Enteromorpha compressa Macroalgal Biomass Nanoparticles as Eco-Friendly Biosorbents for the Efficient Removal of Harmful Metals from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
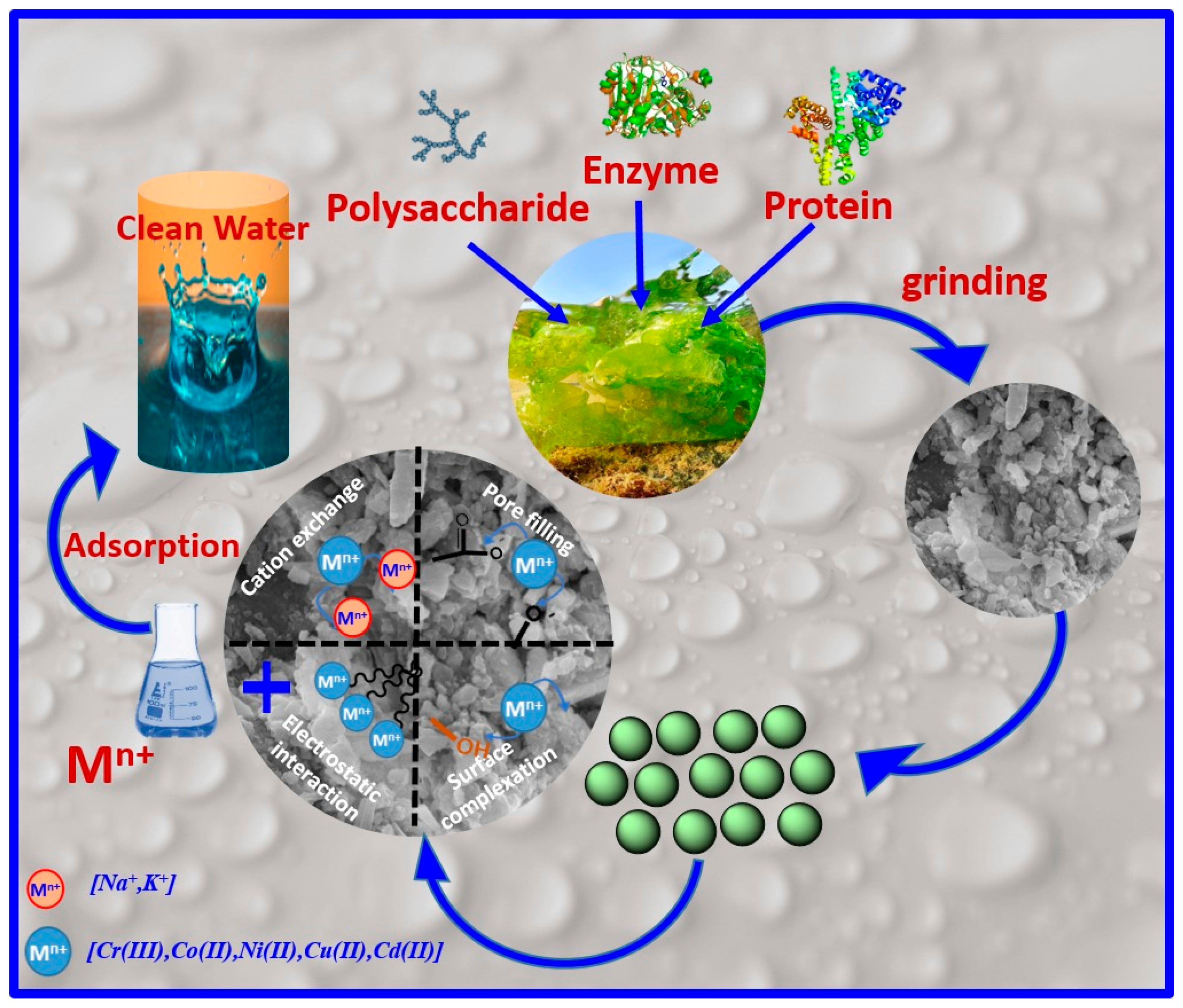
2.1. Preparation of Biosorbent
2.2. Metal Solution Preparation
2.3. Biosorbent Characterization
2.4. Investigation of Metal Biosorption
2.4.1. Kinetic and Adsorption Isotherm Investigations
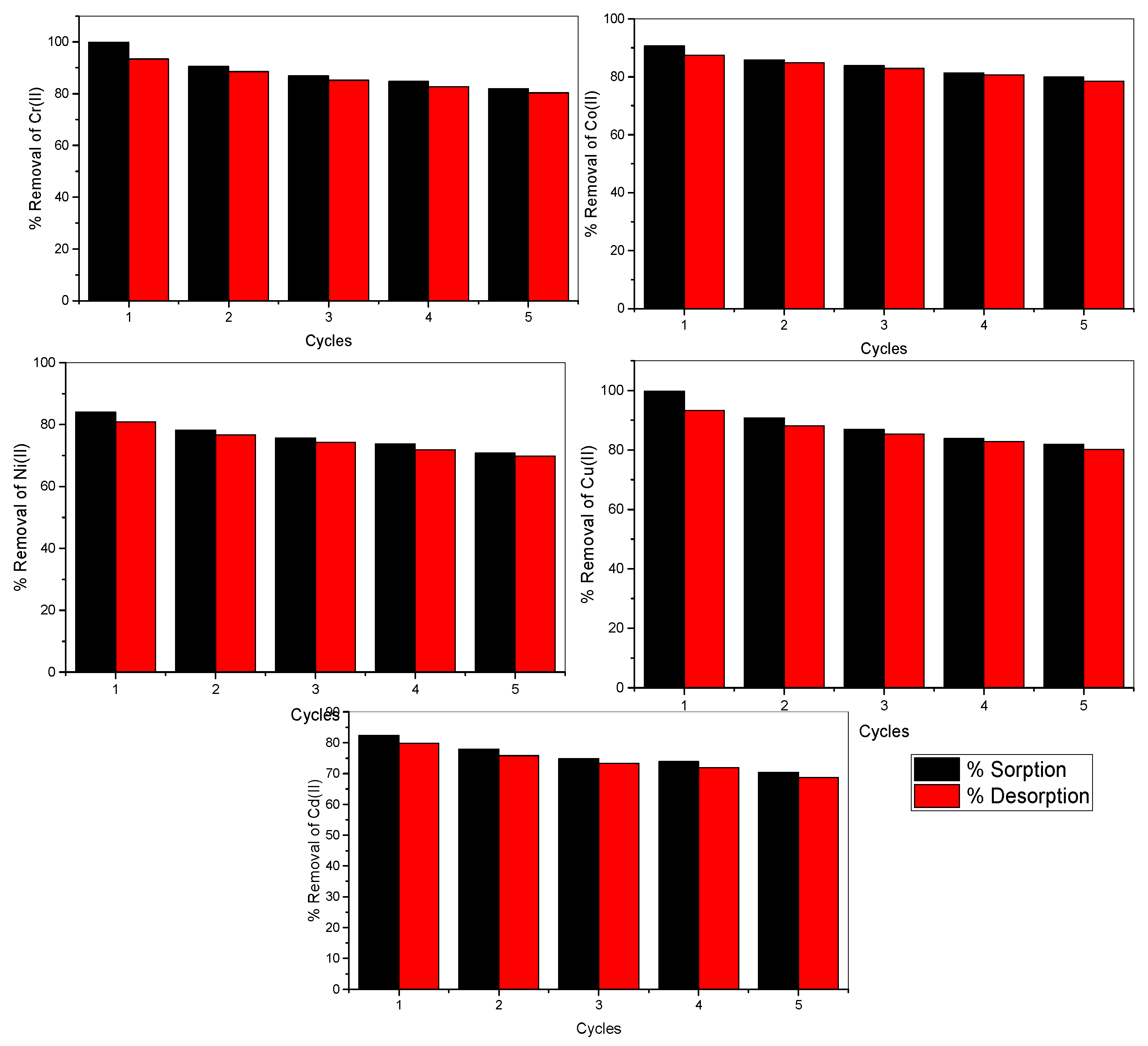
2.4.2. Regeneration and Desorption
3. Results and Discussion
3.1. Biomass Characterization
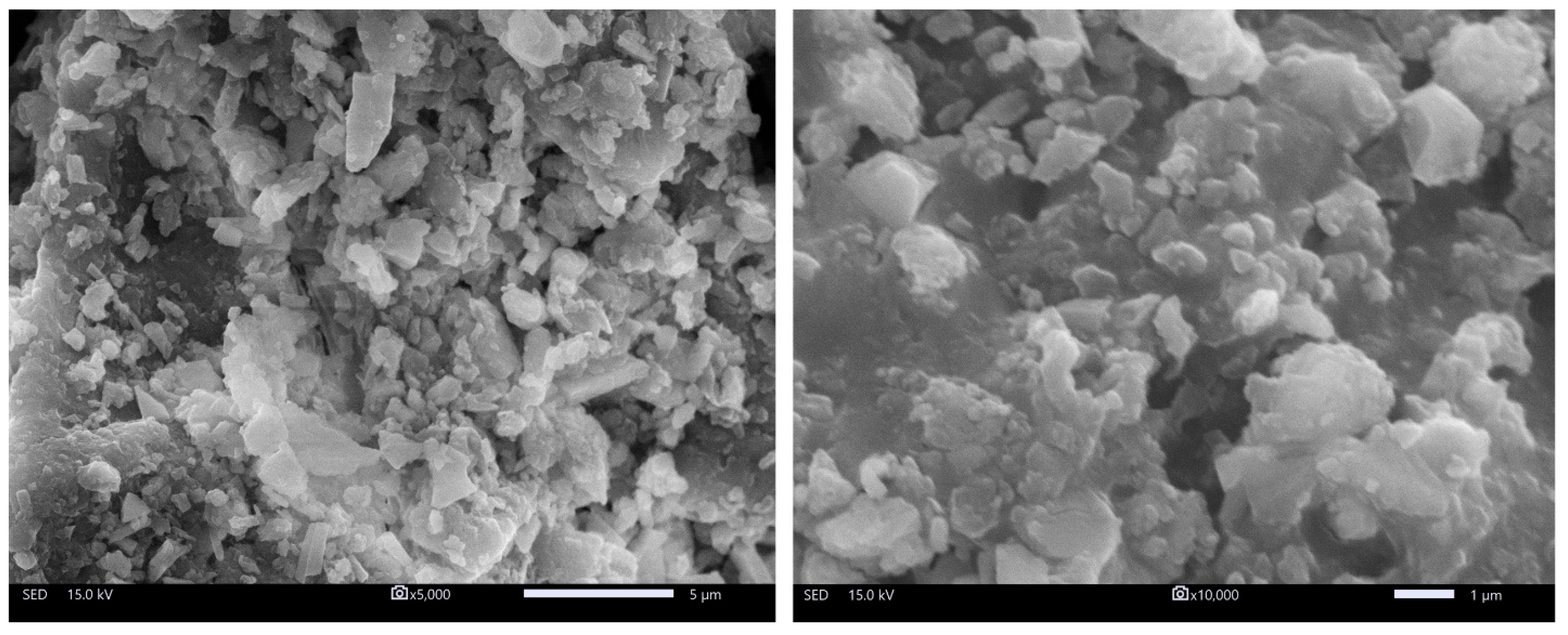
3.1.1. Surface Morphology Assessment through SEM Analysis
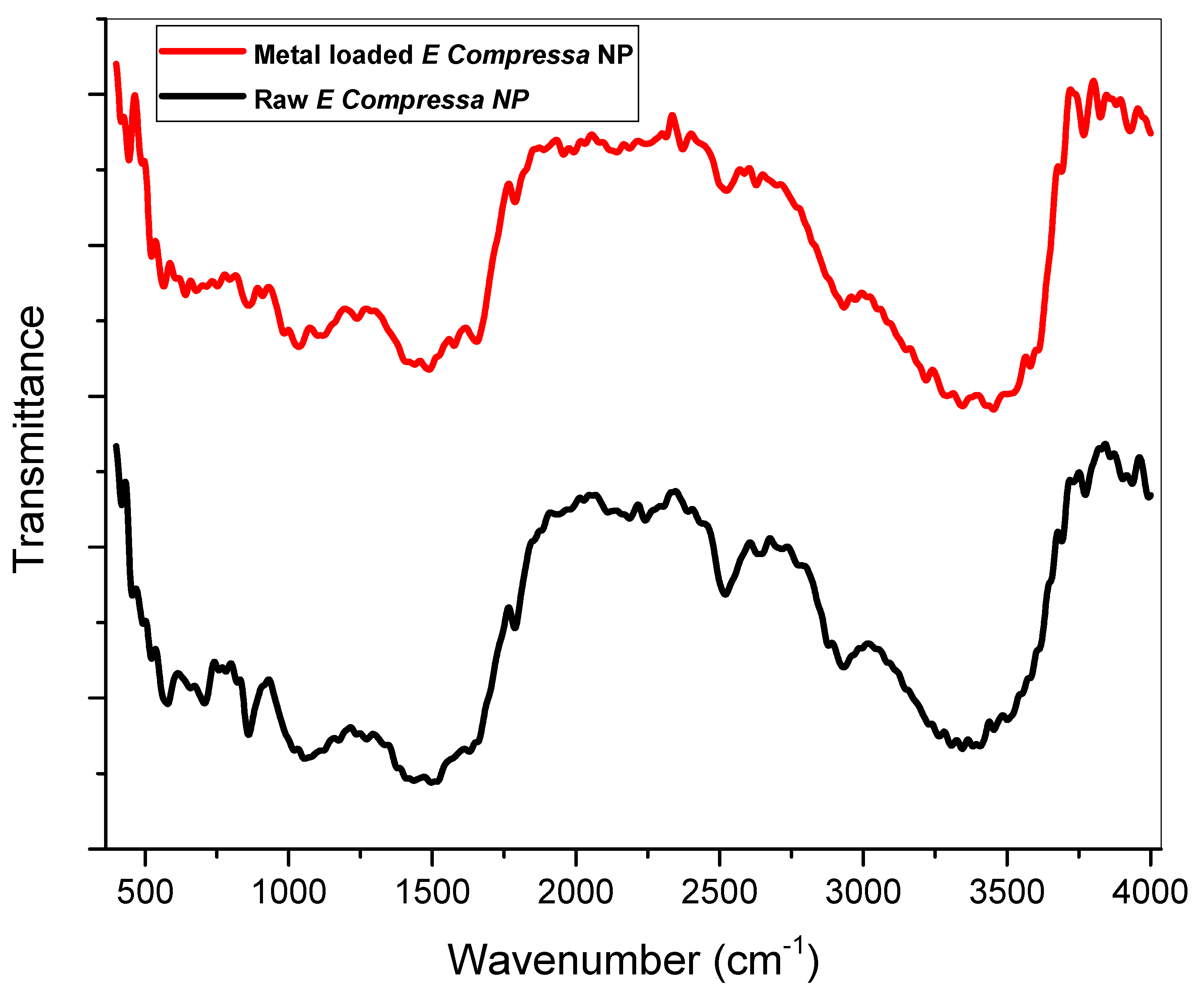
3.1.2. Fourier Transform Infrared Spectroscopy (FT-IR)
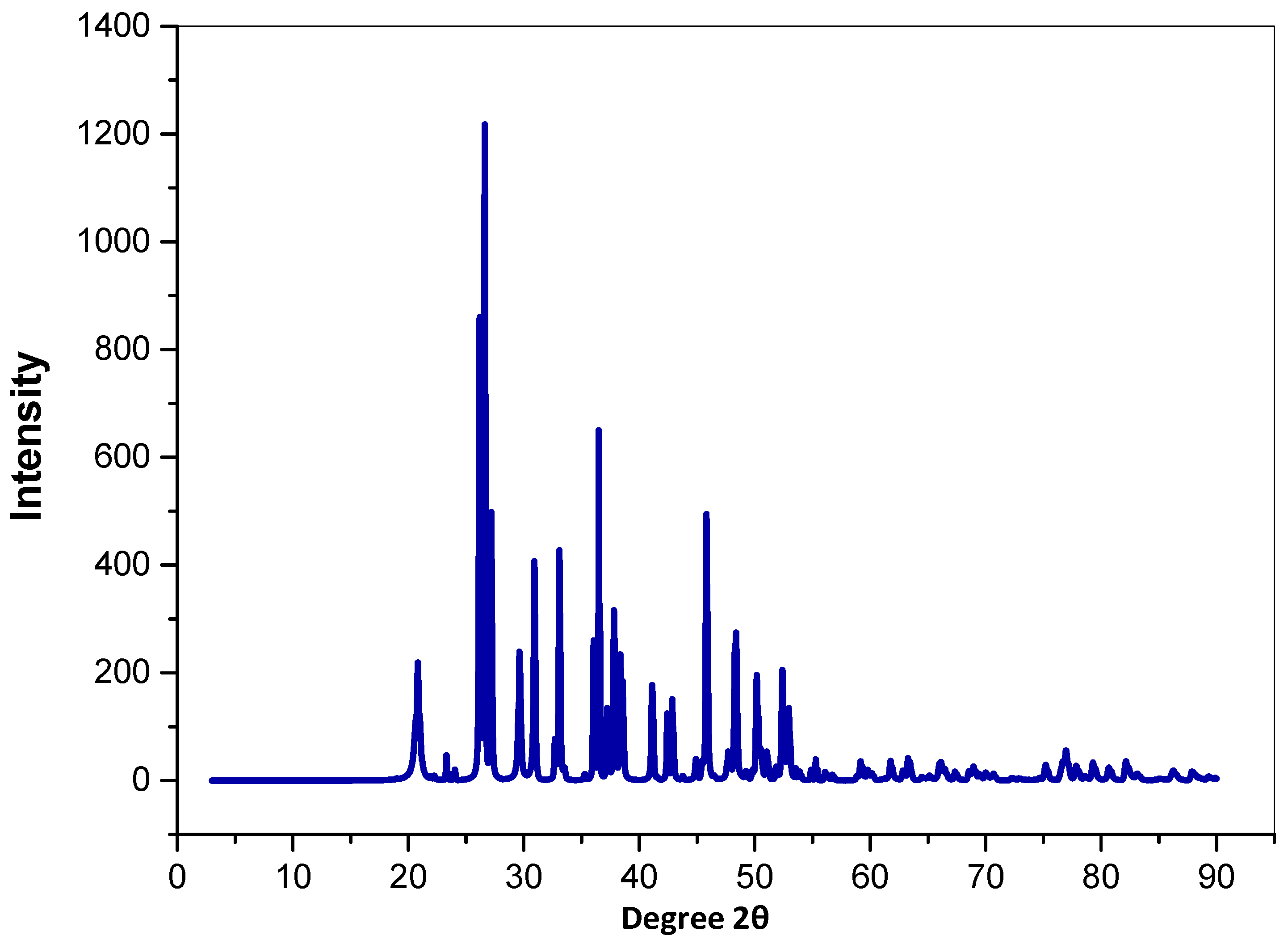
3.1.3. X-ray Diffraction (XRD)
3.2. Investigating Biosorption
3.2.1. pH Effects on Metal Biosorption
3.2.2. Implications of Biosorbent Dosage
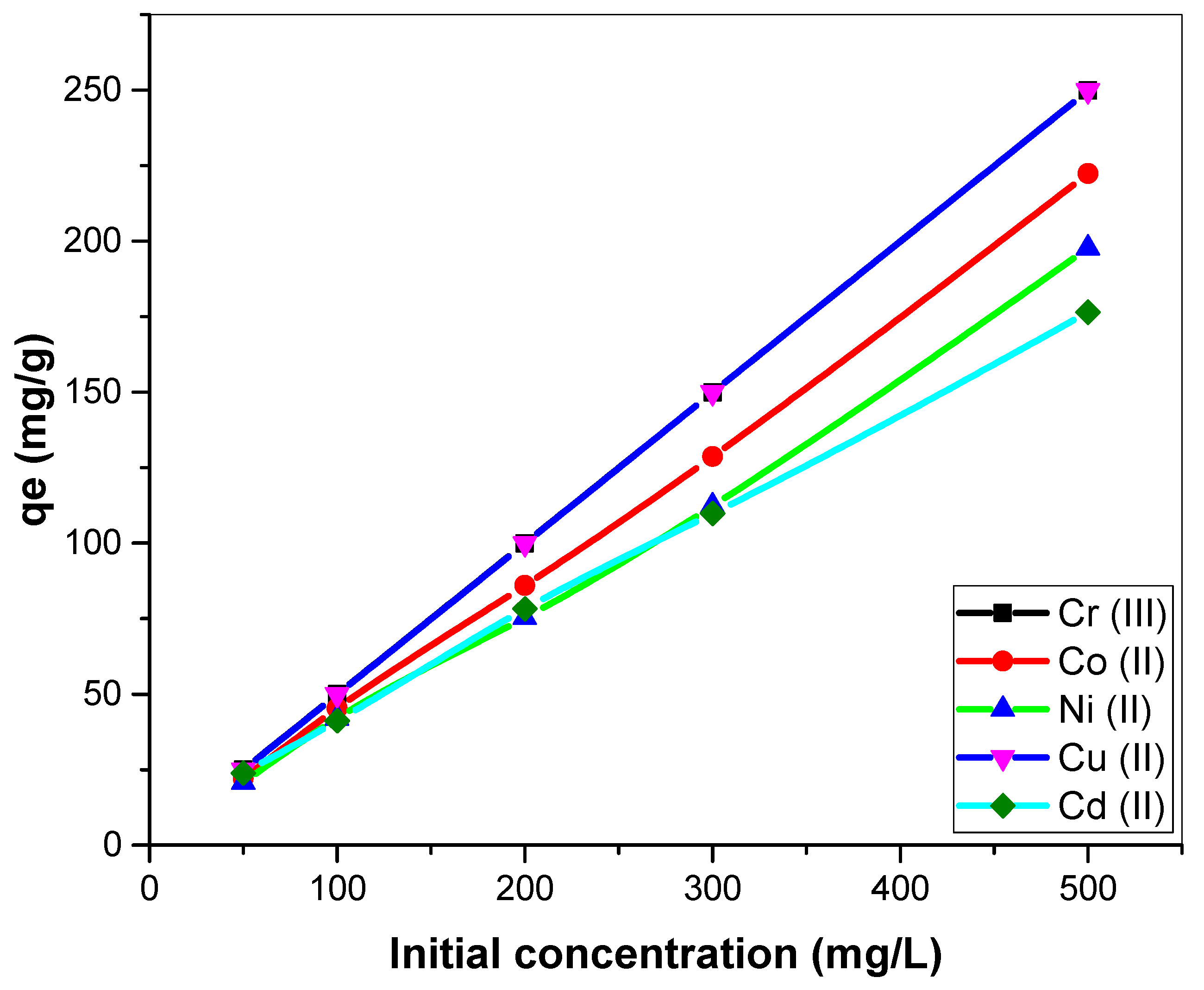
3.2.3. Effect of Initial Metal Ion Concentration on Biosorption
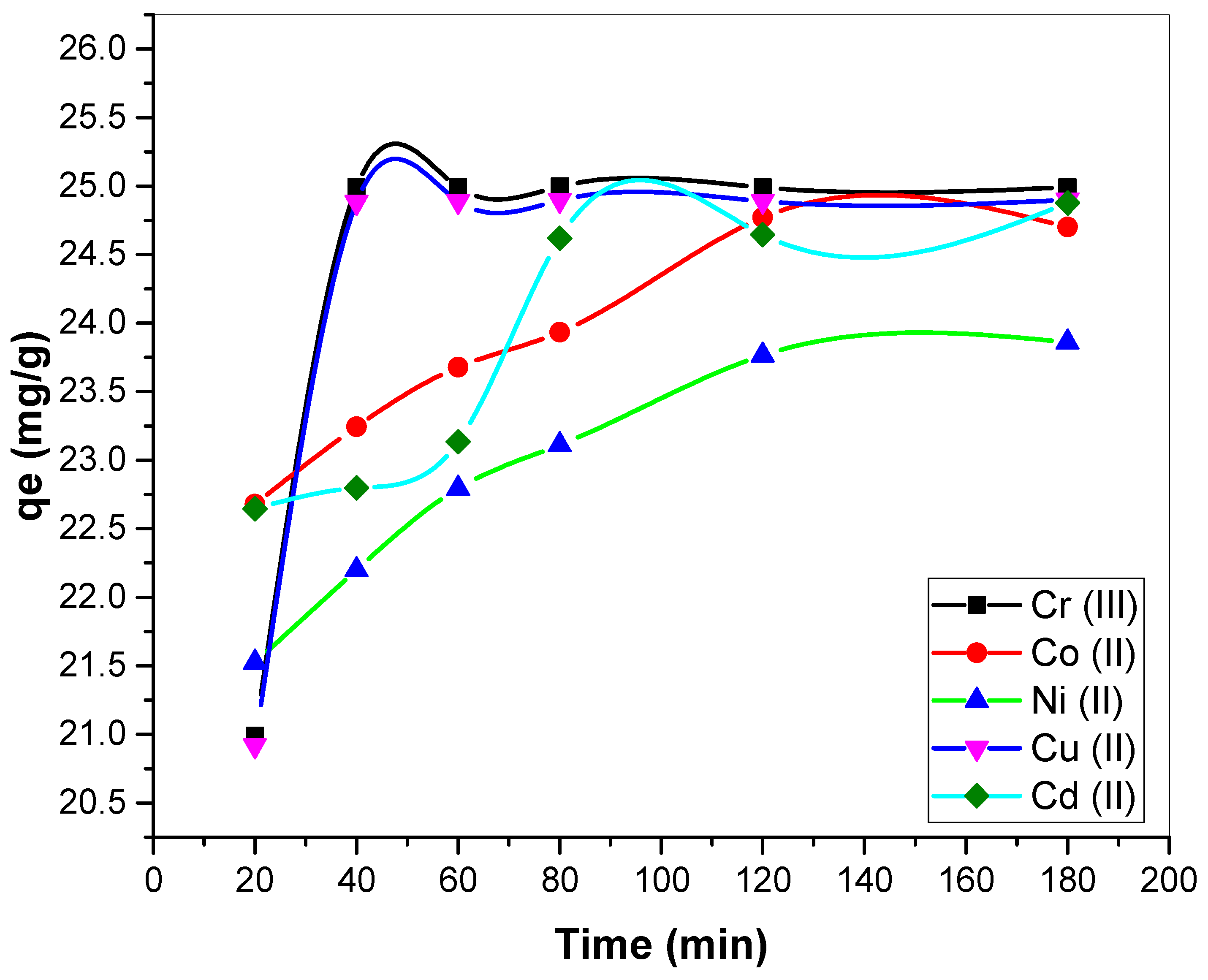
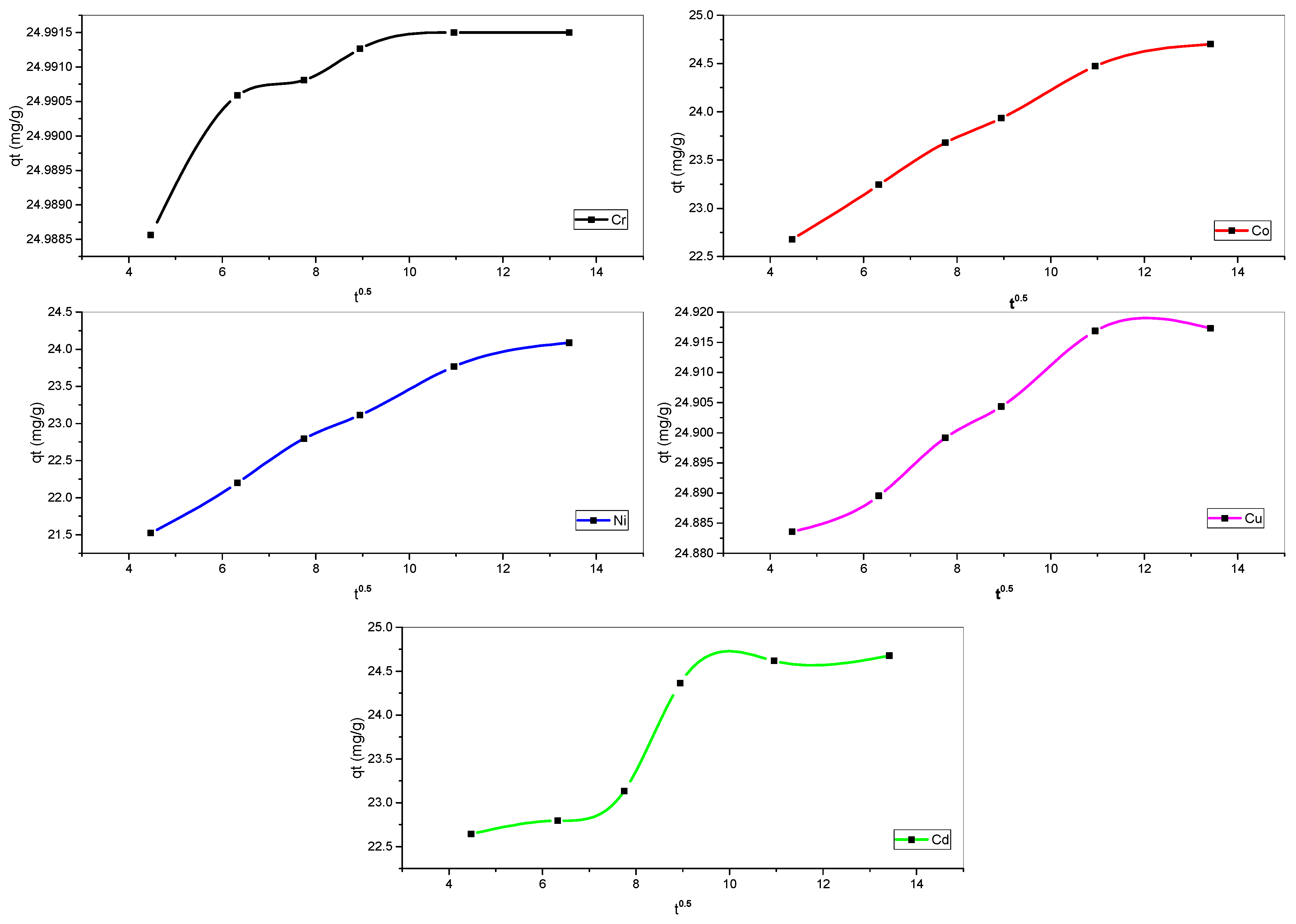
3.2.4. Influence of Reaction Time on Metal Biosorption
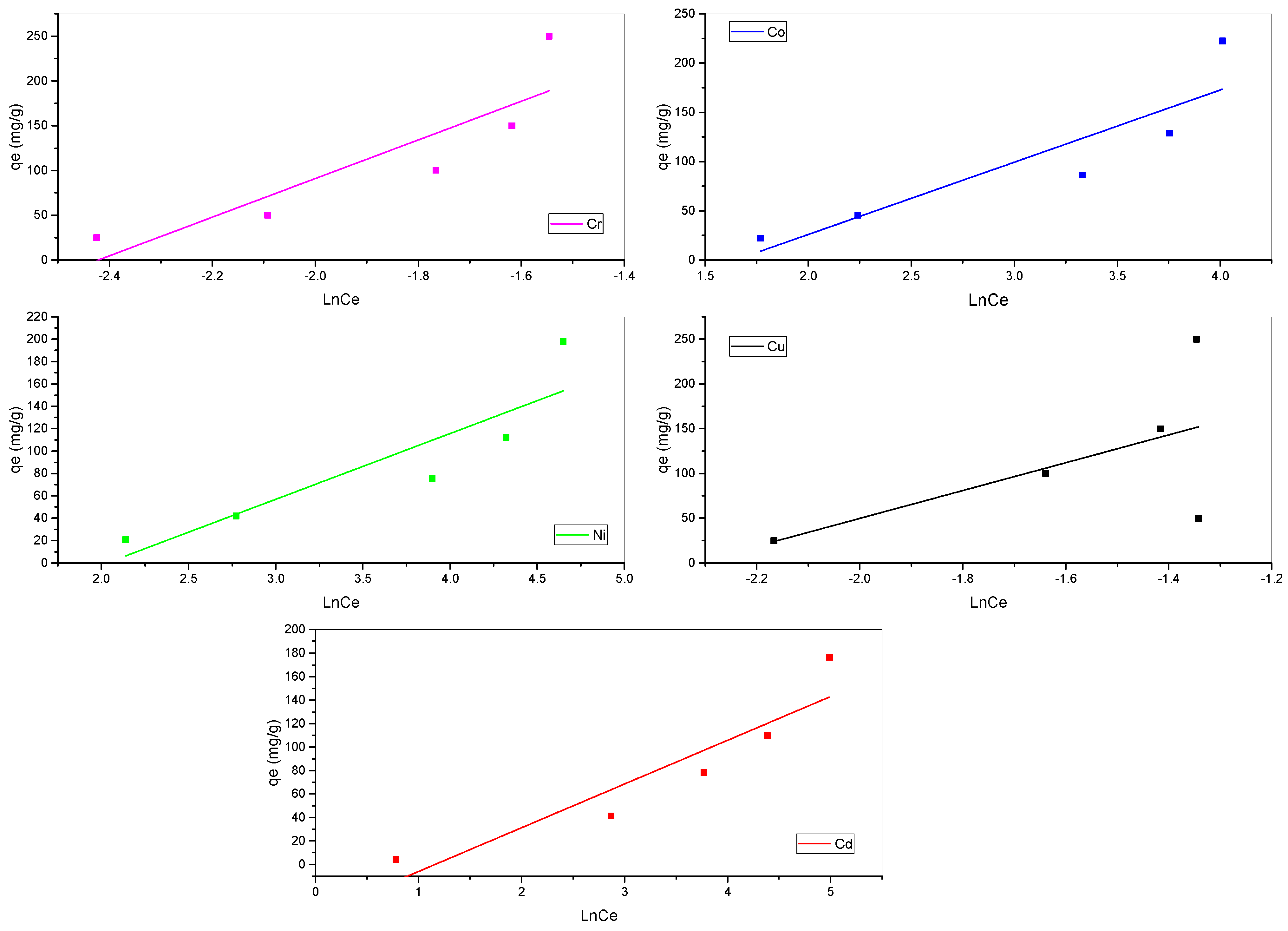
3.3. Biosorption Isotherm Models
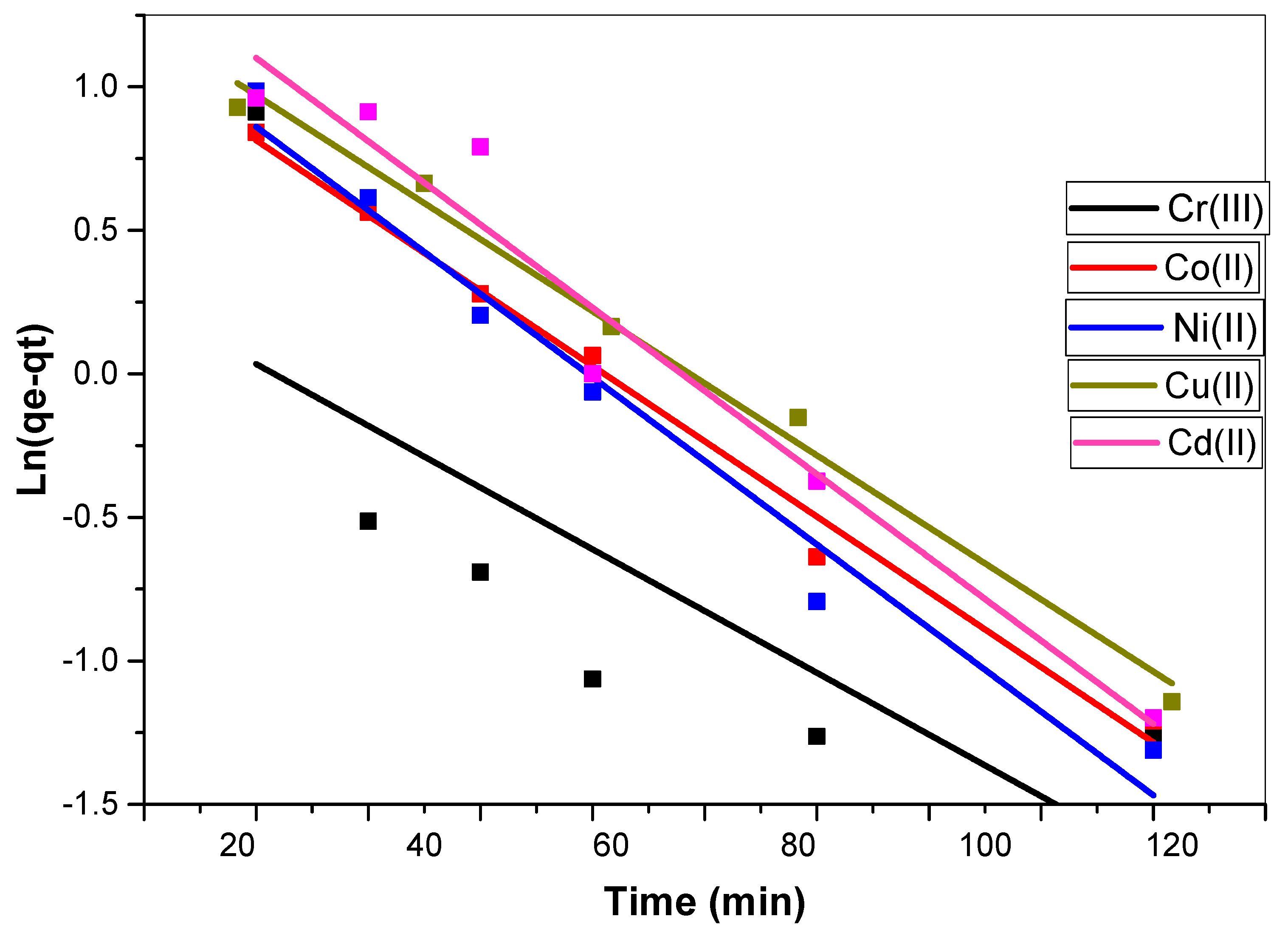
3.3.1. Kinetic Models of Biosorption
3.3.2. Adsorption Mechanism
3.3.3. Desorption and Reusability of E. compressa Biomass Nanoparticles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younis, A.M. Accumulation and rate of degradation of organotin compounds in coastal sediments along the Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 413–436. [Google Scholar] [CrossRef]
- Hanafy, S.; Younis, A.M.; El-Sayed, A.; Ghandour, M.A. Spatial, seasonal distribution and ecological risk assessment of Zn, Cr, and Ni in Red Sea surface sediments, Egypt. Egypt. J. Aquat. Biol. Fish. 2021, 25, 413–438. [Google Scholar]
- USEPA. National Center for Environmental Assessment; USEPA: Washington, DC, USA, 1999.
- Lakherwal, D. Adsorption of heavy metals: A review. Int. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Younis, A.M.; Hanafy, S.; Elkady, E.M.; Ghandour, M.A.; El-Sayed, A.-A.Y.; Alminderej, F.M. Polycyclic aromatic hydrocarbons (PAHs) in Egyptian red sea sediments: Seasonal distribution, source Identification, and toxicological risk assessment. Arab. J. Chem. 2023, 16, 104999. [Google Scholar] [CrossRef]
- Elkady, E.M.; Younis, A.M.; El-Naggar, M.H. Investigating the Biosorption Potential of Ulva intestinalis Linnaeus for Efficient Removal of Phenol from Aqueous Solutions. Egypt. J. Aquat. Biol. Fish. 2023, 27, 411. [Google Scholar] [CrossRef]
- Boretti, A.; Rosa, L. Reassessing the projections of the world water development report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Sweetly, D.J.; Sangeetha, K.; Suganthi, B. Biosorption of heavy metal lead from aqueous solution by non-living biomass of Sargassum myriocystum. Inter. J. Appl. Innov. Eng. Manag. 2014, 3, 39–45. [Google Scholar]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Iordache, M.; Iordache, A.M.; Sandru, C.; Voica, C.; Zgavarogea, R.; Miricioiu, M.G.; Ionete, R.E. Assessment of heavy metals pollution in sediments from reservoirs of the olt river as tool for environmental risk management. Rev. Chim. 2019, 70, 4153–4162. [Google Scholar]
- Khan, S.; Waqas, M.; Ding, F.; Shamshad, I.; Arp, H.P.H.; Li, G. The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J. Hazard. Mater. 2015, 300, 243–253. [Google Scholar] [CrossRef]
- Türker, A.R. Separation, preconcentration and speciation of metal ions by solid phase extraction. Sep. Purif. Rev. 2012, 41, 169–206. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Na, Z.; Lu, D.; Liu, Z. Adsorption, concentration, and recovery of aqueous heavy metal ions with the root powder of Eichhornia crassipes. Ecol. Eng. 2013, 60, 160–166. [Google Scholar] [CrossRef]
- Al-Ghanim, K.; Mahboob, S.; Vijayaraghavan, P.; Al-Misned, F.; Kim, Y.O.; Kim, H.-J. Sub-lethal effect of synthetic pyrethroid pesticide on metabolic enzymes and protein profile of non-target Zebra fish, Danio rerio. Saudi J. Biol. Sci. 2020, 27, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, P.; Lourthuraj, A.A.; Arasu, M.V.; AbdullahAl-Dhabi, N.; Ravindran, B.; WoongChang, S. Effective removal of pharmaceutical impurities and nutrients using biocatalyst from the municipal wastewater with moving bed packed reactor. Environ. Res. 2021, 200, 111777. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, Y.; Rezgui, A.; Dekhil, A.B.; Boubaker, T. Removal of cadmium (II) from aqueous solutions by biosorption onto the brown macroalga (Dictyota dichotoma). Desalination Water Treat. 2015, 54, 1663–1673. [Google Scholar] [CrossRef]
- Younis, A.M.; Kolesnikov, A.V.; Elkady, E.M. Phycoremediation of Phenolic Compounds in Wastewater: Ecological Impacts, Mitigation Strategies, and Process Mechanisms. Egypt. J. Aquat. Biol. Fish. 2023, 27, 1133–1170. [Google Scholar] [CrossRef]
- Younis, A.M.; Elkady, E.M.; El-Naggar, M. Biosorption of Arsenic (III) and Arsenic (V) from Aqueous Solutions: Equilibrium and Kinetic Studies using Mangrove Leaf Biomass (Avicennia marina). Egypt. J. Aquat. Biol. Fish. 2023, 27, 477–497. [Google Scholar] [CrossRef]
- Henriques, B.; Rocha, L.S.; Lopes, C.B.; Figueira, P.; Duarte, A.; Vale, C.; Pardal, M.; Pereira, E. A macroalgae-based biotechnology for water remediation: Simultaneous removal of Cd, Pb and Hg by living Ulva lactuca. J. Environ. Manag. 2017, 191, 275–289. [Google Scholar] [CrossRef]
- Mazur, L.P.; Cechinel, M.A.; de Souza, S.M.G.U.; Boaventura, R.A.; Vilar, V.J. Brown marine macroalgae as natural cation exchangers for toxic metal removal from industrial wastewaters: A review. J. Environ. Manag. 2018, 223, 215–253. [Google Scholar] [CrossRef]
- Ali, H.S.; Kandil, N.; Ibraheem, I. Biosorption of Pb2+ and Cr3+ ions from aqueous solution by two brown marine macroalgae: An equilibrium and kinetic study. Desalination Water Treat. 2020, 206, 250–262. [Google Scholar] [CrossRef]
- Naskar, S.; Biswas, G.; Kumar, P.; De, D.; Das, S.; Sawant, P.B.; Chadha, N.K.; Behera, P. The green seaweed, Enteromorpha intestinalis: An efficient inorganic extractive species for environmental remediation and improved performances of fed species in brackishwater integrated multi-trophic aquaculture (BIMTA) system. Aquaculture 2023, 569, 739359. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Weber Jr, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, A.K.; Sikandar, M. Biosorption of Hg (II) from aqueous solution using algal biomass: Kinetics and isotherm studies. Heliyon 2020, 6, e03321. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.C.; Ungureanu, G.; Volf, I.; Boaventura, R.A.; Botelho, C.M. Macroalgae biomass as sorbent for metal ions. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018; pp. 69–112. [Google Scholar]
- Kyzioł-Komosińska, J.; Augustynowicz, J.; Lasek, W.; Czupioł, J.; Ociński, D. Callitriche cophocarpa biomass as a potential low-cost biosorbent for trivalent chromium. J. Environ. Manag. 2018, 214, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Vafajoo, L.; Cheraghi, R.; Dabbagh, R.; McKay, G. Removal of cobalt (II) ions from aqueous solutions utilizing the pre-treated 2-Hypnea Valentiae algae: Equilibrium, thermodynamic, and dynamic studies. Chem. Eng. J. 2018, 331, 39–47. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P.; Singh, V.K. Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. J. Hazard. Mater. 2006, 135, 280–295. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Khan, T.A.; Ali, I. Equilibrium, kinetic and thermodynamic studies of Cr (VI) adsorption from aqueous solution onto manganese oxide coated sand grain (MOCSG). J. Mol. Liq. 2017, 236, 320–330. [Google Scholar] [CrossRef]
- Deniz, F.; Karabulut, A. Biosorption of heavy metal ions by chemically modified biomass of coastal seaweed community: Studies on phycoremediation system modeling and design. Ecol. Eng. 2017, 106, 101–108. [Google Scholar] [CrossRef]
- Hackbarth, F.V.; Girardi, F.; de Souza, S.M.G.U.; de Souza, A.A.U.; Boaventura, R.A.; Vilar, V.J. Marine macroalgae Pelvetia canaliculata (Phaeophyceae) as a natural cation exchanger for cadmium and lead ions separation in aqueous solutions. Chem. Eng. J. 2014, 242, 294–305. [Google Scholar] [CrossRef]
- Akar, T.; Kaynak, Z.; Ulusoy, S.; Yuvaci, D.; Ozsari, G.; Akar, S.T. Enhanced biosorption of nickel (II) ions by silica-gel-immobilized waste biomass: Biosorption characteristics in batch and dynamic flow mode. J. Hazard. Mater. 2009, 163, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Asnaoui, H.; Laaziri, A.; Khalis, M. Biosorption of chromium (Cr) onto algae (Ulva. lactuca): Application of isotherm and kinetic models. Moroc. J. Chem. 2017, 5, 2186–2195. [Google Scholar]
- Khalifa, E.B.; Rzig, B.; Chakroun, R.; Nouagui, H.; Hamrouni, B. Application of response surface methodology for chromium removal by adsorption on low-cost biosorbent. Chemom. Intell. Lab. Syst. 2019, 189, 18–26. [Google Scholar] [CrossRef]
- Khambhaty, Y.; Mody, K.; Basha, S.; Jha, B. Kinetics, equilibrium and thermodynamic studies on biosorption of hexavalent chromium by dead fungal biomass of marine Aspergillus niger. Chem. Eng. J. 2009, 145, 489–495. [Google Scholar] [CrossRef]
- Nunez-Gomez, D.; Rodrigues, C.; Lapolli, F.R.; Lobo-Recio, M.A. Adsorption of heavy metals from coal acid mine drainage by shrimp shell waste: Isotherm and continuous-flow studies. J. Environ. Chem. Eng. 2019, 7, 102787. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Biosorption performance of date palm empty fruit bunch wastes for toxic hexavalent chromium removal. Environ. Res. 2020, 187, 109694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, H.; Lv, X.; Tang, J.; Xu, X. Removal of Cu (II) from aqueous solution using the rice husk carbons prepared by the physical activation process. Biomass Bioenergy 2011, 35, 464–472. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Suganya, E.; Lity, A.V.; Selvaraju, N. Equilibrium and kinetics studies of hexavalent chromium biosorption on a novel green macroalgae Enteromorpha sp. Res. Chem. Intermed. 2016, 42, 1275–1294. [Google Scholar] [CrossRef]
- Jayakumar, V.; Govindaradjane, S.; Rajasimman, M. Isotherm and kinetic modeling of sorption of cadmium onto a novel red algal sorbent, Hypnea musciformis. Model. Earth Syst. Environ. 2019, 5, 793–803. [Google Scholar] [CrossRef]
- Saleh, S.M.; Younis, A.M.; Ali, R.; Elkady, E.M. A novel and eco-friendly Algae amino-modified nanoparticles with significant environmental effect for the removal of As (III) and As (V) from water. Environ. Adv. 2024, 16, 100550. [Google Scholar] [CrossRef]
- Xing, W.; Liu, Q.; Wang, J.; Xia, S.; Ma, L.; Lu, R.; Zhang, Y.; Huang, Y.; Wu, G. High selectivity and reusability of biomass-based adsorbent for chloramphenicol removal. Nanomaterials 2021, 11, 2950. [Google Scholar] [CrossRef]
- Younis, A.M.; Aly-Eldeen, M.A.; M Elkady, E. Effect of different molecular weights of chitosan on the removal efficiencies of heavy metals from contaminated water. Egypt. J. Aquat. Biol. Fish. 2019, 23, 149–158. [Google Scholar] [CrossRef]
- Jayakumar, V.; Govindaradjane, S.; Rajamohan, N.; Rajasimman, M. Biosorption potential of brown algae, Sargassum polycystum, for the removal of toxic metals, cadmium and zinc. Environ. Sci. Pollut. Res. 2021, 29, 41909–41922. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Shukla, M.K.; Mishra, A.; Kumari, P.; Reddy, C.; Jha, B. Isolation and characterization of exopolysaccharides from seaweed associated bacteria Bacillus licheniformis. Carbohydr. Polym. 2011, 84, 1019–1026. [Google Scholar] [CrossRef]
- El-Wakeel, S.T.; Moghazy, R.; Labena, A.; Husien, S. Algal biosorbent as a basic tool for heavy metals removal; the first step for further applications. J. Mater. Environ. Sci 2019, 10, 75–87. [Google Scholar]
- Farajzadeh, M.A.; Monji, A.B. Adsorption characteristics of wheat bran towards heavy metal cations. Sep. Purif. Technol. 2004, 38, 197–207. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R. Bio-sorption of toxic metals from industrial wastewater by algae strains Spirulina platensis and Chlorella vulgaris: Application of isotherm, kinetic models and process optimization. Sci. Total Environ. 2021, 755, 142654. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.E.; Quesada, A. Nickel biosorption by Acinetobacter baumannii and Pseudomonas aeruginosa isolated from industrial wastewater. Braz. J. Microbiol. 2006, 37, 465–467. [Google Scholar] [CrossRef]
- Bulgariu, L.; Bulgariu, D. Enhancing biosorption characteristics of marine green algae (Ulva lactuca) for heavy metals removal by alkaline treatment. J. Bioprocess. Biotech. 2014, 4, 1. [Google Scholar] [CrossRef]
- Pham, B.N.; Kang, J.-K.; Lee, C.-G.; Park, S.-J. Removal of heavy metals (Cd2+, Cu2+, Ni2+, Pb2+) from aqueous solution using Hizikia fusiformis as an algae-based bioadsorbent. Appl. Sci. 2021, 11, 8604. [Google Scholar] [CrossRef]


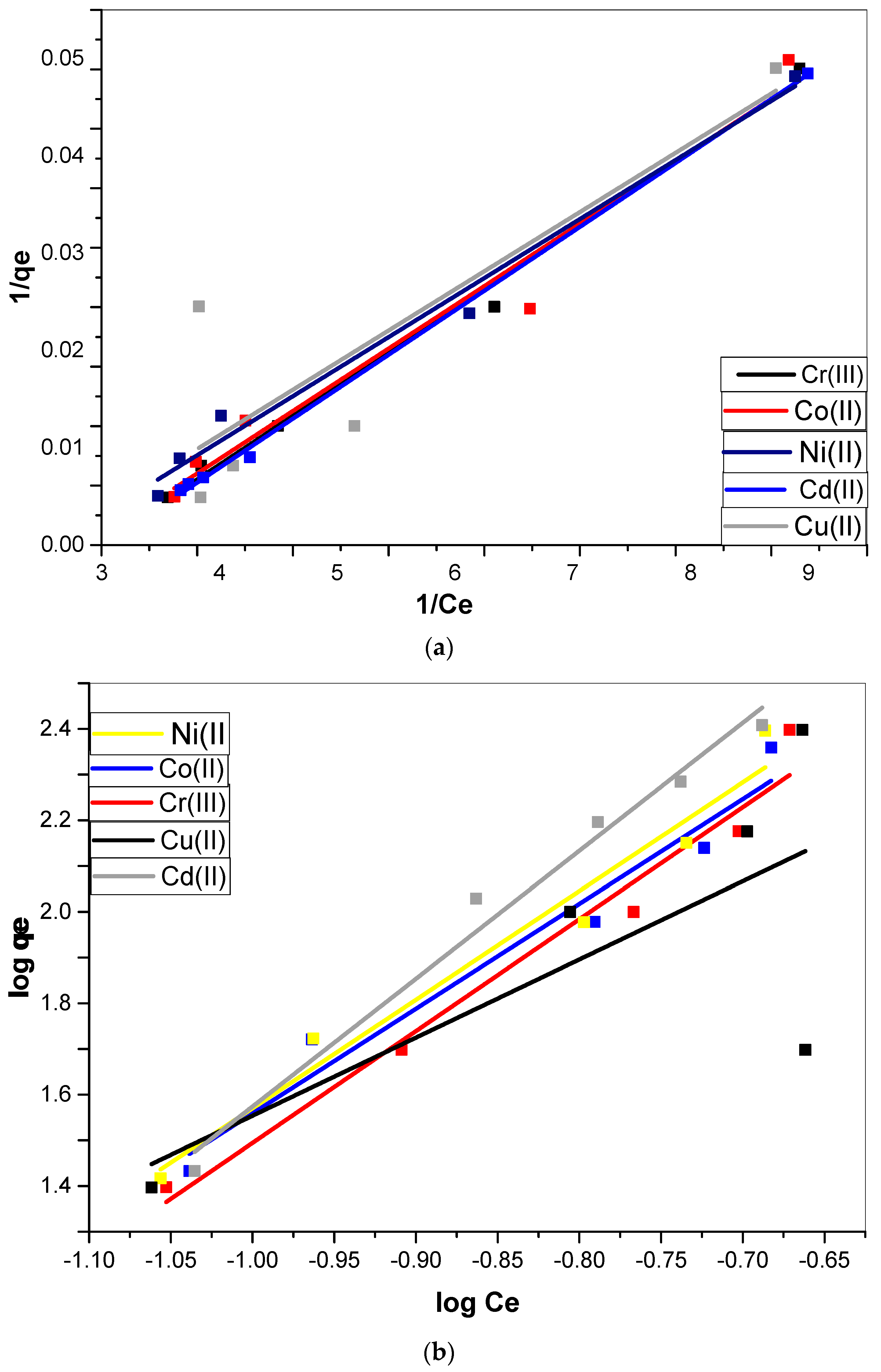

| Equilibrium Model | Parameters | Ni(II) | Co(II) | Cr(III) | Cu(II) | Cd(II) |
|---|---|---|---|---|---|---|
| Langmuir | qm (mg·g−1) | 201.6129 | 217.5668 | 249.1901 | 201.6129 | 172.4138 |
| KL (mg·g−1) | 0.013279 | 0.018718 | 0.752908 | 0.661175 | 0.011124 | |
| RL (L·mg−1) | 0.882773 | 0.842335 | 0.117246 | 0.131376 | 0.899898 | |
| R2 | 0.98 | 0.96 | 0.96 | 0.7 | 0.99 | |
| Freundlich | n | 1.240187 | 1.102086 | 0.871293 | 1.91177 | 1.128643 |
| KF (L·mg−1) | 3.867319 | 4.87147 | 8.751047 | 1765.062 | 2.456009 | |
| R2 | 0.96 | 0.95 | 0.96 | 0.5 | 0.98 | |
| Temkin | B (J·mol−1) | 58.74544 | 73.37301 | 215.8127 | 155.4976 | 37.29655 |
| KT (L·mg−1) | 0.131244724 | 0.192784789 | 11.265276 | 10.18093794 | 0.312696732 | |
| R2 | 0.73 | 0.8 | 0.7 | 0.3 | 0.8 |
| Kinetics Models | Variables | Parameters Unit | Cr(III) | Co(II) | Ni(II) | Cu(II) | Cd(II) |
|---|---|---|---|---|---|---|---|
| PFO | qe | mg/g | 0.010447 | 2.93366 | 3.830978 | 0.072258 | 4.227158 |
| R2 | - | 0.56 | 0.988 | 0.97 | 0.987 | 0.95 | |
| PSO | qe (calculated) | mg/g | 24.99375 | 25.06894 | 24.55796 | 24.97502 | 25.3936 |
| R2 | - | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | |
| IPD | Kid | ((mg/g) min−0.5) | 0.000014 | 0.23106 | 0.29321 | 0.00417 | 0.26992 |
| c | - | 24.98953 | 21.7876 | 20.37882 | 24.86572 | 21.37326 | |
| R2 | - | 0.44321 | 0.95863 | 0.96101 | 0.92284 | 0.80179 |
| Adsorbent | Adsorbed | Capacity (mg g−1) | References | ||||
|---|---|---|---|---|---|---|---|
| D. dichotoma | Cd(II) | 75 | [17] | ||||
| H. clathratus | Cr(III) | 7.19 | [22] | ||||
| Pistachio hull powder | Ni(II) | 14 | [48] | ||||
| C. barbata | 7.30 | [22] | |||||
| Ulva | Cu(II) | 250 | [49] | ||||
| Sargassum | Cu(II) | 125 | [49] | ||||
| Wheat bran | Cd(III) | 21 | [50] | ||||
| Spirulina platensis | Ni(II) | 21.3−49.32 | [51] | ||||
| Chlorella vulgar | Ni(II) | 18.96−43.89 | [51] | ||||
| Spirulina platensis | Cu(II) | 15.01−38.90 | [51] | ||||
| Chlorella vulgar | Cu(II) | 12.54−39.10 | [51] | ||||
| Acinetobacter baumannii UCR-2971 | Ni(II) | 3.5 | [52] | ||||
| Ulva lactuca | Co(II) | 0.2406 | [53] | ||||
| H. fusiformis | Cu(II) | 42.25 | [54] | ||||
| H. fusiformis | Cd(III) | 38.39 | [54] | ||||
| H. fusiformis | Ni(II) | 41.87 | [54] | ||||
| E. compressa | Cr(III) | 24.99375 | Present study | ||||
| E. compressa | Co(II) | 25.06894 | Present study | ||||
| E. compressa | Ni(II) | 24.55796 | Present study | ||||
| E. compressa | Cu(II) | 24.97502 | Present study | ||||
| E. compressa | Cd(III) | 25.3936 | Present study | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younis, A.M.; Saleh, S.M.; Albadri, A.E.A.E.; Elkady, E.M. Enteromorpha compressa Macroalgal Biomass Nanoparticles as Eco-Friendly Biosorbents for the Efficient Removal of Harmful Metals from Aqueous Solutions. Analytica 2024, 5, 322-342. https://doi.org/10.3390/analytica5030021
Younis AM, Saleh SM, Albadri AEAE, Elkady EM. Enteromorpha compressa Macroalgal Biomass Nanoparticles as Eco-Friendly Biosorbents for the Efficient Removal of Harmful Metals from Aqueous Solutions. Analytica. 2024; 5(3):322-342. https://doi.org/10.3390/analytica5030021
Chicago/Turabian StyleYounis, Alaa M., Sayed M. Saleh, Abuzar E. A. E. Albadri, and Eman M. Elkady. 2024. "Enteromorpha compressa Macroalgal Biomass Nanoparticles as Eco-Friendly Biosorbents for the Efficient Removal of Harmful Metals from Aqueous Solutions" Analytica 5, no. 3: 322-342. https://doi.org/10.3390/analytica5030021
APA StyleYounis, A. M., Saleh, S. M., Albadri, A. E. A. E., & Elkady, E. M. (2024). Enteromorpha compressa Macroalgal Biomass Nanoparticles as Eco-Friendly Biosorbents for the Efficient Removal of Harmful Metals from Aqueous Solutions. Analytica, 5(3), 322-342. https://doi.org/10.3390/analytica5030021









