Insights into Analytical Precision: Understanding the Factors Influencing Accurate Vitamin A Determination in Various Samples
Abstract
1. Introduction
- Historical progress in vitamin A analysis techniques.
- Quantitative vitamin A analysis: diverse analytical approaches.
- Deciphering the factors: unveiling the complexities of analytical precision.
- The crucial role of quality control: navigating the path to reliable results.
- Reflection and future prospects: charting the course for enhanced analytical precision.
2. Historical Progress in Vitamin A Analysis Techniques
3. Quantitative Vitamin A Analysis: Diverse Analytical Approaches
3.1. Colorimetric Assays
- The Carr and Price assay: This method involves the quantitative evaluation of retinol utilizing antimony trichloride (SbCl3) as a crucial component [43].
- Trifluoroacetic acid-based colorimetric determination: This technique relies on the interaction of a vitamin A solution in food or feed materials with several Lewis acids, resulting in the transient manifestation of a blue color [41].
3.2. Spectrophotometric Analyses
3.3. Chromatographic Techniques
- a.
- High-performance liquid chromatography (HPLC)
- b.
- Gas–liquid chromatography (GLC)
- c.
- Liquid–liquid chromatography (LLC)
- d.
- Waters UltraPerformance Convergence Chromatography (UPC)
- e.
- Ultra-high-performance liquid chromatography–tandem triple quadrupole mass spectrometry (UHPLC-MS/MS)
3.4. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.5. Near-Infrared Spectroscopy (NIRS)
3.6. Enzyme-Linked Immunosorbent Assays (ELISAs) for Biological Tissues
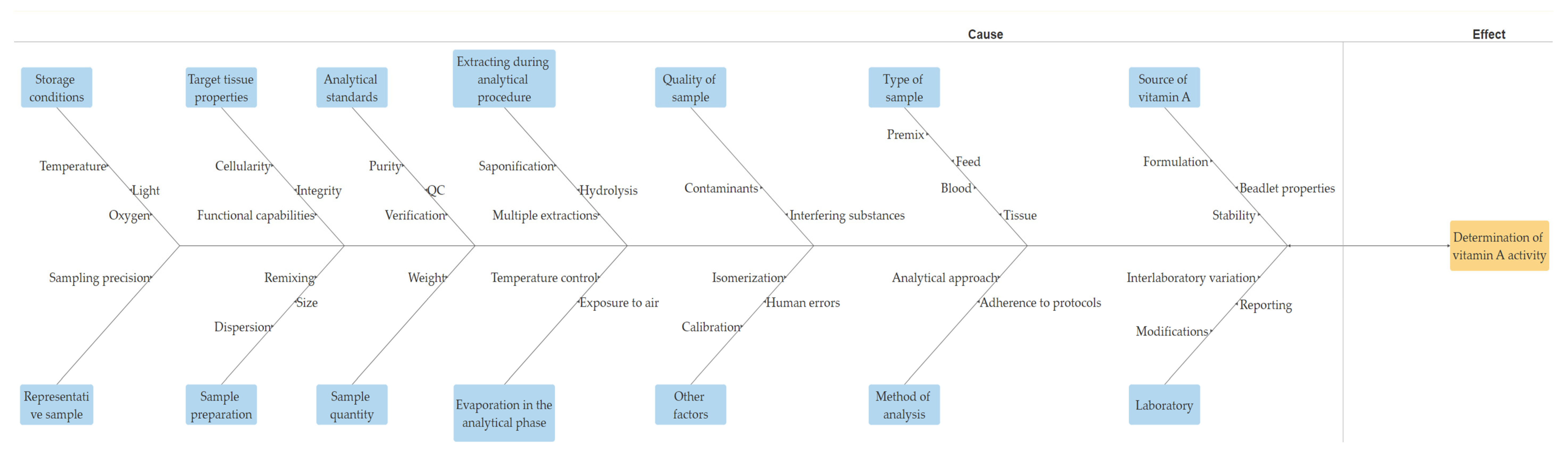
4. Deciphering the Factors: Unveiling the Complexities of Analytical Precision
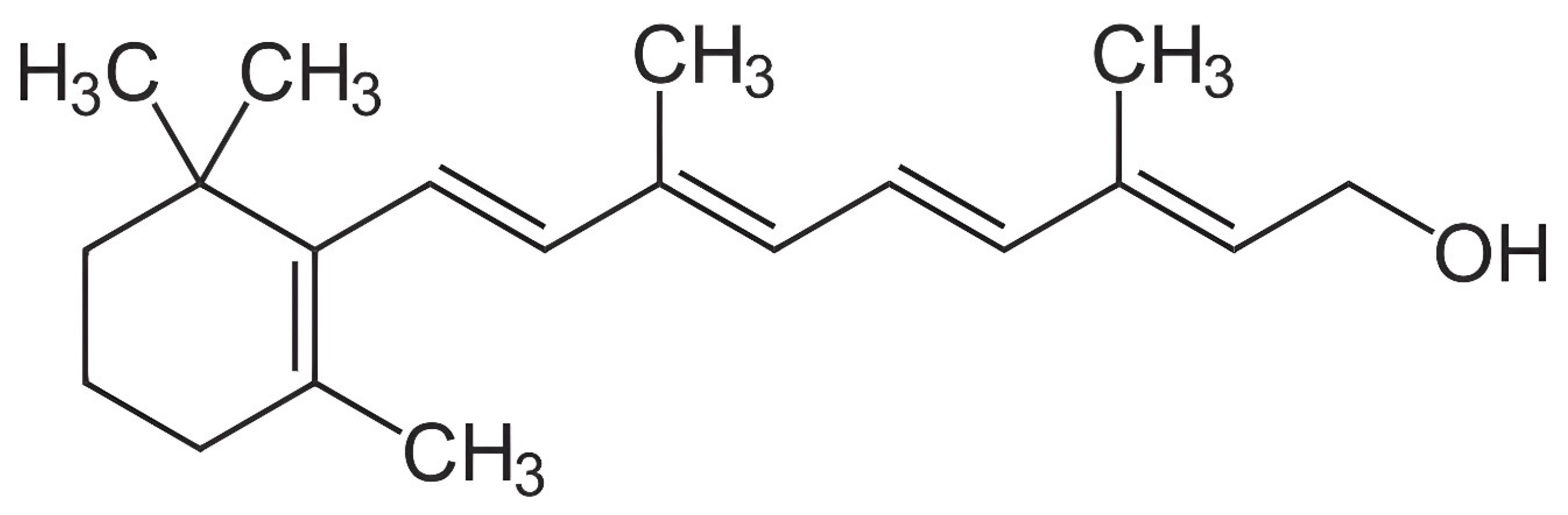
- Source of vitamin A: The susceptibility of various sources or commercial products of vitamin A to degradation can vary significantly due to differences in their formulation [94] (Figure 2). Factors such as light, oxygen, temperature, and moisture play crucial roles in the degradation process. Consequently, these variations can potentially influence the analytical outcomes, even if the initial activity of multiple vitamin A sources in an identical premix composition is similar. Furthermore, repeatability in retinol analysis is influenced by the physical properties of the vitamin A source (beadlet) utilized during the production of the premix or feed [8]. It is inversely correlated with the concentration of vitamin A present in the sample.
- 2.
- Type of sample: Different types of samples, such as premix, feed, blood, or other tissues, may necessitate distinct analytical methodologies [43].
- 3.
- Quality of the sample: The accuracy of analytical procedures can be significantly impacted by the quality of the sample. Contaminants or interfering substances within the sample can exert substantial influence on the physicochemical processes utilized during analysis [23].
- 4.
- Representativeness of the sample: Ensuring a representative sample is imperative. Ideally, the laboratory should only determine the amount of vitamin A present in the sample. If the sample does not accurately reflect the entire batch, the precision achieved is rendered ineffective.
- 5.
- Method of analysis: The precision of the outcomes can be influenced by the analytical approach employed [95]. Various methodologies may exhibit varied sensitivities to distinct configurations of vitamin A [23]. Furthermore, variations in the adherence of analysts to established and sanctioned protocols within a specific methodology may also exert an influence [96].
- 6.
- Laboratory: An empirical analysis reveals that the discrepancy in the precision of vitamin A analysis among different laboratories surpasses the variation attributed to differences in analytical methods [96]. Certain techniques or procedures can significantly contribute to substantial interlaboratory variation [96]. Examples of such techniques include the inconsistent reporting or calculation of results, particularly when comparing retinol palmitate with retinyl acetate. Furthermore, modifications made to the vitamin A analysis procedure, which lack validation through rigorous interlaboratory collaborative studies or statistically sound within-laboratory comparisons with validated test methods, can also be a source of significant variability. Additionally, within-laboratory sampling techniques may further compound this issue.
- 7.
- Storage conditions: The stability of vitamin A is known to be influenced by various storage conditions, including temperature, light exposure, and oxygen levels [97]. The improper storage of laboratory samples under such conditions can significantly impact the precision and reliability of the analysis.
- 8.
- Target tissue cellularity, integrity, and function (for biological tissues): Vitamin A status is characterized by the cellular structure, integrity, and functional capabilities of the target tissues. Unlike some biochemical indicators, any compromise in these aspects may require several weeks of restoration following vitamin A repletion or depletion [98].
- 9.
- Sample preparation approaches: It is crucial to emphasize the importance of obtaining an adequately sized initial sample for the evaluation. Moreover, it is essential to refrain from presuming uniform dispersion of vitamin A throughout the sample during the analysis [11]. Following the grinding process, it is imperative to ensure comprehensive remixing of the ground sample and repeat this process before proceeding with the weighing of a test portion [11].
- 10.
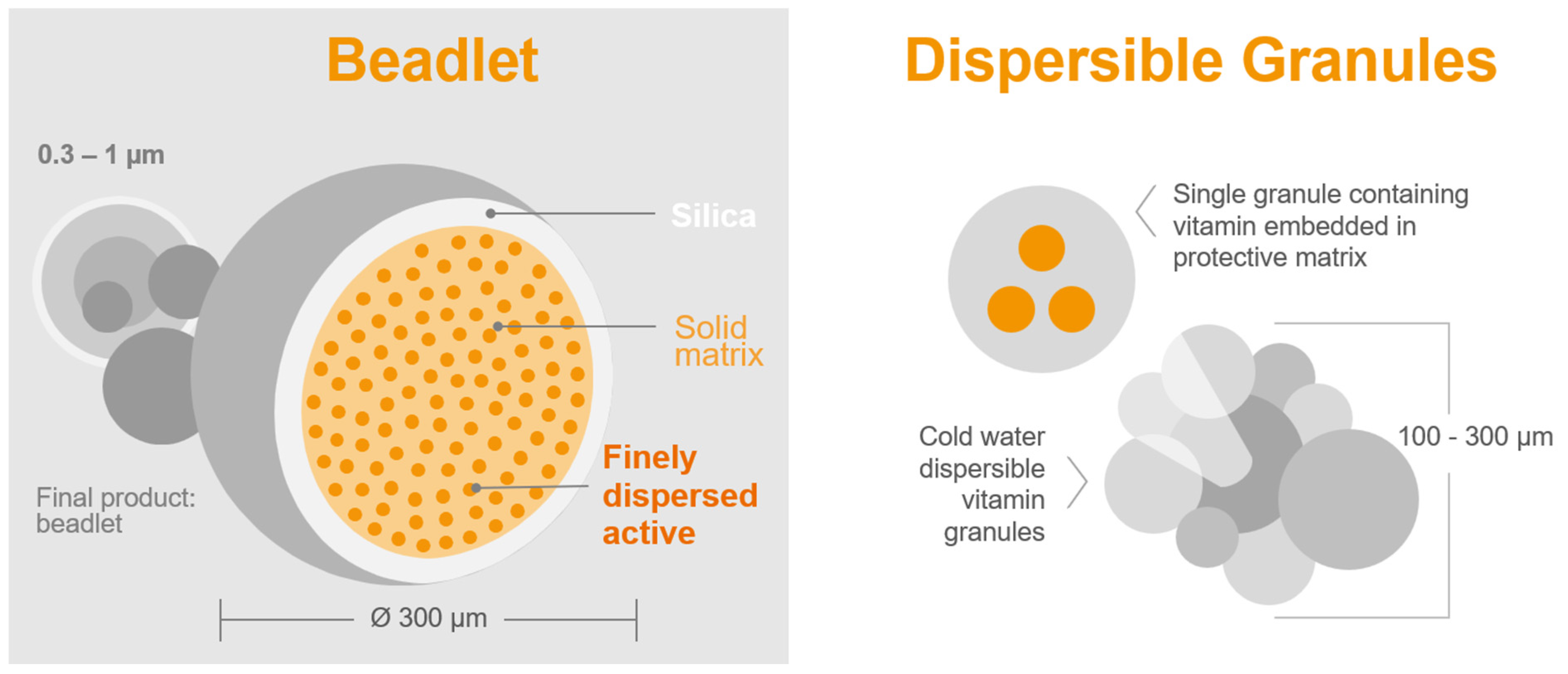
- Sample quantity for analysis: The precision of the chemical analysis of vitamin A in feed or premix samples is significantly affected by the weight of the sample. Dry vitamin A supplements are composed of beadlets (Figure 3 and Table 2) that contain multiple units of retinyl acetate [99]. When assessing a small sample of the feed, there might be a limited number of particles per sample [100]. A recent study by Inerowicz et al. [8] indicated that the relative standard deviations for vitamin A determinations in feed varied between 10.5–24.7% and 2.26–10.7% for sample sizes of 10 g and 100 g, respectively (Table 3). The findings of the study suggest that the mass of the sample can considerably influence the accuracy of vitamin A testing in animal feed materials.
- 11.
- Analytical standards as benchmarks for the identification and quantification of retinol: The variability in the purity of these standards is a critical factor contributing to the observed inconsistencies among laboratories engaged in vitamin A analysis. In the comparison with the recognized US Pharmacopeia (USP) standard retinyl acetate, varying standards often display significant disparities in measurements, with values fluctuating between 50% and 140% of the officially stated value [11]. Regrettably, certain laboratories fail to validate the vitamin A content of the reference materials and exhibit insufficient quality control protocols for their analytical methods [96]. Laboratories exhibiting exemplary accuracy and precision continuously validate reference materials and incorporate in-house quality control samples, employing robust statistical methodologies to ensure and confirm the reliability of their results [96].
- 12.
- Extraction during analytical procedure: In certain instances, the presence of significant quantities of carotenoids following hydrolysis in the solution, coupled with a low concentration of vitamin A, might necessitate the implementation of multiple extraction procedures [102]. In the context of high-fat samples, the formation of extra soaps during the saponification process has the potential to influence the partition coefficient, thereby favoring the aqueous alcohol phase. Consequently, in such scenarios, it becomes imperative to conduct multiple extractions to ensure the efficient separation of retinol into the solvent [11]. According to Moore et al. [96], the primary cause of variation in retinol analysis in feed among various laboratories is, in fact, the vitamin extraction procedure.
- 13.
- Evaporation in the analytical phase: During the process of solvent evaporation, the thermal degradation of retinol solutions can occur, particularly at temperatures exceeding 40 degrees Celsius [11]. Thus, it is vital to control and maintain the temperature below this threshold to prevent the degradation of retinol. Additionally, it is essential to minimize the exposure of the retinol residues to ambient air, as this could potentially compromise the stability of the solution [11].
- 14.
- Other factors: During the analytical process, other factors, such as the isomerization of all-trans retinol, quality control protocols, precise equipment calibration, potential human errors, systematic and bias errors, and various other influences, could potentially impact the final analytical results [11].
5. The Crucial Role of Quality Control: Navigating the Path to Reliable Results
5.1. The Pivotal Role of Quality Control
5.2. Implementing Stringent Protocols and Standard Operating Procedures
5.3. Navigating the Challenges of Consistency and Accuracy
6. Reflection and Future Prospects: Charting the Course for Enhanced Analytical Precision
7. Conclusions
- The accurate determination of vitamin A is crucial for animal health and product quality.
- Historical advancements in analysis techniques have evolved from basic methods to sophisticated chromatographic and spectroscopic approaches, improving precision and sensitivity.
- Various factors, including sample quality, the method of analysis, and storage conditions, significantly impact analytical precision in retinol determination, necessitating a comprehensive understanding and careful consideration.
- Emphasizing the critical role of quality control through stringent protocols and regular proficiency testing is essential for ensuring consistent and reliable results.
- Future progress in analytical precision lies in the integration of advanced technologies, such as miniaturized devices and data-driven approaches, promising to overcome current challenges and enhance accuracy in vitamin A analysis.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nation). Quality assurance for animal feed analysis laboratories. In FAO Animal Production and Health Manual No. 14; FAO: Rome, Italy, 2011. [Google Scholar]
- Khan, R.U.; Khan, A.; Naz, S.; Ullah, Q.; Puvača, N.; Laudadio, V.; Mazzei, D.; Seidavi, A.; Ayasan, T.; Tufarelli, V. Pros and Cons of Dietary Vitamin A and Its Precursors in Poultry Health and Production: A Comprehensive Review. Antioxidants 2023, 12, 1131. [Google Scholar] [CrossRef]
- Shastak, Y.; Gordillo, A.; Pelletier, W. The relationship between vitamin A status and oxidative stress in animal production. J. Appl. Anim. Res. 2023, 51, 546–553. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. The role of vitamin A in non-ruminant immunology. Front. Anim. Sci. 2023, 4, 1197802. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Delving into Vitamin A Supplementation in Poultry Nutrition: Current Knowledge, Functional Effects, and Practical Implications. Worlds Poult. Sci. J. 2023, 79. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Vitamin A supply in swine production: Current science and practical considerations. Appl. Anim. Sci. 2023, 39, 289–305. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Balancing Vitamin A Supply for Cattle: A Review of the Current Knowledge. In Advances in Animal Science and Zoology 21; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2023; Chapter 2; Available online: https://novapublishers.com/wp-content/uploads/2023/11/Advances-in-Animal-Science-and-Zoology.-Volume-21-Chapter-2.pdf (accessed on 14 December 2023).
- Inerowicz, H.D.; Novotny, L.; Ramsey, C.A.; Riter, K.L.; Swarbrick, M.; Thiex, N. Effect of Test Portion Mass on Vitamin A Testing in Animal Feed Materials. J. AOAC Int. 2022, 105, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Stroka, J.; Dehouck, P.; Bouten, K.; Serano, F.; Stroh, A.; von Holst, C. Determination of the Content of Vitamin A in Compound Feed; Proficiency Testing Report FAC-22/01; European Commission: Brussels, Belgium, 2023. [CrossRef]
- Czuba, L.C.; Zhong, G.; Yabut, K.C.; Isoherranen, N. Analysis of vitamin A and retinoids in biological matrices. Methods Enzymol. 2020, 637, 309–340. [Google Scholar] [CrossRef] [PubMed]
- Thiex, N.; Smallidge, R.; Beine, R. Sources of Error in Vitamin A Analysis. J. AOAC Int. 1996, 79, 1269–1276. [Google Scholar] [CrossRef][Green Version]
- AAFCO (The Association of American Feed Control Officials). Determination of Vitamin A in Animal Feed by HPLC/UV. In Proceedings of the AAFCO Annual Meeting, Bellevue, WC, USA, 10–12 August 2017; Available online: https://www.aafco.org/wp-content/uploads/2023/01/201708_Determination_of_Vitamin_A_in_Animal_Feed.pdf (accessed on 22 October 2023).
- Munsell, H.E. Vitamin A: Methods of assay and sources in food. JAMA 1938, 111, 245–252. [Google Scholar] [CrossRef]
- Parrish, D.B. Determination of vitamin A in foods-a review. CRC Crit. Rev. Food Sci. Nutr. 1977, 9, 375–394. [Google Scholar] [CrossRef]
- Kaur, H.; Kewalramani, N.; Garg, M.R.; Kumar, P. Methodology for simultaneous estimation of vitamin A and E in animal feeds using high performance liquid chromatography. Indian J. Anim. Sci. 2004, 74, 1236–1238. [Google Scholar]
- Semba, R.D. On the ‘Discovery’ of Vitamin, A. Ann. Nutr. Metab. 2012, 61, 192–198. Available online: https://www.jstor.org/stable/48508230 (accessed on 20 October 2023). [CrossRef]
- Anonymous. Isolation of Vitamin A. Nature 1932, 129, 88. [Google Scholar] [CrossRef]
- Holmes, H.N.; Corbet, R.E. The Isolation of Crystalline Vitamin A1. J. Am. Chem. Soc. 1937, 59, 2042–2047. [Google Scholar] [CrossRef]
- Brown, P.; Blum, W.; Stern, M. Isomers of vitamin A in fish liver oils. Nature 1959, 184, 1377–1379. [Google Scholar] [CrossRef]
- Braekkan, O.; Myklestad, H.; Njaa, L.; Utne, F. Vitamin A Isomers in the Liver of Rats and Chicks. Nature 1960, 186, 312. [Google Scholar] [CrossRef]
- Pitt, G. Chemical structure and vitamin A activity. Proc. Nutr. Soc. 1965, 24, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Steuerle, H. Untersuchungen zur Bildung von cis-Isomeren aus all-trans-Vitamin-A-Acetat während des alkalischen Aufschlusses bei der Bestimmung von Vitamin A in Futtermitteln. Z. Lebensm. Unters. Forsch. 1985, 181, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.A.; Moran, J.J. An improved colorimetric procedure for the analysis of vitamin A. Can. J. Chem. 1976, 54, 1757–1764. [Google Scholar] [CrossRef]
- Rutkowski, M.; Grzegorczyk, K. Modifications of spectrophotometric methods for antioxidative vitamins determination convenient in analytic practice. Acta Sci. Pol. Technol. Aliment. 2007, 6, 17–28. [Google Scholar]
- Md Noh, M.F.; Gunasegavan, R.D.; Mustafa Khalid, N.; Balasubramaniam, V.; Mustar, S.; Abd Rashed, A. Recent Techniques in Nutrient Analysis for Food Composition Database. Molecules 2020, 25, 4567. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.E.; Yan, J.Q.; Liu, M.; Zhou, Y.; Shen, X.; Ma, Y.L.; Feng, X.S.; Yang, J.; Li, G.H. A Review of the Extraction and Determination Methods of Thirteen Essential Vitamins to the Human Body: An Update from 2010. Molecules 2018, 23, 1484. [Google Scholar] [CrossRef]
- Bilek, M.; Namieśnik, J. Chromatographic techniques in pharmaceutical analysis in poiand: History and the presence on the basis of papers published in selected polish pharmaceutical journals in xx century. Acta Pol. Pharm. 2016, 73, 605–612. [Google Scholar] [PubMed]
- Köseoğlu, K.; Ulusoy, H.I.; Yilmaz, E.; Soylak, M. Simple and sensitive determination of vitamin A and E in the milk and egg yolk samples by using dispersive solid phase extraction with newly synthesized polymeric material. J. Food Compos. Anal. 2020, 90, 103482. [Google Scholar] [CrossRef]
- Sheppard, A.J.; Prosser, A.R.; Hubbard, W.D. Gas chromatography of the fat-soluble vitamins: A review. J. Am. Oil Chem. Soc. 1972, 49, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Bohman, O.; Engdahl, K.-A.; Johnsson, H. High performance liquid chromatography of vitamin A. J. Chem. Educ. 1982, 59, 251. [Google Scholar] [CrossRef]
- Smidt, C.R.; Jones, A.D.; Clifford, A.J. Gas chromatography of retinol and alpha-tocopherol without derivatization. J. Chromatogr. 1988, 434, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ryndakova, I.A.; Grigor’eva, M.P.; Veshchikov, V.V.; Zolotareva, S.I.; Dracheva, O.V. Use of high performance liquid chromatography for analysis of vitamin A in food products. Vopr. Pitan. 1991, 1991, 64–68. [Google Scholar]
- Lee, B.L.; Chua, S.C.; Ong, H.Y.; Ong, C.N. High-performance liquid chromatographic method for routine determination of vitamins A and E and beta-carotene in plasma. J. Chromatogr. 1992, 581, 41–47. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Huang, C.R. High-performance liquid chromatography-electrospray mass spectrometry of retinoids. FASEB J. 1996, 10, 1098–1101. [Google Scholar] [CrossRef]
- Goetz, H.J.; Kopec, R.E.; Riedl, K.M.; Cooperstone, J.L.; Narayanasamy, S.; Curley, R.W., Jr.; Schwartz, S.J. An HPLC-MS/MS method for the separation of α-retinyl esters from retinyl esters. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 6, 1029–1030. [Google Scholar] [CrossRef]
- Ertugrul, S.; Yucel, C.; Sertoglu, E.; Ozkan, Y.; Ozgurtas, T. Development and optimization of simultaneous determination of fat soluble vitamins by liquid chromatography tandem mass spectrometry. Chem. Phys. Lipids 2020, 230, 104932. [Google Scholar] [CrossRef]
- Dunning, M.W. Quantification and Profiling of Hepatic Retinoids in Freshwater Fishes by Liquid Chromatography Tandem Mass Spectrometry. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2018. Available online: https://uwspace.uwaterloo.ca/bitstream/handle/10012/13059/Dunning_Michael.pdf?sequence=3 (accessed on 23 October 2023).
- Bodin, J.C.; Dussert, L.; D’alfonso, T. Near Infrared Spectroscopic Analysis of Vitamins. World Intellectual Property Organization Patent WO2000039562A1, 22 December 1999. Application PCT/EP1999/010502. [Google Scholar]
- Eiff, J.; Monakhova, Y.B.; Diehl, B.W. Multicomponent analysis of fat- and water-soluble vitamins and auxiliary substances in multivitamin preparations by qNMR. J. Agric. Food Chem. 2015, 63, 3135–3143. [Google Scholar] [CrossRef]
- Carazo, A.; Macákova, K.; Matoušová, K.; Krčmová, K.; Protti, M.; Mladěnka, P. Vitamin A update: Forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Dugan, R.E.; Frigerio, N.A.; Siebert, J.M. Colorimetric Determination of Vitamin A and Its Derivatives with Trifluoroacetic Acid. Anal. Chem. 1964, 36, 114–117. [Google Scholar] [CrossRef]
- Dary, O.; Arroyave, G. Manual for Sugar Fortification with Vitamin A Part 3: Analytical Methods for the Control and Evaluation of Sugar Fortification with Vitamin A; INCAP, USAID/OMNI: Arlington, VA, USA, 1996.
- Egbuonu, R. Evaluation of Colorimetric Determination of Vitamin A in Foods. Master’s Thesis, Texas Tech University, Lubbock, TX, USA, 1986. [Google Scholar]
- Sobel, A.E.; Werbin, H. Spectrophotometric study of a new colorimetrie reaction of vitamin A. J. Biol. Chem. 1945, 159, 681–691. [Google Scholar] [CrossRef]
- Cortés-Herrera, C.; Artavia, G.; Leiva, A.; Granados-Chinchilla, F. Liquid Chromatography Analysis of Common Nutritional Components, in Feed and Food. Foods. 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Mueller, V.; Lengerken, V.; Wetterau, H. Vitamin A determination in industrially produced feeds. Part 1. Determination of vitamin A in pre-mixes and mixes of active ingredients. Nahrung 1974, 18, 425–437. [Google Scholar] [CrossRef]
- Alqahtani, N.S. Vitamin A Quantification using Photometric Methods. J. Nutr. Weight. Loss. 2021, 6, 140. [Google Scholar]
- Huey, S.L.; Krisher, J.T.; Morgan, D.; Mkambula, P.; Srinivasan, B.; Gannon, B.M.; Mbuya, M.N.N.; Mehta, S. Portable Devices for Measurement of Vitamin A Concentrations in Edible Oil: Field Readiness of Available Options. ACS Omega 2022, 7, 17502–17518. [Google Scholar] [CrossRef] [PubMed]
- Koehn, C.J.; Sherman, W.C. The determination of vitamin a and carotene with the photoelectric colorimeter. J. Biol. Chem. 1940, 132, 527–538. [Google Scholar] [CrossRef]
- Bayfield, R.; Cole, E. Colorimetric estimation of vitamin A with trichloroacetic acid. Methods Enzymol. 1980, 67, 189–195. [Google Scholar] [CrossRef]
- U.S.P. The Pharmacopeia of the United States of America; 18th Revision; Mack Printing Co.: Easton, PA, USA, 1970; pp. 775, 914. [Google Scholar]
- Arroyave, G.; de Funes, C. Enriquecimiento de azUcar con vitamina A. Metodo para la determinacion cuantitativa de retinol en azUcar blanca de mesa. Arch. Latinoam. Nutr. 1974, 24, 147–153. [Google Scholar]
- Rathi, D.N.G.; Rashed, A.A.; Noh, M.F.M. Determination of retinol and carotenoids in selected Malaysian food products using high-performance liquid chromatography (HPLC). SN Appl. Sci. 2022, 4, 93. [Google Scholar] [CrossRef]
- Coskun, O. Separation techniques: Chromatography. North Clin. Istanb. 2016, 3, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, A.; Smith, F.J. Theoretical Considerations in Chromatographic Methods; Springer: Dordrecht, The Netherlands, 1985; pp. 24–84. [Google Scholar] [CrossRef]
- Rodriguez, E.L.; Poddar, S.; Iftekhar, S.; Suh, K.; Woolfork, A.G.; Ovbude, S.; Pekarek, A.; Walters, M.; Lott, S.; Hage, D.S. Affinity chromatography: A review of trends and developments over the past 50 years. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1157, 122332. [Google Scholar] [CrossRef]
- Bower, P. High-Performance Liquid Chromatography. J. Vis. Exp. 2023. Available online: https://app.jove.com/cn/v/10156/operation-of-high-performance-liquid-chromatography-hplc (accessed on 29 October 2023).
- Al-Abdulaly, A.B. Determination of Retinol by Aqueous Reverse Phase Open Column System. Ph.D. Thesis, University of Rhode Island, Kingston, RI, USA, 1986. Available online: https://digitalcommons.uri.edu/oa_diss/543 (accessed on 5 December 2023).
- Hosain, M.Z.; Islam, S.M.S.; Kamal, M.M.; Rahman, M.M. Quantitative Analysis of Fat-Soluble Vitamins in Feed Additives Using an In-House Developed and Validated HPLC Method. Austin. J. Anal. Phar. M Chem. 2022, 9, 1143. [Google Scholar]
- GMI. Exploring the Key Components of an HPLC System: A Comprehensive Guide. HPLC, Chromatography. 2023. Available online: https://www.gmi-inc.com/exploring-the-key-components-of-an-hplc-system-a-comprehensive-guide/#:~:text=The%20column%20is%20where%20the,for%20achieving%20the%20desired%20separation (accessed on 29 October 2023).
- Boettcher, J.; Margraf, M.; Monks, K. HPLC Basics—Principles and parameters. VSP0019, KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin. 2023. Available online: https://www.knauer.net/Application/application_notes/VSP0019_HPLC%20Basics%20-%20principles%20and%20parameters_final%20-web-.pdf (accessed on 29 October 2023).
- Kim, Y.K.; Quadro, L. Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol. Biol. 2010, 652, 263–275. [Google Scholar] [CrossRef]
- Howells, D.W.; Brown, I.R.; Brooke, O.G.; Newey, V. A simple automated injection technique for the high-pressure liquid chromatographic determination of plasma retinol. Ann. Clin. Biochem. 1983, 20, 308–311. [Google Scholar] [CrossRef]
- Kane, M.A.; Folias, A.E.; Napoli, J.L. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal. Biochem. 2008, 378, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Falaki, F. Sample Preparation Techniques for Gas Chromatography; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Ryhage, R. Use of a mass spectrometer as a detector and analyzer for effluents emerging from high temperature gas liquid chromatography columns. Anal. Chem. 1964, 36, 759–764. [Google Scholar] [CrossRef]
- Pitt, J.J. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem. Rev. 2009, 30, 19–34. [Google Scholar]
- Minceva, M. Liquid-Liquid Chromatography. Wiley Analytical Science. 2016. Available online: https://analyticalscience.wiley.com/content/article-do/liquid-liquid-chromatography (accessed on 29 October 2023).
- Maryutina, T.A.; Savonina, E.Y.; Fedotov, P.S.; Smith, R.M.; Siren, H.; Hibbert, D.B. Terminology of separation methods (IUPAC Recommendations 2017). Pure Appl. Chem. 2018, 90, 181–231. [Google Scholar] [CrossRef]
- Gu, G.; Brosig, K.; Kennedy, L.; Oglobline, A.; Richardson, G.; Walker, B. Simultaneous Analysis of Vitamin A and D3 in Vitamin Premixes and Concentrates by Convergence Chromatography/PDA Detection; Dairy Technical Services Ltd.: Waters Corporation, MA, USA, 2019; Available online: https://www.waters.com/content/dam/waters/en/app-notes/2014/720005220/720005220-en.pdf (accessed on 30 October 2023).
- Turner, J.E.; Jenkins, K.M. The Evolution of Ultra High-Performance Liquid Chromatography: Expanding the Future of Separation Technologies. Chromatography Today May/June 2018. 2018. Available online: https://www.chromatographytoday.com/article/hplc-uhplc/31/waters-corporation/pthe-evolution-of-ultra-high-performance-liquid-chromatography-expanding-the-future-of-separation-technologiesp/2376 (accessed on 30 October 2023).
- Chen, X.; Gong, Z.; Shen, S. Determination of vitamin A and vitamin E in human serum by ultra-high performance liquid chromatography-tandem triple quadrupole mass spectrometry. Wei Sheng Yan Jiu 2021, 50, 301–307. [Google Scholar] [CrossRef]
- Eitenmiller, R.R. Strengths and weaknesses of assessing vitamin content of foods. J. Food Qual. 1990, 13, 1–69. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; Moliner-Martínez, Y.; Molins-Legua, C.; Campíns-Falcó, P. Trends for the Development of In Situ Analysis Devices. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, H.K.; Wilson, E.G.; Erkelens, C.; Trijzelaar, B.; Verpoorte, R. Quantitative analysis of retinol and retinol palmitate in vitamin tablets using 1H-nuclear magnetic resonance spectroscopy. Anal. Chim. Acta 2004, 512, 141–147. [Google Scholar] [CrossRef]
- Koshani, R.; Jafari, S.M.; van de Ven, T.G.M. Going deep inside bioactive-loaded nanocarriers through Nuclear Magnetic Resonance (NMR) spectroscopy. Trends Food Sci. Technol. 2020, 101, 198–212. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. TrAC Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Williamson, K.; Hatzakis, E. NMR Spectroscopy as a Robust Tool for the Rapid Evaluation of the Lipid Profile of Fish Oil Supplements. J. Vis. Exp. 2017, 123, 55547. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Guimarães, J.T.; Rocha, R.S.; Pimentel, T.C.; Neto, R.P.C.; Tavares, M.I.B.; Graça, J.S.; Filho, E.G.A.; Freitas, M.Q.; Esmerino, E.A.; et al. Nuclear magnetic resonance as an analytical tool for monitoring the quality and authenticity of dairy foods. Trends Food Sci. Technol. 2021, 108, 84–91. [Google Scholar] [CrossRef]
- Bec, K.B.; Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Genkawa, T.; Futami, Y. Near-infrared spectroscopy. In Encyclopedia of Spectroscopy and Spectrometry; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 40–49. [Google Scholar] [CrossRef]
- Jaren, C.; Lopez, A.; Arazuri, S. Advanced analytical techniques for quality evaluation of potato and its products. In Advances in Potato Chemistry and Technology Book; Jaspreet, S., Lovedeep, K., Eds.; Academic Press: Cambridge, MA, USA, 2016; Chapter 19; pp. 563–602. [Google Scholar]
- Pires, F.F.; Lemos, M.C.; Petersen, J.C.; Kessler, A.M. Use of Near-Infrared Reflectance Spectroscopy to Analyze Vitamin Content. J. Appl. Poult. Res. 2001, 10, 412–418. [Google Scholar] [CrossRef]
- Jia, L.P.; Tian, S.L.; Zheng, X.C.; Jiao, P.; Jiang, X.P. Application of near-infrared spectroscopy in the detection of fat-soluble vitamins in premix feed. In Proceedings of the Fourth Seminar on Novel Optoelectronic Detection Technology and Application, Nanjing, China, 24–26 October 2017; Volume 10697, p. 106971U. [Google Scholar] [CrossRef]
- Tamura, Y.; Inoue, H.; Takemoto, S.; Hirano, K.; Miyaura, K. A Rapid Method to Measure Serum Retinol Concentrations in Japanese Black Cattle Using Multidimensional Fluorescence. J. Fluoresc. 2021, 31, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Alhajj, M.; Zubair, M.; Farhana, A. Enzyme Linked Immunosorbent Assay; StatPearls Publishing LLC: Tampa, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555922/ (accessed on 3 November 2023).
- Lequin, R.M. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Osmekhina, E.; Neubauer, A.; Klinzing, K.; Myllyharju, J.; Neubauer, P. Sandwich ELISA for quantitative detection of human collagen prolyl 4-hydroxylase. Microb. Cell Fact. 2010, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Innovative Research. 2023. Available online: https://www.innov-research.com/products/vitamin-a-elisa-kit (accessed on 27 November 2023).
- Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O. Advantages, Disadvantages and Modifications of Conventional ELISA. In Enzyme-Linked Immunosorbent Assay (ELISA); Springer Briefs in Applied Sciences and Technology; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- AAFCO (The Association of American Feed Control Officials). Official Publication. 2011, pp. 298–299. Available online: https://www.aafco.org/resources/official-publication/ (accessed on 2 December 2023).
- VDLUFA (Association of German Agricultural Analytic and Research Institutes e. V.). Analysis Leeways (ASR) Version 13 (2022); VDLUFA-Verlag: Darmstadt, Germany, 2022; p. 13. [Google Scholar]
- Hirai, R.A.; De Leon, D.; Randig-Biar, M.; Silva, A.; Sanchez, E.; McElroy, A.P.; Bailey, C.A.; Martinez, N.; Sokale, A.; Music, L. Evaluation of the stability of vitamin A acetate concentrates mixed in a vitamin-trace mineral premix over a 56-day high temperature and humidity storage stress. In Proceedings of the 2023 the International Poultry Scientific Forum, Atlanta, GA, USA, 23–24 January 2023; p. 112. [Google Scholar]
- Parrish, D.B. Study of the Method for Vitamin A in Mixed Feeds. J. AOAC Int. 1960, 43, 30–34. [Google Scholar] [CrossRef]
- Moore, W.R.; DeVries, J.; MacDonald, J.; Hare, L.; Carson, J.; Chaudhari, P.; DeVries, J.; Fontana, J.; Golz, P.; King, J.; et al. Assessing analytical variability of measurement of vitamin A in corn-soy blend. J. AOAC Int. 2010, 93, 638–649. [Google Scholar] [CrossRef]
- Duarte Fávaro, R.M.; Iha, M.H.; Mazzi, T.C.; Fávaro, R.; de Lourdes Pires Bianchi, M. Stability of vitamin A during storage of enteral feeding formulas. Food Chem. 2011, 126, 827–830. [Google Scholar] [CrossRef]
- NASEM (The National Academies of Sciences, Engineering, and Medicine). Dietary Reference Intakes for Vitamin, A.; Vitamin, K.; Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Institute of Medicine (US) Panel on Micronutrients. 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222318/ (accessed on 31 October 2023).
- Lehman, R.W. A Statistical Procedure for Estimating Vitamin A Assay Variation Caused by Particulate Distribution of Dry Vitamin A in Feed Samples. J. Assoc. Off. Agric. Chem. 1960, 43, 15–20. [Google Scholar] [CrossRef]
- Tinkler, F.H.; Hanley, J.B.; Lehman, R.W. The Use of Large Samples in the Determination of Vitamin A in Mixed Feeds Fortified with Dry Vitamin A Supplements. J. AOAC Int. 1960, 43, 25–28. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Nutritional Balance Matters: Assessing the Ramifications of Vitamin A Deficiency on Poultry Health and Productivity. Poultry 2023, 2, 493–515. [Google Scholar] [CrossRef]
- Parrish, D.B. Vitamin A in Mixed Feeds, Premixes, and Foods: Development of Method. J. AOAC Int. 1974, 57, 897–902. [Google Scholar] [CrossRef]
- Taverniers, I.; Van Bockstaele, E.; De Loose, M. Trends in quality in the analytical laboratory. I. Traceability and measurement uncertainty of analytical results. Trends Anal. Chem. 2004, 23, 480–490. [Google Scholar] [CrossRef]
- IYTE (Izmir Yüksek Teknoloji Enstitüsü). Chapter 5: Errors in Chemical Analyses. 2023. Available online: https://web.iyte.edu.tr/~serifeyalcin/lectures/chem201/cn_5.pdf (accessed on 31 October 2023).
- Yadav, V. Food Analysis and Quality Control. Government Polytechnic, Mandi Adampur, Hisar, Haryana, India. 2023. Available online: https://gpadampur.files.wordpress.com/2011/11/6-2-faqc-class-notes-08022014.pdf (accessed on 31 October 2023).
- Galyean, M.L. Laboratory Procedures in Animal Nutrition Research; Department of Animal and Food Sciences Texas Tech University: Lubbock, TX, USA, 2010; Available online: https://www.depts.ttu.edu/agriculturalsciences/vetSciences/mgalyean/lab_man.pdf (accessed on 1 November 2023).
- Krishna, G. Livestock Nutrition: Analytical Techniques; New India Pub Agency: New Delhi, India, 2012; p. 763. [Google Scholar]
- de Jonge, L.H.; Jackson, F.S. The feed analysis laboratory: Establishment and quality control. Setting up a feed analytical laboratory, and implementing a quality assurance system compliant with ISO/IEC 17025:2005. In Animal Production and Health Guidelines No. 15; Makkar, H.P.S., Ed.; FAO: Rome, Italy, 2005. [Google Scholar]
- Holland, I.; Davies, J.A. Automation in the Life Science Research Laboratory. Front. Bioeng. Biotechnol. 2020, 8, 571777. [Google Scholar] [CrossRef]
- Hurst, W.J. Automation in Food and Agricultural Laboratories. JALA J. Assoc. Lab. Automat. 2009, 14, A9–A15. [Google Scholar] [CrossRef]
- von Holst, C.; Boix, A.; Vincent, U. Addressing a multidisciplinary challenge from animal nutrition to analytical science. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 1271. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Strnad, I.; Mittendorfer, J. Proficiency Testing of Feed Constituents: A Comparative Evaluation of European and Developing Country Laboratories and Its Implications for Animal Production. J. Agric. Food Chem. 2016, 64, 7679–7687. [Google Scholar] [CrossRef] [PubMed]
- Dragacci, S.; Grosso, F.; Pfauwathel-Marchond, N.; Fremy, J.M.; Venant, A.; Lombard, B. Proficiency testing for the evaluation of the ability of European Union-National Reference laboratories to determine aflatoxin M1 in milk at levels corresponding to the new European Union legislation. Food Addit. Contam. 2001, 18, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Thiex, N.J.; Ramsey, C.A. Piloting a Proficiency Testing Program for Laboratory Sampling of Animal Feed Materials. J. AOAC Int. 2023, 106, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Earnshaw, A.; Smith, R.A.; Owen, L. How proficiency testing can improve the quality of analytical data using vitamin analysis as an example. Food Chem. 2009, 113, 781–783. [Google Scholar] [CrossRef]
- Sykes, M.; Croucher, J.; Smith, R.A. Proficiency testing has improved the quality of data of total vitamin B2 analysis in liquid dietary supplement. Anal. Bioanal. Chem. 2011, 400, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Nemser, S.; Lindemann, S.; Chen, Y.; Lopez, S.; Pickens, S.; Ulaszek, J.; Kmet, M.; Powers, C.; Ensley, S.; Schrunk, D.; et al. A review of proficiency exercises offered by the Veterinary Laboratory Investigation and Response Network (Vet-LIRN) and Moffett Proficiency Testing Laboratory from 2012 to 2018. Accredit. Qual. Assur. 2021, 26, 143–156. [Google Scholar] [CrossRef]
- Waugh, C.; Clark, G. Factors affecting test reproducibility among laboratories. Rev. Sci. Tech. 2021, 40, 131–143. [Google Scholar] [CrossRef]
- Malomo, G.A.; Ihegwuagu, N.E. Some Aspects of Animal Feed Sampling and Analysis [Internet]. In Ideas and Applications Toward Sample Preparation for Food and Beverage Analysis; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Greaves, R.; Jolly, L.; Woollard, G.; Hoad, K. Serum vitamin A and E analysis: Comparison of methods between laboratories enrolled in an external quality assurance programme. Ann. Clin. Biochem. 2010, 47 Pt 1, 78–80. [Google Scholar] [CrossRef]
- Binkley, N.; Sempos, C.T. Vitamin D Standardization Program (VDSP). Standardizing vitamin D assays: The way forward. J. Bone Min. Res. 2014, 29, 1709–1714. [Google Scholar] [CrossRef]
- Greaves, R.F.; Woollard, G.A.; Hoad, K.E.; Walmsley, T.A.; Johnson, L.A.; Briscoe, S.; Koetsier, S.; Harrower, T.; Gill, J.P. Laboratory medicine best practice guideline: Vitamins a, e and the carotenoids in blood. Clin. Biochem. Rev. 2014, 35, 81–113. [Google Scholar]
- AOAC (the Association of Official Agricultural Chemists). AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals. 2002. Available online: https://s27415.pcdn.co/wp-content/uploads/2020/01/64ER20-7/Validation_Methods/d-AOAC_Guidelines_For_Single_Laboratory_Validation_Dietary_Supplements_and_Botanicals.pdf (accessed on 2 November 2023).
- Thompson, L.B.; Schimpf, K.J.; Stiner, L.A.; Schmitz, D.J. Determination of vitamin A (retinol) in infant and medical nutritional formulas with AOAC method 992.06 using a modified extraction procedure: Single-laboratory validation. J. AOAC Int. 2010, 93, 1523–1529. [Google Scholar] [CrossRef]
- Cuadros-Rodriguez, L.; Gamiz-Gracia, L.; Almansa-Lopez, E.; Bosque-Sendra, J.M. Calibration in chemical measurement processes. II. A methodological approach. TrAC Trends Anal. Chem. 2001, 20, 620–636. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Gamba, G.; Riondato, I.; Beccaro, G.L. Analytical Strategies for Fingerprinting of Antioxidants, Nutritional Substances, and Bioactive Compounds in Foodstuffs Based on High Performance Liquid Chromatography-Mass Spectrometry: An Overview. Foods 2020, 9, 1734. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Patel, J.K.; Patel, M.P.; Rajput, G.C.; Patel, H.A. Introduction to hyphenated techniques and their applications in pharmacy. Pharm. Methods 2010, 1, 2–13. [Google Scholar] [CrossRef]
- Corradini, D.; Nicoletti, I. High performance separation techniques for identification, characterization and quantification of plant secondary metabolites with health-promoting properties. In Proceedings of the 30th International Symposium on the Chemistry of Natural Products, Athens, Greece, 25–29 November 2018. [Google Scholar]
- Betz, J.M.; Rimmer, C.A.; Saldanha, L.G.; Phillips, M.M.; Andrews, K.W.; Wise, S.A.; Wood, L.J.; Kuszak, A.J.; Gusev, P.A.; Pehrsson, P.R. Challenges in Developing Analytically Validated Laboratory-Derived Dietary Supplement Databases. J. Nutr. 2018, 148 (Suppl. 2), 1406S–1412S. [Google Scholar] [CrossRef]
- Alonso, N.; Zelzer, S.; Eibinger, G.; Herrmann, M. Vitamin D Metabolites: Analytical Challenges and Clinical Relevance. Calcif Tissue Int. 2023, 112, 158–177. [Google Scholar] [CrossRef]
- Ferranti, P. The future of analytical chemistry in foodomics. Curr. Opin. Food Sci. 2018, 22, 102–108. [Google Scholar] [CrossRef]
- Valdés, A.; Álvarez-Rivera, G.; Socas-Rodríguez, B.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Foodomics: Analytical Opportunities and Challenges. Anal. Chem. 2022, 94, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Słowik-Borowiec, M.; Głąb, N.; Stach, S.; Szpyrka, E. A Miniaturized Sample Preparation Method for the Determination of Vitamins A and E in Food Products. Molecules 2023, 28, 3449. [Google Scholar] [CrossRef]
- Ahmadi, M.; Amouzegar, Z.; Khalili, S.; Asadi, S.; Aghajani, S.; Aryanrad, P.; Afkhami, A.; Madrakian, T.; Thomas, S.; Nguyen, T.A. Chapter 1—Miniaturization—An introduction to miniaturized analytical devices. In Micro and Nano Technologies, Micro- and Nanotechnology Enabled Applications for Portable Miniaturized Analytical Systems; Thomas, S., Ahmadi, M., Nguyen, T.A., Afkhami, A., Madrakian, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–16. [Google Scholar] [CrossRef]
- Szymańska, E. Modern data science for analytical chemical data—A comprehensive review. Anal. Chim. Acta 2018, 1028, 1–10. [Google Scholar] [CrossRef]
- Dotzert, M. The Power of Algorithms in Analytical Chemistry. Creating a Positive Lab Culture 15(6). 2020. Available online: https://www.labmanager.com/the-power-of-algorithms-in-analytical-chemistry-23167 (accessed on 2 November 2023).

| Ascertained Level (c), IU/kg | Relative Leeway | Absolute Leeway | Extrapolated Leeway |
|---|---|---|---|
| 2000–<3720 | - | - | 2.1696·c0.8495 IU |
| 3720–<7800 | - | - | 2340 IU |
| 7800–<100,000 | 30% | - | - |
| 100,000–<125,000 | - | 30,000 IU | - |
| 125,000–<375,000 | 24% | - | - |
| 375,000–<450,000 | - | 90,000 IU | - |
| 450,000–<1,020,000 | 20% | - | - |
| 1,020,000–<7,570,000 | - | - | 20% |
| 7,570,000–≤460,000,000 | - | - | 2.1696·c0.8495 IU |
| >460,000,000 | - | - | 2309·c0.5 IU |
| Vitamin A Source | Initial Mass, g | Density, g/cm3 @ 21.9 °C c | Number of Particles Measured | Particle Size Measurements a,b | ||||
|---|---|---|---|---|---|---|---|---|
| Average, mm | Median, mm | Minimum, mm | Maximum, mm | SD | ||||
| 1 | 221.0 | 0.60 | 2074 a | 0.466 | 0.456 | 0.065 | 1.179 | 0.156 |
| 2 | 109.5 | 0.63 | 2415 b | 0.333 | 0.323 | 0.047 | 0.738 | 0.102 |
| Sample | Vitamin A, IU/kg | |||||
|---|---|---|---|---|---|---|
| Poultry Feed (Conditioner) | Poultry Feed (Texturized) | Mineral Mix | ||||
| Quantity, g | 10 | 100 | 10 | 100 | 10 | 100 |
| 1 | 6112 | 5898 | 20,875 | 24,476 | 164,762 | 176,587 |
| 2 | 5230 | 5748 | 18,851 | 18,443 | 163,719 | 171,192 |
| 3 | 5352 | 5654 | 24,575 | 21,794 | 184,971 | 173,370 |
| 4 | 4875 | 5779 | 15,140 | 22,853 | 176,246 | 177,037 |
| 5 | 6736 | 6223 | 23,810 | 19,640 | 180,409 | 168,987 |
| 6 | 7801 | 6346 | 22,685 | 19,642 | 203,235 | 180,078 |
| 7 | 6575 | 6430 | 34,162 | 16,716 | 138,778 | 173,772 |
| 8 | 7294 | 5923 | 26,550 | 19,186 | 184,375 | 180,673 |
| 9 | 6818 | 4926 | 22,687 | 23,999 | 170,451 | 170,817 |
| 10 | 5768 | 5490 | 18,112 | 19,503 | 151,056 | 171,569 |
| 11 | 5682 | 5153 | 25,915 | 21,724 | 185,745 | 180,100 |
| 12 | 4646 | 5283 | 31,337 | 18,976 | 168,742 | 181,044 |
| 13 | 6904 | 6181 | 22,925 | 19,556 | 190,880 | 170,998 |
| 14 | 5907 | 5228 | 28,815 | 18,182 | 205,806 | 177,503 |
| 15 | 5746 | 5870 | 16,339 | 20,145 | 183,401 | 177,366 |
| 16 | 5389 | 5400 | 14,127 | 22,279 | 204,112 | 175,273 |
| Average 1 | 6052 | 5721 | 22,930 | 20,450 | 178,500 | 175,400 |
| SD | 893 | 448 | 5673 | 2181 | 18,670 | 3972 |
| RSD, % | 14.8 | 7.82 | 24.7 | 10.7 | 10.5 | 2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shastak, Y.; Pelletier, W.; Kuntz, A. Insights into Analytical Precision: Understanding the Factors Influencing Accurate Vitamin A Determination in Various Samples. Analytica 2024, 5, 54-73. https://doi.org/10.3390/analytica5010004
Shastak Y, Pelletier W, Kuntz A. Insights into Analytical Precision: Understanding the Factors Influencing Accurate Vitamin A Determination in Various Samples. Analytica. 2024; 5(1):54-73. https://doi.org/10.3390/analytica5010004
Chicago/Turabian StyleShastak, Yauheni, Wolf Pelletier, and Andrea Kuntz. 2024. "Insights into Analytical Precision: Understanding the Factors Influencing Accurate Vitamin A Determination in Various Samples" Analytica 5, no. 1: 54-73. https://doi.org/10.3390/analytica5010004
APA StyleShastak, Y., Pelletier, W., & Kuntz, A. (2024). Insights into Analytical Precision: Understanding the Factors Influencing Accurate Vitamin A Determination in Various Samples. Analytica, 5(1), 54-73. https://doi.org/10.3390/analytica5010004






