. Cations
3.4. Color Measurement
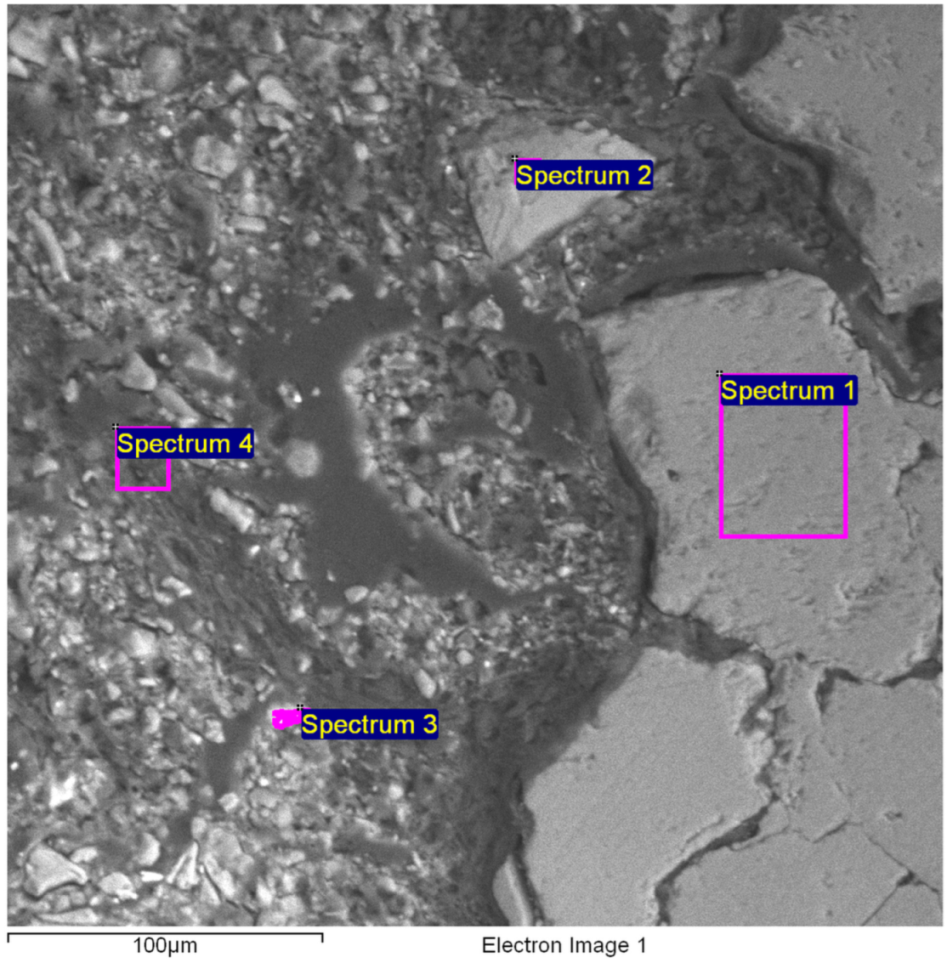
3.5. SEM Image and X-Ray Microanalysis by Energy Dispersive Spectroscopy (EDS)
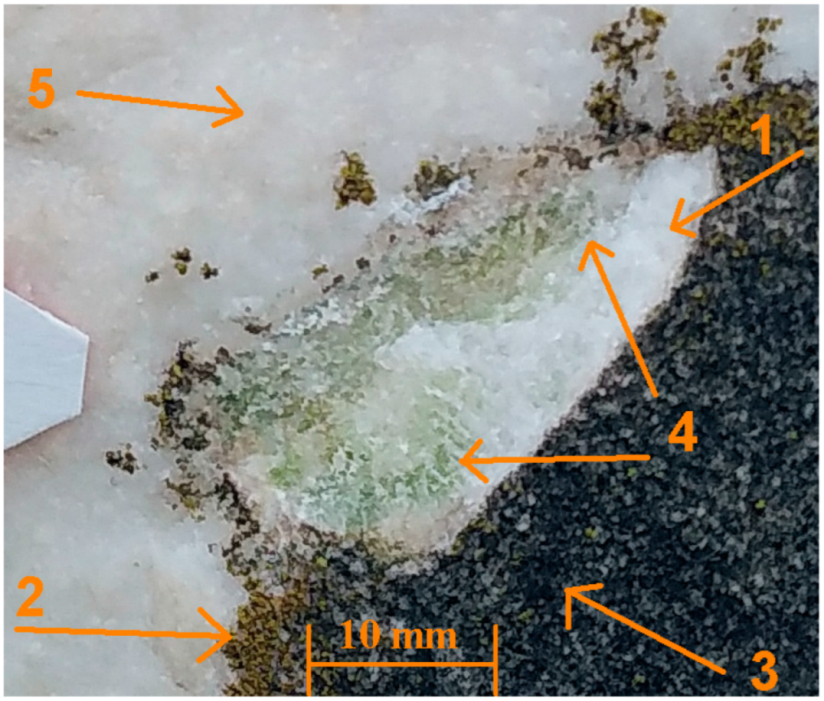
3.6. Macrophotography and Optical Microscopy (OM)
4. Discussion of Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavalli, E. Cippo funerario di Dolichas. Axon 2017, 1, 31–43. [Google Scholar] [CrossRef]
- Sowa, W. The Dialectology of Greek. In Handbook of Comparative and Historical Indo-European Linguistics; Klein, J., Joseph, B., Fritz, M., Eds.; De Gruyter Mouton: Berlin, Germany, 2017; Volume 1, pp. 710–716. [Google Scholar] [CrossRef]
- Bonamici, M. Cippo a clava con viaggio all’aldilà. In Studi Etruschi; Istituto Nazionale di Studi Etruschi ed Italici: Florence, Italy, 2019; Volume 82, pp. 87–97. ISBN 03917762StEtr82. [Google Scholar]
- Antonelli, F.; Lazzarini, L. The Use of White Marble in the Central and Upper Adriatic Between Greece and Rome: Hellenistic Stelae from the Necropolis of Ancona (Italy). Camb. Archaeol. J. 2013, 23, 149–162. [Google Scholar] [CrossRef]
- Haack, M.-L. Ritual and Cults, 250–89 BCE. In Etruscology; Naso, A., Ed.; De Gruyter: Berlin, Germany, 2017; pp. 1203–1214. [Google Scholar] [CrossRef]
- Steiner, A.P. Epitaph from Roma. Epigraphica 1981, 43, 213–216, ISBN-13: 9788875940478. Available online: https://edh.ub.uni-heidelberg.de/edh/inschrift/HD001255 (accessed on 9 March 2025).
- De Cristofaro, A. Da via Cornelia a Via di Boccea: Storia, Percorso e Paesaggi di una Strada Suburbana. In Atlante Tematico di Topografia Antica; L’Erma di Bretschneider: Rome, Italy, 2021; Volume 31, pp. 201–218. [Google Scholar] [CrossRef]
- Koch, T.; Fischer, C.; Schad, F.; Siegesmund, S. Quantification of surface changes and volume losses of selected rock types due to different cleaning processes. Environ. Earth Sci. 2023, 82, 132. [Google Scholar] [CrossRef]
- Diakumaku, E.; Gorbushina, A.A.; Krumbein, W.E.; Panina, L.; Soukharjevski, S. Black fungi in marble and limestones—An aesthetical, chemical and physical problem for the conservation of monuments. Sci. Total Environ. 1995, 167, 295–304. [Google Scholar] [CrossRef]
- Torralba, M.G.; Kuelbs, C.; Moncera, K.J.; Roby, R.; Nelson, K.E. Characterizing Microbial Signatures on Sculptures and Paintings of Similar Provenance. Microb. Ecol. 2021, 81, 1098–1105. [Google Scholar] [CrossRef]
- Romani, M.; Warscheid, T.; Nicole, L.; Marcon, L.; Di Martino, P.; Suzuki, M.T.; Lebaron, P.; Lami, R. Current and future chemical treatments to fight biodeterioration of outdoor building materials and associated biofilms: Moving away from ecotoxic and towards efficient, sustainable solutions. Sci. Total Environ. 2022, 802, 149846. [Google Scholar] [CrossRef]
- Lineback, C.B.; Nkemngong, C.A.; Tongyu Wu, S.; Li, X.; Teska, P.J.; Oliver, H.F. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob. Resist. Infect. Control 2018, 7, 447. [Google Scholar] [CrossRef]
- Reale, R.; Campanella, L.; Devreux, G.; Dell’Aglio, E. Study of microbial melanin decolorization from carbonatic stones surfaces. J. Cult. Herit. 2021, 47, 21–27. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Q.; Geng, J.; Dai, J.; Ge, Z.; Wang, S. Cyclic heating of saturated steam changes the surface properties of sandstone. J. Therm. Anal. Calorim. 2023, 148, 5325–5334. [Google Scholar] [CrossRef]
- Barbut, F.; Menuet, D.; Verachten, M.; Girou, E. Comparison of the efficacy of a hydrogen peroxide dry-mist disinfection system and sodium hypochlorite solution for eradication of Clostridium Difficile spores. Infect. Control. Hosp. Epidemiol. 2009, 30, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Konkol, N.; McNamara, C.; Sembrat, J.; Rabinowitz, M.; Mitchell, R. Enzymatic decolorization of bacterial pigments from culturally significant marble. J. Cult. Herit. 2009, 10, 362–366. [Google Scholar] [CrossRef]
- Crisci, G.M.; La Russa, M.F.; Macchione, M.; Malagodi, M.; Palermo, A.M.; Ruffolo, S.A. Study of archaeological underwater finds: Deterioration and conservation. Appl. Phys. A Mater. Sci. Process. 2010, 100, 855–863. [Google Scholar] [CrossRef]
- Marszałek, M. Application of Optical Microscopy and Scanning Electron Microscopy to the Study of Stone Weathering: A Cracow Case Study. Int. J. Archit. Herit. 2008, 2, 83–92. [Google Scholar] [CrossRef]
- Güleç, A.; Acun, S.; Ersen, A. A characterization method for the fifth-century traditional mortars in the land walls of Constantinople, Yedikule. Stud. Conserv. 2005, 50, 295–306. [Google Scholar] [CrossRef]
- Germinario, L.; Andrian, G.F.; Laviano, R. Petrography, mineralogy, chemical and technical properties of the building stone of Ostuni Cathedral (Italy): Inferences on diagnostics and conservation. Period Miner. 2014, 83, 379–400. [Google Scholar] [CrossRef]
- Astolfi, M.L. Advances in Analytical Strategies to Study Cultural Heritage Samples. Molecules 2023, 28, 6423. [Google Scholar] [CrossRef]
- Sanjurjo Sánchez, J.; Vidal Romaní, J.R.; Fernández Mosquera, D.; Alves, C.A. Study of origin and composition of coatings in a monument built with granitic rocks, by SEM, XRD, XRF and DTA-TGA. X-Ray Spectrom. Int. J. 2008, 37, 346–354. [Google Scholar] [CrossRef]
- Falcone, F.; Cinosi, A.; Siviero, G.; Rosatelli, G. Innovative methodological approach integrating SEM-EDS and TXRF microanalysis for characterization in materials science: A perspective from cultural heritage studies. Spectrochim. Acta Part B At. Spectrosc. 2024, 218, 106980. [Google Scholar] [CrossRef]
- UNI 11087:2003; Beni Culturali—Materiali Lapidei Naturali ed Artificiali—Determinazione del Con-Tenuto di Sali Solubili. Ente Nazionale Italiano di Unificazione: Rome, Italy, 2003.
- EN 16455:2014; Conservation of Cultural Heritage—Extraction and Determination of Soluble Salts in Natural Stone and Related Materials Used in and from Cultural Heritage. CEN: Brussels, Belgium, 2014.
- Tomassetti, M.; Castrucci, M.; Dell’Aglio, E.; Sammartino, M.P.; Visco, G.; Martini, E.; Innocenzi, F.; Reale, R.; Ronca, S. Analytical-petrographic Study of Bugnato Degradation of an Ancient Milan Building. Cur. Anal. Chem. 2024, 20, e130624231005. [Google Scholar] [CrossRef]
- Fontanella, L.U.; Tomassetti, M.; Visco, G.; Sammartino, M.P. Characterization of Rome’s rainwater in the early of 2018 aiming to find correlations between chemical-physical parameters and sources of pollution: A statistical study. J. Atmos. Chem. 2021, 78, 1–16. [Google Scholar] [CrossRef]
- Woldemichael, G.; Tulu, T.; Flechsig, G. Solar UV Photooxidation as Pretreatment for Stripping Voltammetric Trace Metal Analysis in River Water. Int. J. Electrochem. 2011, 2011, 481370. [Google Scholar] [CrossRef]
- Kiprop, A.K.; J-Coumon, M.C.; Pourtier, E.; Kimutai, S.; Kirui, S. Synthesis of Humic and Fulvic Acids and their Characterization using Optical Spectroscopy (ATR-FTIR and UV-Visible). Int. J. Appl. Sci. Technol. 2013, 3, 28–35. [Google Scholar]
- Tsadilas, C. Nitrate Handbook: Environmental, Agricultural, and Health Effects; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–426. [Google Scholar]
- Caliamanis, A.; McCormick, M.J.; Carpente, P.D. Enhanced conductometric detection of cyanide in suppressed ion chromatography. J. Chromatogr. A 2000, 884, 75–80. [Google Scholar] [CrossRef]
- ISO 5725-1:2023; Accuracy (Trueness and Precision) of Measurement Methods and Results. ISO: Geneva, Switzerland, 2023.
- Ivanova, V.; Surleva, A.; Koleva, B. Validation of Ion Chromatographic Method for Determination of Standard Inorganic Anions in Treated and Untreated Drinking Water. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012053. [Google Scholar] [CrossRef]
- Lazzarini, L.; Laurenzi Tabasso, M. Il Restauro della Pietra; CEDAM: Padua, Italy, 1986; pp. 166–264. [Google Scholar]
- Ogino, T.; Suzuki, T.; Sawada, K. The formation and transformation mechanism of calcium carbonate in water. Geochim. Cosmochim. Acta 1987, 51, 2757–2767. [Google Scholar] [CrossRef]
- Prasad Gopi, S.; Subramanian, V.K. Polymorphism in CaCO3—Effect of temperature under the influence of EDTA (di sodium salt). Desalination 2012, 297, 38–47. [Google Scholar] [CrossRef]
- Amadori, M.L.; Santi, P.; Frapiccini, N.; Rinaldi Tufi, S.; Gorgoni, C.; Pallante, P. Roman White Marbles in Northern Marche Region (Italy): An Archaeometric Provenance Study. Geoheritage 2024, 16, 122. [Google Scholar] [CrossRef]
- Huesca-Tortosa, J.A.; Spairani-Berrio, Y.; Coviello, C.G.; Sabbà, M.F.; Rizzo, F.; Foti, D. Evaluation of Eco-Friendly Consolidating Treatments in Pugliese Tuff (Gravina Calcarenite) Used in Italian Heritage Buildings. Buildings 2024, 14, 940. [Google Scholar] [CrossRef]
- Cescon, M. Valutazione di Prodotti Inorganici per il Consolidamento di Materiali Lapidei Carbonatici. Master’s Thesis, Universita’ Ca’ Foscari, Venice, Italy, 2017. [Google Scholar]
- Hansen, E.; Doehne, E.; Fidler, J.; Larson, J.; Martin, B.; Matteini, M.; Rodriguez-Navarro, C.; Pardo, E.S.; Price, C.; de Tagle, A.; et al. A review of selected inorganic consolidants and protective treatments for porous calcareous materials. Stud. Conserv. 2003, 48 (Suppl. 1), 13–25. [Google Scholar] [CrossRef]
- Otero, J.; Carola, A.E.; Starinieri, V. Preliminary Investigations of Compatible Nanolime Treatments on Indiana Limestone and Weathered Marble Stone. Int. J. Archit. Herit. Conserv. Anal. Restor. 2022, 16, 394–404. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Montojo, C.; López de Silanes, M.E.; de Rosario, I.; Rivas, T. In situ evaluation by colour spectrophotometry of cleaning and protective treatments in granitic Cultural Heritage. Int. Biodeterior. Biodegrad. 2017, 123, 251–261. [Google Scholar] [CrossRef]






| Sample n. | Dissolved (mg) | pH | ORP (mV) | Conductivity (µS) | Temperature (°C) |
|---|---|---|---|---|---|
| n 1 | 108.8 | 9.64 | 152.6 | 43.1 | 22.5 |
| n 2 | 108.2 | 8.85 | 144.0 | 29.0 | 21.7 |
| n 3 | 108.5 | 9.69 | 149.8 | 34.4 | 22.2 |
| n 4 | 107.8 | 9.83 | 310.6 | 81.8 | 22.1 |
| Sample n. | Abs at 200 nm | Abs at 254 nm | Abs at 440 nm | Abs at 550 nm | Abs at 664 nm | Abs at 750 nm |
|---|---|---|---|---|---|---|
| n 1 | 0.2492 | 0.0718 | 0.0251 | 0.0126 | 0.0078 | 0.0039 |
| n 2 | 0.2948 | 0.1051 | 0.0376 | 0.0204 | 0.0122 | 0.0068 |
| n 3 | 0.2351 | 0.1020 | 0.0337 | 0.0178 | 0.0088 | 0.0044 |
| n 4 | 0.1129 | 0.0266 | 0.0117 | 0.0066 | 0.0052 | 0.0041 |
| Sample n. | F− (ppm) | CH3COO− (ppm) | Cl− (ppm) | NO2− (ppm) | NO3− (ppm) | SO42− (ppm) | HCO3− (ppm) * |
|---|---|---|---|---|---|---|---|
| n 1 | 0.004 | 0.057 | 0.359 | <LOD | 0.244 | 0.281 | 41.50 |
| n 2 | 0.005 | <LOD | 0.174 | <LOD | <LOD | 1.381 | 46.10 |
| n 3 | 0.011 | <LOD | 0.041 | <LOD | <LOD | 0.040 | 35.70 |
| n 4 | 0.001 | <LOD | 0.007 | 0.023 | 0.016 | 0.059 | 14.60 |
| Sample n. | Na+ (ppm) | NH4+ (ppm) | K+ (ppm) | Mg+2 (ppm) | Zn+2 (ppm) | Ca+2 (ppm) |
|---|---|---|---|---|---|---|
| n 1 | 0.15 | 0.19 | 0.28 | 0.29 | 0.04 | 13.0 |
| n 2 | 0.10 | 0.07 | 0.17 | 0.29 | <LOD | 14.76 |
| n 3 | 0.03 | 0.10 | <LOD | 0.23 | <LOD | 10.96 |
| n 4 | 0.00 | 0.08 | <LOD | 0.08 | 0.09 | 4.55 |
| Test Point or Sample | Sikkens Code | RGB | CIELAB (L a* b*) | Color |
|---|---|---|---|---|
| test n.1 | F2.07.88 | R = 253 G = 241 B = 224 | L = 95.78 a* = 1.56 b* = 8.53 | |
| test n.2 | F2.05.85 | R = 245 G = 235 B = 220 | L = 93.64 a* = 1.21 b* = 7.41 | |
| test n.3 | FN.02.88 | R = 246 G = 240 B = 231 | L = 95.18 a* = 0.83 b* = 4.04 | |
| test n.4 | ON.00.90 | R = 242 G = 240 B = 237 | L = 95.03 a* = 0.74 b* = 0.90 |
| Spectrum | C | O | Al | Si | S | K | Ca | Mn | Fe | Cu | Sn | Sb | I | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spectrum 1 | 8.4 | 52.1 | 32.9 | 1.5 | 5.0 | 100.0 | ||||||||
| Spectrum 2 | 14.2 | 42.0 | 0.0 | 32.8 | 9.1 | 1.8 | 100.0 | |||||||
| Spectrum 3 | 42.1 | 2.9 | 6.9 | 2.0 | 2.2 | 42.9 | 0.9 | 100.0 | ||||||
| Spectrum 4 | 68.7 | 6.1 | 14.3 | 2.2 | 1.3 | 4.6 | 2.7 | 100.0 | ||||||
| Max. | 14.2 | 68.7 | 6.1 | 14.3 | 2.2 | 1.3 | 32.9 | 2.2 | 42.9 | 0.9 | 1.5 | 9.1 | 1.8 | |
| Min. | 8.4 | 42.0 | 2.9 | 0.0 | 2.2 | 1.3 | 2.0 | 2.2 | 2.7 | 0.9 | 1.5 | 5.0 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrucci, M.; Tomassetti, M.; Dell’Aglio, E.; Visco, G.; Sammartino, M.P.; Castracane, M. Chemical, Diagnostic, and Instrumental Analysis of an Ancient Roman Cippus funebris from the First Century AD. Analytica 2025, 6, 11. https://doi.org/10.3390/analytica6010011
Castrucci M, Tomassetti M, Dell’Aglio E, Visco G, Sammartino MP, Castracane M. Chemical, Diagnostic, and Instrumental Analysis of an Ancient Roman Cippus funebris from the First Century AD. Analytica. 2025; 6(1):11. https://doi.org/10.3390/analytica6010011
Chicago/Turabian StyleCastrucci, Mauro, Mauro Tomassetti, Emanuele Dell’Aglio, Giovanni Visco, Maria Pia Sammartino, and Marco Castracane. 2025. "Chemical, Diagnostic, and Instrumental Analysis of an Ancient Roman Cippus funebris from the First Century AD" Analytica 6, no. 1: 11. https://doi.org/10.3390/analytica6010011
APA StyleCastrucci, M., Tomassetti, M., Dell’Aglio, E., Visco, G., Sammartino, M. P., & Castracane, M. (2025). Chemical, Diagnostic, and Instrumental Analysis of an Ancient Roman Cippus funebris from the First Century AD. Analytica, 6(1), 11. https://doi.org/10.3390/analytica6010011








