Abstract
Paracoccidioidomycosis (PCM) is a systemic mycosis caused by fungi of the genus Paracoccidioides. Serological tests are auxiliary in the diagnosis of PCM. However, the lack of standardization is a central problem in serodiagnosis and antibody titration. The objective of this study was to propose a methodology based on Fourier transform infrared spectroscopy (FTIR) for predicting antibody titers in patients with PCM. A total of 118 serum samples from patients with PCM were included, for which antibody titration using double immunodiffusion (DID) was previously performed. Serum samples were analyzed by attenuated total reflection (ATR)-FTIR and a supervised analysis with partial least squares regression (PLS) was used to predict the antibody titers. The PLS model with two latent variables and with the use of one orthogonal signal correction (OSC) showed a determination coefficient (R2) higher than 0.9999 for both the calibration and prediction set. The model was able to predict the antibody titers from patients with PCM with a minimal error. Therefore, modeling with FTIR/ATR and multivariate calibration proved to be a fast and highly accurate method for antibody titration, replacing the need for antigen production and performance of traditional serological tests.
1. Introduction
Paracoccidioidomycosis (PCM) is a systemic granulomatous mycosis endemic to Latin America, occurring from Mexico to Argentina, caused by the thermodimorphic fungi of the genus Paracoccidioides. The country with the highest occurrence of PCM is Brazil, with 80% of all reported cases, mainly in the Southeast, Center-West and South regions [1]. However, human occupation of new areas, together with environmental interventions, has modified the epidemiology of PCM [2], causing outbreaks of the disease in urban areas [3] and the emergence of new hyperendemic regions [4].
Paracoccidioides spp. is classified in the medium priority group in the WHO fungal priority pathogens list [5]. However, PCM is still not recognized by the World Health Organization as a neglected tropical disease (NTD), although it fulfills all the requirements to be in that list, due to its great social impact in Latin America [6]. PCM is responsible for 50% of deaths caused by systemic mycoses in Brazil [7], and is the eighth cause of death among chronic infectious diseases in the country [8].
Fungi of the genus Paracoccidioides are soil saprophytes, in addition to being commonly found in mammal reservoirs, mainly armadillos [9]. Thus, the major risk factor for PCM is soil exposure, being considered an occupational disease in people who work with the soil, such as farmers [10]. The habit of smoking is another important risk factor, and 90% of patients with the chronic form of PCM are smokers. The disease in the chronic form is more common in males aged between 30 and 60 years. However, in the acute form, it commonly to affects children, adolescents and young adults up to 30 years old of both sexes [2,11]. What differentiates the two forms (chronic and acute) is mainly the age of the affected individuals, the duration of symptoms and the organs involved. In the acute form, the symptoms develop over a short period of time and the main organs involved are the lymph nodes, liver and spleen. In the chronic form, symptoms develop on average for at least six months, and involvement of the lungs and upper aerodigestive tract mucosa is usually present [1].
The infection starts in the lungs, through the inhalation of fungal propagules. From them, the disease can spread to any organ or body system, resulting in a wide spectrum of clinical manifestations. If not diagnosed and treated correctly, PCM can lead to hospitalizations and generate serious sequelae such as pulmonary fibrosis and emphysema [12,13].
Thus, the early diagnosis of PCM is essential. It is established by demonstrating Paracoccidioides spp. in clinical samples, mainly through direct mycological examination of sputum, lesion scrapings and bronchoalveolar lavage (BAL) fluid. Histopathological examination is also a frequent diagnostic method, through visualization of the typical yeast cells in tissue fragments [1,11]. Isolation and cultivation of the fungus from clinical specimens is difficult and can take between 3 and 6 weeks of growth [10]; therefore, it is not a routinely used diagnostic tool.
There are other auxiliary diagnostic methods, such as the serological tests for the detection of anti-Paracoccidioides antibodies [1]. The visualization of the fungus in clinical samples is not always possible, making serological tests a good option [14]. Positivity in serological tests does not establish the diagnosis; however, it makes the case probable. In addition to being useful for diagnosis, the titer of specific anti-Paracoccidioides antibodies correlates with the severity of the case and can be used as a criterion for cure [15]. The most used technique is double immunodiffusion (DID), whose specificity and sensitivity can vary from 65% to 100%, depending on the antigen used [14]. The lack of standardization of techniques and antigens is a central problem in serological tests [16].
Recently, our research group developed a method for diagnosing PCM in serum using Fourier transform infrared spectroscopy (FTIR) and partial least squares-discriminant analysis (PLS-DA) [17]. Considering that FTIR is a technique that allows to determine the “molecular fingerprint” of a sample [18], we hypothesized that it would be possible to apply this technique for antibody titration, which is traditionally done by DID. Therefore, the aim of the present study was to propose an innovative methodology based on FTIR spectroscopy for predicting antibody titers in patients with PCM, facilitating the clinical management of this neglected endemic mycosis.
2. Materials and Methods
2.1. Patients and Serum Samples
A total of 118 retrospective serum samples from patients with PCM were analyzed. These samples were provenient from a repository at the School of Medicine of Botucatu, Universidade Estadual de São Paulo (UNESP). The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committees of the universities participating in the study (CAAE 37684720.2.1001.5343). All patients included were characterized as to sex, age, affected organs and clinical form/severity, following the criteria by Mendes et al. (2017) [1].
All the patients were diagnosed and treated at the Infectious Diseases Service of the School of Medicine of Botucatu (UNESP), from the Botucatu region, São Paulo, Brazil. The cases were diagnosed by clinical suspicion followed by visualization of the fungus of the genus Paracoccidioides in clinical samples, by direct mycological examination and/or culture and/or histopathology and/or cytopathology by cyto-inclusion [1].
For all cases, antibody titration was performed at the Infectious Diseases Service. An in-house produced exoantigen was used, based on the methodology by Camargo et al. (2003) [19], from the sample of P. brasiliensis B-339 (=ATCC 200273; American Type Culture Collection, Manassas, VA, USA). Antibody titration was performed using the double immunodiffusion technique (DID), with antibody titers ranging from 1:1024 to 1:1 (Table 1).

Table 1.
Antibody titration from the double immunodiffusion test (DID) of 118 serum samples from patients with paracoccidioidomycosis.
2.2. FTIR-ATR Analysis
Serum samples were analyzed in triplicate by attenuated total reflection (ATR) on Spectrum 400 FT-IR/FT-NIR (Perkin Elmer, Waltham, MA, USA) spectrometer, coupled to standard Universal ATR Sampling Accessory (UATR, PerkinElmer Inc., Waltham, MA, USA; Registration number L1250050).
Triplicates of 1 µL of each sample were deposited on the crystal of the instrument and dehydrated in airstream (60–65 °C) for one minute. The acquisition range was from 4000 to 650 cm−1 using the spectral resolution of 4 cm−1 and 8 scans. Between the spectral acquisitions of each of the serum samples and their triplicates, a blank acquisition was performed under the same conditions.
2.3. Chemometrics
All chemometric analyses were conducted in the software Pirouette 4.5 (Infometrix, Bothell, WA, USA), and figures were constructed in the software OriginPro70 (OriginLab, Northampton, MA, USA).
Antibody titers were transformed by the expression log2 (1/Antibody titer), obtaining inverse powers of 2, as described in Table 1. The average serum spectra were presented in two sets to analyze differences between samples with higher antibody titers and those with lower titers. Considering the mean value of the titers in log2 (1/Antibody titer), which was 4.6, one of the sets grouped the samples with values of titers less than or equal to 4.6 (n = 64), and the other grouped the samples with titer values greater than 4.6 (n = 54). Averaging was obtained by calculating the arithmetic mean of absorption for each frequency of the three min–max and vector normalized spectra from each sample, followed by the arithmetic mean of all samples in the set. A t-test with a significance level of 0.05 was performed between the sets to determine their differences [20].
Supervised analysis with partial least squares regression (PLS) was used to predict the antibody titers of the serum samples from patients with PCM. The average spectrum of the triplicates of each serum sample was obtained after amplitude normalization (0–1, min–max normalization) followed by vector normalization. PLS models were initially developed with the total dataset, different regions of the spectra, different pre-processing techniques (raw data, mean centering or autoscaling) and different variable transformation algorithms (1st or 2nd derivative) to determine the best models.
After defining the best conditions for the model, the dataset was systematically divided in a 1:1 ratio into a calibration set (CS) and a prediction set (PS). The maximum number of latent variables (LV) allowed in the PLS models was defined according to the recommendation of the ASTM E1655–05 guidelines, using the equation n = 6 (A + 1), where n is the number of CS samples and A is the maximum number of LV allowed in the model. Considering n = 59 samples, the maximum number of LV is 8 [21]. The CS was submitted to leave-one-out cross-validation with one to eight latent variables. External validation was performed, including each mean spectrum of a serum sample from PS in the PLS model obtained with CS (training set), and the respective antibody titer value was predicted. The performance of the PLS model was evaluated by the determination coefficient (R2), root mean square error of cross-validation (RMSECV), root mean square error of prediction (RMSEP) and tbias.
3. Results and Discussion
The 118 patients included in the study had a mean age of 42.6 ± 17.3 years, ranging from 4 to 85 years old. The predominant sex was male, with a male/female ratio of 3.33:1. The patients had a wide range of clinical presentations, predominantly the moderate chronic form, in 53.39% of the cases (n = 63) (Table 2). In total, 13 organs were involved in the cases of PCM (lungs, skin, upper aerodigestive tract mucosa, lymph nodes, trachea, larynx, liver, spleen, adrenal glands, intestine, bones, central nervous system and genitals), with 73.73% of the patients (n = 87) having involvement of more than one organ. The most affected organs were the lungs, in 88 cases, the lymph nodes, in 45 cases, and the upper aerodigestive tract mucosa, in 37 cases. The inclusion of patients with different clinical presentations was important to make the model more robust, considering that PCM is a disease that presents a wide spectrum of manifestations [1].

Table 2.
Distribution of 118 patients with paracoccidioidomycosis according to sex, clinical form and severity.
The average spectra of the 118 serum samples are shown in Figure 1A. The main contributions in the spectra were related to proteins and lipids, and the main bands were amide I and amide II, at approximately 1652 cm−1 and 1543 cm−1, respectively [22]. The main differences between the groups (antibody titer ≤ 4.6 and antibody titer > 4.6) were found in the region from 1727 to 1403 cm−1, which presented the lowest p values, highlighted in Figure 1B. In this region, the absorption of immunoglobulins IgG2, IgG3, and IgG4 occurred [23], which could explain its contribution in differentiating samples with lower and higher concentrations of antibodies.
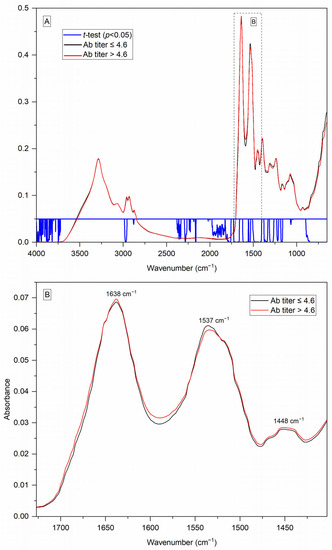
Figure 1.
(A) Average FTIR/ATR spectra of serum from patients with paracoccidioidomycosis, considering two sets (antibody titer ≤ 4.6 and antibody titer > 4.6), showing regions with p < 0.05 in the t-test performed between the two groups. Averaging was obtained by calculating the arithmetic mean of absorption for each frequency of the three min–max and vector normalized spectra from each sample, followed by the arithmetic mean of all the samples of the set; (B) 1727–1403 cm−1 region enlarged, showing the differences between the two groups and the main bands found.
Despite the differences found in the region from 1727 to 1403 cm−1, the best PLS models for the antibody titer prediction were obtained with the use of the full spectrum, evidencing that contributions showing antibody concentrations were present across the entire spectrum, not just in that specific region [17].
As can be seen in Figure 2, the best models were obtained using the first or second derivative. Derivatives are powerful methods both for baseline correction and for resolving overlapping spectral bands, being very suitable for improving the quality of ATR-FTIR spectra of biofluids [24]. However, even so, the models showed insufficient predictive power, because the RMSECV values were still too high. The predictive power was only optimized with the application of one orthogonal signal correction (OSC) component (Figure 2). OSC was proposed in 1998 by Wold et al., where it was developed for application in near-infrared (NIR) spectra. This filter removed from the spectral data (X matrix) only those that were totally unrelated to the property of interest to be inferred from the spectra (Y matrix). This was carried out through orthogonalization, ensuring that the information from the X matrix that was removed was mathematically orthogonal to Y. Thus, in the work in which it was initially proposed, the OSC was able to considerably improve the quality of the multivariate calibration, compared to the raw data and with other signal correction techniques [25].
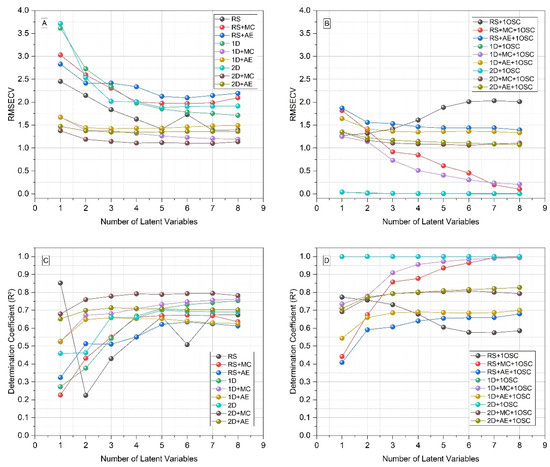
Figure 2.
Figures of merit of PLS models for antibody titer prediction with different pre-processing methods, from one to eight latent variables, considering the total set of the samples (N = 118). (A) Root mean square error of cross-validation (RMSECV), without orthogonal signal correction (OSC); (B) RMSECV with OSC; (C) determination coefficient (R2) without OSC; (D) R2 with OSC. RS: raw spectra; MC: mean centering; AE: autoscaling; 1D: 1st derivative; 2D: 2nd derivative.
Several studies have already used OSC in different techniques and analyses in order to improve the predictive quality of multivariate calibration models. In the field of medical mycology, our research group already successfully applied OSC to identify fungi that cause chromoblastomycosis, using FTIR and PLS-DA [26], and for predicting the antifungal susceptibility of Fonsecaea pedrosoi isolates against itraconazole, using FTIR and PLS [27]. In the same way, in the present work, we were able to optimize the results with the use of one OSC component, together with the use of first derivative. Although the figures of merit were very similar with the use of first or second derivative and one OSC component (they are even superimposed in Figure 2B,D), the values were slightly better with the first derivative. From the definition of the best conditions, the samples were divided 1:1 in the calibration and prediction sets and the modeling was carried out. Under these conditions, the models with one to eight latent variables showed determination coefficients and root mean square errors compatible with high performance (Figure 3).
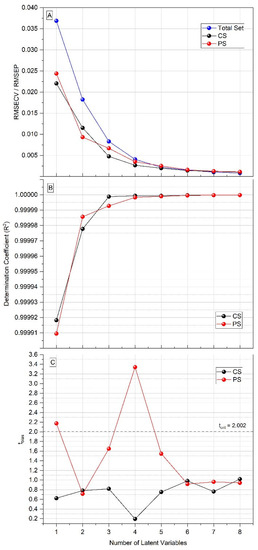
Figure 3.
Figures of merit of PLS models for antibody titer prediction, from one to eight latent variables. (A) Root mean square error of cross-validation (RMSECV) of total set and calibration set (CS), and root mean square error of prediction (RMSEP) of prediction set (PS). (B) Determination coefficient (R2) of CS and PS. (C) tbias of CS and PS.
As can be seen in Figure 3, the tbias value in the prediction set using one latent variable was greater than the tcrit value (=2.002). The tbias value is important, as it represents the mean bias between the predicted and actual values of the antibody titers and, therefore, must be below tcrit. The tbias value was less than 2.002 in the PLS model with two latent variables, and considering that, with two LVs, the prediction error (=0.0093) was already about a hundred times smaller than the difference between the antibody titers (when considered as potency rather than fractions, the interval between titers equals 1), this was the chosen number of latent variables to be used in the PLS model (Table 3). In the developed model, to be considered negative, a sample must have a negative predicted value less than 2×the RMSECV value (i.e, predicted value ≤ −0.023), which can be a problem for samples with a titer of 1:1, or 0, if they have a predicted value equal or less than −0.023. However, the two samples with antibody titer 0 included in the study had a prediction error within the established limit, being correctly predicted with titer 1:1 (Figure 4). Thus, it was possible to predict the antibody titers of the serum samples from patients with PCM with a minimal error (Figure 4).

Table 3.
Figures of merit of ATR/PLS model for antibody titer prediction with two latent variables, after min–max normalization followed by vector normalization, 1st derivative by Saviztky–Golay (5 points), and one component of orthogonal signal correction (OSC).
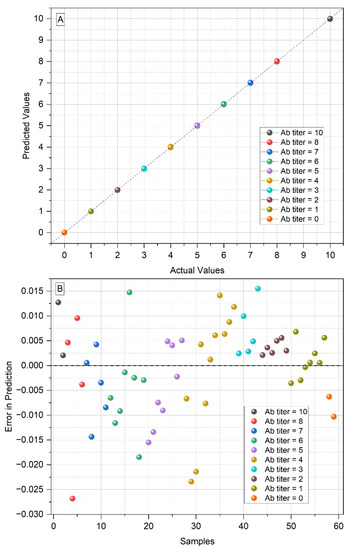
Figure 4.
(A) Predicted values vs. actual values of the antibody titers from the serum samples of patients with paracoccidioidomycosis, in the PLS prediction set with two latent variables (the points representing the samples are overlapped); (B) errors in predicted values for each serum sample, in the PLS prediction set with two latent variables.
To the best of our knowledge, this was the first study to propose FTIR spectroscopy as an antibody titer prediction tool in serum. Reference values for antibody titers were highly correlated with the values predicted from FTIR spectra, with R2 higher than 0.9999 in the PLS models. However, negative control samples from individuals without PCM were not included in the model. Thus, the model proposed here had no diagnostic validity, and the diagnosis must be previously performed using another method. As already shown by our research group, FTIR and multivariate calibration (specifically PLS-DA) can be used for PCM diagnosis, differentiating serum from these patients from healthy individuals and from patients with other systemic mycoses [17]. Thus, one possibility would be to use FTIR for both approaches, i.e., the PLS-DA model for diagnosis and the PLS model for antibody titration.
However, it is important to highlight that this diagnostic limitation is already inherent to serological tests. According to the current guidelines for the clinical management of PCM, a case is only confirmed by the presence of two criteria together: clinical manifestations compatible with the disease and observation of Paracoccidioides sp. in clinical specimens (secretions, fluids or lesion materials). In turn, a probable case presents clinical manifestations compatible with PCM and the detection of serum antibodies, preferably through quantitative DID [15]. However, despite not confirming the diagnosis, antibody titration has other important uses, as it is useful for classifying the severity of PCM, in addition to being essential in monitoring the evolution of the disease during treatment and being used as a cure criterion [1].
It is important to note that DID, which is the most used technique for serodiagnosis and antibody titration, can take more than three days to be performed, in addition to involving the preparation and subsequent disposal of several reagents [28]. Furthermore, the most important limitation of serological methods for PCM is the need to use antigens and their lack of standardization. The most used antigen is the one produced from the standard B-339 strain of P. brasiliensis, whose main component is the 43-kDa glycoprotein (gp43) [29]. There is no standard protocol for its production, and the culture medium and incubation conditions, such as initial inoculum size, time and temperature, vary depending on the laboratory [11]. Additionally, different laboratories use different techniques to produce antigens, such as sonic extracts or lyophilized filtrates, and for none of these is there an official standardization. Thus, standards can vary even in different batches produced in the same laboratory [30].
The antigen preparation method is directly related to the accuracy of serodiagnosis and antibody titration, and the lack of standardization leads to discordant results, also facilitating the occurrence of cross-reaction with other fungal diseases [1]. In addition, reproducibility is compromised. This has already been proven by a study that compared the results of immunodiffusion from six different Brazilian centers that were referenced in the serological diagnosis of PCM. In that study, discordant results (antibody titers) were found in all centers, with an average of 20% of inconsistencies [16]. These differences were caused by the high variability in antigens, considering that each center used its own antigen preparation. A review on PCM serology listed the lack of standardization in the antigens used as one of the most important issues to be studied [14], and the method proposed here can avoid this problem, as it rules out the need to use the gp43 antigen from B-339 strain.
Another issue in relation to antigens is the taxonomy of the genus Paracoccidioides, considered for 75 years to be formed by only P. brasiliensis. Now, the genus Paracoccidioides is divided into at least four phylogenetic species within the P. brasiliensis complex (P. brasiliensis sensu stricto, P. americana, P. restrepiensis and P. venezuelensis), in addition to the species P. lutzii [31,32,33]. Recent studies have classified P. americana as a separate species of the P. brasiliensis complex, and have included in the genus Paracoccidioides two uncultivable species that cause lobomycosis (P. ceti and P. lobogeorgii) [34]. Furthermore, the high genetic diversity found in Paracoccidioides isolates suggests that there may be a greater number of lineages [35].
Based on proteomic studies, it is known that P. lutzii has a different antigenic composition than P. brasiliensis sensu stricto [36]. The region with the highest occurrence of P. lutzii is the Central-West region of Brazil, and it has already been found that sera from patients in this region do not always react with antigens produced from P. brasiliensis sensu stricto, generating false-negative results in immunodiffusion [37]. The species of the P. brasiliensis complex have similar protein profiles, and in these cases, there are no problems regarding the antigen used in the serodiagnosis, as already shown for P. restrepiensis and P. americana. Sera from patients infected with these species of Paracoccidioides react with antigens produced from P. brasiliensis cells [38,39]. As for P. lutzii infections, there is a need to develop and use specific antigens, produced by cells of this species [40,41]. The method proposed here is not able to avoid this problem, as the models were developed based on antigens from P. brasiliensis reference strain B-339. Thus, a future perspective is to develop a model with a reference method based on immunodiffusion using P. lutzii antigens.
4. Conclusions
Here, we developed a fast, reproducible and highly accurate method to determine the antibody titers of patients with PCM. This new method replaces the need to use antigens, which is a problematic question in the serodiagnosis of PCM, due to the lack of standardization of antigen production. Additionally, the method replaces the traditional DID test, which can take several days to be performed. As limitations, we highlight the need to include negative control samples in the model. Furthermore, a model based on sera from patients infected with P. lutzii and antigens of this species should be developed, considering that the model based on P. brasiliensis cannot be transposed to this species. Despite these limitations, the method here proposed has potential to be transposed into clinical practice, and to be developed for other neglected fungal diseases.
Author Contributions
Conceptualization, A.K. and V.A.C.; methodology, A.K. and V.A.C.; software, A.K.; validation, P.C.d.M.; formal analysis, A.K.; investigation, A.K.; resources, B.A.S.P., R.d.S.C. and R.P.M.; data curation, B.A.S.P., R.d.S.C. and R.P.M.; writing—original draft preparation, A.K.; writing—review and editing, M.L.S. and V.A.C.; visualization, A.K. and P.C.d.M.; supervision, M.L.S. and V.A.C.; project administration, M.L.S. and V.A.C.; funding acquisition, M.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (Projeto: Processo CNPq number 442448/2019-8—Chamada Doenças Negligenciadas).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to still being used for further studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mendes, R.P.; Cavalcante, R.S.; Marques, S.A.; Marques, M.E.A.; Venturini, J.; Sylvestre, T.F.; Paniago, A.M.M.; Pereira, A.C.; Silva, J.F.; Fabro, A.T.; et al. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol. J. 2017, 11, 224–282. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R. New trends in paracoccidioidomycosis epidemiology. J. Fungi 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.C.F.; Macedo, P.M.; Almeida-Paes, R.; Romão, A.R.; Lazéra, M.S.; Wanke, B. Paracoccidioidomycosis after highway construction, Rio de Janeiro, Brazil. Emerg. Infect. Dis. 2017, 23, 1917–1919. [Google Scholar] [CrossRef]
- Krakhecke-Teixeira, A.G.; Yamauchi, D.H.; Rossi, A.; Sousa, H.R.; Garces, H.G.; Júnior, J.L.; Júnior, A.O.S.; Felipe, M.S.S.; Bagagli, E.; Andrade, H.F., Jr.; et al. Clinical and Eco-Epidemiological Aspects of a Novel Hyperendemic Area of Paracoccidioidomycosis in the Tocantins-Araguaia Basin (Northern Brazil), Caused by Paracoccidioides sp. J. Fungi 2022, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022.
- Griffiths, J.; Colombo, A.L.; Denning, D.W. The case for paracoccidioidomycosis to be accepted as a neglected tropical (fungal) disease. PLoS Negl. Trop. Dis. 2019, 13, e0007195. [Google Scholar] [CrossRef]
- Prado, M.; Silva, M.B.; Laurenti, R.; Travassos, L.R.; Taborda, C.P. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: A review from 1996 to 2006. Mem. Inst. Oswaldo Cruz 2009, 104, 513–521. [Google Scholar] [CrossRef]
- Bittencourt, J.I.M.; Oliveira, R.M.; Coutinho, Z.F. Paracoccidioidomycosis mortality in the State of Paraná, Brazil, 1980/1998. Cad. Saúde Pública 2005, 21, 1856–1864. [Google Scholar] [CrossRef]
- Bagagli, E.; Matute, D.R.; Garces, G.; Tenório, B.G.; Garces, A.G.; Alves, L.G.B.; Yamauchi, D.H.; Hrycyk, M.F.; Barker, B.M.; Teixeira, M.M. Paracoccidioides brasiliensis Isolated from Nine-Banded Armadillos (Dasypus novemcinctus) Reveal Population Structure and Admixture in the Amazon Basin. J. Fungi 2021, 7, 54. [Google Scholar] [CrossRef]
- Peçanha, P.M.; Peçanha-Pietrobom, P.M.; Grão-Velloso, T.R.; Júnior, M.R.; Falqueto, A.; Gonçalves, S.S. Paracoccidioidomycosis: What We Know and What Is New in Epidemiology, Diagnosis, and Treatment. J. Fungi 2022, 8, 1098. [Google Scholar] [CrossRef]
- Hahn, R.C.; Hagen, F.; Mendes, R.P.; Burger, E.; Nery, A.F.; Siqueira, N.P.; Guevara, A.; Rodrigues, A.M.; Camargo, Z.P. Paracoccidioidomycosis: Current Status and Future Trends. Clin. Microbiol. Rev. 2022, 35, e00233-21. [Google Scholar] [CrossRef]
- Giacomazzi, J.; Baethgen, L.; Carneiro, L.C.; Millington, M.A.; Denning, D.W.; Colombo, A.L.; Pasqualotto, A.C. The burden of serious human fungal infections in Brazil. Mycoses 2016, 59, 145–150. [Google Scholar] [CrossRef] [PubMed]
- González, Á. The therapy of pulmonary fibrosis in paracoccidioidomycosis: What are the new experimental approaches? J. Fungi 2020, 6, 217. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Oliveira, H.C.; Marcos, C.M.; Assato, P.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Advances and challenges in paracoccidioidomycosis serology caused by Paracoccidioides species complex: An update. Diag. Microbiol. Infect. Dis. 2016, 84, 87–94. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; Queiroz-Telles, F.; Kono, A.S.G.; Paniago, A.M.M.; Nathan, A.; Valle, A.C.F.; Bagagli, E.; Benard, G.; et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.S.M.; Del Negro, G.M.B.; Vicentini, A.P.; Svidzinksi, T.I.E.; Mendes-Giannini, M.J.; Almeida, A.M.F.; Martinez, R.; Camargo, Z.P.; Taborda, C.P.; Benard, G. Serological Diagnosis of Paracoccidioidomycosis: High Rate of Inter-laboratorial Variability among Medical Mycology Reference Centers. PLoS Negl. Trop. Dis. 2014, 8, e3174. [Google Scholar] [CrossRef]
- Koehler, A.; Scroferneker, M.L.; Pereira, B.A.S.; Souza, N.M.P.; Cavalcante, R.S.; Mendes, R.P.; Corbellini, V.A. Using infrared spectroscopy of serum and chemometrics for diagnosis of paracoccidioidomycosis. J. Pharm. Biomed. Anal. 2022, 221, 115021. [Google Scholar] [CrossRef]
- Balan, V.; Mihai, C.T.; Cojocaru, F.D.; Uritu, C.M.; Dodi, G.; Botezat, D.; Gardikiotis, I. Vibrational Spectroscopy Fingerprinting in Medicine: From Molecular to Clinical Practice. Materials 2019, 12, 2884. [Google Scholar] [CrossRef]
- Camargo, Z.P.; Berzaghi, R.; Amaral, C.C.; Silva, S.H.M. Simplified method for producing Paracoccidioides brasiliensis exoantigens for use in immunodiffusion tests. Med. Mycol. 2003, 41, 539–542. [Google Scholar] [CrossRef][Green Version]
- Trevisan, J.; Angelov, P.P.; Carmichael, P.L.; Scott, A.D.; Martin, F.L. Extracting biological information with computational analysis of Fourier transform infrared (FTIR) biospectroscopy datasets: Current practices to future perspectives. Analyst 2012, 137, 3202–3215. [Google Scholar] [CrossRef]
- ASTM—American Society for Testing and Materials. Standard Practices for Infrared Multivariate Quantitative Analysis, ASTM International E1655–17; West Conshohocken: Montgomery County, PA, USA, 2017. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Petibois, C.; Cazorla, G.; Cassaigne, A.; Déléris, G. Plasma protein contents determined by Fourier-transform infrared spectroscopy. Clin. Chem. 2001, 47, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Butler, H.J.; Smith, B.R.; Fritzsch, R.; Radhakrishnan, P.; Palmer, D.S.; Baker, M.J. Optimised spectral pre-processing for discrimination of biofluids via ATR-FTIR spectroscopy. Analyst 2018, 143, 6121–6134. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Antti, H.; Lindgren, F.; Öhman, J. Orthogonal signal correction of near-infrared spectra. Chemometr. Intell. Lab. Syst. 1998, 44, 175–185. [Google Scholar] [CrossRef]
- Heidrich, D.; Koehler, A.; Ramírez-Castrillón, M.; Pagani, D.M.; Ferrão, M.F.; Scroferneker, M.L.; Corbellini, V.A. Rapid classification of chromoblastomycosis agents genera by infrared spectroscopy and chemometrics supervised by sequencing of rDNA regions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 254, e119647. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.; Corbellini, V.A.; Heidrich, D.; Scroferneker, M.L. Prediction of itraconazol minium inhibitory concentration for Fonsecaea pedrosoi using Fourier Transform Infrared Spectroscopy (FTIR) and chemomectris. PLoS ONE 2020, 15, e0243231. [Google Scholar] [CrossRef] [PubMed]
- Camargo, Z.P.; Unterkircher, C.; Campoy, S.P.; Travassos, L.R. Production of Paracoccidioides brasiliensis Exoantigens for Immunodiffusion Tests. J. Clin. Microbiol. 1988, 26, 2147–2151. [Google Scholar] [CrossRef] [PubMed]
- Puccia, R.; Schenkman, S.; Gorin, P.A.J.; Travassos, L.R. Exocellular Components of Paracoccidioides brasiliensis: Identification of a Specific Antigen. Infec. Immun. 1986, 53, 199–206. [Google Scholar] [CrossRef]
- Camargo, Z.P. Serology of paracoccidioidomycosis. Mycopathologia 2008, 165, 289–302. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Hagen, F.; Puccia, R.; Hahn, R.C.; Camargo, Z.P. Paracoccidioides and Paracoccidioidomycosis in the 21st Century. Mycopathologia 2023, 188, 129–133. [Google Scholar] [CrossRef]
- Turissini, D.A.; Gomez, O.M.; Teixeira, M.M.; McEwen, J.G.; Matute, D.R. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 2017, 106, 9–25. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Theodoro, R.C.; Carvalho, M.J.A.; Fernandes, L.; Paes, H.C.; Hahn, R.C.; Mendoza, L.; Bagagli, E.; San-Blas, G.; Felipe, M.S.S. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol. Phylogenet. Evol. 2009, 52, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Vilela, R.; de Hoog, S.; Bensch, K.; Bagagli, E.; Mendoza, L. A taxonomic review of the genus Paracoccidioides, with focus on the uncultivable species. PLoS Negl. Trop. Dis. 2023, 17, e0011220. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Campanini, E.B.; Nishikaku, A.S.; Puccia, R.; Marques, M.; Bialek, R.; Rodrigues, A.M.; Batista, W.L. PbGP43 Genotyping Using Paraffin-Embedded Biopsies of Human Paracoccidioidomycosis Reveals a Genetically Distinct Lineage in the Paracoccidioides brasiliensis Complex. Mycopathologia 2022, 187, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Júnior, L.P.Q.; Camargo, Z.P.; Tadano, T.; Rodrigues, A.M.; Takahara, D.T.; Gegembauer, G.; Araujo, L.M.; Hahn, R.C. Serological and antigenic profiles of clinical isolates of Paracoccidioides spp. from Central Western Brazil. Mycoses 2014, 57, 466–472. [Google Scholar] [CrossRef]
- Gegembauer, G.; Araujo, L.M.; Pereira, E.F.; Rodrigues, A.M.; Paniago, A.M.M.; Hahn, R.C.; Camargo, Z.P. Serology of Paracoccidioidomycosis Due to Paracoccidioides lutzii. PLoS Negl. Trop. Dis. 2014, e2986. [Google Scholar] [CrossRef]
- Cocio, T.A.; Martinez, R. Serological diagnosis of paracoccidioidomycosis using a Paracoccidioides spp. comercial antigen and the counterimmunoelectrophoresis method. Braz. J. Infect. Dis. 2021, 25, 101607. [Google Scholar] [CrossRef]
- Macedo, P.M.; Teixeira, M.M.; Barker, B.M.; Zancopé-Oliveira, R.M.; Almeida-Paes, R.; Valle, A.C.F. Clinical features and genetic background of the sympatric species Paracoccidioides brasiliensis and Paracoccidioides americana. PLoS Negl. Trop. Dis. 2019, 13, e0007309. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Kubitschek-Barreira, P.H.; Pinheiro, B.G.; Teixeira-Ferreira, R.; Hahn, R.C.; Camargo, Z.P. Immunoproteomic Analysis Reveals Novel Candidate Antigens for the Diagnosis of Paracoccidioidomycosis Due to Paracoccidioides lutzii. J. Fungi 2020, 6, 357. [Google Scholar] [CrossRef]
- Grossklaus, D.A.; Takahara, D.T.; Kruger, C.S.; Gonzaga, A.M.; Camargo, Z.P.; Nery, A.F.; Almeida, H.L.; Hahn, R.C. Profile of exoantigens from clinical isolates of Paracoccidioides lutzii in Mato Grosso, Brazil. Rev. Patol. Trop. 2016, 45, 265–272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).