Abstract
This work describes a two-stage technique of X-ray fluorescence (XRF) analysis of rare earth niobates. A comparison between the two approaches revealed that the Fundamental Parameters Method (FPM) can be employed for a rapid preliminary assessment of the composition of the resulting material and the construction of calibration curves can be used to determine the contents of the major elements with precision. The results of the relative standard deviation (RSD) for FPM were no more than 7%, while the approach to construct calibration curves had an RSD of no more than 1%. Calibration samples were prepared using the same synthesis method as the study samples to construct the calibration curves. The possibility of constructing calibration dependencies using mixtures of oxides was assessed, but this approach could not provide the desired accuracy. The obtained results have been shown to have a good correlation with inductively coupled plasma optical emission spectrometry. The developed technique enables the determination of the major components in niobates containing two and three rare earth elements, which are used as optical materials and medium-entropy ceramics.
1. Introduction
Rare earth niobates have a wide range of applications. They are used as phosphors [1,2,3,4,5], magnetic materials [6,7,8], materials with photocatalytic properties [9,10,11], dielectrics [12], thermal barrier coatings [13,14,15,16,17], and electrolytes in various devices such as fuel cells, hydrogen sensors, humidity sensors [18,19], etc.
The ceramics of the Y3−xYbxNbO7 type represent an example of monosubstitution in the starting yttrium niobate and can also act as a matrix for the production of up-conversion phosphors [20]. Cation substitution of ytterbium for yttrium in Yb3NbO7 with the structure of defect fluorite improves the thermal-insulating properties of the material and its use as a thermal barrier coating [21]. However, nowadays, there is an important challenge to increase the efficiency of phosphors capable of processing shorter wavelength infrared radiation. Traditionally, for that purpose, a dopant ion (for example, Yb3+) is added to the main luminescent ion (Er3+, Ho3+, Tm3+, or Y3+), which acts as a sensitizer and promotes a more efficient transfer of excitation energy. The optical properties of YNbO4 doped with Eu3+ phosphor have been previously reported; the characteristic emission of Eu3+ ions was observed, as was the total or partial quenching of the NbO43− emission, which occurred due to the energy transfer from the matrix to the dopant [22,23,24].
The usage of rare earth niobates is also possible as thermal barrier materials [15] and high-entropy ceramics [16] (HECs). The niobates with three rare earth elements (REEs) in their structure, which were studied in this research, are an example of medium-entropy ceramics (MECs). When compared to their high-entropy counterparts, the MECs show significantly lower thermal conductivity, making them a continued research priority. According to recent studies [25,26], MECs exhibit mechanical properties and thermal conductivity that are at least equivalent to, and often surpass, those of HECs.
The gradual enrichment of the composition with the production of MECs (at least three different REEs) and HECs requires special control of the composition at each stage of complexity of the initial niobate (Y3NbO7) and the development of analytical techniques for the determination of the element composition. For such materials, it is extremely important to control the chemical composition, the major component, and the ratio of dopants for the final product and at the intermediate stages of its production.
Niobates are typically analyzed using X-ray powder diffraction (XRD) [2,3,4,5,6,8,9,11,12,14,15,16,17,19,27] to confirm the presence of the expected phases. Moreover, X-ray photoelectron spectroscopy (XPS) [18] and scanning electron microscopy (SEM) with energy-dispersive X-ray spectrometry (EDX) are employed [2,3,4,5,12,14,15,16,17,18]. SEM-EDX is used for microstructure analysis, local elemental analysis, and elemental mapping. In all the cited works, chemical analysis primarily serves an identification function and does not give an idea of the quantitative characteristics of the objects. To confirm the composition, inductively coupled plasma optical emission spectrometry (ICP-OES) is used, but the analysis technique has not been described in detail [11]. Niobates with several REEs are a difficult object for analytical studies. In order to determine the elements present in rare earth niobates, it is necessary to develop a technique that employs statistical evaluation and has been tested on real samples.
In the field of analytical chemistry, multi-element methods such as ICP-OES, XRF spectrometry, and inductively coupled plasma mass spectrometry (ICP-MS) are used in the development of new functional materials. For this type of object, it is preferable to use XRF analysis, since it does not require the dissolution of sparingly soluble rare earth niobates. To analyze such objects, it is important to use XRF spectrometry as it is an analytical method that is accurate and has a wide range of detectable contents and a speed that does not require sample dissolution. However, XRF spectrometry, like any analytical method, has limitations. First of all, the superposition of the spectral lines of the analytes on each other represents a significant challenge in the analysis of materials based on REEs [28,29,30]. This problem is usually solved by conducting a set of studies aimed at a detailed analysis of the obtained spectra, selection of experimental conditions, and searching for an approach to take into account the spectral background.
The use of modern XRF equipment makes it possible to quickly obtain preliminary data on the elemental composition of the sample under investigation using the Fundamental Parameters Method (FPM) [29,31], as well as flow control of the composition at different stages of technological progress. Precise quantitative determination is realized by constructing calibration curves. It is essential to note that there are no standard samples and calibration series of comparison samples for complex rare earth niobates, which require their production and certification. In such cases, it is possible to prepare reference samples that mimic the matrix by mixing and homogenizing the individual substances containing the analytes [32]. In our case, metal oxides can be used.
An advanced solution to the problem of analyzing rare earth niobates, which have no analogs, is the development of a two-stage technique for X-ray fluorescence analysis of rare earth niobates with two and three REEs in the composition. XRF analysis with FPM can rapidly determine an approximate composition. This approach can accompany synthesis at all stages of material development. For a more precise determination, unique calibration samples with the same composition as the sample have been synthesized for the first time.
2. Materials and Methods
2.1. Instrumentation
A wavelength-dispersive X-ray fluorescence spectrometer SPECTROSCAN MAX-GVM (Spectron Ltd., St. Petersburg, Russia) was used to analyze the rare earth niobates. This instrument uses a palladium X-ray with a maximum power of 160 W. The maximum tube voltage is 40 kV, and the tube current can be 0.5–3.5 mA. The spectrometer is equipped with 4 crystal-analyzers: a single crystal of lithium fluoride LiF200 (first reflection order (1): 821–3000 mÅ; second reflection order (2): 408–1664 mÅ), pentaerythrinol PET (first reflection order (1): 5000–7218 mÅ; second reflection order (2): 2500–3609 mÅ), rubidium biphthalate RbAP (first reflection order (1): 7000–12511 mÅ; second reflection order (2): 3500–6256 mÅ), and graphite C002 (first reflection order (1): 2500–5585 mÅ; second reflection order (2): 1250–2773 mÅ). SPECTROSCAN MAX-GVM uses a two-chamber proportional detector as a detector that converts X-ray light quanta into voltage pulses. The X-ray window is made of a beryllium film with a thickness of 15 µm; the entrance window is 12 µm thick.
The atlas of spectral lines is included in the Spectr-Quant software (4.0.0) for the spectrometer.
Laboratory scales VL-224V (Gosmeter, St. Petersburg, Russia) were used to take samples.
The results obtained by the XRF analysis were confirmed by an ICP-OES spectrometer ICAP PRO XP (Thermo Electron Corp., Waltham, MA, USA).
2.2. Samples of Rare Earth Niobates
The rare earth niobates with 2 (Y2YbNbO7, Y1.5Yb1.5NbO7, and YYb2NbO7) and 3 REEs (ErYYbNbO7, synthesized by different methods) were considered in this study. The formulas of the compounds are given based on the assumption of a stoichiometric composition. The determined elemental composition of the samples will be presented below.
Samples with two rare earth elements, Y2YbNbO7 (2Y1Yb), Y1.5Yb1.5NbO7 (1Y1Yb), and YYb2NbO7 (1Y2Yb), were obtained by reverse precipitation in an ammonia aqueous solution (aqueous solutions of yttrium and ytterbium nitrates, alcoholic solution of niobium chloride). The resulting mixture of metal hydroxides was centrifuged, dried at 100 °C, and then calcined at 1000 °C and 1500 °C for 16 h. The process is similar to the synthesis of tantalates [33,34].
ErYYbNbO7 samples with three rare earth elements were synthesized by three methods: reverse precipitation (30S), solid-state reaction (30T1, 30T2, and 10T), and sol-gel synthesis (SER). For synthesis by the reverse precipitation method (30S), solutions of yttrium, ytterbium, and erbium nitrates and an alcohol solution of niobium chloride were used. The process is similar to the synthesis of niobates with 2 REEs. Solutions of RE nitrates were added to an aqueous solution of ammonia, then a solution of niobium chloride was added to the mixture. This resulted in the precipitation of hydroxides. The formed precipitate remained in the mother liquor for 12 h. The precipitate was separated and washed with distilled water by centrifugation. This procedure was carried out at least three times. The resulting precipitate was dried in an oven at a temperature of 100 °C. It was also calcined in several stages at temperatures of 1000, 1500, and 1600 °C for 8, 16, and 6 h, respectively.
A mixture of niobium, erbium, yttrium, and ytterbium oxides was used for the synthesis of rare earth niobates by the solid-state method (30T1, 30T2, and 10T) [35,36]. The starting substances were homogenized in a laboratory ball mill for 90 min at a speed of 100 rpm with the addition of alcohol. The mixed oxide powder was dried in an oven at a temperature of 70 °C and calcined in three stages at temperatures of 1000, 1500, and 1600 °C for 24, 16, and 6 h, respectively.
For the sol-gel synthesis (SER), the same starting components were used as for the precipitation synthesis [9]. In the first stage, a solution of rare earth nitrates was prepared, to which an alcohol solution of NbCl5 was added based on the ratio Y:Yb:Er:Nb = 1:1:1:1. Next, anhydrous citric acid was added to the solution in excess relative to the metals and evaporated to a gel state, which was kept in an oven at 115 °C for 16 h. As a result, a highly dispersed powder was formed. Annealing of the highly dispersed precursor also took place in several stages at temperatures of 1000, 1500, and 1600 °C for 8, 16, and 6 h, respectively.
2.3. Preliminary Characterization Using SEM-EDX
The niobate samples are highly dispersed powders. The particle size of the samples was determined using scanning electron microscopy using a TESCAN AMBER GMH scanning electron microscope (TESCAN, Brno, Czech Republic) with ultra-high resolution. As a result of preliminary observations, areas were identified that most fully characterize the microrelief features of the grain surface of the studied samples. After establishing acceptable contrast and brightness, the images were photographed. EDX analysis and elemental mapping of microstructures were also performed.
2.4. Sample Preparation
For XRF analyses, samples were prepared as pressed powder pellets using a laboratory hydraulic press machine PLG-12 (Lab Tools, St. Petersburg, Russia). A previously pressed boric acid powder was covered with a small amount of the powder sample (0.43 ± 0.01 g) and pelletized again to form a double pellet. The pressure did not exceed 160 bar = 1.6 × 107 Pa for niobates with two REEs and 120 bar = 1.2 × 107 Pa for niobates with three REEs. If the force was exceeded, the fragility of the tablet increased critically, and its destruction followed.
2.5. Spectral Overlap
One of the most important XRF parameters is the spectral lines. Despite the relatively simple X-ray spectrum, line interferences still occur, especially for elements that differ in atomic number by only a few units, as is the case for a series of REEs. The considerations in selecting the analytical lines of the determined elements were the absence of spectral overlap among the lines, mutual inter-element influences, and the possibility of their resolution, as well as taking into account the background in the vicinity of the analytical lines of the determined elements.
The SPECTROSCAN MAX-GVM spectrometer has 4 crystal analyzers with 2 reflection orders: LiF200, RbAP, PET, and C002. They have different values of interplanar distances, which allows for optimizing the conditions for elemental determination in different wavelength ranges.
Typically, in XRF analysis, the crystal with the most intense K-series element lines is preferred for recording, unless these lines are distorted by various effects, rendering them unsuitable for analysis. The K-series lines are used for the determination of Y. Almost all analysts recommend lines in the L-series for REE determination [37]. Lines of the α- or β-series of the first or second reflection order were predominantly chosen as characteristic lines. When determining most REEs (Yb and Er), it is impossible to measure the analytical signal using the K-series line, since very high energies are required to excite such heavy elements. In these cases, L-series lines are used.
The main spectral overlaps of the analytical lines for the studied samples are presented in Table 1 and Figure 1.

Table 1.
Selected analytical lines.
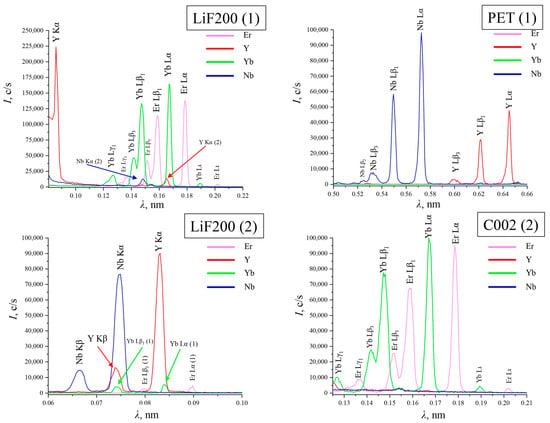
Figure 1.
The main spectral overlays of Er, Y, Yb, and Nb on crystal analyzers LiF200 (1), LiF200 (2), PET (1), and C002 (2).
According to the data listed above, it can be concluded that in the case of yttrium, it is rational to choose the Y Kα line on the LiF200 (1). The Y Kα and Y Kβ lines on LiF200 (2) are subject to spectral overlaps from Yb (penetrating from the first reflection order) and Nb. When choosing between the K-series and the L-series, it is better to give preference to the bright K-series, so the spectral lines on the PET crystal analyzer are not involved in the analysis.
For ytterbium and erbium, Yb Lα1 and Er Lα1 were selected on the C002 crystal analyzer, as they have the most intense lines without spectral interference.
To determine niobium, it is preferable to choose the Kβ line on LiF200 (2) because the Nb Kα line overlaps with the Y Kβ line.
Table 2 shows selected analytical lines.

Table 2.
Selected analytical lines and experimental conditions for XRF analysis.
2.6. Selection of Conditions for XRF Analysis
In order to perform an efficient and accurate analysis using XRF, various analytical parameters must be optimized. It is optimal to use a variable tube current and measuring time of the line to obtain the highest count rates from each element. However, a high tube current setting may destroy the X-ray tube. The intensity count rate of selected analytical lines was measured at a voltage at the anode of the X-ray tube of 40 kV. The current directly determines the intensity of the radiation recorded by the detector. The tube current was set according to the content of the elements being determined and the intensity of the measured analytical line in the range from 0.1 to 3.5 mA. The choice of exposure time was set depending on the selected current strength.
All selected analysis conditions are shown in Table 2.
2.7. Background Correction
The content of the major components was monitored in the niobate samples. The peak-to-background X-ray count ratios were notably high. However, in order to construct calibration dependencies that take the background into account, it is necessary to perform a background correction before the calculation of the calibration lines. In this study, two methods of background correction were investigated for the construction of calibration dependencies—using a blank sample and using two points. Samples that did not contain the analyte to be determined were used as blanks, and for niobium, a mixture of rare earth oxides was used. The minimum error of the obtained results was set with a background correction at two points. When using the FPM, automatic background accounting was sufficient.
2.8. XRF Measurements
2.8.1. XRF Analysis with the Fundamental Parameters Method
Samples 2Y1Yb, 1Y1Yb, 1Y2Yb, 30S, 30T1, 30T2, 10T, and SER were analyzed using FPM. Three replicate measurements of each sample were made under the conditions given in Table 2. The compressed tablets were placed in an aluminum sample with a hole diameter of 15 mm. All samples were measured with removal from irradiation at least twice.
The standard FPM setting was used (hardware functions were calculated based on oxides). The quantification of composition was made by calculating the peak areas of the measured spectrum of each element relative to each other. The result of the analysis was then calculated using the “type-matching” method. This method is based on the comparison of the measurement results of the sample with the measurement results of a reference material of known elemental composition. Details of the operation of the FPM are presented in [38].
2.8.2. XRF Analysis with Calibration Curves Using Oxide Mixtures
The possibility of carrying out the analysis using mixtures of oxides for niobates with the composition ErYYbNbO7 has been investigated, since there are no reference samples for rare earth niobates, and the FPM does not always meet the accuracy requirements. The oxides of erbium, yttrium, ytterbium, and gadolinium were mixed in the ratios indicated in Table 3.

Table 3.
The weight of mixed powders for making calibration samples and the composition of calibration samples.
The measurements were conducted in accordance with the conditions specified in Table 2 with an increasing exposure time (40 s for all elements) to accumulate the count rates. When constructing the calibration dependence for each element, the choice of regression model parameters was made with consideration of background compensation at points equidistant from the characteristic line and the inter-element effect. The curves obtained were evaluated using the linear correlation coefficients (r2). Statistical evaluations, such as the mean, relative standard deviation (RSD), and error (±Δ), were calculated for each sample. XRF and ICP-OES results were compared using a t-test [39].
2.8.3. XRF Analysis with Calibration Curves Using Synthesized Calibration Samples
To determine elements in samples of rare earth niobates, it is necessary to construct calibration dependencies using a series of standard samples synthesized in the same way as the samples under investigation. The problem has been solved by the preparation of a calibration series. The composition of the calibration samples is presented in Table 4. It is important to note that the synthesis of the calibration series is similar to the synthesis of the studied samples by reverse precipitation.

Table 4.
The composition of synthesized calibration samples.
The analysis procedure is similar to the one in Section 2.8.2
2.9. ICP-OES Measurements
The results obtained by X-ray fluorescence analysis were compared to those obtained by ICP-OES. First, (0.1 ± 0.01) g of a sample of rare earth niobates is dissolved using microwave-assisted mineralization (MARS6 system, CEM Corp., Matthews, NC, USA). After mineralization, the sample was quantitatively transferred to a 100 mL flask and diluted to 100 mL with deionized water (18.2 MΩ cm at 25 °C). Single-element standard solutions (High-Purity Standards, Charleston, SC, USA) were used for calibration. The completeness of sample dissolution was assessed visually.
3. Results and Discussion
3.1. SEM-EDX
The SEM micrographs of the surfaces of the rare earth niobates are shown in Figure 2. The niobate sample with two rare earth elements, 2Y1Yb, exhibits spherical grain morphology, with an average size of less than 1–2 microns. These grains aggregate to form agglomerates of approximately 10–15 microns in size. The similarity of the relief was noted for the double and triple rare earth niobates obtained by the methods under consideration. The solid-phase method is characterized by a greater tendency for particles to agglomerate.
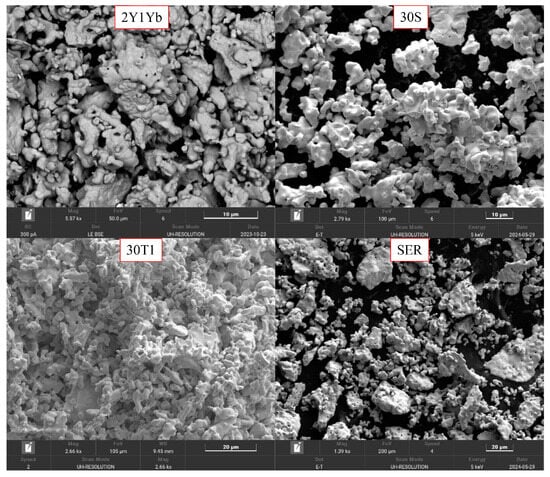
Figure 2.
The SEM micrographs of the surfaces of rare earth niobates.
The SEM-EDX mapping data are presented in Figure 3. The element distributions are relatively uniform. Er and Yb are distributed more uniformly than other elements.
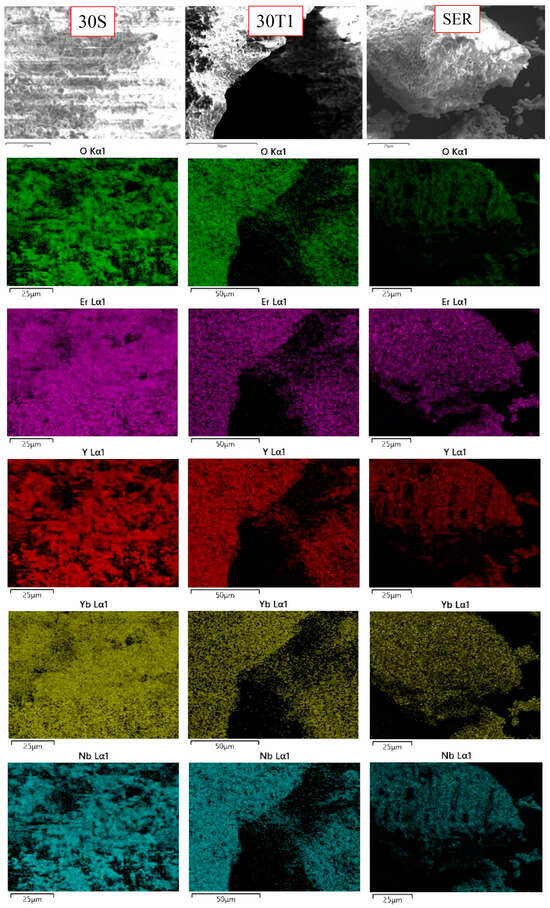
Figure 3.
The SEM-EDX mapping.
The local elemental analysis carried out at different points of the sample is presented in Table 5. The results obtained confirm the presence of the major elements in the sample and their ratio is close to the expected. The results show that the samples are heterogeneous and require careful averaging before analysis. In order to obtain a homogeneous sample, the material under study was subjected to mixing. The most homogeneous sample was obtained by the 30S reverse precipitation method. This can be seen from the RSD values presented in Table 5.

Table 5.
The composition of synthesized calibration samples.
3.2. The Results of XRF Measurements
3.2.1. The Results of XRF Analysis with FPM
The results of the determination of elements in rare earth niobates by XRF with FPM are shown in Table 6. The results of FPM measurements are presented as a ratio of metals without taking oxygen into account in order to avoid unnecessary errors. The results obtained were compared with the results obtained by ICP-OES.

Table 6.
Results of the determination of elements in niobates with 2 REEs (a) and 3 REEs (b) using XRF analysis with FPM and the results of ICP-OES. The results are presented in %wt. and do not include oxygen.
The RSD of the determination using FPM was no greater than 7%. It should be noted that XRF-FPM is recommended for identification, as well as for semi-quantitative determination of the preliminary composition of ceramics containing REEs. The total analysis time did not exceed 10 min. For a more accurate quantitative determination of the elements, it is necessary to construct calibration curves.
3.2.2. The Results of XRF Analysis with Calibration Curves Using Oxide Mixtures
The calibration curve (Figure 4) shows that the intensity of the analytical signal for erbium, ytterbium, and niobium is subject to significant fluctuations. This is explained by insufficient mixing of the samples and the influence of particle size. The linear correlation coefficients are 0.9773 for Er, 0.9965 for Y, 0.9780 for Yb, and 0.9576 for Nb.
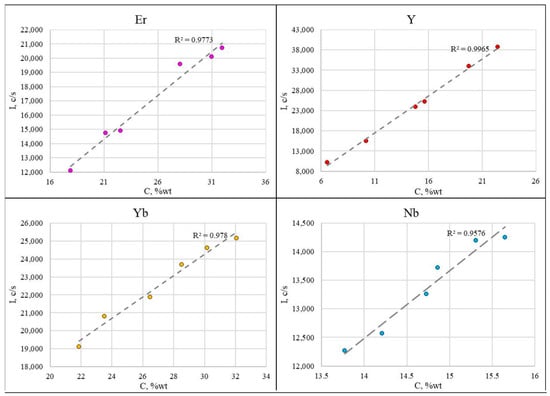
Figure 4.
The calibration curves obtained using oxide mixtures.
The results of the analysis of niobates with three REEs according to calibration curves using oxide mixtures are presented in Table 7.

Table 7.
Results of the determination of elements in niobates with 3 REEs using XRF analysis with calibration curves using oxide mixtures and the results of ICP-OES (n = 10; p = 0.95).
Calibration samples based on oxide mixtures do not fully meet the requirements for adequacy in the construction of calibration dependencies, and they are not consistent with ICP-OES. This is confirmed by t-test calculations and comparisons with the table value (t-test > 2.26 in all cases). It was decided to produce a calibration series of reference samples, which were synthesized in the same way as the study samples.
3.2.3. The Results of XRF with Calibration Curves Using Synthesized Calibration Samples
The calibration curves for the analysis of rare earth niobates are presented in Figure 5. The linear correlation coefficients are not less than 0.9965 for all elements.
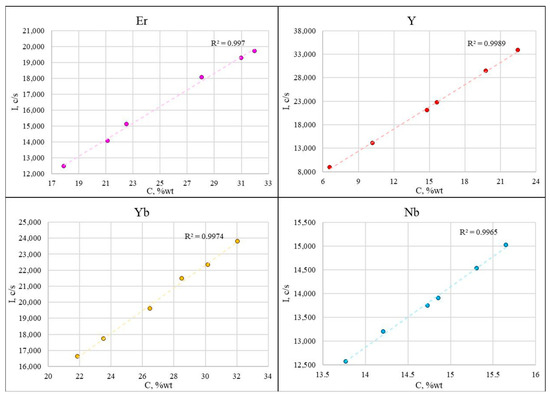
Figure 5.
The calibration curves obtained using synthesized calibration samples for analysis of niobates with 3 REEs.
Table 8 presents the results of the determination of Er, Y, Yb, and Nb in rare earth niobates, obtained by constructing calibration curves using synthesized calibration samples.

Table 8.
Results of the determination of elements in niobates with 2 (a) and 3 (b) REEs by using XRF analysis with calibration curves using synthesized calibration samples and the results of ICP-OES (n = 10; p = 0.95).
The accuracy assessment was carried out by comparing the results of determining the mass fraction of the analyzed components by comparing the results of the analysis of real samples with the data of an independent method (ICP-OES). The difference between the XRF and ICP-OES results was not statistically significant. This is confirmed by comparing the calculated t-tests with the table value (t-test < 2.62 for all elements).
The RSD of the obtained XRF measurements with the construction of a calibration dependence for the analysis of niobates with two REEs is in the range of 0.19–0.37% for Y, 0.29–0.47% for Yb, and 0.22–0.50% for Nb. The RSD for the analysis of niobates with three REEs is 0.19–0.39% for Er, 0.24–0.45% for Y, 0.28–0.42% for Yb, and 0.31–0.45% for Nb. The proposed methods for preparing calibration samples and constructing calibration curves for rare earth niobates make it possible to determine the major components in samples with sufficient precision and sensitivity for a given task.
4. Conclusions
Wavelength-dispersive XRF analysis can be applied to determine major elements in rare earth niobates. Er, Y, Yb, and Nb can be determined simultaneously. The Fundamental Parameter Method provides rapid semi-quantitative analysis. The synthesis of calibration samples contributes to the precision of the determination. It is essential that the calibration series be synthesized in the same way as the samples being studied, as this is the only way to ensure that the desired results will be obtained. The use of oxide mixtures to construct calibration curves does not guarantee the precision of the determination. A two-stage technique for elemental analysis was developed, and the conditions for performing XRF analysis (spectral lines, X-ray tube current, and exposure time) were selected. RSD did not exceed 0.50%.
Author Contributions
Conceptualization, A.A.A., G.E.M. and V.B.B.; methodology, M.S.D., G.E.M. and V.B.B.; software, M.S.D. and N.A.K.; validation, A.A.A., G.E.M. and M.S.D.; formal analysis, A.A.A., N.A.K. and T.D.K.; investigation, A.A.A., G.E.M. and V.B.B.; resources, M.A.R. and V.B.B.; data curation, A.A.A., G.E.M. and V.B.B.; writing—original draft preparation, A.A.A. and G.E.M.; writing—review and editing, A.A.A., N.A.K., T.D.K. and V.B.B.; visualization, M.S.D.; supervision, M.A.R. and V.B.B.; project administration, M.S.D.; funding acquisition, V.B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Russian Science Foundation, project № 20-13-00180-P.
Data Availability Statement
All data used to support the findings of this study are included within the article.
Acknowledgments
This research was performed using the equipment of the Research Equipment Sharing Center of Physical Methods for Studying Substances and Materials IGIC RAS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ege, A.; Özkan, A. Thermoluminescence kinetic parameters of yttrium niobate. Optik 2022, 259, 168983. [Google Scholar] [CrossRef]
- Oliveira, L.R.; Moscardini, S.B.; Molina, E.F.; Nassar, E.J.; Verelst, M.; Rocha, L.A. Effect of gadolinium incorporation on the structure and luminescence properties of niobium-based materials. Nanotechnology 2018, 29, 235204. [Google Scholar] [CrossRef] [PubMed]
- Matias, C.R.; Nassar, E.J.; Verelst, M.; Rocha, L.A. Synthesis and characterization of Nb2O5:La3+,Eu3+ phosphors obtained by the non-hydrolytic sol-gel process. J. Braz. Chem. Soc. 2015, 26, 2558–25570. [Google Scholar] [CrossRef]
- Xiao, X.; Yan, B. In-situ chemical co-precipitation composition of hybrid precursors and luminescence of Y1−xGdxNbO4:RE3+ (RE = Tb, Eu) micron crystalline phosphors. J. Alloys Compd. 2008, 456, 447–451. [Google Scholar] [CrossRef]
- Hsiao, Y.J.; Fang, T.H.; Chang, Y.S.; Chang, Y.H.; Liu, C.H.; Ji, L.W.; Jywe, W.Y. Structure and luminescent properties of LaNbO4 synthesized by sol–gel process. J. Lumin. 2007, 126, 866–870. [Google Scholar] [CrossRef]
- Doi, Y.; Harada, Y.; Hinatsu, Y. Crystal structures and magnetic properties of fluorite-related oxides Ln3NbO7 (Ln = lanthanides). J. Solid State Chem. 2009, 182, 709–715. [Google Scholar] [CrossRef]
- Wakeshima, M.; Hinatsu, Y. Magnetic properties and structural transitions of orthorhombic fluorite-related compounds Ln3MO7 (Ln = rare earths, M = transition metals). J. Solid State Chem. 2010, 183, 2681–2688. [Google Scholar] [CrossRef]
- Vente, J.F.; Helmholdt, R.B.; IJdo, D.J.W. The Structure and Magnetic Properties of Pr3MO7 with M = Nb, Ta, and Sb. J. Solid State Chem. 1994, 108, 18–23. [Google Scholar] [CrossRef]
- Abe, R.; Higashi, M.; Zou, Z.; Sayama, K.; Abe, Y.; Arakawa, H. Photocatalytic water splitting into H2 and O2 over R3TaO7 and R3NbO7 (R = Y, Yb, Gd, La): Effect of crystal structure on photocatalytic activity. J. Phys. Chem. B 2004, 108, 811–814. [Google Scholar] [CrossRef]
- Masloboeva, S.M.; Biryukova, I.V.; Efremov, I.N.; Teplyakova, N.A.; Palatnikov, M.N. Obtaining and studying photorefractive and optical properties of lithium niobate single crystal co-doped with gadolinium and boron. Opt. Quantum Electron. 2024, 56, 835. [Google Scholar] [CrossRef]
- Tang, X.-D.; Ye, H.-Q.; Liu, H.; Ma, C.-X.; Zhao, Z. A novel visible-light-driven photocatalyst Sm2InNbO7 for H2 or O2 evolution. Chem. Phys. Lett. 2009, 484, 48–53. [Google Scholar] [CrossRef]
- Cai, L.; Nino, J.C. Structure and dielectric properties of Ln3NbO7 (Ln = Nd, Gd, Dy, Er, Yb and Y). J. Eur. Ceram. Soc. 2007, 27, 3971–3976. [Google Scholar] [CrossRef]
- Wu, F.; Wu, P.; Zong, R.; Feng, J. Investigation on thermo-physical and mechanical properties of Dy3(Ta1-xNbx)O7 ceramics with order-disorder transition. Ceram. Int. 2019, 45, 15705–15710. [Google Scholar] [CrossRef]
- Wu, F.; Wu, P.; Zhou, Y.; Chong, X.; Feng, J. The thermo-mechanical properties and ferroelastic phase transition of RENbO4 (RE = Y, La, Nd, Sm, Gd, Dy, Yb) ceramics. J. Am. Ceram. Soc. 2020, 103, 2727–2740. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zheng, Q.; Feng, J. Structures, and Thermophysical Properties Characterizations of (La1-xHox)3NbO7 Solid Solutions as Thermal Barrier Coatings. Front. Mater. 2021, 8, 703098. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Hu, M.; Zhang, L.; Wang, J.; Zhang, Z.; Liang, X.; Guo, J.; Feng, J. Achieved limit thermal conductivity and enhancements of mechanical properties in fluorite RE3NbO7 via entropy engineering. Appl. Phys. Lett. 2021, 118, 071905. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Xiang, H.; Dai, F.-Z.; Wang, X.; Xu, W.; Sun, K.; Peng, Z.; Zhou, Y. High entropy defective fluorite structured rare earth niobates and tantalates for thermal barrier applications. J. Adv. Ceram. 2020, 9, 303–311. [Google Scholar] [CrossRef]
- Priscillal, I.J.D.; Wang, S.-F. Nanoengineered lanthanum niobate nanocaviar anchored carbon nanofibers for trace level detection of menadione in environmental samples. Environ. Res. 2023, 227, 115794. [Google Scholar] [CrossRef] [PubMed]
- Mielewczyk-Gryn, A.; Gdula-Kasica, K.; Kusz, B.; Gazda, M. High temperature monoclinic-to-tetragonal phase transition in magnesium doped lanthanum ortho-niobate. Ceram. Int. 2013, 39, 4239–4244. [Google Scholar] [CrossRef]
- Liao, J.; Nie, L.; Liu, S.; Liu, B.; Wen, H. Yb3+ concentration dependence of upconversion luminescence in Y2Sn2O7:Yb3+/Er3+ nanophosphors. J. Mater. Sci. 2014, 49, 6081–6086. [Google Scholar] [CrossRef]
- Song, D.; Kim, J.; Lyu, G.; Pyeon, J.; Jeon, H.-B.; Oh, Y.-S.; Song, T.; Paik, U.; Jung, Y.-G. Effect of cation substitution on thermophysical properties of fluorite A3BO7 ceramics. J. Alloys Compd. 2021, 883, 160848. [Google Scholar] [CrossRef]
- Li, B.; Gu, Z.; Lin, J.; Su, M.-Z. Photoluminescence of Eu3+-activated GdTaO4 with both M type and M′ type structures. J. Mater. Sci. 2000, 35, 1139–1143. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Photoluminescent Efficiency of Phosphors with Electronic Transitions in Localized Centers. J. Electrochem. Soc. 1968, 115, 1067. [Google Scholar] [CrossRef]
- Massabni, A.M.G.; Montandon, G.J.M.; Couto dos Santos, M.A. Synthesis and Luminescence Spectroscopy of YNbO4 Doped with Eu(III). Mater. Res. 1998, 1, 1–4. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Huang, C.; Nieto, A.; Chen, R.; Luo, J. From high-entropy ceramics to Compositionally Complex ceramics: A case study of fluorite oxides. J. Eur. Ceram. Soc. 2020, 40, 2120–2129. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Ko, S.-T.; Chung, K.M.; Chen, R.; Luo, J. Size disorder as a descriptor for predictingreduced thermal conductivity in medium- and high-entropy pyrochlores. Scr. Mater. 2020, 181, 76–81. [Google Scholar] [CrossRef]
- Siqueira, K.P.F.; Soares, J.C.; Granado, E.; Bittar, E.M.; de Paula, A.M.; Moreira, R.L.; Dias, A. Synchrotron X-ray diffraction and Raman spectroscopy of Ln3NbO7 (Ln = La, Pr, Nd, Sm-Lu) ceramics obtained by molten-salt synthesis. J. Solid State Chem. 2014, 209, 63–68. [Google Scholar] [CrossRef]
- Schramm, R. Use of X-ray Fluorescence Analysis for the Determination of Rare Earth Elements. Phys. Sci. Rev. 2016, 1, 20160061. [Google Scholar] [CrossRef]
- Sitko, R.; Zawisza, B.; Czaja, M. Fundamental parameters method for determination of rare earth elements in apatites by wavelength-dispersive X-ray fluorescence spectrometry. J. Anal. At. Spectrom. 2005, 20, 741–745. [Google Scholar] [CrossRef]
- Wu, W.; Xu, T.; Hao, Q.; Wang, Q.; Zhang, S.; Zhao, C. Applications of X-ray fluorescence analysis of rare earths in China. J. Rare Earths 2010, 28, 30–36. [Google Scholar] [CrossRef]
- Bondarenko, A.V.; Belonovsky, A.V.; Katzman, Y.M. Application of fundamental parameter methodin X-ray fluorescence analysis of pulp productsin ore concentration. Mining 2021, 5, 84–88. [Google Scholar]
- Abramov, A.V.; Polovov, I.B. Method for Determining Mass Fractions of Principal and Impurity Elements in Salt Fluoride Systems by X-ray Fluorescence Analysis. RU Patent 2772103, 16 May 2022. [Google Scholar]
- Guskov, A.V.; Gagarin, P.G.; Guskov, V.N.; Tyurin, A.V.; Khoroshilov, A.V.; Gavrichev, K.S. Thermodynamic Properties of Gadolinium Tantalate Gd3TaO7. Russ. J. Phys. Chem. A 2022, 96, 1195–1203. [Google Scholar] [CrossRef]
- Gagarin, P.G.; Guskov, A.V.; Guskov, V.N.; Nikiforova, G.E.; Khoroshilov, A.V.; Tyurin, A.V.; Gavrichev, K. S Sm3TaO7: Heat capacity and thermal expansion. Russ. J. Inorg. Chem. 2022, 67, 2183–2192. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Hu, C.; Yeh, Y.-T.; Chen, R.; Luo, J. Single-phase duodenary high-entropy fluorite/pyrochlore oxides with an order-disorder transition. Acta Mater. 2021, 211, 116858. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Yeh, Y.-T.; Zhang, D.; Everett, M.; Neuefeind, J.; Chen, R.; Luo, J. Short-range order and origin of the low thermal conductivity in compositionally complex rare-earth niobates and tantalates. Acta Mater. 2022, 235, 118056. [Google Scholar] [CrossRef]
- DeKalb, E.L.; Fassel, V.A. Optical Atomic Emission and Absorption Methods; Gschneidner, K.A., Jr., Eyring, L., Eds.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1979; Volume 4, pp. 405–440. [Google Scholar] [CrossRef]
- Jalas, P.; Ruottinen, J.-P.; Hemminki, S. XRF Analysis of jewelry using fully standardless fundamental parameter approach. Gold Technol. 2022, 35, 28–34. [Google Scholar]
- Otto, M. Chemometrics: Statistics and Computer Application in Analytical Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2023; 432p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).