1. Introduction
Feed is the most important factor in animal production, accounting for over 50% of the production costs. Thus, it is natural that it is one of the aspects that deserves the most attention in the effort to make the best use of available resources [
1]. For ruminants, pasture is the predominant food source, with forage crops also being preserved, for example, as silage, for use in periods of greater scarcity of food. Monitoring forage quality, whether pasture or silage, thus becomes an important vehicle for pasture management and the design of diets that maximize performance, animal welfare and longevity [
2,
3].
Until the 1860s, the assessment of the nutritional value of foodstuff was based on the concept of hay equivalent, a subjective method that used a standard hay as a reference [
4]. In 1865, Wilhelm Henneberg and Friedrich Stohmann defined a chemical system of analysis that quantified the nutritional value of foods in a more systematic manner. This system became known as the Weende System [
4], and has since been complemented and improved, with important qualitative leaps taking place a century later when, in the 1960s, Peter van Soest proposed an alternative approach for determining biological parameters related to food digestibility based on a system of detergent solutions for food analysis, and Tilley and Terry presented in vitro methods. Yet, all these developments rely on laboratory analysis, which tends to be impractical, time-consuming and expensive, with possible negative impacts on the environment due to the chemicals they rely on. It is, therefore, difficult to set up a quality monitoring system that provides results on a regular, timely, and affordable basis [
5].
It was in this context that, in the 1970s, the application of NIR spectroscopy appeared. Since then, it has been investigated in combination with chemometric methods for obtaining quick estimates, and with good precision of various food parameters [
6]. At the limit, this method only requires the application of a light beam on the sample under analysis to obtain the desired results almost instantaneously. To reach this level, it is necessary first carry out a calibration process, whereby a relation is established between the absorbance of the light beam by the sample in the near-infrared zone and the properties of interest [
7]. This calibration is sensitive to the agroclimatic particularities where the samples are collected. This means that, to obtain the best possible results, it is important that the calibration obey some criteria and be performed with samples from similar agroclimatic zones.
This paper presents an overview of the historical context in which NIR spectroscopy emerged and its evolution in agriculture and subsequently in animal nutrition, as well as insights into the basic principles of NIR spectroscopy and associated calibration techniques.
2. A Consistent Evolution in Agriculture
In 1983, Professor David Wetzel wrote an article for the journal Analytical Chemistry in which he described near-infrared spectroscopic (NIR) analysis as a “sleeping technique” [
8]. Decades later, NIR spectroscopy has rapidly developed into an important and extremely useful method of analysis not only in the field of animal nutrition.
After the foundations established during the 19th century, including the first publication on infrared radiation by W. Herschel (1800) [
9], the first half of the 20th century saw the emergence of several papers that increase the knowledge about the spectra of organic compounds and extend the use of NIR spectroscopy to quantitative analysis.
In 1960, Karl Norris used NIR spectroscopy to measure moisture in grains and seeds [
10], initiating the development of analytical methods based on NIR spectroscopy, which were deepen during the 1970s by the American Department of Agriculture (USDA), and whose success drew the attention of several other industries [
11].
The first practical applications of NIR, by Karl Norris and Phil Williams, were performed by the American Department of Agriculture, in the 1960s and 1970s. The application, by Williams, of NIR spectroscopy to the rapid analysis of wheat shipments in Canada during that period enabled NIR spectroscopy to be brought into the limelight, making it a feasible option for the large-scale monitoring of production and transport quality [
12]. Later, the studies of John Shenk and others on the application of NIR spectroscopy to the analysis of forages contributed to the constitution of a forage network in the United States in 1978 [
13]. This process promoted the marketing of forages and prediction of animal feed requirements based on analytical data derived from NIR.
In the last three decades, NIR spectroscopy has been consolidated as a tool capable of ensuring the close monitoring of the quality of the resources most involved in agricultural production (
Figure 1).
Not only is the application of NIR spectroscopy more efficient and capable of determining multiple parameters in minutes, as opposed to the scale of weeks of traditional methods, but a new generation of portable instruments has allowed these analyses to be less and less dependent on specialized, necessarily one-off laboratory work, allowing them to be applied efficiently and repeatedly to concrete samples. This opens the door to close the monitoring of all aspects that influence production [
14]. It is now commonplace to resort to NIR spectroscopy for the analysis, sometimes in the context of systems with dozens or hundreds of NIRS instruments operating in a network, of such diverse dimensions as soil properties [
15], crops, forage and feces [
16], as well as agricultural products such as fruit [
17], dairy [
18], and meat [
19,
20], among others.
3. Basic Principles of NIR Spectroscopy
The term spectroscopy is applied to analytical methods in which the interaction of electromagnetic radiation with molecules is studied [
21].
The region of the electromagnetic spectrum called infrared is situated after the visible region and comprises radiation with a wavelength between 780 and 1 mm (1,000,000 nm). The infrared region is further subdivided into three sub-regions: FIR (far infrared), MIR (middle infrared) and NIR (near infrared). Different chemical components have been found to absorb light differently in each of the three regions, due to differences in the interaction between light and matter [
22,
23]. It is important to note that the term “NIR” can vary in definition depending on the context. In physics, NIR typically refers to wavelengths between 780 nm and 2500 nm [
23], while in chemical and biological applications, slightly different ranges might be used [
6,
21]. These variations can influence how NIR spectroscopy is applied in specific fields, including animal nutrition.
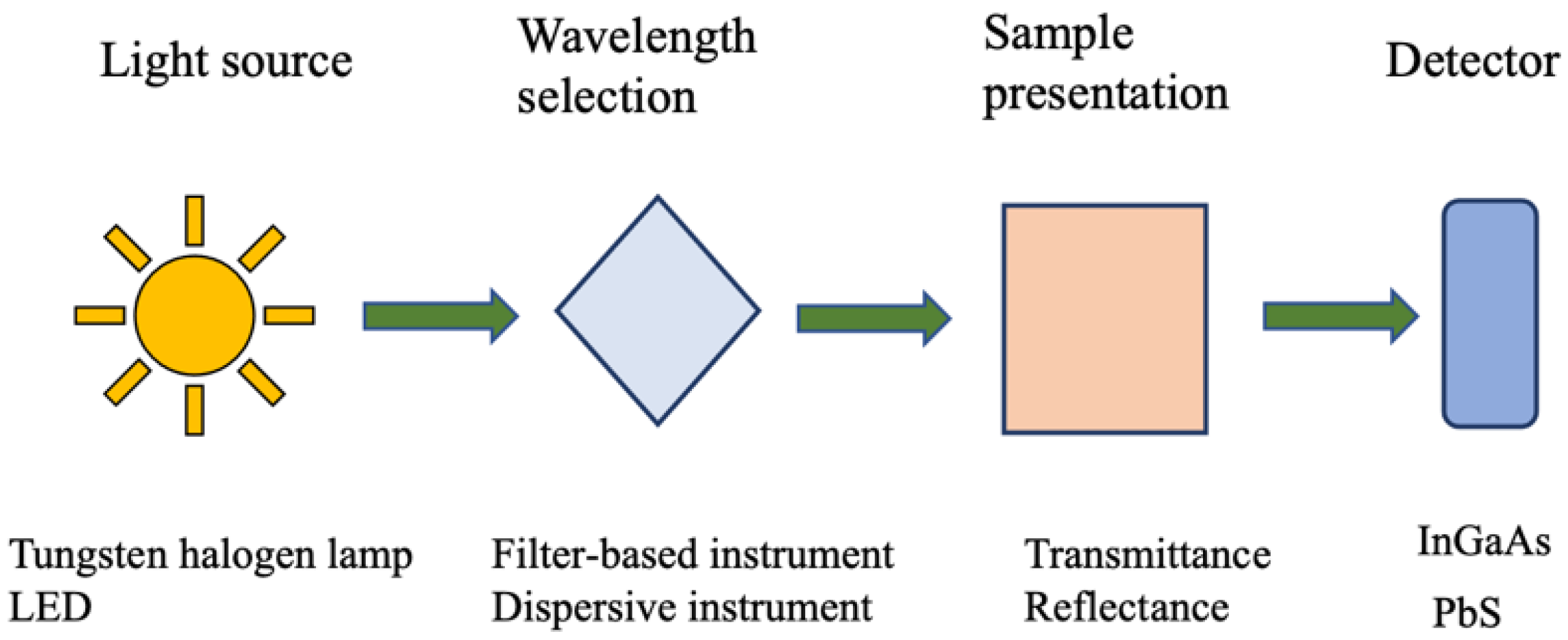
3.1. Instrumentation
NIR instruments used for assessing animal nutrition typically consist of a light source, a sample holder, a detector, and a monochromator. The first NIR instrument, developed by William Herschel, used thermometers to detect radiation in this spectral region [
11]. Modern NIR instruments were significantly advanced during World War II for military purposes, but their potential in analyzing organic materials was fully realized later by Karl Norris, who overcame the initial skepticism of chemists [
11,
24].
In current applications, NIR instruments share a fundamental structure but have evolved to become smaller, more portable, and more efficient due to recent technological advancements [
25,
26].
All optical instruments consist of at least three components:
A light source capable of emitting radiation at the desired wavelengths;
A suitable position to place the sample;
A detector, which may be instrumental.
For an instrument to function as a spectrophotometer, it must also include a monochromator to isolate specific wavelengths, allowing for the construction of a spectrum by measuring light at different wavelengths [
11]. This process, repeated across the region of interest, produces the sample’s spectrum, which is crucial for analysis (
Figure 2).
Today, various configurations of NIR spectrophotometers exist, all based on this core structure. Differences in how light interacts with the sample can affect the level of sample damage, especially for light-sensitive materials [
27,
28,
29].
3.1.1. Light Source
In NIR spectroscopy, the most common light source is a tungsten halogen lamp, which uses thermal radiation generated by heating a tungsten filament within a halogen gas-filled casing. This design prevents the filament from darkening, ensuring a stable and durable light output [
11,
28,
30].
Alternatively, light-emitting diodes (LEDs) are increasingly used, especially in portable instruments due to their low cost and energy efficiency. LEDs, made from materials like gallium arsenide (GaAs), offer specific emission peaks in the near-infrared region [
28,
31].
The most advanced light sources are tunable diode lasers, also known as superluminescent LEDs (SLEDs). These are compact, cost-effective, and provide excellent wavelength resolution, making them ideal for high-precision measurements and use in miniature NIR instruments [
32].
3.1.2. Wavelength Selection
Of the components that make up a spectrophotometer, the wavelength selection system is the one with the most varied technical solutions [
11]. The choice of technique to be used in combination with a given instrument is determined by the intended cost of the device, the level of requirement of spectral resolution, and the need for mobility, among other things. The simplest form of wavelength selector is an interference filter, which involves a non-dispersive technique, and it consists primarily of a sheet of glass, quartz, or other transparent materials [
33].
In a dispersive spectrophotometer, the different wavelengths of radiation are spatially separated [
34]. A classical approach, which is still employed today, is the use of a prism [
11]. A typical diffraction grating consists of a substrate of optical material with a large number of parallel grooves arranged on a planar surface, covered with a reflective material, such as aluminum or gold, capable of separating light into its constituent wavelengths. A prototype was first designed by Fraunhofer in 1814, and diffraction gratings have been commercially available since 1945 [
11]. While the spectral characteristics of a prism depend on its physical properties (refractive index), those of a diffraction grating are determined only by its geometric structure [
11], making this technique more versatile and more commonly applied.
Beyond these traditional methods, other sophisticated techniques such as Fourier Transform, Linear Variable Filter (LVF), and Fabry–Perot etalon are also used for wavelength selection. Fourier Transform instruments are known for their high spectral resolution and ability to capture the entire spectrum in a single measurement, making them suitable for a broad range of applications. LVFs offer compactness and efficiency, often being used in miniaturized devices for their ability to selectively transmit a narrow range of wavelengths. A Fabry–Perot etalon, on the other hand, provides high spectral resolution and is particularly valuable in applications requiring fine spectral detail. Each of these methods offers distinct advantages depending on the application and required precision, as reviewed in [
35].
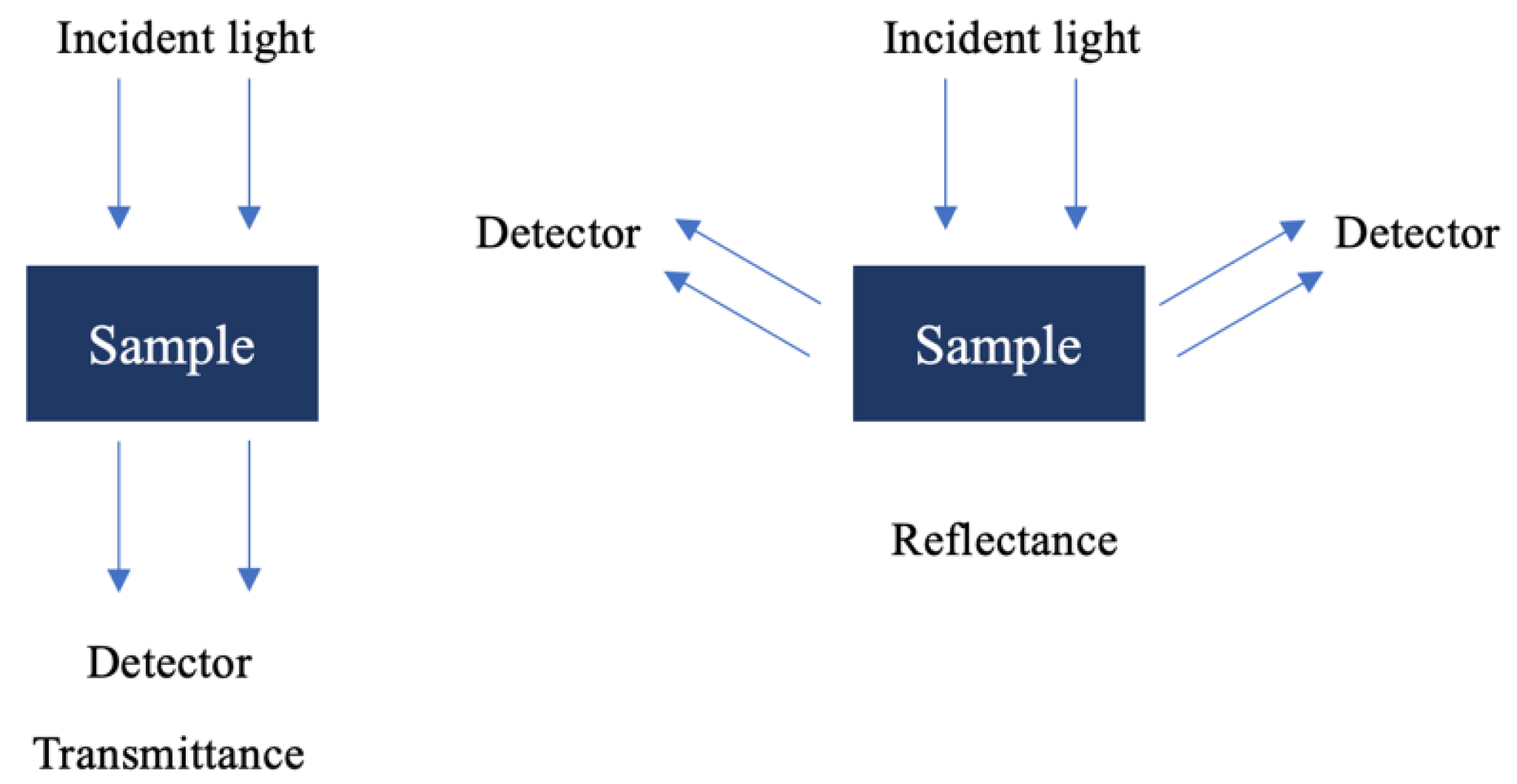
3.1.3. Sample Analysis Methods
One of the great advantages of NIR spectroscopy is its adaptability to the analysis of different types of samples without the need for complex pre-treatments to record the spectrum. Therefore, the versatility of a particular instrument depends largely on the type of accessories that can be fitted to the apparatus. The accessories can be divided into two types, depending on the type of sample to be studied: transmittance (T) and reflectance (R). The units of the absorbance spectra (A) are given by A = Log(1/T) and A = Log(1/R), respectively [
36].
Under the transmittance method (
Figure 3), light passes through the sample to the detector, making it ideal for application to transparent samples such as gases, liquids, and some thin solids [
33]. When transmittance is not feasible, reflectance (
Figure 3) techniques are used, often through diffuse reflection rather than Attenuated Total Reflectance (ATR), which is typically employed in the mid-infrared (MIR) region. Diffuse reflection measures the decrease in light intensity as it interacts with the sample, providing insights into the sample’s absorbance characteristics.
3.1.4. Detectors
Early NIR detectors, like thermionic valves, have largely been replaced by semiconductor-based detectors due to their smaller size, lighter weight, lower cost, and operability without high voltages [
11]. These modern detectors, however, vary in type since no single detector can cover the entire NIR range [
37].
Silicon (Si) photodiodes, sensitive to the 700–1100 nm range, are commonly used for quantitative analysis, especially for samples with high water content, such as in measuring sugar content in fruits. They require filters to minimize interference from visible light [
11].
Gallium indium arsenide (InGaAs) detectors, which operate within the 900–1600 nm range and can extend up to 2600 nm, are noted for their rapid response, making them suitable for high-speed measurements [
38].
Lead sulfide (PbS) detectors, sensitive to the 900–2500 nm range, are widely used in NIR spectrometers. However, their performance depends on consistent light exposure across the sample area, necessitating careful design to ensure a uniform beam diameter [
37].
4. Calibration Model Development
Calibration is the process by which a mathematical relationship is established between the values obtained by a measuring instrument for a set of samples and previously known values for the same samples [
39]. These values could be the physical and chemical properties of the sample [
40,
41]. The mathematical expression that establishes this relationship is known as the calibration equation [
42].
The calibration process (
Figure 4) starts with the determination of reference values, using conventional methods, for a set of selected samples called the calibration set.
4.1. Reference Values, Spectrum Data
NIR spectroscopy can provide rapid and highly consistent results, offering precision and accuracy comparable to traditional wet chemistry methods [
43,
44]. However, it is important to note that NIRS calibrations are initially dependent on the accuracy of the reference values provided by these traditional methods. Therefore, the first step to a successful calibration is the careful selection of a suitable calibration set [
43]. The importance of this step is often underestimated and is not sufficiently addressed in the literature. An ideal calibration set should cover the chemical, spectral and physical characteristics of the analyte population and avoid future extrapolations when predicting new samples [
45].
There is no fixed number or rule used to determine the number of samples to include in a calibration set, but the most robust calibrations may use a few hundred samples, since when we talk about, for example, forage samples, we are talking about samples of high complexity and heterogeneity of composition [
32].
Most of the absorption bands in the near-infrared region are overtones or combinations of fundamental absorption bands in the infrared region of the electromagnetic spectrum, due to vibrational and rotational transitions [
6]. As these types of bonds are frequently observed in organic matter, NIR spectroscopy becomes particularly well suited to the analysis of the chemical composition of forages [
28].
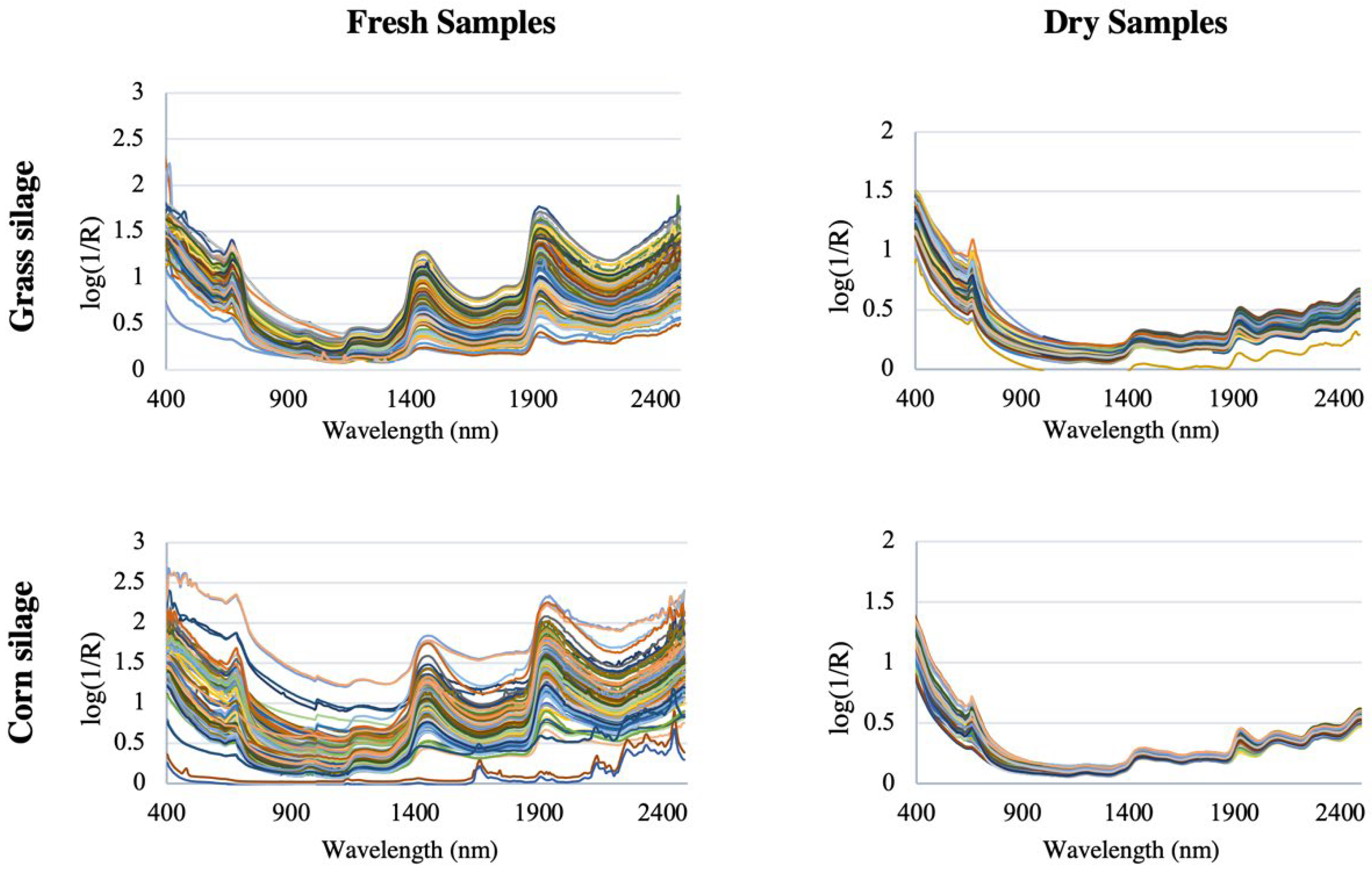
Figure 5 shows the spectra of grass silage and corn silage for both dry and therefore homogenized samples, and for fresh non-homogenized samples, from a study of 400 grass silage samples and 400 corn silage samples [
46]. The spectra are clearly very similar and are dominated by the water spectrum, with overtone bands of OH bonds at 1450 nm and a combination band at 1950 nm, which corresponds to the moisture content [
47,
48]. It is also possible to see the most intense peaks in fresh and therefore non-homogenized samples [
49].
Thus, inhomogeneous particles (for example, due to significant variations in their degree of compaction and size or in their surface finish) can lead to a misaligned baseline [
50] and cause significant differences between the spectra. Not surprisingly, dried and ground samples are much more homogeneous than fresh ones, so the baseline is naturally closer. Obtaining a spectrum is especially complicated due not only to the inhomogeneity of the sample, but also to wavelength-dependent scattering effects, instrumental noise, environmental effects, and other sources of variability. Consequently, it is difficult to assign specific absorption bands to particular functional groups, let alone chemical components [
51]. Multivariate statistical techniques (also called chemometrics) are therefore needed to extract the information about quality attributes that is hidden in the NIR spectrum (“model calibration”). Chemometrics, a term widely used in NIRS-related literature, has made it possible to treat NIR units at the resolution of broad, highly overlapping peaks, with high sensitivity to physical characteristics of the sample, and high redundancy of information [
52].
Essentially, this involves regression techniques coupled with spectral pre-treatments [
53].
This only illustrates the importance of pre-treatments in obtaining consistent results for varied samples.
4.2. Data Pre-Processing
Data pre-processing is crucial in NIR spectroscopy to enhance the quality of spectral data before applying multivariate models [
54]. The main goal is to remove systematic variations so that the spectral signals adhere more closely to Beer–Lambert’s law, which relates the concentration of constituents in a sample to the absorbance of light (1):
where
Aλ is the absorbance at wavelength
λ,
ελ is the absorbance coefficient of the constituent material at that wavelength, l is the length of the path through the sample, and c is the concentration of the constituent of interest. What is intended is that the ελ × l term should be as close as possible to a constant for the entire dataset, so that the relationship between Aλ and c tends to linearity [
55].
The most common NIR spectra-pre-processing techniques fall into two broad groups [
55]: (1) scatter correction methods, e.g., the Multiplicative Scatter Correction (MSC), and the Standard Normal Variate (SNV); and (2) spectral derivatives, e.g., the Savitzky–Golay algorithm. Knowing the available pre-processing steps and their purpose is therefore essential to obtaining the best possible results from multivariate analysis.
Data Pre-Processing Techniques
Data pre-processing in NIR spectroscopy is essential to improve the quality of the spectral data by removing systematic variations and ensuring that the data adhere to Beer–Lambert’s law. Common techniques include mean centering and autoscaling, where the average spectrum is subtracted from each individual spectrum, and the data are then scaled to ensure equal variance across all variables [
21,
56]. Another widely used method is Multiplicative Scatter Correction (MSC), which corrects for nonlinearities in the data caused by variations in sample density or light scattering. MSC uses reference spectra to estimate correction coefficients, although it can be sensitive to outliers [
57,
58,
59]. Similarly, the Standard Normal Variate (SNV) method normalizes each spectrum individually, offering robustness against data inconsistencies without requiring a reference spectrum [
60,
61].
Baseline correction is another crucial pre-processing step, aiming to eliminate the baseline effects introduced by the spectrophotometer, ensuring the spectrum accurately represents constituent absorption. Techniques like de-trending [
60] and more sophisticated methods such as wavelets [
62] and iterative polynomial baseline fitting (IPBF) are employed for this purpose. Additionally, applying derivatives, such as those calculated by the Savitzky–Golay (SG) algorithm [
55,
63], helps enhance spectral resolution by removing additive and multiplicative effects, emphasizing peaks, and reducing baseline effects [
33,
64].
4.3. Multivariate Regression
In NIR spectroscopy, multivariate calibration is essential for accurately predicting multiple parameters of a sample from complex spectral data. This calibration typically uses multiple linear regression techniques, which build models by approximating the value of each dependent variable (concentrations) as a function of the independent variables (absorbance measurements). The general equation for this model (in matrix form) (2) is as follows:
where
is the vector of the regression coefficients, the target parameters to be determined,
X is the matrix with the spectra for the various samples, and
Y refers to the concentrations determined for the same samples [
56]. However, applying direct multiple linear regression in NIR spectroscopy can give rise to issues due to multicollinearity among the spectral variables. To address these limitations, techniques such as Principal Component Regression (PCR) and Partial Least Squares Regression (PLSR) are widely used. These techniques reduce the dimensionality of the data by compressing the spectral variables into a smaller set of independent variables (factors) without a significant loss of information, facilitating the creation of more robust and accurate models [
65,
66,
67].
4.3.1. Principal Component Regression (PCR) and Partial Least Squares Regression (PLSR)
Principal Component Regression (PCR) and Partial Least Squares Regression (PLSR) are two widely used techniques for multivariate calibration in NIR spectroscopy, particularly when dealing with complex spectral data. PCR performs regression on principal components derived from Principal Component Analysis (PCA), aiming to reduce the dimensionality of the data by selecting a set of vectors that best explain the variance in the original matrix X. However, a limitation of PCR is that these principal components may not be the most effective for predicting concentrations, often leading to less reliable results compared to PLSR [
51,
68,
69].
PLSR, on the other hand, is specifically designed to handle high collinearity in the data by simultaneously decomposing the spectral data X and the concentration matrix Y. This approach ensures that the most significant variance in both matrices is captured, directly linking the spectral data to the constituent concentrations during the calibration process. This makes PLSR generally more robust and accurate [
70] for use in predictive modeling in NIR applications, despite its increased complexity compared to PCR. As a result, PLSR is often preferred for use in building calibration models with higher predictive capacity [
41,
56,
71,
72].
4.3.2. Outlier Detection
Outliers, which are data points that significantly deviate from the majority, can bias regression results if not properly identified and addressed, making their detection crucial in calibration processes. Commonly, 5 to 10% of calibration data may be outliers, arising from legitimate variations or errors in sample handling, instrument performance, or environmental conditions [
39]. A popular method for detecting outliers is the Mahalanobis distance, which calculates how far a data point is from the cluster mean, adjusting for variance. Points falling more than three standard deviations from the mean are typically flagged as potential outliers [
41].
4.4. Model Fit
To assess how well a model fits a data set, several statistical indicators are commonly used, such as the Determination Coefficient (R
2), Standard Error of Calibration (SEC), and Root Mean Squared Error (RMSE). These indicators, however, primarily reflect the model’s performance on the calibration set, and do not necessarily guarantee good predictive ability for new samples. Therefore, while these metrics are important for understanding how well a model explains the calibration data, they should be complemented with other methods to evaluate the model’s predictive accuracy on independent data sets [
46].
4.5. Validation
The last phase of the calibration process is the validation of the produced model. Validation is in essence a process of gauging the predictive ability of the model against new samples [
51]. The validation process consists, fundamentally, in testing the performance of the model against samples that were not used in the calibration. Depending on the number of samples available, this may imply either a set of samples used only for validation (external validation) or the alternate use of the same samples for validation and calibration (internal validation).
External validation relies on the explicitness of a validation set and is the simplest and most robust solution [
73] to validate a model. Given a sample universe, a subset is chosen to be used for calibration (typically 2/3 of the available samples) and the rest is used in the validation process.
Sometimes, however, the number of samples available is not large enough to make it feasible to separate it without loss of representativeness in any of the sets. In that case, we can resort to the so-called internal or cross-validation [
11], whereby each sample can be used for both calibration and validation.
This predictive ability can be assessed using the Standard Error of Prediction (SEP), which involves nothing more than applying the SEC formula over the validation set.
Once a value for the SEP has been determined, the Residual Prediction Deviation (RPD), originally introduced by [
74], can be calculated to assess the quality of an NIR calibration in the context of agriculture and food products (3), as follows:
where
SD is the standard deviation of the data obtained by conventional analysis for all samples. Williams indicated that values above 3 allow the use of calibration in practical applications [
74].
Once a robust calibration model is developed, it should be regularly validated and update to ensure model prediction performance [
75].
5. NIR Spectroscopy in Animal Nutrition: Bridging Technical Advances with Practical Implementation
The performance and health of ruminant animals directly depend on the quality of their nutrition, which, if inadequate, can limit their genetic potential [
76]. Forages, both fresh and preserved, are the cornerstone of ruminant production systems worldwide [
2,
3], with their nutritional value being determined by both their nutritive value and the animals’ voluntary intake propensity [
77]. The ability to chemically characterize forages is therefore a crucial tool for farmers aiming to maximize production potential [
78].
5.1. Evolution of NIRS Applications in Forage Analysis
Initially, forage analysis required labor-intensive, time-consuming, and expensive laboratory methods that were not always accessible. The advent of NIR spectroscopy, however, has significantly democratized this capability. Norris’ pioneering work [
79] marked the beginning of modern forage analysis using NIRS, successfully predicting the in vitro and in vivo digestibility of forages. Since then, NIR spectroscopy has been widely adopted for determining a broad range of chemical parameters across various forages, including grass silages [
77,
80,
81,
82,
83], grains, and pastures [
84,
85]. The tables included (
Table 1 and
Table 2) summarize the studies and parameters analyzed, highlighting the broad applicability and effectiveness of NIRS in this field.
5.2. Critical Evaluation of NIRS in Forage Analysis
NIR spectroscopy has revolutionized forage analysis by providing rapid, non-destructive assessments of key nutritional parameters. However, its effectiveness heavily relies on the quality of the calibration models, which must be robust and representative of real-world forage variability. As shown in
Table 1 and
Table 2, prediction accuracy varies significantly, depending on the forage type and chemical parameter, with R
2 values and SEP/SECV indicating the degree of precision achieved.
For example, predicting crude protein (CP) content in corn silage consistently yields high R
2 values, often above 0.90 [
91], reflecting the ease of calibrating this parameter due to its stable spectral signature. Similarly, acid detergent fiber (ADF) is relatively straightforward to calibrate, with similarly high R
2 values [
88]. On the other hand, predicting neutral detergent fiber (NDF) is more challenging due to its greater susceptibility to sample heterogeneity and environmental factors, which can lead to variability in calibration results. For instance, while some forages provide satisfactory R
2 values for NDF prediction, others show lower accuracy, necessitating more extensive calibration datasets and frequent model updates.
More complex or less homogeneous parameters, such as pH, present even greater calibration challenges. In corn silage, for example, the prediction of pH yielded a much lower R
2 of 0.51 [
91], highlighting the difficulty of developing reliable calibration models for such parameters.
This variability underscores the importance of continuously refining and validating NIRS calibration models to ensure their accuracy over time. Calibration models must be updated regularly as new forage types and environmental conditions emerge. Accurate calibration equations are foundational to the reliability of NIRS predictions, particularly for straightforward parameters like CP and ADF. For more challenging parameters like pH, frequent recalibration and a diverse calibration dataset are essential to accommodate the full range of variability encountered in practical applications.
In summary, while NIR spectroscopy offers significant advantages in forage analysis, its success depends on the ongoing refinement and maintenance of calibration models.
5.3. Integration with Precision Agriculture
Beyond traditional laboratory applications, NIR spectroscopy is increasingly integrated with precision agriculture technologies. The miniaturization of NIR devices has enabled real-time, on-site analysis, significantly enhancing the efficiency and practicality of forage quality monitoring. These advancements are transforming forage management, allowing rapid and precise adjustments to animal diets based on data collected directly in the field.
6. Challenges and Limitations of NIR Spectroscopy in Animal Nutrition
Despite the numerous advantages of NIR spectroscopy, several challenges and limitations must be considered to ensure the accuracy and effectiveness of analyses in animal nutrition. Below are detailed the main challenges and limitations associated with the use of NIRS in this field.
Sample variability is one of the main challenges in the application of NIRS. Factors such as forage type, maturity stage, harvesting conditions, and storage can significantly influence NIR spectra and, consequently, prediction accuracy. Intra-sample variability can also be high, especially in heterogeneous forages. Including a wide variety of samples in the calibration database can help improve the robustness of predictive models. Representative samples from different conditions and variabilities should be collected to create a comprehensive calibration [
6].
The proper calibration of NIR instruments is crucial for obtaining accurate results. However, the need for specific calibrations for different types of samples and agro-climatic conditions can be a significant challenge. Additionally, frequent calibrations are needed to maintain the accuracy of the instruments over time [
52]. Parameters that are difficult to calibrate include lignin and mineral components at low levels [
102]. Developing and maintaining specific calibrations for different regions, types of forages, and environmental conditions is essential. Using advanced calibration techniques, such as transferable calibration, can help improve accuracy and reduce the frequency of recalibrations.
NIRS is sensitive to physical variations in samples [
46], such as density and compaction. These variations can affect NIR light penetration and, consequently, measurement accuracy. Standardizing sample preparation methods to ensure consistency in density and compaction and using appropriate measurement techniques that consider the physical variations of samples are crucial.
Multivariate regression models are essential for interpreting NIRS data. However, the accuracy of these models depends on the quality and representativeness of the samples used for calibration. The continual updating and validation of models with new samples are also crucial.
Integrating NIRS with agricultural and livestock management systems can be complex. Synchronizing NIRS data with other management data such as weather information and productivity data is necessary for comprehensive analysis. Developing integrated software platforms that can collect, analyze, and interpret data from multiple sources is essential. Adopting precision agriculture technologies can facilitate this integration.
Overcoming these challenges and limitations is essential to maximize the potential use of NIR spectroscopy in animal nutrition. With the continuous advancement of technologies and calibration methods, NIRS can offer an increasingly accurate, efficient, and accessible solution for analyzing forages and feeds. Ongoing research and technological innovation are crucial order to address these limitations and expand the applications of NIRS in the future.
7. Concluding Remarks and Future Perspectives
NIR spectroscopy, a technique that has significantly evolved since its origins in the 19th century, is now widely recognized for its diverse applications in science and, more specifically, in animal nutrition. Initially, NIR technology was limited to benchtop spectrometers in laboratory environments. However, over the past decade, it has expanded to include compact and portable devices that are revolutionizing analytical practices in the field. Despite these promising advancements, challenges remain, particularly in balancing miniaturization with performance. The diversity in design and functionality among portable NIR spectrometers directly impacts their accuracy and practical utility.
One of the most promising areas for the future of NIRS in animal nutrition is the miniaturization and portability of devices. These advancements will enable real-time, in-field analyses without the need to send samples to laboratories, significantly increasing the efficiency and practicality of assessments. However, this also highlights the need for systematic evaluations of the various devices available on the market to ensure that these advancements do not compromise the precision and quality of analyses [
103].
Integrating NIR spectroscopy with other precision agriculture technologies, such as soil sensors, drones, and climate monitoring systems, has the potential to transform agricultural management. By combining data from multiple sources, a more holistic and accurate view of crop and forage health and quality can be achieved, allowing for real-time adjustments in management practices [
104].
The development of advanced software and algorithms is another critical area, where machine learning and artificial intelligence techniques can enhance the robustness of predictive models, allowing the better handling of sample variability and other interferences. The creation of universal and transferable calibrations could further expand the use of NIRS, making it an even more valuable tool in animal nutrition [
105].
Finally, the broader dissemination and adoption of NIRS technology depend on developing more accessible and user-friendly technologies, as well as educating and training producers on its benefits and use. Partnerships between researchers, technology companies, and producers will be crucial to accelerating this adoption. Collaboration across different sectors will be essential to ensuring that NIRS technology reaches its full potential [
103,
104,
105].
In conclusion, while NIR spectroscopy already plays a crucial role in animal nutrition, the future of this technology is extremely promising, with numerous opportunities for innovation and improvement. Continuing to explore and understand the capabilities and limitations of NIR devices, particularly miniaturized ones, will be essential to ensure that this technology continues to provide significant value in practical applications.
Author Contributions
Conceptualization, C.M.D., H.N. and A.B.; methodology, C.M.D.; formal analysis, C.M.D.; investigation, C.M.D.; resources, A.B.; data curation, C.M.D. and A.B.; writing—original draft, C.M.D.; preparation, C.M.D.; writing—review and editing, H.N. and A.B.; supervision, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Cristiana Maduro Dias and Helder Nunes were funded by FCT—Fundação para a Ciência e a Tecnologia, I.P., under the framework of the Contract-Program for the Institute of Agricultural Research and Environmental Technologies (IITAA), through programmatic funding with reference UIDP/00153/2020.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Givens, D.I.; Deaville, E.R. The current and future role of near infrared reflectance spectroscopy in animal nutrition: A review. Aust. J. Agric. Res. 1999, 50, 1131–1145. [Google Scholar] [CrossRef]
- Wilkins, R.J.; Givens, D.I.; Owen, E.; Axford, R.F.E.; Omed, H.M. Forages and Their Role in Animal Systems. In Forage Evaluation in Ruminant Nutrition; CABI: Wallingford, UK, 2000; pp. 1–14. [Google Scholar]
- Dale, L.M.; Thewis, A.; Boudry, C.; Rotar, I.; Pacurar, S.F.; Abbas, Q.; Dardenne, P.; Baeten, V.; Pfister, J. Discrimination of grassland species and their classification in botanical families by laboratory scale NIR hyperspectral imaging: Preliminary results. Talanta 2013, 116, 149–154. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Rumen; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Bruno-Soares, A.M.; Murray, I.; Paterson, R.M.; Abreu, J.M.F. Use of near infrared reflectance spectroscopy (NIRS) for the prediction of chemical composition and nutritional attributes of green crop cereals. Anim. Feed Sci. Technol. 1998, 75, 15–25. [Google Scholar] [CrossRef]
- Reich, G. Near-infrared spectroscopy and imaging: Basic principles and pharmaceutical applications. Adv. Drug Deliv. Rev. 2005, 7, 1109–1143. [Google Scholar] [CrossRef]
- Barton, F.E.; Coleman, S.W. Potential of near infrared reflectance spectroscopy for measuring forage quality. Anim. Sci. Res. Rep. 1981, 108, 73–76. [Google Scholar]
- Wetzel, D.L. Near-infrared reflectance analysis: Sleeper among spectroscopic techniques. Anal. Chem. 1983, 55, 1165A–1176A. [Google Scholar] [CrossRef]
- Herschel, W. Investigation of the powers of the prismatic colours to heat and illuminate objects; with remarks, that prove the different refrangibility of radiant heat. To which is added, an inquiry into the method of viewing the sun advantageously, with telescopes of large apertures and high magnifying powers. Philos. Trans. R. Soc. 1800, 90, 255–283. [Google Scholar]
- Givens, D.I.; De Boever, J.L.; Deaville, E.R. The principles, practices and some future applications of near infrared spectroscopy for predicting the nutritive value of foods for animals and humans. Nutr. Res. Rev. 1997, 10, 83–114. [Google Scholar] [CrossRef]
- Ciurczak, E.W.; Igne, B.; Workman, J.; Burns, D.A. Handbook of Near-Infrared Analysis, 4th ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2021. [Google Scholar]
- Williams, P.; Norris, K. Near-Infrared Technology: In the Agricultural and Food Industries; American Association of Cereal Chemists: St. Paul, MN, USA, 2001. [Google Scholar]
- Shenk, J.S.; Westerhaus, M.O. Analysis of Agriculture and Food Products by Near Infrared Reflectance Spectroscopy; Monograph, NIRSSystems: Silver Spring, MD, USA, 1995. [Google Scholar]
- Jespersen, B.M.; Munck, L. Cereals and Cereal Products. In Infrared Spectroscopy for Food Quality Analysis and Control; Sun, D., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 13–28. [Google Scholar]
- Genot, V.; Bock, L.; Dardenne, P.; Colinet, G. Use of near-infrared reflectance spectroscopy in soil analysis: A review. Biotechnol. Agron. Soc. Environ. 2014, 18, 247–261. [Google Scholar]
- Andueza, D.; Picard, F.; Dozias, D.; Aufrère, J. Fecal Near-Infrared Reflectance Spectroscopy Prediction of the Feed Value of Temperate Forages for Ruminants and Some Parameters of the Chemical Composition of Feces: Efficiency of Four Calibration Strategies. Appl. Spectrosc. 2017, 71, 2165–2176. [Google Scholar] [CrossRef]
- Zhang, B.; Gu, B.; Tian, G.; Zhou, J.; Huang, J.; Xiong, Y. Challenges and solutions of optical-based nondestructive quality inspection for robotic fruit and vegetable grading systems: A technical review. Trends Food Sci. Technol. 2018, 81, 213–231. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Dehareng, F.; Hammida, M.; Baeten, V.; Froidmont, E.; Soyeurt, H.; Niemöller, A.; Dardenne, P. Potential of near infrared spectroscopy for on-line analysis at the milking parlour using a fibre-optic probe presentation. NIR News 2011, 22, 11–13. [Google Scholar] [CrossRef]
- Huawei, S.; Kun, S.; Li, Z.; Qian, Z.; Yuling, X.; Rong, Z.; Haipeng, L.; Baozhong, S. Development of near infrared reflectance spectroscopy to predict chemical composition with a wide range of variability in beef. Meat Sci. 2014, 98, 110–114. [Google Scholar]
- Maduro Dias, C.S.A.M.; Nunes, H.P.; Melo, T.M.M.V.; Rosa, H.J.D.; Silva, C.C.G.; Borba, A.E.S. Application of Near Infrared Reflectance (NIR) spectroscopy to predict the moisture, protein, and fat content of beef for gourmet hamburger preparation. Livest. Sci. 2021, 254, 104772. [Google Scholar] [CrossRef]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, M. Near-Infrared Spectroscopy: Principles, Instruments, Applications; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Workman, J. The Concise Handbook of Analytical Spectroscopy: Theory, Applications, And Reference Materials; World Scientific: Singapore, 2016. [Google Scholar]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; Wiley: New York, NY, USA, 2019. [Google Scholar]
- Ciurczak, E. Near-infrared spectroscopy. Mod. Instrum. Anal. 2006, 47, 157–170. [Google Scholar]
- Wetzel, D.L. Analytical Near-Infrared Spectroscopy. In Instrumental Methods in Food and Beverage Analysis; Wetzel, D.L., Charalambous, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Wetzel, D.L. Contemporary Near-Infrared Instrumentation. In Near-Infrared Technology in the Agricultural and Food Industries; Williams, P., Norris, K., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 2001. [Google Scholar]
- Blanco, M.; Villarroya, I. NIR spectroscopy: A rapid-response analytical tool. Trends Anal. Chem. 2002, 21, 240–250. [Google Scholar] [CrossRef]
- Osborne, B.G.; Fearn, T.; Hindle, P.H. Practical Near Infrared Spectroscopy with Applications in Food and Beverage Analysis; Longman Scientific and Technical: Harlow, UK, 1993. [Google Scholar]
- Barton, F.E. Theory and principles of near-infrared spectroscopy. Spectrosc. Eur. 2002, 14, 12–18. [Google Scholar]
- McClure, W.F. Near-Infrared Instrumentation. In Near-Infrared Technology in the Agricultural and Food Industries; Williams, P., Norris, K., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 2001. [Google Scholar]
- Ozaki, Y.; Huck, C.; Tsuchikawa, S.; Engelsen, S.B. Near-Infrared Spectroscopy: Theory, Spectral Analysis, Instrumentation, and Applications; Springer Nature Singapore: Singapore, 2021. [Google Scholar]
- Agelet, L.E.; Hurburgh, C.R. A Tutorial on Near Infrared Spectroscopy and Its Calibration. Crit. Rev. Anal. Chem. 2010, 40, 246–260. [Google Scholar] [CrossRef]
- Osborne, B.G. Near-Infared Spectroscopy in Food Analysis. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Chichester, UK, 2000. [Google Scholar]
- Kellner, R.; Mermet, J.-M.; Otto, M.; Valcárcel, M.; Widmer, H.M. Analytical Chemistry: A Modern Approach to Analytical Science; Wiley-VCH: Hoboken, NJ, USA, 2004. [Google Scholar]
- Yan, H.; De Gea Neves, M.; Noda, I.; Guedes, G.M.; Silva Ferreira, A.C.; Pfeifer, F.; Chen, X.; Siesler, H.W. Handheld Near-Infrared Spectroscopy: State-of-the-Art Instrumentation and Applications in Material Identification, Food Authentication, and Environmental Investigations. Chemosensors 2023, 11, 272. [Google Scholar] [CrossRef]
- Kawano, S. Sampling and sample presentation. In Handbook of Near-Infrared Analysis; Siesler, H.W., Ozaki, Y., Kawata, H.M., Eds.; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Workman, J.J.; Burns, D.A. Commercial NIR Instruments. In Handbook of Near Infrared Spectroscopy Analysis; Burns, D.A., Ciurczak, E.W., Eds.; Marcel Dekker, Inc: New York, NY, USA, 2008. [Google Scholar]
- Stark, E.; Luchter, K. Diversity in NIR Instrumentation. In Proceedings of the 11th International Conference on Near Infrared Spectroscopy, Cordoba, Spain, 6–11 April 2004; Davies, A.M.C., Garrido-Varo, A., Eds.; NIR Publications: Cordoba, Spain, 2004. [Google Scholar]
- Patoprsty, V.; Valkova, M. Remarks to Calibration in Chemistry. Int. J. Meas. Technol. Instrum. Eng. 2012, 2, 67–76. [Google Scholar] [CrossRef][Green Version]
- Martens, H.; Martens, M. Multivaried Analysis of Quality: An Introduction; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Naes, T.; Isaksson, T.; Fearn, T.; Davies, T. A User-Friendly Guide to Multivariate Calibration and Classification; NIR Publications: Chichester, UK, 2002. [Google Scholar]
- Mark, H.; Workman, J. Statistics in Spectroscopy; Elsevier: London, UK, 2003. [Google Scholar]
- Maduro Dias, C.; Nunes, H.; Borba, A. Near-Infrared Spectroscopy Integration in the Regular Monitorization of Pasture Nutritional Properties and Gas Production. Agriculture 2023, 13, 1398. [Google Scholar] [CrossRef]
- Coats, D.B. Is near infrared spectroscopy only as good as the laboratory reference values? An empirical approach. Spectrosc. Eur. 2002, 14, 24–26. [Google Scholar]
- Fearn, T. Chemometrics: An enabling tool for NIR. NIR News 2005, 16, 17–19. [Google Scholar] [CrossRef]
- Maduro Dias, C.S.A.M.; Nunes, H.P.B.; Borba, A.E.S. Influence of the Physical Properties of Samples in the Use of NIRS to Predict the Chemical Composition and Gas Production Kinetic Parameters of Corn and Grass Silages. Fermentation 2023, 9, 418. [Google Scholar] [CrossRef]
- Cozen, J.P. Multivariate Calibration: A Practical Guide for Developing Methods in Quantitative Analytical Chemistry; Bruker Optik: Ettlingen, Germany, 2014. [Google Scholar]
- Cozzolino, D.; Fassio, A.; Gimenez, A. The use of near infrared reflectance spectroscopy (NIRS) to predict the composition of whole maize plants. J. Sci. Food Agric. 2000, 81, 142–146. [Google Scholar] [CrossRef]
- Murray, I. The NIR spectra of homologous series of organic compounds. In NIR/NIT Conference; Hollo, J., Kaffka, K.J., Gonczy, J.L., Eds.; Akademiai Kiado: Budapest, Hungary, 1986; pp. 13–28. [Google Scholar]
- Metrohm. NIR Spectroscopy: A Guide to Near-Infrared Spectroscopic Analysis of Industrial Manufacturing Processes; Metrohm: Herisau, Switzerland, 2013. [Google Scholar]
- Sørensen, K.M.; Van den Berg, F.; Engelsen, S.B. NIR Data Exploration and Regression by Chemometrics—A Primer. In Near-Infrared Spectroscopy Theory, Spectral Analysis, Instrumentation, and Applications; Ozaki, Y., Huck, C., Tsuchikawa, S., Engelsen, S.B., Eds.; Springer: Basingstoke, UK, 2021. [Google Scholar]
- Burns, D.A.; Ciurczak, E.W. Handbook of Near-Infrared Analysis; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- García-Ciudad, A.; Garcia-Criado, B.; Perez-Corona, M.E.; Vasquez de Aldana, B.R.; Ruano-Ramos, A.M. Application of near-infrared reflectance spectroscopy to protein analysis of grassland herbage samples. J. Sci. Food Agric. 1993, 50, 479–484. [Google Scholar] [CrossRef]
- Hanrahan, G.; Udeh, F.; Patil, D.G. Chemometrics and Statistics, Multivariate Calibration Techniques. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Rinnan, Å.; Nørgaard, L.; van den Berg, F.; Thygesen, J.; Bro, R.; Engelsen, S.B. Data Pre-Processing. In Infrared Spectroscopy for Food Quality Analyses and Control; Sun, D., Ed.; Elsevier Inc.: New York, NY, USA, 2009. [Google Scholar]
- Kramer, R. Chemometric Techniques for Quantitative Analysis; Marcel Dekker, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Martens, H.; Jensen, S.A.; Geladi, P. Multivariate Linearity Transformations for Near Infrared Spectroscopy. In Proceeding of the Nordic Symposium on Applied Statistics; Christie, O.H.J., Ed.; Stokkand Forlag: Stavanger, Norway, June 12 to 14 1983.
- Geladi, P.; MacDougal, D.; Martens, H. Linearization and Scatter Correction for Near-Infrared Reflectance Spectra of meat. Appl. Spectrosc. 1985, 30, 491–500. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; Barnes, R.J.; López, S.; Sanderson, R.; France, J.; Lister, S.J.; Ellis, J.L. Methodology Adjusting for Least Squares Regression Slope in the Application of Multiplicative Scatter Correction to Near-Infrared Spectra of Forage Feed Samples. J. Chemom. 2023, 37, e3511. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and de-Trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi- and Megavariate Data Analysis: Basic Principles and Applications; Umetrics: Malmö, Sweden, 2013. [Google Scholar]
- Coblentz, W.W. Investigations of Infrared Spectra Part I; Carnegie Institute of Washington: Washington, DC, USA, 1905. [Google Scholar]
- Norris, K.H.; Williams, P.C. Optimization of Mathematical Treatments of Raw Near-Infrared Signal in the Measurement of Protein in Hard Red Spring Wheat. I. Influence of Particle Size. Cereal Chem. 1984, 62, 158–165. [Google Scholar]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Esbensen, K.H.; Guyot, D.; Westad, F.; Houmoller, L.P. Multivariate Data Analysis: In Practice, An Introduction to Multivariate Data Analysis; Camo Process AS: Oslo, Norway, 2010. [Google Scholar]
- Elvin, P.J.; Meehan, E.J.; Kolthoff, I.M. Treatise on Analytical Chemistry, Part 1; Wiley: New York, NY, USA, 1981; Volume 7. [Google Scholar]
- Martens, H.; Stark, E. Extended multiplicative signal correction and spectral interference subtraction: New preprocessing methods for near infrared spectroscopy. J. Pharm. Biomed. Anal. 1991, 625, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Romía, M.B.; Bernàrdez, M.A. Multivariate Calibration for Quantitative Analysis. In Infrared Spectroscopy for Food Quality Analyses and Control; Sun, D., Ed.; Elsevier Inc.: New York, NY, USA, 2009. [Google Scholar]
- Massart, D.L.; Vandegiste, B.G.M.; Deming, S.N.; Michotte, Y.; Kaufman, L. Chemometrics: A Textbook; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Wold, H. Soft Modeling by Latent Variables: Non-Linear Iterative Partial Least Squares (NIPALS) Approach. In Perspectives in Probability and Statistics; Gani, M.S.B., Ed.; Academic Press: London, UK, 1975. [Google Scholar]
- Varmuza, P.; Filzmoser, P. Introduction to Multivariate Statistical Analysis in Chemometrics; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- De Jong, S. SIMPLS: An alternative approach to partial least squares regression. Chemom. Intell. Lab. Syst. 1993, 18, 251–263. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: Fundamentals, practical aspects and analytical applications. J. Braz. Chem. Soc. 2003, 14, 198–219. [Google Scholar] [CrossRef]
- Williams, P.; Sobering, D.C. How Do We Do It: A Brief Summary of the Methods we Use in Developing Near Infrared Calibrations. In Near Infrared Spectroscopy: Future Waves; NIR Publications: Chichester, UK, 1996; pp. 185–188. [Google Scholar]
- Pu, Y.; O’Donnell, C.; Tobin, J.T.; O’Shea, N. Review of near-infrared spectroscopy as a process analytical technology for real-time product monitoring in dairy processing. Int. Dairy J. 2020, 103, 104623. [Google Scholar] [CrossRef]
- Ulyatt, M.J.; Waghorn, G.C. Proceedings of a workshop on improving the quality and intake of pasture-based diets for lactating cows. Dep. Anim. Sci., Massey Univ. 1993, 1, 11–32. [Google Scholar]
- Park, R.S.; Agnew, R.E.; Gordon, F.J.; Steen, R.W.J. The use of near infrared reflectance spectroscopy (NIRS) on undried samples of grass silage to predict chemical composition and digestibility parameters. Anim. Feed Sci. Technol. 1998, 72, 155–167. [Google Scholar] [CrossRef]
- Soldado, A.; Fearn, T.; Martínez-Fernández, A.; De La Roza-Delgado, B. The Transfer of NIR Calibrations for undried grass silage from the laboratory to on-site instruments: Comparison of two approaches. Talanta 2013, 105, 8–14. [Google Scholar] [CrossRef]
- Norris, K.H.; Barnes, R.F.; Moore, J.E.; Shenk, J.S. Predicting forage quality by infrared reflectance spectroscopy. J. Anim. Sci. 1976, 43, 889–897. [Google Scholar] [CrossRef]
- Shenk, J.S.; Workman, J.J.; Westerhaus, M.O. Application of NIR Spectroscopy to Agricultural Products. In Handbook of Near-Infrared Analysis; Burns, D.A., Ciurczak, E.W., Eds.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Tilmann, P.; Horst, H.; Danier, J.; Dieterle, P.; Philipps, P. Analysis of Mixed Feeds and Their Components Using Spectroscopy. In Proceedings of the 10th International Conference on Near Infrared Spectroscopy; Davies, A.M.C., Cho, R.K., Eds.; NIR Publications: Chichester, UK, 2002. [Google Scholar]
- Villamarín, B.; Fernández, E.; Mendéz, J. Analysis of grass silage from northwestern Spain by near-infrared reflectance spectroscopy. J. AOAC Int. 2002, 85, 541–545. [Google Scholar] [CrossRef]
- Barber, G.D.; Givens, D.I.; Krisdis, M.S.; Offer, N.W.; Murray, I. Prediction of the organic matter digestibility of grass silage. Anim. Feed Sci. Technol. 1990, 28, 115–128. [Google Scholar] [CrossRef]
- Cozzolino, D.; Moron, A. Exploring the use of near infrared reflectance spectroscopy (NIRS) to predict trace minerals in legumes. Anim. Feed Sci. Technol. 2004, 111, 161–173. [Google Scholar] [CrossRef]
- Garnsworthy, P.C.; Wiseman, J.; Fegeros, K. Prediction of chemical, nutritive, and agronomic characteristics of wheat by near infrared spectroscopy. J. Agric. Sci. 2000, 135, 409–417. [Google Scholar] [CrossRef]
- Reeves, J.B. Near infrared spectroscopic analysis of lignin components in sodium chlorite-treated and untreated forages and forage by-products. J. Dairy Sci. 1988, 72, 388–397. [Google Scholar] [CrossRef]
- Smith, K.F.; Flinn, S.E. Measurements of the magnesium concentration in Perennial Ryegrass (Lolium perenne) using near infrared reflectance spectroscopy. Aust. J. Agric. Res. 1991, 42, 1399–1404. [Google Scholar] [CrossRef]
- Kennedy, C.A. NIRS Analysis of Intact Grass Silage and Fresh Grass for the Prediction of Dry Matter, Crude Protein and Acid Detergent Fibre. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 1996. [Google Scholar]
- Corson, D.C.; Waghorn, G.C.; Ulyatt, M.J.; Lee, J. NIRS: Forage analysis and livestock feeding. Proc. N. Zealand Grassl. Assoc. 1999, 61, 127–132. [Google Scholar] [CrossRef]
- Sørensen, L.K. Prediction of fermentation parameters in grass and corn silage by near infrared spectroscopy. J. Dairy Sci. 2004, 87, 3826–3835. [Google Scholar] [CrossRef]
- Cozzolino, D.; Fassio, A.; Fernández, E.; Restaino, E.; La Manna, A. Measurement of chemical composition in wet whole maize silage by visible and near infrared reflectance spectroscopy. Anim. Feed Sci. Technol. 2006, 129, 329–336. [Google Scholar] [CrossRef]
- Fassio, A.; Fernández, E.G.; Restaino, E.A.; La Manna, A.; Cozzolino, D. Predicting the nutritive value of high moisture grain corn by near infrared reflectance spectroscopy. Comput. Electron. Agric. 2009, 67, 59–63. [Google Scholar] [CrossRef]
- Burns, G.A.; O’Kiely, P.; Grogan, D.; Gilliland, T.J. A note on the comparison of three near infrared reflectance spectroscopy calibration strategies for assessing herbage quality of ryegrass. Irish J. Agric. Food Res. 2014, 53, 199–204. [Google Scholar]
- De Boever, J.L.; Vanacker, J.M.; De Brabander, D.L. Rumen degradation characteristics of nutrients in compound feeds and the evaluation of tables, laboratory methods and NIRS as predictors. Anim. Feed Sci. Technol. 2003, 107, 29–43. [Google Scholar] [CrossRef]
- Foskolos, A.; Calsamiglia, S.; Chenková, M.; Weisbjerg, M.R.; Albanell, E. Prediction of rumen degradability parameters of a wide range of forages and non-forages by NIRS. Animal 2015, 9, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Wilman, D.; Field, M.L.; Lister, S.J.; Givens, D.I. The use of near infrared spectroscopy to investigate the composition of silages and the rate and extent of cell-wall degradation. Anim. Feed Sci. Technol. 2000, 88, 3–4. [Google Scholar] [CrossRef]
- Andrés, S.; Murray, I.; Calleja, A.; Giráldez, F.J. Nutritive Evaluation of Forages by near Infrared Reflectance Spectroscopy. J. Near Infrared Spectrosc. 2005, 13, 301–311. [Google Scholar] [CrossRef]
- Thomson, A.L.; Humphries, D.J.; Rymer, C.; Archer, J.E.; Grant, N.W.; Reynolds, C.K. Assessing the accuracy of current near infra-red reflectance spectroscopy analysis for fresh grass-clover mixture silages and development of new equations for this purpose. Anim. Feed Sci. Technol. 2018, 239, 94–106. [Google Scholar] [CrossRef]
- Decruyenaere, V.; Planchon, V.; Dardenne, P.; Stilmant, D. Prediction error and repeatability of near infrared reflectance spectroscopy applied to faeces samples in order to predict voluntary intake and digestibility of forages by ruminants. Anim. Feed Sci. Technol. 2015, 205, 49–59. [Google Scholar] [CrossRef]
- Olsoy, P.J.; Griggs, T.C.; Ulappa, A.C.; Gehlken, K.; Shipley, L.A.; Shewmaker, G.E.; Forbey, J.S. Nutritional analysis of sagebrush by near-infrared reflectance spectroscopy. J. Arid Environ. 2016, 134, 125–131. [Google Scholar] [CrossRef]
- Nousiainen, J.; Ahvenjärvi, S.; Rinne, M.; Hellämäki, M.; Huhtanen, P. Prediction of indigestible cell wall fraction of grass silage by near infrared reflectance spectroscopy. Anim. Feed Sci. Technol. 2004, 115, 3–4. [Google Scholar] [CrossRef]
- Parrini, S.; Acciaioli, A.; Franci, O.; Pugliese, C.; Bozzi, R. Near infrared spectroscopy technology for prediction of chemical composition of natural fresh pastures. J. Appl. Anim. Res. 2019, 47, 514–520. [Google Scholar] [CrossRef]
- Modroño, S.; Soldado, A.; Martínez-Fernández, A.; Roza-Delgado, B. Handheld NIRS sensors for routine compound feed quality control: Real time analysis and field monitoring. Talanta 2016, 162, 62–68. [Google Scholar] [CrossRef]
- Trotter, M.G. Precision Agriculture for Pasture, Rangeland and Livestock Systems; CABI: Wallingford, UK, 2011. [Google Scholar]
- Samadi, S.; Wajizah, S.; Munawar, A.A. Near infrared spectroscopy (NIRS) data analysis for a rapid and simultaneous prediction of feed nutritive parameters. Data Brief 2020, 29, 105623. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).