Chemical Characterization and Biological Activity of Varronia curassavica Jacq. Essential Oil (Boraginaceae) and In Silico Testing of α-Pinene
Abstract
1. Introduction
2. Materials and Methods
2.1. Botanical Material Collection
2.2. Essential Oil Extraction
2.3. Phytochemical Analysis of Essential Oil by Gas Chromatography
2.4. Antibacterial Activity
2.4.1. Strains, Culture Media, and Drugs
2.4.2. Minimum Inhibitory Concentration (MIC)
2.4.3. Activity Modifying the Action of Antibiotics
2.5. Toxicity Test with Drosophila melanogaster
2.5.1. Breeding and Storage of D. melanogaster
2.5.2. Toxicological Tests
2.5.3. Negative Geotaxis Assessment
2.6. In Silico Prediction of ADMET
2.7. Statistical Analysis
3. Results
3.1. Chemical Composition
3.2. Antibacterial Activity
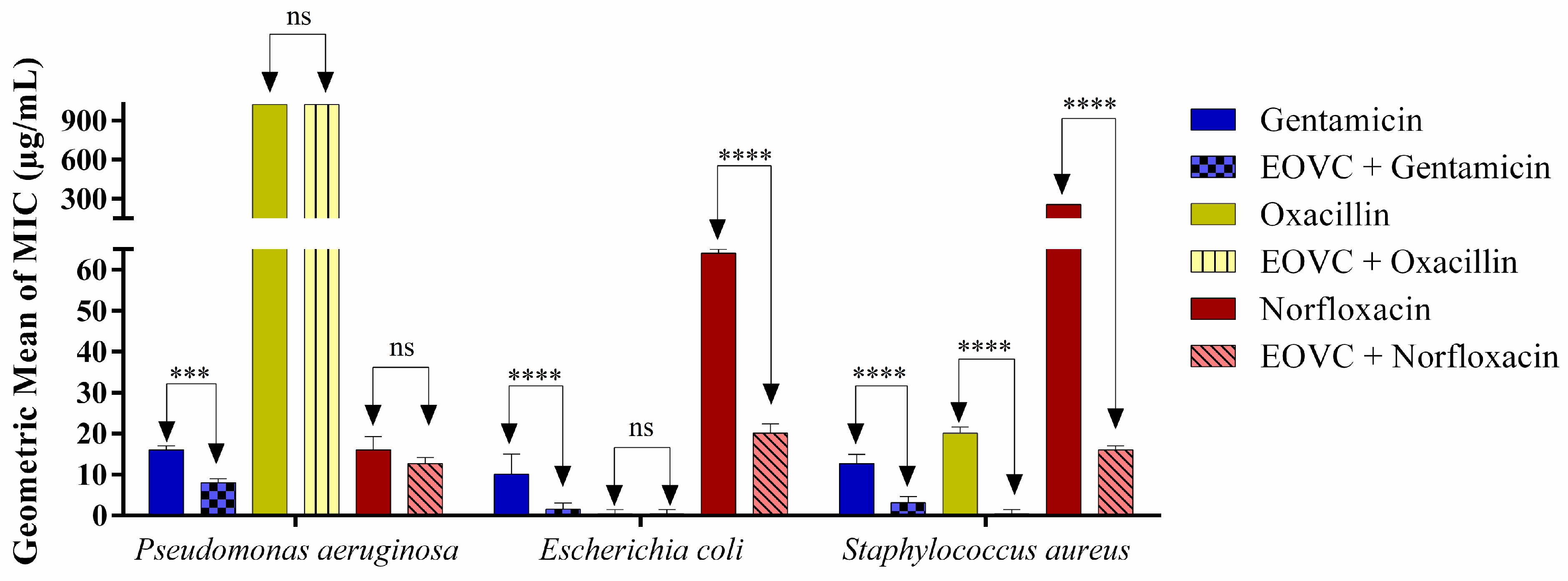
3.3. Activity Modifying the Action of Antibiotics
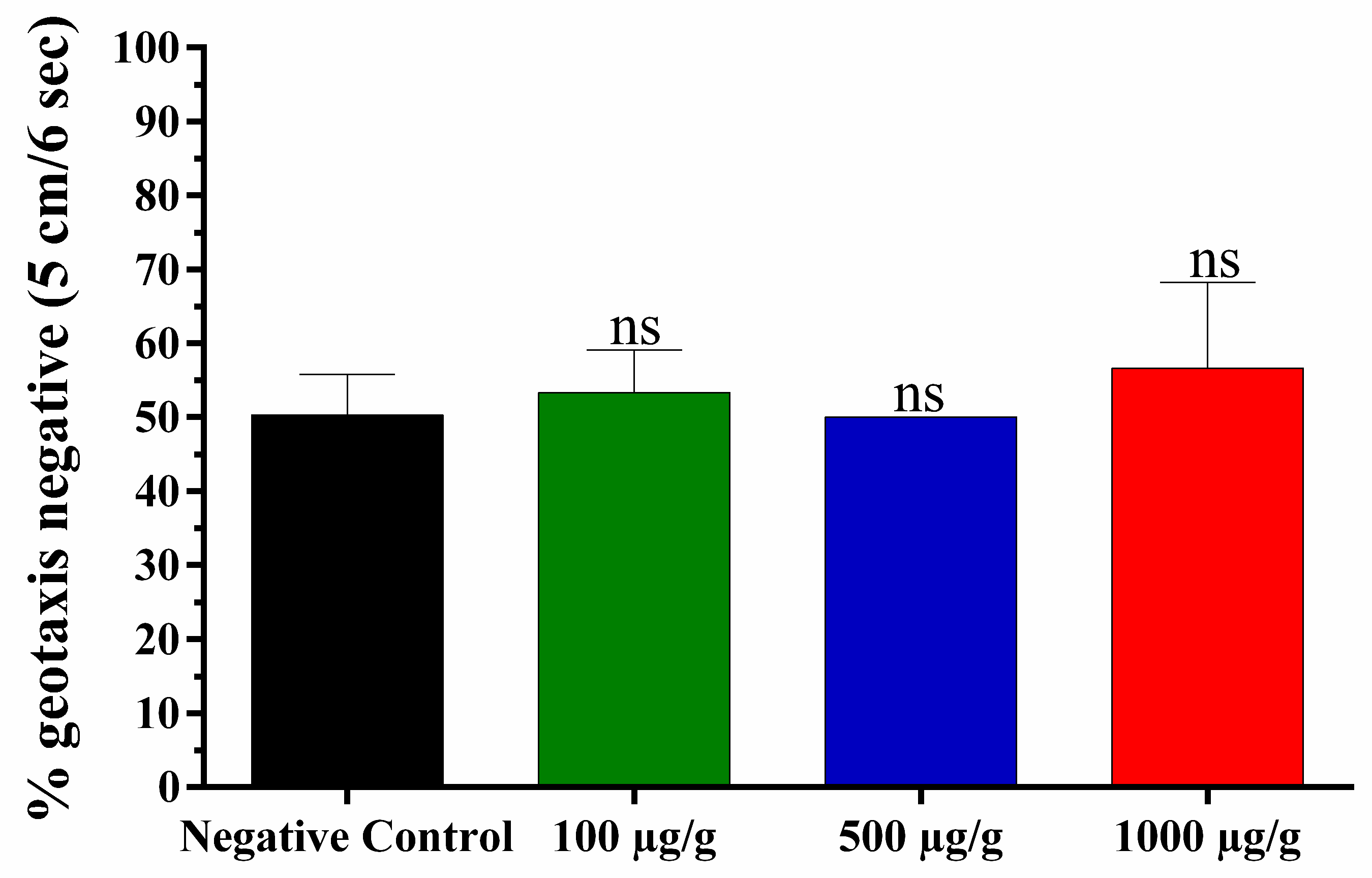
3.4. Toxicity against Drosophila melanogaster
3.5. In Silico Analysis (pkCMS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freires, M.S.; Junior, O.M.R. Bacterial Resistance to Indiscriminate Use of Azithromycin versus COVID-19: An Integrative Review. Res. Soc. Dev. 2022, 11, e31611125035. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA J. 2021, 19, 1–179. [Google Scholar] [CrossRef]
- Arbab, S.; Iqbal, M.K.; Hussain, T.; Yasmeen, R.; Bano, R.; Abbas, G. Antimicrobial drug resistance against Escherichia coli and its harmful effect on animal health. Vet. Med. Sci. 2022, 8, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Avershina, E.; Shapovalova, V.; Shipulin, G. Fighting antibiotic resistance in hospital-acquired infections: Current state and emerging technologies in disease prevention, diagnostics and therapy. Front. Microbiol. 2021, 12, 707330. [Google Scholar] [CrossRef]
- Teixeira, A.R.; Figueiredo, A.F.C.; França, R.F. Resistência bacteriana relacionada ao uso indiscriminado de antibióticos. Rev. Saúde Foco 2019, 11, 853–875. [Google Scholar]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial resistance in bacteria: Mechanisms, evolution, and persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef]
- Jian, Z.; Li, Y.; Xiao, X.; Zhang, S.; Zhao, Y.; Zhang, L.; Dong, L.; Wang, Y. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. J. Basic Microbiol. 2021, 61, 1049–1070. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Macedo, D.G.; Oliveira, L.G.S.; Santos, M.O.; Almeida, B.V.; Macedo, J.G.F.; Macêdo, M.J.F.; Souza, R.K.D.; Araújo, T.M.S.; Almeida-Souza, M.M. Conservation priorities for medicinal woody species in a cerrado area in the Chapada do Araripe, northeastern Brazil. J. Environ. Sustain. 2019, 21, 61–77. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Santos, A.T.L.; Machado, A.J.T.; Bezerra, C.F.; Freitas, T.S.; Coutinho, H.D.M.; Morais-Braga, M.F.B.; Almeida-Bezerra, J.W.; Duarte, A.E.; Kamdem, J.P.; et al. Chemical composition and anti-Candida potencial of the extracts of Tarenaya spinosa (Jacq.) Raf. (Cleomaceae). Comp. Immunol. Microbiol. Infect. Dis. 2019, 64, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, M.M.; Schmitt, V.; Mazur, C.E. Effect of herbal medical plants on anxiety: A brief review. Res. Soc. Dev. 2020, 9, e02911504. [Google Scholar] [CrossRef]
- Nuță, D.C.; Limban, C.; Chiriță, C.; Chifiriuc, M.C.; Costea, T.; Ioniță, P.; Nicolau, L.; Zarafu, I. Contribution of essential oils to the fight against microbial biofilms—A review. Processes 2021, 9, 537. [Google Scholar] [CrossRef]
- Alves, N.V.; Peres, M.M.; Lopes, L.A.; Rodrigues, T.G.L. Potencial farmacológico dos óleos essenciais: Uma atualização. In Práticas Integrativas e Complementares: Visão Holística e Multidisciplinar. Ed. Científica Digit. 2022, 13, 144–160. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Martim, J.K.; Maranho, L.T.; Costa-Casagrande, T.A. Role of the chemical compounds present in the essential oil and in the extract of Cordia verbenacea DC as an anti-inflammatory, antimicrobial and healing product. J. Ethnopharmacol. 2021, 265, 113300. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Abdullah, F.O.; Hassan, A.O.; Galali, Y.; Hassan, R.R.; Rashid, E.Q.; Salih, M.I.; Aziz, K.F. Ethnobotanical, Phytochemistry, and Pharmacological Activity of Onosma (Boraginaceae): An Updated Review. Molecules 2022, 27, 8687. [Google Scholar] [CrossRef]
- Nigussie, G.; Ibrahim, F.; Neway, S. A Phytopharmacological Review on a Medicinal Plant: Cordia africana Lam. J. Trop. Pharm. Chem. 2021, 5, 254–263. [Google Scholar] [CrossRef]
- Toghlobi, G.S.S.; Arantes, R.A.; Knudsen, B.G.; Tabach, R.; Pereira, M.A.A.; Carvalho, R.G.; Ferraz, R.R.N.; Rodrigues, F.S.M. Usos clínicos do fitoterápico da erva-baleeira (Varronia curassavica jacq.): Revisão da literatura. Int. J. Health Manag. Rev. 2022, 8, 1–10. [Google Scholar] [CrossRef]
- Marques, A.P.S.; Bonfim, F.P.G.; Dantas, W.F.C.; Puppi, R.J.; Marques, M.O.M. Chemical composition of essential oil from Varronia curassavica Jacq. accessions in different seasons of the year. Ind. Crops Prod. 2019, 140, 111656. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Almeida, R.S.; Freitas, P.R.; Araújo, A.C.J.; Alencar Menezes, I.R.; Santos, E.L.; Tintino, S.R.; Moura, T.F.; Ribeiro-Filho, J.; Ferreira, V.A.; Silva, A.C.A.; et al. GC-MS profile and enhancement of antibiotic activity by the essential oil of Ocotea odorífera and safrole: Inhibition of Staphylococcus aureus efflux pumps. Antibiotics 2020, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.D.S.; Pereira, R.L.S.; Santos, A.T.L.D.; Coutinho, H.D.M.; Morais-Braga, M.F.B.; Silva, V.B.; Costa, A.R.; Generino, M.E.M.; Oliveira, M.G.; Menezes, S.A.; et al. Phytochemical analysis, antibacterial activity and modulating effect of essential oil from Syzygium cumini (L.) skeels. Molecules 2022, 27, 3281. [Google Scholar] [CrossRef]
- Almeida-Bezerra, J.W.; Cruz, R.P.; Pereira, R.L.S.; Silva, V.B.; Sousa, D.D.O.B.; Neto, J.X.D.S.; Souza, L.A.L.; Araújo, N.M.S.; Silva, R.G.G.; Lucetti, D.L.; et al. Caryocar coriaceum fruits as a potential alternative to combat fungal and bacterial infections: In vitro evaluation of methanolic extracts. Microb. Pathog. 2023, 181, 106203. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, H.D.; Costa, J.G.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.R.; Lima-Silva, J.R.; Oliveira, T.J.S.; Silva, T.G.; Pereira, P.S.; Oliveira-Borba, E.F.; Brito, E.S.; Ribeiro, P.R.V.; Almeida-Bezerra, J.W.; Júnior, J.T.C.; et al. Phytochemical profile of Anacardium occidentale L. (cashew tree) and the cytotoxic and toxicological evaluation of its bark and leaf extracts. S. Afr. J. 2020, 135, 355–364. [Google Scholar] [CrossRef]
- Azzam, K.A.L. SwissADME and pkCSM webservers predictors: An integrated online platform for accurate and comprehensive predictions for in silico ADME/T properties of artemisinin and its derivatives. Complex Use Miner. Resour. 2023, 325, 14–21. [Google Scholar] [CrossRef]
- Bristot, S.F.; Della-Colle, M.P.; Rossato, A.E.; Citadini-Zanette, V. Uso medicinal de Varronia curassavica Jacq. “erva-baleeira”(Boraginaceae): Estudo de caso no sul do Brasil. Braz. J. Anim. Environ. Res. 2021, 4, 170–182. [Google Scholar] [CrossRef]
- Sciarrone, D.; Rigano, F.; Carnovale, C.; Cimino, F.; Dugo, P. Quali-quantitative characterization of the volatile constituents in Cordia verbenacea DC essential oil exploiting advanced chromatographic approaches and nuclear magnetic resonance analysis. J. Chromatogr. A 2017, 1524, 246–253. [Google Scholar] [CrossRef]
- Silva, K.P.; de Carvalho Santos, T.A.; Moutinho, B.L.; da Silva, R.S.; dos Santos Pinto, V.; Blank, A.F.; Corrêa, C.B.; Scher, R.; Fernandes, R.P.M. Using Varronia curassavica (Cordiaceae) essential oil for the biocontrol of Phytomonas serpens. Ind. Crops Prod. 2019, 139, 111523. [Google Scholar] [CrossRef]
- Pereira, P.S.; de Oliveira, L.G.; de Lima, V.F.; da Silva, M.A.F.; Rodrigues, I.A.; Almeida, E.M. Cytotoxicity of essential oil Cordia verbenaceae against Leishmania brasiliensis and Trypanosoma cruzi. Molecules 2021, 26, 4485. [Google Scholar] [CrossRef]
- Farias, J.P.; Barros, A.L.A.N.; Araújo-Nobre, A.R.; Sobrinho-Júnior, E.P.C.; Alves, M.M.D.M.; Carvalho, F.A.D.A.; Rodrigues, K.A.F.; Andrade, I.M.; Silva-Filho, F.A.; Moreira, D.C.; et al. Influence of Plant Age on Chemical Composition, Antimicrobial Activity and Cytotoxicity of Varronia curassavica Jacq. Essential Oil Produced on an Industrial Scale. Agriculture 2023, 13, 373. [Google Scholar] [CrossRef]
- Rodrigues, F.F.G.; Costa, J.G.M.; Rios, K.A.; Carvalho, M.G.; Carvalho, F.A.A. Chemical composition, antibacterial and antifungal activities of essential oil from Cordia verbenacea DC leaves. Pharmacogn. Res. 2012, 4, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.P.; Venzon, M.; Dôres, R.G.R.; Franzin, M.L.; Martins, E.F.; Araújo, G.J.; Fonseca, M.C.M. Toxicity of Varronia curassavica Jacq. essential oil to two arthropod pests and their natural enemy. Neotrop. Entomol. 2021, 50, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Castro-Nizio, D.A.; Blank, A.F.; de Andrade Brito, F.; Gagliardi, P.R.; Alves, E.; Arrigoni-Blank, M.D.F. A comparative study of the antifungal activity of essential oils of Varronia curassavica Jacq. obtained by different distillation methods. Biosci. Res. 2020, 36, 1856–1865. [Google Scholar] [CrossRef]
- Rodrigues, F.A.F.; Silva, G.C.D.; Santana, M.F.; Bazzolli, D.M.S.; Rossi, C.C.; Diaz, M.A.N. Essential oils isolated from popular medicinal plants and spices as alternative antimicrobial and antibiofilm compounds against the pig pathogen Actinobacilluspleuro pneumoniae. Ciência Rural 2023, 53, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Oliveira, B.M.; Melo, C.R.; Santos, A.C.; Nascimento, L.F.; Nízio, D.A.; Cristaldo, P.F.; Blank, A.F.; Bacci, L. Essential oils from Varronia curassavica (Cordiaceae) accessions and their compounds (E)-caryophyllene and α-humulene as an alternative to control Dorymyrmex thoracius (Formicidae: Dolichoderinae). Environ. Sci. Pollut. Res. Int. 2019, 26, 6602–6612. [Google Scholar] [CrossRef]
- Matias, E.F.F.; Santos, K.K.A.; Almeida, T.S.; Costa, J.G.M.; Coutinho, H.D.M. Seasonal variation, chemical composition and biological activity of the essential oil of Cordia verbenacea DC (Boraginaceae) and the sabinene. Ind. Crops Prod. 2016, 87, 45–53. [Google Scholar] [CrossRef]
- Tonial, C.H.; Rodrigues, M.F.; Bosse, M.A.; Sousa, I.M.; Lima, J.D.; Cunha, M.A.A.; Foglio, M.A.; Marques, M.O.M.; Marchese, J.A. Technical and economic evaluation of cultivation and obtaining of Varronia curassavica Jacq. essential oil. Ind. Crops Prod. 2020, 154, 112650. [Google Scholar] [CrossRef]
- Lima, F.J.A.; Sousa, R.O.; Artur, F.; Araújo-Mendes, M.R.; Val, A.D.B. Characterization of the growth and production of essential oil from “erva baleeira” (Varronia curassavica Jaqc). Res. Soc. Dev. 2021, 10, e5810716204. [Google Scholar] [CrossRef]
- Meccia, G.; López, S.; Gutiérrez, J.; Echeverría, J.; Suárez, L.; Porras, N.; Delgado, G.; Stashenko, E.; Bazzocchi, I.L. Chemical composition and antibacterial activity of the essential oil of Cordia verbenacea from the Venezuelan Andes. Nat. Prod. Commun. 2009, 4, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.R.A.; Freitas, P.R.; Alencar Menezes, I.R.; Tintino, S.R.; Santos, E.L.; Lima, M.A.; Coutinho, H.D.M.; Junior, W.J.F.L. Antibiotic-Modifying Activity and Chemical Profile of the Essential Oil from the Leaves of Cordia verbenacea DC. J. Essent. Oil Bear. Plants 2017, 20, 337–345. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Tasir, S.; Seven, A.; Akgun, N.; Karbancioglu-Guler, F. Evaluation of the single and combined antibacterial efficiency of essential oils for controlling Campylobacter coli, Campylobacter jejuni, Escherichia coli, Staphylococcus aureus, and mixed cultures. Flavour Fragr. J. 2019, 34, 280–287. [Google Scholar] [CrossRef]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.R.; Araújo, A.C.J.; Santos-Barbosa, C.R.; Muniz, D.F.; Silva, A.C.A.; Rocha, J.E.; Oliveira-Tintino, C.D.M.; Ribeiro-Filho, J.; Silva, L.E.; Confortin, C.; et al. GC-MS-FID and potentiation of the antibiotic activity of the essential oil of Baccharis reticulata (ruiz & pav.) pers. and α-pinene. Ind. Crops Prod. 2020, 145, 112106. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Eduardo, L.S.; Silva, R.O.; Silva, K.M.; Moreira, G.O.; Costa, J.G.M.; Coutinho, H.D.M.; Almeida, V.M.; Menezes, I.R.A.; Tintino, S.R. Antibacterial activity and time-kill kinetics of positive enantiomer of α-pinene against strains of Staphylococcus aureus and Escherichia coli. Curr. Top. Med. Chem. 2018, 18, 917–924. [Google Scholar] [CrossRef]
- Yang, C.; Hu, D.-H.; Feng, Y. Antibacterial activity and mode of action of the Artemisia capillaris essential oil and its constituents against respiratory tract infection-causing pathogens. Mol. Med. Rep. 2015, 11, 2852–2860. [Google Scholar] [CrossRef]
- Leite-Sampaio, N.F.; Nogueira, C.E.S.; Silva, A.F.M.; Sampaio, B.L.; Andrade, L.N.; Fernandes, P.C.; Rocha, J.E.; Oliveira, M.R.; Quintans-Júnior, L.J.; Coutinho, H.D.M.; et al. Potentiation of the Activity of Antibiotics against ATCC and MDR Bacterial Strains with (+)-α-Pinene and (-)-Borneol. BioMed Res. Int. 2022, 2022, 8217380. [Google Scholar] [CrossRef]
- Utegenova, G.A.; Pallavi, R.; Fukin, A.M.; Kondratenko, E.; Bagryanskaya, I.Y.; Atazhanova, G.A.; Adekenov, S.M.; Zibareva, L.N.; Desenko, S.M.; Sharma, R.; et al. Chemical composition and antibacterial activity of essential oils from Ferula L. species against methicillin-resistant Staphylococcus aureus. Molecules 2018, 23, 1679. [Google Scholar] [CrossRef] [PubMed]
- Melkina, O.E.; Plyuta, V.A.; Khmel, I.A.; Zavilgelsky, G.B. The mode of action of cyclic monoterpenes (−)-limonene and (+)-α-pinene on bacterial cells. Biomolecules 2021, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Murakami, S.; Hayashi, S.; Koike, K.; Sengoku, T. Mouse brain concentrations of α-pinene, limonene, linalool, and 1,8-cineole following inhalation. Flavour Fragr. J. 2016, 32, 36–39. [Google Scholar] [CrossRef]
- Khoshnazar, M.; Parvardeh, S.; Bigdeli, M.R. Alpha-pinene exerts neuroprotective effects via anti-inflammatory and anti-apoptotic mechanisms in a rat model of focal cerebral ischemia-reperfusion. J. Stroke Cerebrovasc. Dis. 2020, 29, 104977. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, M.; Mahardika, A.M.; Fattah, F.A.; Krisnadi, D. Alpha-pinene attenuates microglial NF-κB activation and iNOS expression in gp120-induced neuroinflammation. Malang Neurol. J. 2021, 7, 80–84. [Google Scholar] [CrossRef]
- Rahimi, K.; Ghanbari, A.; Badi, H.N.; Asadi, F.; Sadeghi, S.; Azadi, H. The effects of alpha-pinene on the Nrf2-HO1 signaling pathway in gastric damage in rats. Mol. Biol. Rep. 2023, 50, 8615–8622. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Tang, F.-D.; Mao, G.-G.; Bian, R.-L. Effect of alpha-pinene on nuclear translocation of NF-kappa B in THP-1 cells. Acta Pharmacol. Sin. 2004, 25, 480–484. [Google Scholar]
- Yanaka, A. Role of NRF2 in protection of the gastrointestinal tract against oxidative stress. J. Clin. Biochem. Nutr. 2018, 63, 18–25. [Google Scholar] [CrossRef]
- Santos, E.L.; de Lima, M.A.; de Almeida, R.S.; de Menezes, I.R.A.; Tintino, S.R.; Rodrigues, M.A.V.; Rocha, J.E.; Júnior, W.J.F.L. Phytochemical characterization and antibiotic potentiating effects of the essential oil of Aloysia gratissima (Gillies & Hook.) and beta-caryophyllene. S. Afr. J. Bot. 2021, 143, 1–6. [Google Scholar] [CrossRef]
- Santos, E.L.; de Lima, M.A.; de Almeida, R.S.; de Menezes, I.R.A.; Tintino, S.R.; Rodrigues, M.A.V.; Rocha, J.E.; Júnior, W.J.F.L. Enhanced antibacterial effect of antibiotics by the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. and its major constituent beta-caryophyllene. Phytomed. Plus 2021, 1, 100100. [Google Scholar] [CrossRef]
- Yoo, H.-J.; Jwa, S.-K. Inhibitory effects of β-caryophyllene on Streptococcus mutans biofilm. Arch. Oral Biol. 2018, 88, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Moo, C.L.; Yang, S.K.; Osman, M.A.; Yuswan, M.H.; Loh, J.Y.; Lim, W.M.; Lim, S.H.E.; Lai, K.S. Antibacterial Activity and Mode of Action of β-caryophyllene on Bacillus cereus. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef] [PubMed]

| Multi-Resistant Bacterial Strains | Origin/Source | Resistance Profile | Reference |
|---|---|---|---|
| Escherichia coli 06 | Urine | Cephalexin, cefoxitin, cefadroxil, ceftriaxone, cefepime, and ampicillin/sulbactam. | [23] |
| Pseudomonas aeruginosa 24 | Urine culture | Amikacin, imipenem, ciprofloxacin, levofloxacin, piperacillin/tazobactam, ceftazidime, merpenem, and cefepime. | |
| Staphylococcus aureus 10 | Rectal swab culture | Cefadroxil, cephalexin, cephalothin, oxacillin, penicillin, ampicillin, amoxicillin, moxifloxacin, ciprofloxacin, levofloxacin, ampicillin/sulbactam, amoxilin/clavulanic acid, erythromycin, clarithromycin, azithromycin, and clindamycin. |
| Components | RI | (%) |
|---|---|---|
| α-Pinene | 976 | 42.26 |
| β-Pinene | 980 | 2.82 |
| β-Elemene | 1375 | 1.13 |
| β-Caryophyllene | 1428 | 22.08 |
| α-humulene | 1460 | 2.77 |
| Zingiberene | 1492 | 1.16 |
| Biciclogermacrene | 1496 | 13.24 |
| cis-α-Bisabolene | 1778 | 2.87 |
| Nerolidol | 1961 | 3.26 |
| Caryophyllene oxide | 2023 | 1.92 |
| Juniper Camphor | 2205 | 1.15 |
| Hydrocarbon Monoterpene | 45.08 | |
| Oxygenated Monoterpene | 1.15 | |
| Hydrocarbon Sesquiterpene | 43.25 | |
| Oxygenated Sesquiterpene | 5.18 | |
| Total (%) | 94.66% | |
| Descriptor | Value |
|---|---|
| Molecular formula | C10H16 |
| Molecular weight | 136.238 |
| LogP | 2.9987 |
| Number of rotatable bonds | 0 |
| Number of hydrogen acceptors | 0 |
| Number of hydrogen donors | 0 |
| Surface area | 63.322 |
| Property | Model Name | Predicted Value |
|---|---|---|
| Absorption | Water solubility | −3.733 mol/L |
| Caco2 permeability | 1.38 × 10−6 cm/s | |
| Intestinal absorption (human) | 96.041 | |
| Skin permeability (log Kp) | −1.827 | |
| P-glycoprotein substrate | No | |
| P-glycoprotein I inhibitor | No | |
| P-glycoprotein II inhibitor | No | |
| Distribution | VDss (human) | 0.667 L/kg |
| Fraction unbound (human) | 0.425 | |
| BBB permeability | 0.791 | |
| CNS permeability | −2.201 | |
| Metabolism | CYP2D6 substrate | No |
| CYP3A4 substrate | No | |
| CYP1A2 inhibitor | No | |
| CYP2C19 inhibitor | No | |
| CYP2C9 inhibitor | No | |
| CYP2D6 inhibitor | No | |
| CYP3A4 inhibitor | No | |
| Excretion | Total clearance | 0.043 mL/min/kg |
| Renal OCT2 substrate | No | |
| Toxicity | AMES toxicity | No |
| Max. tolerated dose (human) | 0.48 mg/kg/day | |
| hERG I inhibitor | No | |
| hERG II inhibitor | No | |
| Oral rat acute toxicity (LD50) | 1.77 mol/kg | |
| Oral rat chronic toxicity (LOAEL) | 2.262 | |
| Hepatotoxicity | No | |
| Skin sensitization | No | |
| T.Pyriformis toxicity | 0.45 ug/L | |
| Minnow toxicity | 1.159 mM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.T.d.C.; Silva, V.B.d.; Silva, S.B.d.; Rocha, M.I.; Costa, A.R.; Silva, J.R.d.L.; Santos, M.A.F.d.; Generino, M.E.M.; Souza, J.H.d.; Oliveira, M.G.d.; et al. Chemical Characterization and Biological Activity of Varronia curassavica Jacq. Essential Oil (Boraginaceae) and In Silico Testing of α-Pinene. Analytica 2024, 5, 499-511. https://doi.org/10.3390/analytica5040034
Silva JTdC, Silva VBd, Silva SBd, Rocha MI, Costa AR, Silva JRdL, Santos MAFd, Generino MEM, Souza JHd, Oliveira MGd, et al. Chemical Characterization and Biological Activity of Varronia curassavica Jacq. Essential Oil (Boraginaceae) and In Silico Testing of α-Pinene. Analytica. 2024; 5(4):499-511. https://doi.org/10.3390/analytica5040034
Chicago/Turabian StyleSilva, José Thyálisson da Costa, Viviane Bezerra da Silva, Sabrina Bezerra da Silva, Maria Ivaneide Rocha, Adrielle Rodrigues Costa, Jailson Renato de Lima Silva, Marcos Aurélio Figueirêdo dos Santos, Maria Elizete Machado Generino, Jeovane Henrique de Souza, Maraiza Gregorio de Oliveira, and et al. 2024. "Chemical Characterization and Biological Activity of Varronia curassavica Jacq. Essential Oil (Boraginaceae) and In Silico Testing of α-Pinene" Analytica 5, no. 4: 499-511. https://doi.org/10.3390/analytica5040034
APA StyleSilva, J. T. d. C., Silva, V. B. d., Silva, S. B. d., Rocha, M. I., Costa, A. R., Silva, J. R. d. L., Santos, M. A. F. d., Generino, M. E. M., Souza, J. H. d., Oliveira, M. G. d., Lima, C. M. G., Pereira, R. L. S., Santana, R. F., Araujo, I. M., Morais-Braga, M. F. B., Emran, T. B., Coutinho, H. D. M., & Almeida-Bezerra, J. W. (2024). Chemical Characterization and Biological Activity of Varronia curassavica Jacq. Essential Oil (Boraginaceae) and In Silico Testing of α-Pinene. Analytica, 5(4), 499-511. https://doi.org/10.3390/analytica5040034










