A Novel ppb-Level Sensitive and Highly Selective Europium-Based Diketone Luminescent Sensor for the Quantitative Detection of Aluminum Ions in Water Samples
Abstract
:1. Introduction
2. Experimental Section
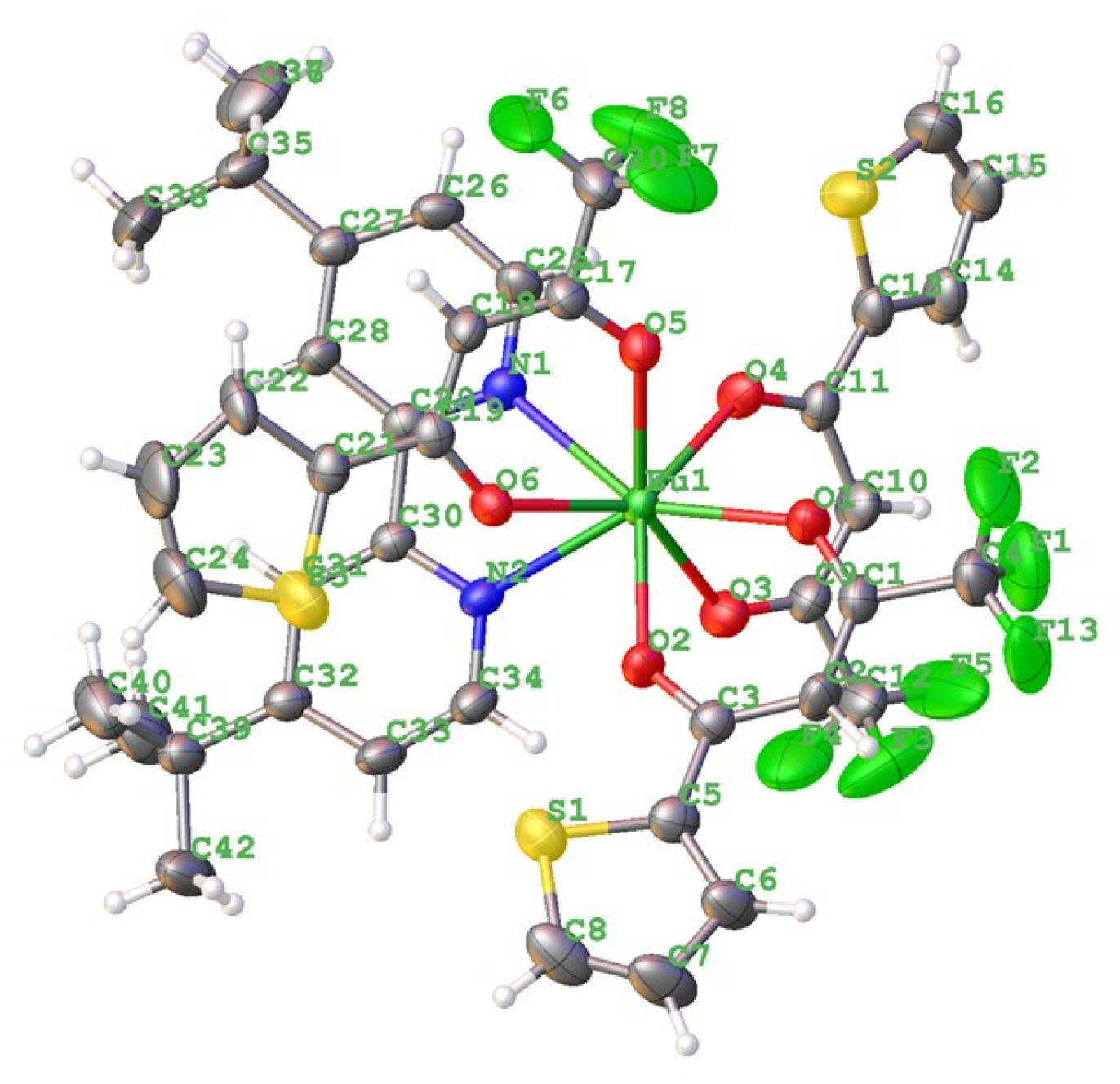
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Kim, H.; Yoon, J. A selective ‘Off-On’ fluorescent sensor for Zn2+ based on hydrazone-pyrene derivative and its application for imaging of intracellular Zn2+. Bioorganic Med. Chem. Lett. 2010, 20, 125–128. [Google Scholar] [CrossRef]
- Xu, W.; Qi, D.; You, J.; Hu, F.; Bian, J.; Yang, C.; Huang, J. Coumarin-based ‘turn-off’ fluorescent chemosensor with high selectivity for Cu2+ in aqueous solution. J. Mol. Struct. 2015, 1091, 133–137. [Google Scholar] [CrossRef]
- Xu, T.; Duan, H.; Wang, X.; Meng, X.; Bu, J. Fluorescence sensors for Zn2+ based on conjugated indole Schiff base. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Zhang, C.; Wu, Y.; Liu, Z. Coumarin-hydrazone based high selective fluorescence sensor for copper(II) detection in aqueous solution. Inorg. Chem. Commun. 2013, 34, 8–11. [Google Scholar] [CrossRef]
- Wang, L.; Ye, D.; Cao, D. A novel coumarin Schiff-base as a Ni(II) ion colorimetric sensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 40–44. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mergu, N.; Kumawat, L.K.; Singh, A.K. A reversible fluorescence “off–on–off” sensor for sequential detection of aluminum and acetate/fluoride ions. Talanta 2015, 144, 80–89. [Google Scholar] [CrossRef]
- Ma, J.; Shi, W.; Feng, L.; Chen, Y.; Fan, K.; Hao, Y.; Hui, Y.; Xie, Z. A highly selective and sensitive acylhydrazone-based turn-on optical sensor for Al3+. RSC Adv. 2016, 6, 28034–28037. [Google Scholar] [CrossRef]
- Goswami, S.; Aich, K.; Das, S.; Das, A.K.; Sarkar, D.; Panja, S.; Mondal, T.K.; Mukhopadhyay, S. A red fluorescence ‘off-on’ molecular switch for selective detection of Al3+, Fe3+ and Cr3+: Experimental and theoretical studies along with living cell imaging. Chem. Commun. 2013, 49, 10739–10741. [Google Scholar] [CrossRef]
- Suryawanshi, V.D.; Gore, A.H.; Dongare, P.R.; Anbhule, P.V.; Patil, S.R.; Kolekar, G.B. A novel pyrimidine derivative as a fluorescent chemosensor for highly selective detection of Aluminum (III) in aqueous media. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 681–686. [Google Scholar] [CrossRef]
- Shinde, S.; Kim, D.; Saratale, R.G.; Syed, A.; Ameen, F.; Ghodake, G. A Spectral Probe for Detection of Aluminum (III) Ions Using Surface Functionalized Gold Nanoparticles. Nanomaterials 2017, 7, 287. [Google Scholar] [CrossRef]
- Huang, M.; Lai, J.; Sun, H.; Wu, W. A simple, highly selective and ultra-sensitive “off-on-off” fluorescent chemosensor for successive detection of aluminum ion and phosphate in water samples. Microchem. J. 2019, 151, 104195. [Google Scholar] [CrossRef]
- Liu, Y.; Bi, A.; Gao, T.; Cao, X.; Gao, F.; Rong, P.; Wang, W.; Zeng, W. A novel self-assembled nanoprobe for the detection of aluminum ions in real water samples and living cells. Talanta 2019, 194, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Han, Y.; Lin, N.; Liu, H. Two pyridine-derived Schiff-bases as turn-on fluorescent sensor for detection of aluminium ion. Opt. Mater. 2019, 95, 109210. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, Y.; Huang, D.; Su, M.; Wang, K.; Hong, M. A Highly Sensitive and Selective Fluorescent Sensor for Detection of Al3+ Using a Europium(III) Quinolinecarboxylate. Inorg. Chem. 2014, 53, 6497–6499. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, X.; Zhu, Y.; Zhou, B.; Zang, S.; Tang, M.; Zhang, H.; Mak, T.C.W. A new fluorescent probe for Al3+ based on rhodamine 6G and its application to bioimaging. Dalton Trans. 2014, 43, 12624. [Google Scholar] [CrossRef]
- Polle, E.; Konzak, C.F.; Kattrick, J.A. Visual Detection of Aluminum Tolerance Levels in Wheat by Hematoxylin Staining of Seedling Roots. Crop Sci. 1978, 18, 823–827. [Google Scholar] [CrossRef]
- Martin, J.W.; Stark, T.D.; Thalhamer, T.; Gerbasi-Graf, G.T.; Gortner, R.E. Detection of Aluminum Waste Reactions and Waste Fires. J. Hazard. Toxic Radioact. Waste 2013, 17, 164–174. [Google Scholar] [CrossRef]
- Park, H.; Kim, W.; Kim, M.; Lee, G.; Lee, W.; Park, J. Eco-friendly and enhanced colorimetric detection of aluminum ions using pectin-rich apple extract-based gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 245, 118880. [Google Scholar] [CrossRef]
- Mohan, N.; Sreejith, S.S.; Begum, P.M.S.; Kurup, M.R.P. A modern approach for the sensing of aqueous Al(III) ion by Ni(II) Salen-type Schiff base complexes. Appl. Organomet. Chem. 2019, 33, e5064. [Google Scholar] [CrossRef]
- Benton, E.N.; Perera, N.A.K.R.; Nesterov, V.N.; Perera, W.; Omary, M.A.; Marpu, S.B. A europium-based optical sensor for the detection of carbon dioxide and its application for a fermentation reaction. Chemosensors 2023, 11, 5. [Google Scholar] [CrossRef]
- Song, H.; Liu, G.; Fan, C.; Pu, S. A novel fluorescent sensor for Al3+ and Zn2+ based on a new europium complex with a 1,10-phenanthroline ligand. J. Rare Earths 2021, 39, 460–468. [Google Scholar] [CrossRef]
- Weitz, E.A.; Pierre, V.C. A ratiometric probe for the selective time-gated luminescence detection of potassium in water. Chem. Commun. 2011, 47, 541–543. [Google Scholar] [CrossRef]
- Bodman, S.E.; Butler, S.J. Advances in anion binding and sensing using luminescent lanthanide complexes. Chem. Sci. 2021, 12, 2716–2734. [Google Scholar] [CrossRef]
- Parker, D. Luminescent lanthanide sensors for pH, pO2 and selected anions. Coord. Chem. Rev. 2000, 205, 109. [Google Scholar] [CrossRef]
- Cable, M.L.; Levine, D.J.; Kirby, J.P.; Gray, H.B.; Ponce, A. Luminescent lanthanide sensors. Inorg. Photochem. 2011, 63, 1. [Google Scholar]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Gan, Z.; Hu, X.; Huang, X.; Li, Z.; Zou, X.; Shi, J.; Zhang, W.; Li, Y.; Xu, Y. A dual-emission fluorescence sensor for ultrasensitive sensing mercury in milk based on carbon quantum dots modified with europium (III) complexes. Sens. Actuators B Chem. 2021, 328, 128997. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, C.; Tamiaki, H.; Chen, H. Emission response towards three anions (F−, HSO4− and AcO−) by a luminescent europium ternary complex with a 2-arylimidazole-1,10-phenanthroline conjugate. Photochem. Photobiol. Sci. 2010, 9, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ma, X.; Zhang, Y.; Liu, J.; Zhou, X.; Xiang, H. Optical Chemosensors Based on Transmetalation of Salen-Based Schiff Base Complexes. Inorg. Chem. 2014, 53, 3210–3219. [Google Scholar] [CrossRef] [PubMed]
- Royzen, M.; Dai, Z.; Canary, J.W. Ratiometric Displacement Approach to Cu(II) Sensing by Fluorescence. J. Am. Chem. Soc. 2005, 127, 1612–1613. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Govindaraju, T. A differentially selective sensor with fluorescence turn-on response to Zn2+ and dual-mode ratiometric response to Al3+ in aqueous media. Chem. Commun. 2012, 48, 259–261. [Google Scholar] [CrossRef]
- Wang, J.; Qi, Q.; Zhang, L.; Li, S. Turn-On Luminescent Sensing of Metal Cations via Quencher Displacement: Rational Design of a Highly Selective Chemosensor for Chromium(III). Inorg. Chem. 2012, 51, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Choi, S.H.; Lee, J.; Huh, J.O.; Do, Y.; Churchill, D.G. Highly Selective Fluorescence Detection of Cu2+ in Water by Chiral Dimeric Zn2+ Complexes through Direct Displacement. Inorg. Chem. 2009, 48, 1799–1801. [Google Scholar] [CrossRef]
- You, Y.; Tomat, E.; Hwang, K.; Atanasijevic, T.; Nam, W.; Jasanoff, A.P.; Lippard, S.J. Manganese displacement from Zinpyr-1 allows zinc detection by fluorescence microscopy and magnetic resonance imaging. Chem. Commun. 2010, 46, 4139–4141. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhao, Y.; He, C.; Liu, Y.; Duan, C. “Turn-On” Fluorescent Sensor for Hg2+ via Displacement Approach. Inorg. Chem. 2008, 47, 5169–5176. [Google Scholar] [CrossRef]
- Xue, L.; Liu, Q.; Jiang, H. Ratiometric Zn2+ fluorescent sensor and new approach for sensing Cd2+ by ratiometric displacement. Org. Lett. 2009, 11, 3454–3457. [Google Scholar] [CrossRef]
- Lee, J.W.; Jung, H.S.; Kwon, P.S.; Kim, J.W.; Bartsch, R.A.; Kim, Y.; Kim, S.; Kim, J.S. Chromofluorescent Indicator for Intracellular Zn2+/Hg2+ Dynamic Exchange. Org. Lett. 2008, 10, 3801–3804. [Google Scholar] [CrossRef] [PubMed]
- De Silva, C.R.; Maeyer, J.R.; Wang, R.; Nichol, G.S.; Zheng, Z. Adducts of europium β-diketonates with nitrogen p, p′-disubstituted bipyridine and phenanthroline ligands: Synthesis, structural characterization, and luminescence studies. Inorg. Chim. Acta 2007, 360, 3543–3552. [Google Scholar] [CrossRef]
- Sakur, A.A.; Chalati, T.; Fael, H. Selective spectrofluorimetric method for the determination of perindopril erbumine in bulk and tablets through derivatization with dansyl chloride. J. Anal. Sci. Technol. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- El-Didamony, A.M.; Saad, M.Z.; Ramadan, G.M. Charge-transfer complexes of chlorphenoxamine hydrochloride with chloranilic acid, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone and 7,7,8,8-tetracyanoquinodimethane as π-acceptors. Int. J. Appl. Pharm. 2019, 11, 117–123. [Google Scholar] [CrossRef]
- Jumean, F.; El-Dakiky, M.; Manassra, A.; Kareem, M.A.; Alhaj, M.A.; Khamis, M. Complexing Properties of Acid Alizarin Violet with Copper, Cobalt and Nickel in Micellar Media Containing SDS, CTAB and TX-100. Am. J. Anal. Chem. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C. Simple and Accurate Quantification of Quantum Yield at the Single-Molecule/Particle Level. Anal. Chem. 2013, 85, 2000–2004. [Google Scholar] [CrossRef]
- Nagaraja, D.; Melavanki, R.M.; Patil, N.R.; Geethanjali, H.S.; Kusanur, R.A. Solvent effect on the relative quantum yield and fluorescence quenching of a newly synthesized coumarin derivative. Luminescence 2015, 30, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Tsaryuk, V.I.; Zhuravlev, K.P.; Szostak, R.; Vologzhanina, A.V. Structure, luminescence, and Raman spectroscopy of europium and terbium dipivaloylmethanates and other β-diketonates with 2,2′-bipyridine. J. Struct. Chem. 2020, 61, 1026–1037. [Google Scholar] [CrossRef]
- Misra, A.; Bist, H.D.; Navati, M.S.; Thareja, R.K.; Narayan, J. Thin film of aluminum oxide through pulsed laser deposition: A micro-Raman study. Mater. Sci. Eng. B 2001, 79, 49. [Google Scholar] [CrossRef]
- Tsaryuk, V.; Zolin, V.; Legendziewicz, J.; Szostak, R.; Sokolnicki, J. Effect of ligand radicals on vibrational IR, Raman and vibronic spectra of europium β-diketonates. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2005, 61, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.G.; Chen, X.L.; Lan, Y.C.; Li, J.Y.; Xu, Y.P.; Xu, T.P.; Liu, Q.L.; Liang, J.K. Blue emission and Raman scattering spectrum from AlN nanocrystalline powders. J. Cryst. Growth 2000, 213, 198. [Google Scholar] [CrossRef]
- Liu, L.; Liu, B.; Edgar, J.H.; Rajasingam, S.; Kuball, M. Raman characterization and stress analysis of AlN grown on SiC by sublimation. J. Appl. Phys. 2002, 92, 5183–5188. [Google Scholar] [CrossRef]
- Decarlo, S.; Mayo, D.H.; Tomlinson, W.; Hu, J.; Hooper, J.; Zavalij, P.; Bowen, K.; Schnöckel, H.; Eichhorn, B. Synthesis, Structure, and Properties of Al(Rbpy)3 Complexes (R = t-Bu, Me): Homoleptic Main-Group Tris-bipyridyl Compounds. Inorg. Chem. 2016, 55, 4344. [Google Scholar] [CrossRef]
- Ng, S.M.; Narayanaswamy, R. Fluorescence sensor using a molecularly imprinted polymer as a recognition receptor for the detection of aluminium ions in aqueous media. Anal. Bioanal. Chem. 2006, 386, 1235–1244. [Google Scholar] [CrossRef]
- Hill, J.P.; El-Khouly, M.E.; Charvet, R.; Subbaiyan, N.K.; Ariga, K.; Fukuzumi, S.; D’Souza, F. Effect of anion binding on charge stabilization in a bis-fullerene–oxoporphyrinogen conjugate. Chem. Commun. 2010, 46, 7933–7935. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.P.; Schumacher, A.L.; D’Souza, F.; Labuta, J.; Redshaw, C.; Elsegood, M.R.J.; Aoyagi, M.; Nakanishi, T.; Ariga, K. Chromogenic Indicator for Anion Reporting Based on an N-Substituted Oxoporphyrinogen. Inorg. Chem. 2006, 45, 8288–8296. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bolívar, C.; Takizawa, S.; Nishimura, G.; Montes, V.A.; Anzenbacher, P., Jr. High-Efficiency Tris(8-hydroxyquinoline)aluminum (Alq3) Complexes for Organic White-Light-Emitting Diodes and Solid-State Lighting. Chem. Eur. J. 2011, 17, 9076–9082. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Yadav, P.; Gond, S.; Singh, A.; Singh, V.P. Development of a reversible chromogenic sensor for Cu2+ in aqueous ethanol. Mater. Lett. 2021, 295, 129869. [Google Scholar] [CrossRef]








| Sample | [Al3+], Calibration Curve (Average of 3 Readings, after Back Calculation) | [Al3+], ICP-OES Readings (Average of 3 Readings) |
|---|---|---|
| A | 2.051 ppm | 2.052 ppm |
| B | 3.779 ppm | 3.997 ppm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera, N.A.K.R.; Shankar, S.K.; Archambault, C.M.; Nesterov, V.N.; Marpu, S.B.; Yan, H.; Omary, M.A. A Novel ppb-Level Sensitive and Highly Selective Europium-Based Diketone Luminescent Sensor for the Quantitative Detection of Aluminum Ions in Water Samples. Analytica 2023, 4, 432-446. https://doi.org/10.3390/analytica4040031
Perera NAKR, Shankar SK, Archambault CM, Nesterov VN, Marpu SB, Yan H, Omary MA. A Novel ppb-Level Sensitive and Highly Selective Europium-Based Diketone Luminescent Sensor for the Quantitative Detection of Aluminum Ions in Water Samples. Analytica. 2023; 4(4):432-446. https://doi.org/10.3390/analytica4040031
Chicago/Turabian StylePerera, Nawagamu A. K. Rajitha, Sindhu K. Shankar, Cynthia M. Archambault, Vladimir N. Nesterov, Sreekar B. Marpu, Hao Yan, and Mohammad A. Omary. 2023. "A Novel ppb-Level Sensitive and Highly Selective Europium-Based Diketone Luminescent Sensor for the Quantitative Detection of Aluminum Ions in Water Samples" Analytica 4, no. 4: 432-446. https://doi.org/10.3390/analytica4040031
APA StylePerera, N. A. K. R., Shankar, S. K., Archambault, C. M., Nesterov, V. N., Marpu, S. B., Yan, H., & Omary, M. A. (2023). A Novel ppb-Level Sensitive and Highly Selective Europium-Based Diketone Luminescent Sensor for the Quantitative Detection of Aluminum Ions in Water Samples. Analytica, 4(4), 432-446. https://doi.org/10.3390/analytica4040031






