Abstract
To meet consumer demand for fermented beverages with a wide range of flavors, as well as for quality assurance, it is important to characterize volatiles and their relationships with raw materials, microbial and fermentation processes, and the aging process. Sample preparation techniques coupled with comprehensive 2D gas chromatography (GC×GC) and mass spectrometry (MS) are proven techniques for the identification and quantification of various volatiles in fermented beverages. A few articles discuss the application of GC×GC for the measurement of fermented beverage volatiles and the problems faced in the experimental analysis. This review critically discusses each step of GC×GC-MS workflow in the specific context of fermented beverage volatiles’ research, including the most frequently applied volatile extraction techniques, GC×GC instrument setup, and data handling. The application of novel sampling techniques to shorten preparation times and increase analytical sensitivity is discussed. The pros and cons of thermal and flow modulators are evaluated, and emphasis is given to the use of polar-semipolar configurations to enhance detection limits. The most relevant Design of Experiment (DoE) strategies for GC×GC parameter optimization as well as data processing procedures are reported and discussed. Finally, some consideration of the current state of the art and future perspective, including the crucial role of AI and chemometrics.
1. Introduction
The global alcoholic beverages market is expected to reach USD 1684 billion in value by 2025, growing at a compound annual growth rate (CAGR) of 2.0% starting in 2017 [1]. Two of the main products are expected to reach USD 962.39 billion by 2026 at a CAGR of 4.22%, while wine is expected to reach $444.93 billion by 2027 at a CAGR of 6.05% [2]. The increasing young adult population is the major driver for the growth of the alcoholic beverages market, in line with the overall rise in consumption levels and demand. These young drinkers prefer innovative products with more sophisticated and intense flavors as compared to traditional drinkers [3].
In order to match production with new demands, it is essential to understand the science of flavor [4]. The flavor of beer and wine is determined by the volatile organic compounds (VOCs; acronyms are listed in Supplementary Table S1) from raw materials that are produced during fermentation and aging processes.
In the last 40 years, almost 1000 volatile compounds have been identified in wine, with a concentration range varying from hundreds of mg/L to µg/L or ng/L levels [5]. However, only some of them work as odor-active molecules, mainly in concentrations above their sensory perception threshold (Table S2) but also because of synergistic or masking effects at peri/sub-threshold levels [6].
These substances give fermented alcoholic beverages a fruity, herbal, floral, or off-flavor [7]. Extraction methods and gas chromatography coupled with detectors are the traditional methods for detecting and identifying VOCs [8]. In order to obtain an accurate VOC profile of beverages and to best identify each VOC, an instrument with a higher separation capacity is required. Comprehensive two-dimensional gas chromatography coupled with mass spectrometry (GC×GC-MS) is a promising solution [9,10]. To obtain good results for GC×GC-MS VOC analysis, researchers need to optimize sample extraction, GC×GC separation, and data processing.
This review critically discusses each step of the GC×GC-MS workflow in the specific context of beer and wine VOC research, including VOC extraction, GC×GC separation, and data processing. The extraction part covers the most general techniques, such as dynamic headspace (DHS), solid-phase microextraction (SPME), liquid extraction, etc. GC×GC separation part covers the modulator configuration, column setup, and parameter optimization methods. Many applications of GC×GC in beer and wine analysis have been published since 2005, with an increasing number of publications per year (Table 1). They can be found by searching the term GC×GC or “comprehensive GC” in ScienceDirect or Google. As we can see, the number of articles is increasing year by year. Sample preparation methods and instrument setups are also becoming more diverse. Until now, the most used VOC extraction method is HS-SPME. Most studies use a thermal modulator and a polar-semipolar column setup. And in the last data processing part, different algorithms and their pros and cons are discussed based on the demand for VOC profiling.

Table 1.
Articles on applying GC×GC to beer and wine analysis.
2. Sample Extraction
Direct injection of beer and wine into GC is not suitable because the sample contains large amounts of water and non-volatile components that are harmful to the GC column [59]. Besides, some VOCs need to be concentrated to reach the sensitivity of the detector. However, since the aromas of beverages range in concentration from mg/L to ng/L and from polar to non-polar, no single sample preparation method is universally suitable to screen all volatiles in an untargeted analysis. Hence different sample preparation techniques are involved before the GC injection [60]. The physicochemical properties of the common VOCs in the beverages are listed in Table S2. The choice of the VOC extraction method is based on the physicochemical properties of the interested volatiles. Different methods differ in extraction mechanism and absorption/collection material, meaning they require different optimizations. And, most importantly, applying different extraction methods results in different analytical sensitivity to one volatile compound and different coverage of the entire VOC profile [36,51,61]. In this review, we will discuss five common extraction methods and their possible further applications to beer and wine.
Before introducing the sampling techniques, it is necessary to classify the three types of extraction procedures: (i) single extraction, (ii) repeated stepwise extraction, or (iii) continuous extraction. In the case of a single extraction step, the sample is placed in a closed, multi-phase system. After a long enough time, the system reaches equilibrium, when the exchange of analytes among the phases no longer affects their concentration. The concentration value is determined by the equilibrium constant (e.g., Henry’s law constant) among multiple phases [62]. Most modern sampling techniques can be applied with this approach, for example, headspace (HS) and HS-solid phase microextraction (SPME), in-solution SPME, liquid-liquid extraction (LLE), and stir bar sorptive extraction (SBSE). If exhaustive sampling is the purpose, stepwise extraction or multiple extractions can be applied. In this procedure, the single extraction step is repeated on one sample aliquot. Because the analytes in the sample are completely extracted, the summed peak areas of the individual analyses are directly related to the total amount of the analyte in the sample, regardless of the equilibrium constants. In continuous extraction, equilibrium is impossible to reach. Analytes are continuously removed from the liquid beverage sample by gas (e.g., DHS) or stationary phases (e.g., SPE). The analyte concentration never reaches equilibrium between the sample and gas phases because a carrier gas is constantly being blown in. Finally, all the volatile analytes are removed from the sample and collected [62].
Unfortunately, it is unrealistic to have a method to concentrate and recover all VOCs with different properties. There is no “magic bullet” solution available for such a complex chemical issue. The common sample preparation techniques for VOC analysis today are dynamic headspace (DHS), dispersive liquid-liquid microextraction (DLLME), multiple stir absorptive extraction (mSBSE), solid-phase extraction (SPE), and solid-phase microextraction (SPME). They can be roughly classified into two categories: headspace and in-solution. Headspace techniques are HS-SPME and DHS. In-solution techniques are DLLME, mSBSE, and SPE. SPME can also be applied as in-solution sampling. Applications of headspace analysis benefit either from the simplicity of automation or from removing some of the matrix effects. However, one study found that even dynamic headspace techniques could not eliminate the effect of the sample matrix on analysis if the analytes were incompletely extracted [63]. A table comparing the advantages and disadvantages of these techniques is available in our previous study [51].
2.1. Dynamic Headspace
DHS was initially introduced in 1981 [64]. Because equilibrium is not required, it eliminates the sample matrix effect during the absolute VOC quantification. DHS is universally accepted as a non-polluting, easy-to-use, and flexible process. The central idea of this method is the extraction of VOCs by a continuous inert gas flow into the liquid matrix. An adsorbent or cryogenic trap is used to further enrich the extracted VOCs. The performance of different adsorbent materials on sampling wine VOCs was published in a recent study [65]. Since the concentration of the VOCs in the headspace decreases exponentially over extraction time, with proper mathematical modeling, it is possible to describe a total peak area that is proportional to the total amount of analyte existing in the original sample. In DHS sampling, ideally without a breakthrough, the analyte concentration in the sample can be expressed by the following equation:
The residual analyte concentration in the sample is denoted by Ci. The initial concentration was C0 at the beginning of the sampling. Ci decreases exponentially with increasing sweep time (t). q is a constant that reflects the recovery efficiency. The peak area of the analyte (Ai) is proportional (k) to the concentration of the analyte in the adsorbent (C0 × (1 − e−qt)):
This area can then be linked to concentration by using an appropriate calibration procedure. Chemical traps of different sizes, sorbent particle sizes, and sorbent compositions are available on the market [66]. Tenax TA is the most commonly used adsorbent since it can adsorb a wide range of VOCs and has high thermal stability as well as good hydrophobicity [67].
DHS sampling has been applied to VOC profiling for beer and wine [68,69,70]. DHS is suitable for the extraction of highly volatile compounds. Because of the long-term continuous extraction, the analytes in the sample can be recovered almost completely and injected into the GC. So, sampling with DHS provides good analytical sensitivity. In addition, the DHS sampling process has several adjustable parameters (e.g., temperature, time for incubation, trapping, and drying, and gas flow) that can be optimized according to the characteristics of the sample. Ideally, the sampling volume (gas flow multiplied by the trapping time) is smaller than the sampling breakthrough volume of the first eluting analyte from the sampler. And the real sampling gas flow can be obtained as a function of incubation temperature, sampling temperature, head pressure, outlet pressure, and outlet gas flow [71]. However, this flexibility in the parameters requires more instrumental investment, more maintenance, and complex optimization. Both sampling and GC injection can be automated, making it possible to guarantee reproducible analysis with high throughput.
DHS covers a broader range of volatiles compared to the more commonly used SPME, such as acids, benzenoids, ketones, nitrogen, and sulfur-containing compounds [51]. As the VOCs in beer and wine vary from polar to apolar and from trace to high concentration, exhaustive sampling techniques—DHS coupling with GC×GC would be perfect. However, the additional investment in the focusing equipment, such as the Cooled Injection System (Gerstel) and thermal desorption unit, limited the application of DHS in non-specialized VOC analysis laboratories [71].
2.2. Headspace-Solid Phase Microextraction
The first SPME application was presented in 1990 [72]. It is considered one of the most versatile sample preparation techniques currently available [73]. Its simplicity of optimization, high reproducibility, and low cost have made it widely accepted. Similar to liquid-liquid extraction, absorption extraction is also based on the distribution coefficient. The only difference is that the absorbent fibers are in a rubbery state at room temperature and therefore are solid. The final equilibrium sampling state can be expressed as:
The total amount of analytes in the sample is the original concentration (C0) multiplied by the volume (VS). After equilibrium is reached, it is the sum of the analyte amounts in each phase: CS × VS for the sample phase, CG × VG for the gas phase, and CF × VF for the absorbent phase. CF × VF represents the absorbed amount of an analyte in the fiber coating WF:
KG/S and KF/G are the distribution constants between the gas phase and the sample phase and between the fiber phase and the gas phase. Substituting the CG and CS in Equation (3), the absorbed amount in the fiber coating WF is:
HS-SPME is a highly automated technique. It allows direct sampling and does not require organic solvents [74]. HS-SPME-GC-MS has successfully measured a number of volatiles in beer, such as organic acids, carbonyl compounds, esters, alcohols, fatty acids, and monophenols. A detailed description of the coating materials used for SPME fiber is available in a review paper [75]. A method using multi-coated fiber (Divinylbenzene/Carboxen/Polydimethylsiloxane, DVB-CAR-PDMS) was applied to beer VOC analysis by Rodrigues [76]. In a recent study [41], 329 volatiles were determined from 19 types of lager beers by using DVB-CAR-PDMS fiber for extraction and bidimensional GC-MS for analysis. According to its principles, SPME extraction has some inherent disadvantages. Multiphase equilibria to be achieved in SPME systems are influenced by matrix effects [77]. In practice, SPME requires a certain amount of sampling time. The sample, headspace, and fiber phases are brought to an approximate equilibrium to minimize extraction bias. [78]. This requires sufficient sampling time. To overcome the long extraction time, many research groups have made improvements. The mass transfer efficiency of multiphase systems is significantly improved at high temperatures [79]. Cooling the fibers allows them to maintain good absorption in a thermal sample environment [80]. To improve the extraction efficiency and capacity of the SPME device, new geometries of the sorbent phase were used, like SPME Arrow and thin-film SPME (TF-SPME). By applying a larger extraction phase volume and molecular exchange surface area, higher analytical sensitivity and faster extraction speeds were achieved [73,79]. Physical assistive technologies, like vacuum and ultrasound, have also been applied to SPME sampling. With the proper setup, the extraction processing can be faster, milder on temperature, more selective, and more sensitive [81,82,83,84]. The basic principle and the method development strategy are well explained in a recent review [85]. It is worth noting that, in a complex system such as a fermented beverage, which contains hundreds or thousands of components, VOCs might compete for binding to the sorbent, affecting absorption. In this context, the role of ethanol is crucial since it is the most abundant volatile compound in fermented beverages and has been reported as an important interfering molecule during the HS-SPME extraction of minor compounds. This well-known phenomenon, which impacts the overall extraction efficiency, especially for polar phases, leads to decreased signal for semi-volatile analytes due to the faster adsorption of ethanol on the fiber surface [86]. The use of a thin hydrophobic polydimethylsiloxane (PDMS) layer on commercial polar fiber materials is an effective strategy to balance the displacement effect of ethanol in the determination of VOC in fermented beverages [87].
2.3. Dispersive Liquid-Liquid Microextraction
In the last decade, the use of liquid-liquid extraction (LLE) has been limited as much as possible due to its increased environmental impact compared to SPE and SPME [88]. However, these last-mentioned techniques require dedicated tools and, in some cases, involve a pre-treatment step. Because of that, and for all those applications where LLE is the best sample preparation or cannot be replaced, this technique evolved towards the miniaturization and minimization of volumes. The best-in-class alternative to LLE was proposed in 2006 and is dispersive liquid-liquid microextraction (DLLME) [89]. In this protocol, a few mL of sample are treated using a reduced amount of organic non-polar extracting solvent and assisted by a higher volume of organic polar dispersive solvent. The extracting solvent is dispersed in small droplets into the sample using mechanical action or ultra-sound (UA-DLLME) to maximize area/volume ratio and mass transfer. In this step, the role of the dispersive solvent, together with the ethanol present in the matrix, is crucial to allowing analytes to move from the aqueous phase into the organic phase. Another relevant advantage of DLLME is its concentration factor, which enhances method performance.
A further improvement, vortex-assisted LLME (VALLME), was published in 2010 [90]. It requires only basic laboratory equipment and is cost-effective in terms of time and economy [91]. The sample and the organic phase are mixed into an emulsion by vortexing. Because the solvent phase is broken into small droplets, the interfacial area is increased significantly. Based on this, the efficiency of mass transfer increases while the interfacial transfer coefficient remains constant [92]. In the final step of VALLME sample preparation, the emulsion is destabilized by centrifugation using high gravitational acceleration to obtain the separated organic and aqueous phases [93]. Because it involves the dispersion and aggregation of the extractant, its miscibility with the sample phase, and the mass transfer of the analyte between the phases, more investigations are still required to clearly describe the mechanism of this process. Due to this lack of mechanistic understanding, sample preparation parameters should be empirically defined. However, the final state of this in-solution sampling is a simple equilibrium, two-phase system. The concentration of an analyte in the sorbent phase only depends on the volume of the two phases and the distribution coefficient. VALLME has been successfully applied to the VOC analysis of beer and wine. However, basic knowledge of these processes is still lacking, limiting their application. In recent years, other forms of DLLME have progressed. Air-assisted liquid-liquid microextraction (AALLME) using a syringe for sucking and injecting the mixture of the organic phase the and aqueous sample does not require the assistance of dispersant solvents and therefore consumes fewer organic extractants [94]. Switchable hydrophilic solvents expand our understanding of liquid-liquid extraction [95]. It is named switchable polar solvent (SPS) or switchable hydrophilic solvent (SHS) and is controlled by carbon dioxide (CO2). The solvent can adjust the polarity, selectively extracting polar and non-polar substances. All these methods belonging to DLLME are becoming increasingly popular, and new developments, applications, and improvements are expected in the future.
2.4. Stir Bar Sorptive Extraction
The basic principles of stir-bar sorptive extraction (SBSE) are identical to those of SPME. When PDMS was the only phase available for SPME, the quantitative analysis was only obtained for the volatile compounds whose phase/water partition ratio was larger than 105. SBSE was developed to enlarge the application range and boost analytical sensitivity by coating more PDMS on the sorption unit [96]. It can be applied to headspace sampling as HS-SPME, constructing a three-phase system, but mostly the sorbent phase is put into the liquid sample, forming a two-phase equilibrium system. Latterly, multiple SBSEs were proposed to increase the application on polar analytes by adding ethylene glycol/silicone phase during the extraction [97]. Both SBSE and multiple SBSE have been successfully applied to beverage VOC analysis [36,60,64,69,98]. An approach to SBSE in beverages is reverse extraction [60]. After the volatile extraction, analytes on the stir bars can be desorbed by liquid extraction. Back extraction offers possibilities in different aspects, for example, performing the analysis with HPLC, having more choices of sorbent phases [99], downstream odor and taste testing, and allowing free derivatization [100] However, to date, the diffusion of SBSE in laboratory practice has been limited by the need for dedicated and expensive devices for the procedure’s automation. Another relevant gap is the need to dip the stirring bar into the sample, which implies carryover phenomena and an early performance drop if compared to headspace techniques.
2.5. Solid-Phase Extraction
The popularity of solid-phase extraction (SPE) in analytical laboratories is based on its flexibility in application. It is available in a variety of solid and mobile phase combinations. It can be used for concentration and purification and also allows the enzymatic hydrolysis of bound VOCs during sample extraction. In SPE sample preparation, VOCs are retained based on three mechanisms: partition, adsorption, and electronic interactions [101,102,103]. These mechanisms apply to small apolar analytes, large apolar molecules, and polar and polarizable analytes, respectively. Due to the different retention principles, SPE has a high extraction efficiency for both polar and apolar organic analytes. Many studies have been published on applying SPE to beverage analysis. Most of them focus on the quantification of a certain group of VOCs [104,105,106], and the HS-SPE application was also used to obtain extracts with a composition closer to the orthonasal olfaction process [107]. An exciting variety of SPE uses small particles made of nanomaterials for liquid samples. Because of their small size, the nanomaterials have a fairly large surface area and a high surface/volume ratio. Consequently, the extraction time is saved. In a study, a very short exposure time of the sorbent phase to the sample (1 min) was applied to extract biogenic amines in wines [108]. The major drawback of using SPE for beverage VOC analysis is the cumbersome extraction procedure, which can be mitigated by automation. Another expected variety is molecular imprinting-based SPE. The solid-phase polymer preserves the stereochemical properties and synergy of functional groups of target compounds [109]. Increasing selectivity benefits the quantification of target compounds. Firstly, a larger number of samples can be applied to improve the analytical sensitivity. Secondly, less separation power is required, saving analytical time. Molecular imprinting-based SPE has been successfully applied to ester compounds in an aqueous solution [84]. A promising alternative for beverage VOC analysis is membrane-assisted SPME. In this protocol, a miniaturized membrane bag is filled with an organic solvent or solution and inserted into the sample to allow analytes to pass into the extracting phase. The membrane selectively allows only analytes to migrate, overcoming time-consuming procedures. Stirring is used in this step to speed up the mass transfer. The injection volume is finally sampled from the extracting solvent and analyzed with the instrument. In terms of its application in the analysis of VOC in fermented beverages, this method was successfully used for the quantitation of varietal terpenes in white and red wines [110].
3. GC×GC Separation
Beer and wine contain many different volatile compounds. From the identification point of view, direct injection mass spectrometry, lacking separation, will result in a mixed MS signal of all the compounds. Hence, chromatography is used to separate VOCs before detection. Mass spectrometry (MS) can provide information for both identification and quantification in a single measurement. A gas chromatographic separation stage (GC) is commonly used for VOC analysis in beverages, and GC-MS is the technique of choice for most applications. According to the study purpose, there are two types of approaches: untargeted VOC profiling and targeted VOC quantification. A biological phenomenon’s modeling must consider many possible chemical variables. Targeted VOC quantification only requires good separation in the specific chromatographic region. The fast analytical rate can be accomplished in the most efficient way via Fast GC, but not via GC×GC. Fast GC application is characterized by a duration of less than 20 min [111]. GC×GC is more suitable for non-targeted profiling, which focuses on the detection of as many VOCs as possible to obtain patterns or fingerprints of a specific beverage production process. For these reasons, including the unannotated peaks, it only requires semi-quantitative measurement of concentration. The profiling result is usually used to explore the similarities and differences between sample groups [112]. The research question could be, for example, whether there are any volatile biomarkers when a newly developed yeast is used in the fermentation. In this type of study, ensuring analytical coverage is essential for the reliability of the results. Better separation is a key factor in obtaining excellent analytical coverage. Beer and wine contain too many volatile compounds, which overcrowd the chromatogram of a traditional one-dimensional GC (1DGC). When signals from VOCs overlap with each other, it is difficult to proceed with their identification and quantification. GC×GC was developed to conquer this problem [113]. In GC×GC analysis, the analytes are first separated on the first column. The elute of 1D separation is continuously concentrated and injected into the 2D column for further separation. The schematic diagram of GC×GC is simply demonstrated in Figure 1. For a complete and detailed description of the instrument, please refer to a previous review [114].
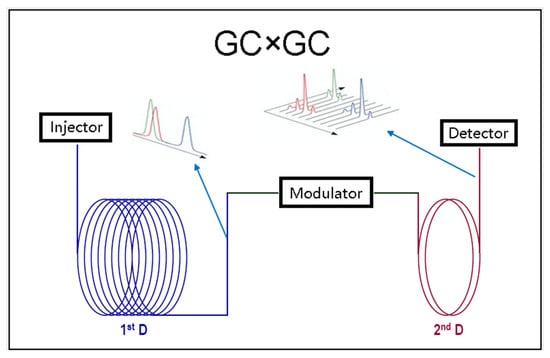
Figure 1.
The basic working scheme of GC×GC.
Compared with 1DGC, GC×GC method development requires more factors to be considered, such as column combination and sequence and modulator parameter adjustment. Additionally, there are secondary or higher-level interactions among these parameters. For this reason, the optimization of GC×GC is complex, and each parameter cannot be simply optimized individually. Currently, most GC×GC studies use the method adapted from 1DGC or rely on the analytical setting recommended by the instrument manufacturer. In doing so, the high separation power of GC×GC is not fully utilized to complete the separation of target analytes. Improper setup may lead to reduced separation power, co-eluting peaks, and distorted peaks, resulting in annotation and quantification errors. Optimizing GC×GC is a challenge to the developer’s knowledge of instrumentation and data handling capabilities.
3.1. Modulator
In order to achieve high-quality GC×GC separations, it is critical to efficiently transfer the eluent from the first dimensional (1D) column to the second dimensional (2D) column while maintaining the separation obtained in the first dimension. The general criterion is that most of the peaks in the first dimension are cut into at least three slices, while no wrap-around occurs in the second dimension [36]. The 1D and 2D columns are connected by a modulator. The modulator periodically collects and focuses the condensable eluent for a short period of time and injects it into the 2D column at once [115]. There are several modulator products on the market; they are either based on temperature regulation or gas flow control to achieve transfer [116]. There are two types of thermal modulator mechanisms: heating and cooling. The heating modulator collects the eluted 1D analytes at temperatures close to those of the oven and performs the injection by raising the temperature. As such, a modulator needs to maintain a temperature difference of at least 100 °C from the GC×GC oven [117], which limits the separation temperature of the GC×GC oven and the boiling point range of the volatiles. It has been used less frequently today. The latter cooling modulator collects analytes with cryogenic cooling and performs the injection at the temperature of the oven. Using this type of modulator, a narrow injection band modulation period can be achieved in the second dimension for C4 to C40+ compounds to provide high detection sensitivity [118]. The flow modulator controls the transfer of analytes from 1D to 2D columns by using a branch line of high-pressure helium. When a 2D column needs to be injected, helium flow will intercept the outflow from the 1D column and inject the analytes stored in the modulator. This principle is known as Dean’s switch and is well described in a paper [119]. When the injection is not required, the helium flow will cut off the path to the 2D column, a portion of the eluates will remain in the modulator, and the excess will be expelled as waste [120]. The flow modulator is suitable for volatiles from C1 to C40+. However, the flow splitting will inevitably lead to a decrease in detection sensitivity.
Both thermal and flow-based modulators have their advantages and disadvantages in areas such as detection limits, volatility range, flexibility of installation, hardware investment, and cost of operation [121]. There is no single modulator that would be the best choice for all purposes. Since non-target VOC profiling is the main task of GC×GC in beverage analysis, a modulator should fit the demands of this task. Due to its technical characteristics, cryogenic thermal modulation is a superior platform for VOC profiling. All eluted analytes from the 1D column are collected, aggregated by the modulator, and injected into the 2D column at extremely high speed. As a result, there is no loss of detection sensitivity and even an improvement in the peak shape if the proper 2D column length is applied [119]. Besides, the method development of thermal modulation is less complicated than that of the flow modulator. It does not require optimization of the auxiliary helium pressure, equilibrium flow rate, or flow volume. The thermal modulator requires fewer connections, reducing potential leakage. The main disadvantage of the thermal modulators is the high level of cryogen consumption per analysis [122]. However, VOC profiling is required once (multiple technical replications) for each biological study, so this cost is affordable.
3.2. Column Setup
Chromatographic columns are the key components of chromatographic systems. GC×GC increases the peak capacity and associated separation capability by introducing two different dimensional separation mechanisms on the two columns [113,123]. According to this concept, the orthogonality of separation is proposed. Separation orthogonality is maximized by using independent retention mechanisms in both dimensions. Previously, this concept was presented by combining polar-apolar (or apolar-polar) columns. However, later studies have reported that enhanced separation resolution can be obtained by using polar or apolar columns in the first dimension and semipolar columns in the second dimension under nonorthogonal conditions [9,124]. The reason for the inefficient performance of combining apolar and polar may be that analytes that are retained excessively in one dimension are not retained at all in the other dimension because the chemistry of the two stationary phases is so different [125]. The choice of stationary phase polarity and column order must consider the analytes contained in the sample. In beer and wine samples, there are more polar compounds than apolar compounds. The most suitable column combination is polar-semipolar for global profiling [24,126].
Other important factors for column selection, especially the second dimension, are column length and diameter. To maintain the separation resolution achieved from the 1D column (short modulation time), the 2D separation time has to reach a sufficiently small value [126]. A short column between 80 and 200 cm long is used to completely elute the analytes within 10 s. Because the columns are shorter in the second dimension, applying a small column diameter to improve the separation efficiency was the first intention [127]. However, using different column diameters induces a flow-mismatch problem, and the two columns cannot both work at the optimal flow rate [128]. Another problem caused by the reduced column diameter in the second dimension is overloading. For a beverage sample, the contents of major and minor compounds may vary by nine orders of magnitude. To cover the trace compounds during VOC profiling, major compounds are over-concentrated. Overloading may lead to distortion of analyte peaks, resulting in data processing errors. Nowadays, both columns usually have matching column diameters. Due to the similar column diameters, the front pressure of both columns can remain similar. Thus, there is no need to sacrifice the performance of the other column in order to maintain the optimal gas flow rate in one column. In this way, the quality of the chromatographic data will be improved, and thus the semi-quantitative results will be enhanced.
3.3. Separation Optimization
Once the GC×GC hardware configuration has been determined, the parameters of this hardware need to be tuned and optimized. Parameters that deserve attention include column pressure, first and second oven temperature programs, and modulation settings. These parameters interact with each other and therefore cannot be optimized separately. The optimization requires statistical experimental design and predictive modeling [36,51]. The quality of the GC×GC separation must be measured quantitatively. The first proposed approach is orthogonality measurement [129]. A number of different metrics were later developed for measuring the orthogonality of 2D separation, such as asterisk equations, bin counting, and convex hull [130,131,132,133]. However, good global orthogonality is not equal to good separation that suits the typical need. Direct measurement distances among the targeted peaks most closely reflect reality. Nearest neighbors’ distances (NND) approaches can be modified according to the specific demands of each study [134,135]. Figure 2 is an example chromatogram of common beverage VOCs obtained at optimized GC×GC conditions. Due to the high concentration of ethanol in the sample, it is common to have the early part of the chromatogram overloaded. In practice, a delay phase should be applied for ionization. The principle of k nearest neighbor (kNN) can also be used for the optimization of GC×GC chromatographic separation. In order to avoid measurement errors introduced by humans, the separated target standards need to be evenly distributed in the entire chromatogram.
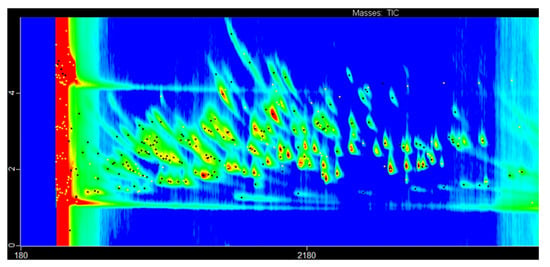
Figure 2.
Chromatogram of common beverage VOCs obtained at optimized GC×GC conditions. Common fermented beverage volatiles (131 compounds), injected at a concentration of 10 mg/L in ethanol, are separated by a polar-semipolar column. A list of VOCs and GC×GC setup can be found in a previous study [51].
As previously mentioned, bidimensional gas chromatography fully exploits its potential in the untargeted analysis of volatiles, which mainly means, for wine and beer, aroma compounds [136]. Even though data processing (which will be extensively discussed in the next paragraph) provides a powerful tool for compound discovery, proper separation is still crucial to avoid signal overlay, which hinders the use of spectral libraries [137]. The following examples are just a snapshot of published GC×GC applications since the main application field is compound discovery and minor differences are present in the workflows. In addition, it must be highlighted the role of multivariate analysis, which was essential in these studies where the amount of data was huge and the approach was qualitative or semi-quantitative [138].
An example of application was the comprehensive mapping of volatile compounds in 70 wines from the two main production areas for Italian sparkling wines (48 wineries and 6 vintages involved) by Carlin et al. (2016). In this research, HS-SPME was coupled to GC×GC-TOF-MS equipped with a thermal modulator and allowed to identify 196 biomarkers within the 1695 compounds detected, which were used to determine the influences of grape cultivar, pedoclimatic conditions, and the metabolomics space of these wines [34].
A similar approach was implemented to determine the effects of various new hybrid yeasts on the aroma profile of beers. In this research, the authors evaluated various yeasts by analyzing the aroma profiles of beers produced with their fermentation [113]. GC×GC was used as a complementary tool to LC-based techniques to give a comprehensive snapshot of yeast performance and allowed proper clone selection to be effectively used in brewing to create new products and to eliminate or increase specific traits.
In addition to untargeted metabolomics, with bidimensional chromatography, it is also possible to solve challenging analytical issues. In a recent publication, Vyviurska et al. optimized the flow modulator settings of a GG×GC method to achieve the chiral separation of enantiomer organic compounds present in botrytized wine samples [139]. Thanks to the use of a multivariate design in the experiment, it was possible to find the optimal setting of a reversed fill/flush (RFF). The enantiomeric composition of chiral compounds allowed the PCA to discriminate their geographical origin, whereas the one-class partial least squares (OC-PLS) model enabled a 93% effectiveness in recognizing the wine samples from the Tokaj region in the presence of other wines.
Another uncommon application of bidimensional gas chromatography was developed by [55]. The authors aimed to overcome the need for a specific instrument (GC-O) to perform olfactory analysis by adapting a more economic GC-FID. A Merlot wine was used in the experiments, where 24 odors emerged with GC-O, 43 volatiles were identified by GC/MS, and GC×GC/MS detected 142 VOCs. Thanks to the 2D, GC×GC/MS indicated an additional set of 14 odor-active molecules hidden by coelution [55]. The combination between the adapted GC-O and GC×GC/MS may be added as a promising tool to describe the aroma profile of wines and boost the improvement of technological practice.
4. Data Processing
At present, the biggest limitation of GC×GC applications is data processing and data mining [140]. When a beverage sample is analyzed by GC×GC in combination with a multi-channel detector such as a time-of-flight mass spectrometer (TOF-MS), a massive amount of information (which takes up hundreds of megabits of disk space per measurement) is obtained. When many sample groups are included in a study, and considering that each measurement requires technical replication to ensure accuracy, the amount of information to be processed is tremendous. As a matter of fact, considering the amount of information contained in a trilinear GC×GC data file [141], the extraction and verification of this information cannot be solved manually but requires the application of signal processing and statistical analysis techniques [142].
The analysis of chromatographic data can be performed in different ways: at the pixel level, on a peak list or peak area basis, by commercial software, or by laboratory-developed code [143,144]. The extraction of useful chemical information from these experimental data requires image signal processing approaches that have to be coupled with linear algebra and statistical concepts to perform a multivariate analysis of the data. These data processing methods can be loosely referred to as “chemometric-based approaches”. A regular data processing flow for GC×GC-MS data and the user input impact for each step are summarized in Figure 3. The complete data processing workflow starts with the experiment design, extracting the accurate peak information from the GC×GC-MS data, multivariate analysis, and statistical model validation. The user has a high impact on the validation of peak information and the statistical model [145]. Among the different aspects of the workflow, many new ideas are under development, particularly in the steps of data preprocessing, peak detection, peak deconvolution, and annotation. However, as often happens, there is no agreement on a “standard” procedure, and there is no clear evidence that one approach is clearly better than others. In addition, even with the most popular commercial GC×GC software, many data processing parameters need to be set by the analyst based on the experimental data and optimized for the matrix. For all these reasons, it is of paramount importance for the analyst to have a basic understanding of these processes. Besides, with a proper understanding of the principles of data processing, an advanced user can customize the workflow according to their needs [146].
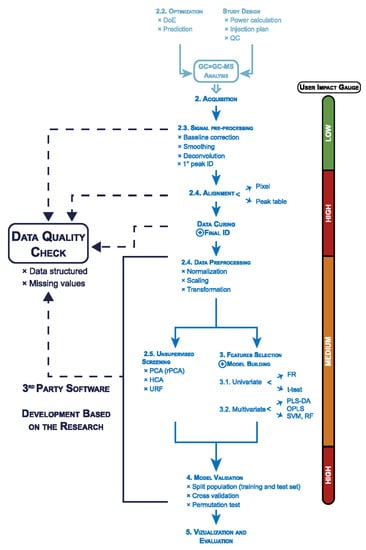
Figure 3.
General data processing workflow for GC×GC-MS data and the user input impact for each step. As quoted from a previous review [145], reproduction is allowed according to STM Permissions Guidelines. Abbreviations: design of experiment (DoE), quality control (QC), identification (ID), robust principal components analysis (rPCA), hierarchical cluster analysis (HCA), unsupervised random forest (URF), fisher ratio (FR), partial least squares discriminant analysis (PLS-DA), orthogonal partial least squares (OPLS), support vector machines (SVM), random forest (RF).
4.1. Background Correction
To accurately identify and quantify analytes, background correction is required. This long-standing problem was first reported in the 1960s [147]. The central idea is to try to remove the signals that are not coming from the ionization of the molecules and that are present either as high-frequency noise or as a smooth background signal. Background correction can be divided into three steps: electronic noise removal, signal smoothing, and baseline correction. Electronic noise may be dominant in GC×GC data, so it should be removed at the beginning. It is generally represented as a low-intensity signal, and its removal only requires estimating the signal intensity and setting a threshold. Signal smoothing can be achieved with a low-pass filter. The last step is the baseline correction. Since the stationary phase in the GC column is not thermally stable, the baseline drift caused by stationary phase leakage with temperature and flow rate changes needs to be corrected. Baseline-drift corrections are usually performed by curve fitting or smoothing strategies. Both methods predict the baseline of the entire chromatogram from the fitted curve of the non-peak signal and then correct for it. Common fitting methods are polynomial fitting and least-squares fitting [148]. Many background correction methods have been developed for GC×GC, such as direct subtraction of mean blanks, local minima values (LMV), asymmetric weighted least squares (AWLS), trilinear decomposition (TD), linear least squares curve fitting methods coupled with moving average smoothing combined with robust orthogonal background correction (ROBC), and singular value decomposition (SVD). Some of them are parametric models (LMV, TD, ROBC), and others are non-parametric (AWLS, SVD). Parametric models assume that the background consists of a fixed combination of terms and can be modeled by applying simple or high-dimensional linear regression. On the contrary, the nonparametric approach does not preset the number of parameters to fit the model. Instead, suitable model parameters are selected based on the experimental data from the baseline. However, both parametric and non-parametric models inevitably make assumptions based on standard chromatograms, which may lead to problems in extreme cases. When a large number of peaks are concentrated in a certain area or even some peaks are connected together, which is common in beverage VOC analysis, the peak signal and background signal become difficult to distinguish [149]. Baseline correction is becoming increasingly difficult, and incorrectly predicted baselines can result in the elimination of true peak signals or the mixing of residual signals with the baseline. A few papers illustrate the mathematical premise of the algorithm in terms of the experimental chemistry of the chromatographic data and test the performance of the model under different extreme conditions, such as heavy co-elution, column saturation, detection saturation, and their coexistence. This makes it difficult for the analyst to choose the most appropriate method based on the experimental chromatogram. In our opinion, the local minima approach respects the experimental data the most. It is more suitable for chromatographic data from complex beverage samples where imperfect chromatographic signals such as saturation may be present [150].
4.2. Peak Detection
Peak detection distinguishes the signals of the analytes from each other and from the background signal, so this step is critical to correctly obtaining the chemical information contained in the chromatographic data. GC×GC-MS peak detection methods were first adapted from the ones used in one-dimensional GC. Regardless of the modulation, the “sample” is continuously fed to the detector. And the feedback of the detector is always a time series of the signal intensity, so 1D integration approaches were first applied to the vector of detector signals from GC×GC, usually with scripts developed by the lab itself. One-dimensional sub-peaks are detected, and then it is manually determined which sub-peaks belong to the same two-dimensional peak. Finally, the sub-peak areas belonging to the same peak will be added [151]. These methods are suitable for the targeted detection of individual analytes. However, when using untargeted analysis, each chromatogram has thousands of subpeaks in one dimension, and manually combining these sub-peaks is not practical. To solve the problem, scripts to automatically recognize and merge sub-peaks in 2D were developed [152,153]. Although the algorithm can automatically merge sub-peaks, errors may occur on the distorted peaks, and the merging criteria must be carefully chosen for crowded chromatograms. Cluster error can be avoided by performing peak detection directly on the 2D demodulated data. During demodulation, the 1D signal vector is rearranged into a 2D matrix. Consequently, multivariate and graphical methods can be applied directly on the 2D plane. Several 2D peak picking methods have been applied to GC×GC-MS data, such as local maximum value combined with parallel factor analysis (PARAFAC), watershed, and peak shape matching [154,155,156]. As usual, they all work well with perfect chromatographic data. Unfortunately, a perfect chromatogram cannot be guaranteed. Especially during beverage VOC profiling, the contents of major and minor compounds may differ by a billion times. It is common to see deformed peaks as the result of column or detector saturation. When saturation occurs, these peak-picking strategies show their limitations by producing false peak splitting, which results in incorrect deconvolution. At this stage, these methods can be used in combination, and the results obtained are validated against each other [130]. Another direction is isolating the unique m/z for each peak, especially when high-resolution mass spectrometry is available, thus eliminating the need to apply complex deconvolution algorithms [157].
4.3. Peak Annotation
After peak detection, peak annotation can be performed using multiple factors. Assist in peak annotation: each peak has two retention times and a normal or high-resolution mass spectrum at the peak apex. Comparing the chromatographic retention time and MS information with a reference standard is always required to achieve level 1 annotation for VOC profiling [158]. Chromatographic peaks can be identified based on their retention times in the 1st and 2nd dimensions. Linear retention indices (LRI) can also be calculated based on analyte retention times and standard retention times. It can be used to cross-check the annotation with the retention time database. A 2D retention index system can be created by using PEG homologs together with n-alkanes [159] and it has recently been modified with the Lee index [160].The identification of unknown peaks can be aided by retention time and/or exponential prediction [161,162]. In most cases, peak annotation requires a comparison of the electron ionization mass spectrum of the chromatographic peak with a standard mass spectrum from a spectral library (NIST, Wiley, etc.). When using high-resolution mass spectra, the mass-to-charge ratio calculation can also be the basis for identification. Many algorithms have been published to compare the mass spectrum of a chromatographic peak with the reference MS spectrum recorded in libraries. Their performances have been extensively studied [163,164,165,166,167,168]. All this requires the simple setting of the appropriate match thresholds, all of which provide accurate annotation results. In most cases, errors in MS identification arise from a non-accurate acquisition of the signals due to analyte characteristic ions in the sample run. This issue affects most signals with low intensities and can be crucial for structural confirmation. In this context, a good alternative is the use of “smart templates”, which are tools whose employment is made possible by prior knowledge of the sample and whose use simplifies peak matching [169]. Besides, most VOCs are common in most types of fermented drinks. For these reasons, it is not necessary to perform a complete, untargeted analysis. Building a library for beverage VOCs and matching their template to the chromatogram peak can dramatically reduce annotation errors. Moreover, in the event that a new compound is found, its template can be simply added to the current library and recognized in future data processing [170]. This target-guided data processing concept has been successfully applied to the imperfect signal correction of GC×GC data [150].
With a perfect chromatogram, the algorithm can reach 97% accuracy for peak annotation of known substances [169]. The remaining 3% can be solved with manual annotation. Manual identification does not need to go through all the peaks but only focuses on the important ones. The important peaks are the peak differences among the chromatograms of different sample classes, which can be measured by the ANOVA, for example. The comparison of the chromatogram may be based on the mathematical features of chromatogram “images”, preventing errors from peak detection and MS construction [171]. This approach is discussed in the next section, “cross-class comparison”, named tile-based fisher ratio. Since manual annotation still plays an important role in metabolomics studies, tools with user-friendly interfaces that support customized peak picking, feature template definition, peak alignment, and result visualization are desired [172]. A tool has been developed to facilitate the user’s evaluation of the peak annotation [173]. Still, GC×GC-MS has its inherent limitations. Typical compounds of interest may elute at close retention times and have similar mass spectra. In such a case, the peak annotation problem cannot be solved by the data processing process. It is necessary to increase the data dimensions; for example, GC×GC coupled with dual detectors [174].
4.4. Between-Class Comparison
4.4.1. Typical Compounds of Interest during Wine and Beer Studies
With VOC profiling results, the conventional learning objective is to find the chemical component characteristics of different sample classes and relate them to their physicochemical properties. This cross-class analysis gives rise to many applications, including chemical fingerprinting, sample clustering analysis, and biomarker discovery. In the case of beverage studies, the above applications can be specified as differences in primary materials, sensory properties, microbial activity, spoilage, aging, etc. For example, monoterpenes are important contributors to the aroma of primary materials such as grapes for wine and hops for beer. Muscat wines have high levels of α-terpineol and linalool [175]. Linalool, at the same time, is an indicator of using hops during beer making [176]. (Z)-Rose oxide is important in Gewürztraminer wines [177]. Flavanoids have correlations with red wine texture [178]. Its concentration depends to a large extent on the contact time of the juice with the exocarp and winemaking practices [179,180]. Furan derivatives, such as 5-methylfurfural and furfural, are sourced from toasted oak. [181]. Premature aging flavors in dry white wines are caused by a chiral furanone, namely, sotolon [182]. The fruitiness of a wine depends on the content of esters and acetate, which changes as the wine matures [81]. Through secondary metabolism via the Ehrlich pathway, amino acids produce higher alcohols and their corresponding acetate esters [183]. Compounds containing one or more carbonyl groups may contribute off-flavor, for example, 2, 3-pentadione and diacetyl [184].
As we know, a GC×GC chromatogram can easily contain hundreds of peaks. Mining useful information manually from such “big data” can quickly become overwhelming for large-scale, untargeted research. Although chemometric strategies do not guarantee the selection of valuable chromatographic information, they can assist in screening out irrelevant information and thus improve the efficiency of manual exploration. A common chemometric cross-class analysis consists of two steps: chromatogram/peak alignment and statistical exploration [185].
4.4.2. Chromatographic Alignment
Chromatographic alignment can significantly reduce errors that occur in across-classes analysis. Retention time locking technique is used to keep the similar retention time after the column replacement [186]. However, even within one study, when the hardware remains the same, because the instrumental conditions change within a long measurement sequence, retention times can vary to some degree among analyses. These retention time shifts usually result from degradation of the stationary phase, unstable carrier gas pressure, oven temperature program, and hardware maintenance. In large-scale studies, unstable chromatography may cause a peak to be detected at different retention times in different measurements. Oscillations of the MS detector can cause peak deconvolution differences on those peaks. Consequently, a peak has different retention times and is quantified in different ion channels. This can create problems for peak rescaling and peak area comparison within an omic study. Therefore, data alignment is a necessary step for cross-class analysis. This alignment is performed either before peak detection on pixel/tile level or after the peak detection on peak table level. In the former case, alignment is performed on the entire chromatogram. Various alignment algorithms are available including wraparound correction, such as the PARAFAC-based modulation time shift correction, the windowed rank minimization, and the indexing schemes cylindrical mapping [187,188,189,190]. The alignment can be achieved from a pixel/tile perspective. With binning and tiling schemes of the chromatogram, the overall size of the data files is reduced [191]. The computer can focus on processing those chromatographic fragments that have significance, thus saving overall data processing time [192]. In the peak table-based alignment process, all peaks detected in different positions or with different mass spectra throughout the processed chromatograms are assigned an identifier. This approach can be completed manually or automatically [193,194].
4.4.3. Statistical Exploration
Extracting valuable information from a high-dimensional data set consisting of hundreds of chemical features and several sample properties is difficult. To simplify this process, statistical tools must be used to reduce the data dimension. Before applying the statistical tools, peak normalization with internal standards is recommended to reduce the systematic error. Then, an intuitive approach is to apply univariate statistics, such as the Fisher ratio (FR), to filter out features that are not significantly different among the classes. In univariate statistical analysis, all variables are assumed to be independent, and therefore the analysis does not consider interactions among variables. Because of its simplicity and effectiveness, the FR method has been built into mainstream GC×GC data analysis software. It is derived from the concept of ANOVA analysis, which compares between-class variance with within-class variance. If the ratio is greater than the F critical value, then the analyte has a significant variation between sample classes [195]. Originally, it was calculated based on the result in the peak table after peak detection. To avoid interference from peak detection error, it has now evolved to image-based comparison, or tile-base FR [172]. A proper tile size selection is important to prevent deteriorated analyte discovery [196]. It is important to mention that many chemicals in the beverage are not independent, so simply applying the FR analysis may discard many relevant features or overlook important features. One should have a scientific background in the sample, knowing the objective of the work and the chemistry relevant to the beverage composition, to supervise the FR test. For example, during taste testing, esteric compounds should not be filtered out even if they do not pass the FR test individually [197].On the other hand, in multivariate statistical analysis, multiple correlated variables can be combined and represented by a new “latent” vector. In such a way, the number of studied volatile features is reduced, and the most informative variables are extracted. Then, selected volatile features can be used for modeling the interested biological phenomena.
Still, in the presence of more variables than samples, correlation can also arise by “chance”. Even with multivariate methods, validation and domain-specific knowledge are required to confirm the results of the experiments. Principal component analysis-based (PCA) tools determine the sources of the greatest variance and are widely used in GC×GC result exploration [198]. Principal component analysis (PCA) is widely used in the exploration of GC×GC results and has been used in many works to characterize wines from different terroirs [56,199] to distinguish the varieties [26], to find chemical markers of wine aging, and also to evaluate the use of different yeasts [35]. PCA can also be applied to graphic data; consider the chromatogram a 3D image and avoid peak identification. With proper binning size applied to the chromatogram, the amount of information for each binned-image comparison is reduced, and the results of image alignment are improved [56]. Other common and useful techniques are discriminant analysis such as partial least squares (PLS-DA) and stepwise linear discriminant analysis (SLDA), which is a supervised multivariate statistical method used for classification purposes and data interpretation, for example. PLS-DA has been used by Robinson et al. (2011) to categorize wines according to geographic origin and SLDA to distinguish grape varieties [17,26,52]. Recently, machine learning methods have been compared based on wine data [42]. It is expected that artificial intelligence (AI) technologies will be applied to mining GC×GC data to facilitate not only feature selection but also feature annotation. In the future, building a suitable database to train predictors of mass spectrometry, retention time, and chemical similarity algorithms will greatly improve the efficiency of feature annotation [200].
5. Conclusions and Perspectives
This review provides an overview of the analytical approach of GC×GC in beer and wine VOC profiling. GC×GC-MS is a well-developed technique at this stage. Although physics and materials scientists are still devoting efforts to innovate instrumental techniques such as 3-dimensional gas chromatography [201], high-resolution mass spectrometry, and higher detection sensitivity, the bottleneck of multidimensional gas chromatography applications is still sample preparation and data analysis. Regarding the sample preparation techniques, we describe their working mechanisms and provide information that is essential for the selection of the appropriate technique and the optimization of their parameters in the experiments. New sample preparation techniques are rapidly evolving, such as adsorbents that can adsorb a wider range of VOCs, assist sample preparation by physical means, and alter the geometry of the adsorbent to increase sample capacity and mass transfer rate. Based on the guidance of green analytical chemistry, organic solvent-free is an important direction. Besides, automation definitely has high demands.
Extracted beverage peaks of interest must be well separated by the GC×GC system. Based on previous studies, there is no doubt about the GC×GC separation hardware setup in wine and beer VOC analysis. A thermal modulator and polar-semipolar column combination with the same diameter are recommended for beer and wine VOC analysis. Optimization of the GC×GC parameter requires a statistical design of the experiment. To have an optimized separation that suits the typical need, defining the peaks of interest and directly measuring distances among them would be a good option. According to our observation, the peak shape of the eluates in the GC×GC analysis is not simply two-dimensional Gaussian [157]. It remains for chromatographers to further refine the separation theory of GC×GC.
Mining valuable information from a large amount of high-dimensional GC×GC data is not an easy task. Analysts need a certain background in signal processing and statistics to choose the appropriate data processing methods and adjust the parameters. There is some vendor-supported software for processing GC×GC data. However, they are not open source. Users do not have a high degree of freedom to customize the data processing process according to their needs. Also, some software does not fully disclose the parameter selection algorithm of the data processing process. Even analysts with relevant backgrounds do not have full control over the data processing. This problem is particularly acute in the case of fermented beverages, where both high and trace analytes are present on the chromatogram. In the future, a tool package based on an open-source platform that integrates published mainstream algorithms and provides a freely customizable process will undoubtedly be favored by most analysts. It has been shown that the application of a sample-specific VOC database can significantly improve the sensitivity of peak detection and the accuracy of analyte identification. Considering the complexity of VOCs in beer and wine, using the same database to train the AI classifier to assist in peak annotation would be a trend. In addition, more and more research is applying AI to the feature selection process of omics studies. Therefore, the analyst should not only understand the beer and wine chemistry and the GC×GC hardware employed but also have sufficient knowledge to perform chemometric and AI learner training.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/analytica4030026/s1. Table S1 acronyms, Table S2 physicochemical properties of common VOCs in the beverages.
Author Contributions
P.Z. and P.F.; conceptualization, P.Z., S.C., M.P. and P.F.; original draft preparation, P.Z. and P.F.; literature review, P.Z., M.P. and S.C.; writing, P.Z., M.P., S.C. and U.V.; review and editing, U.V. and F.M.; supervision, M.P., P.F., S.C., U.V. and F.M.; final editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by European Union’s Horizon 2020, Marie Skłodowska-Curie Innovative Training Networks [Project Aromagenesis grant number 764364].
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank all the Aromagenesis team members for their support and collaboration in our research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prasannan, A. Alcoholic Beverages Market by Type (Beer, Distilled Spirits, Wine, and Others) and Distribution Channel (Convenience Stores, On Premises, Liquor Stores, Grocery Shops, Internet Retailing, and Supermarkets): Global Opportunity Analysis and Industry Forecast, 2018–2025; Allied Market Research: Pune, India, 2018. [Google Scholar]
- Fortune Business Insights. Wine Market Size, Share & Industry Analysis; Fortune Business Insights: Pune, India, 2020. [Google Scholar]
- Jaeger, S.R.; Worch, T.; Phelps, T.; Jin, D.; Cardello, A.V. Preference segments among declared craft beer drinkers: Perceptual, attitudinal and behavioral responses underlying craft-style vs. traditional-style flavor preferences. Food Qual. Prefer. 2020, 82, 103884. [Google Scholar] [CrossRef]
- Paiva, A.C.; Hantao, L.W. Exploring a public database to evaluate consumer preference and aroma profile of lager beers by comprehensive two-dimensional gas chromatography and partial least squares regression discriminant analysis. J. Chromatogr. A 2020, 1630, 461529. [Google Scholar] [CrossRef] [PubMed]
- Pons, A.; Allamy, L.; Schüttler, A.; Rauhut, D.; Thibon, C.; Darriet, P. What is the expected impact of climate change on wine aroma compounds and their precursors in grape? OENO One 2017, 51, 141. [Google Scholar] [CrossRef]
- Ferreira, V.; de la Fuente, A.; Sáenz-Navajas, M.P. 1—Wine aroma vectors and sensory attributes. In Managing Wine Quality, 2nd ed.; Reynolds, A.G., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2022; pp. 3–39. ISBN 978-0-08-102067-8. [Google Scholar]
- Ravasio, D.; Carlin, S.; Boekhout, T.; Groenewald, M.; Vrhovsek, U.; Walther, A.; Wendland, J. Adding Flavor to Beverages with Non-Conventional Yeasts. Fermentation 2018, 4, 15. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, H.; Shioya, S. Beer Volatile Compounds and Their Application to Low-Malt Beer Fermentation. J. Biosci. Bioeng. 2008, 106, 317–323. [Google Scholar] [CrossRef]
- Cordero, C.; Kiefl, J.; Schieberle, P.; Reichenbach, S.E.; Bicchi, C. Comprehensive two-dimensional gas chromatography and food sensory properties: Potential and challenges. Anal. Bioanal. Chem. 2015, 407, 169–191. [Google Scholar] [CrossRef]
- Feizi, N.; Hashemi-Nasab, F.S.; Golpelichi, F.; Saburouh, N.; Parastar, H. Recent trends in application of chemometric methods for GC-MS and GC×GC-MS-based metabolomic studies. TrAC Trends Anal. Chem. 2021, 138, 116239. [Google Scholar] [CrossRef]
- Ryan, D.; Morrison, P.; Marriott, P. Orthogonality considerations in comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2005, 1071, 47–53. [Google Scholar] [CrossRef]
- Mayadunne, R.; Nguyen, T.-T.; Marriott, P.J. Amino acid analysis by using comprehensive two-dimensional gas chromatography. Anal. Bioanal. Chem. 2005, 382, 836–847. [Google Scholar] [CrossRef]
- Junge, M.; Bieri, S.; Huegel, H.; Marriott, P. Fast comprehensive two-dimensional gas chromatography with cryogenic modulation. Anal. Chem. 2007, 79, 4448–4454. [Google Scholar] [CrossRef]
- Rocha, S.M.; Coelho, E.; Zrostlíková, J.; Delgadillo, I.; Coimbra, M.A. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry of monoterpenoids as a powerful tool for grape origin traceability. J. Chromatogr. A 2007, 1161, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Schmarr, H.-G.; Bernhardt, J.; Fischer, U.; Stephan, A.; Müller, P.; Durner, D. Two-dimensional gas chromatographic profiling as a tool for a rapid screening of the changes in volatile composition occurring due to microoxygenation of red wines. Anal. Chim. Acta 2010, 672, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Petronilho, S.; Câmara, J.S.; Rocha, S.M. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid phase microextraction as a powerful tool for quantification of ethyl carbamate in fortified wines. The case study of Madeira wine. J. Chromatogr. A 2010, 1217, 3441–3445. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Boss, P.K.; Heymann, H.; Solomon, P.S.; Trengove, R.D. Development of a sensitive non-targeted method for characterizing the wine volatile profile using headspace solid-phase microextraction comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 504–517. [Google Scholar] [CrossRef]
- Robinson, A.L.; Adams, D.O.; Boss, P.K.; Heymann, H.; Solomon, P.S.; Trengove, R.D. The relationship between sensory attributes and wine composition for Australian Cabernet Sauvignon wines. Aust. J. Grape Wine Res. 2011, 17, 327–340. [Google Scholar] [CrossRef]
- Vestner, J.; Malherbe, S.; Du Toit, M.; Nieuwoudt, H.H.; Mostafa, A.; Górecki, T.; Tredoux, A.G.J.; de Villiers, A. Investigation of the volatile composition of pinotage wines fermented with different malolactic starter cultures using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC-TOF-MS). J. Agric. Food Chem. 2011, 59, 12732–12744. [Google Scholar] [CrossRef]
- Weldegergis, B.T.; Villiers, A.d.; McNeish, C.; Seethapathy, S.; Mostafa, A.; Górecki, T.; Crouch, A.M. Characterisation of volatile components of Pinotage wines using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC–TOFMS). Food Chem. 2011, 129, 188–199. [Google Scholar] [CrossRef]
- Sun, Q.; Gates, M.J.; Lavin, E.H.; Acree, T.E.; Sacks, G.L. Comparison of Odor-Active Compounds in Grapes and Wines from Vitis vinifera and Non-Foxy American Grape Species. J. Agric. Food Chem. 2011, 59, 10657–10664. [Google Scholar] [CrossRef]
- Chin, S.-T.; Eyres, G.T.; Marriott, P.J. Identification of potent odourants in wine and brewed coffee using gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2011, 1218, 7487–7498. [Google Scholar] [CrossRef]
- Perestrelo, R.; Barros, A.S.; Rocha, S.M.; Câmara, J.S. Optimisation of solid-phase microextraction combined with gas chromatography-mass spectrometry based methodology to establish the global volatile signature in pulp and skin of Vitis vinifera L. grape varieties. Talanta 2011, 85, 1483–1493. [Google Scholar] [CrossRef]
- Welke, J.E.; Manfroi, V.; Zanus, M.C.; Lazzarotto, M.; Zini, C.A. Characterization of the volatile profile of Brazilian Merlot wines through comprehensive two dimensional gas chromatography time-of-flight mass spectrometric detection. J. Chromatogr. A 2012, 1266, 124–139. [Google Scholar] [CrossRef]
- Falcao, L.D.; Lytra, G.; Darriet, P.; Barbe, J.-C. Identification of ethyl 2-hydroxy-4-methylpentanoate in red wines, a compound involved in blackberry aroma. Food Chem. 2012, 132, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Welke, J.E.; Manfroi, V.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Differentiation of wines according to grape variety using multivariate analysis of comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection data. Food Chem. 2013, 141, 3897–3905. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Rinaldi, M.; Locatelli, M.; Piana, G.; Travaglia, F.; Coïsson, J.D.; Arlorio, M. Characterization of Muscat wines aroma evolution using comprehensive gas chromatography followed by a post-analytic approach to 2D contour plots comparison. Food Chem. 2013, 140, 57–67. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Dziadas, M.; Majcher, M. Different headspace solid phase microextraction—Gas chromatography/mass spectrometry approaches to haloanisoles analysis in wine. J. Chromatogr. A 2013, 1313, 185–193. [Google Scholar] [CrossRef]
- Dugo, G.; Franchina, F.A.; Scandinaro, M.R.; Bonaccorsi, I.; Cicero, N.; Tranchida, P.Q.; Mondello, L. Elucidation of the volatile composition of Marsala wines by using comprehensive two-dimensional gas chromatography. Food Chem. 2014, 142, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Zini, C.A. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Soares, R.D.; Welke, J.E.; Nicolli, K.P.; Zanus, M.; Caramão, E.B.; Manfroi, V.; Zini, C.A. Monitoring the evolution of volatile compounds using gas chromatography during the stages of production of Moscatel sparkling wine. Food Chem. 2015, 183, 291–304. [Google Scholar] [CrossRef]
- Chin, S.-T.; Eyres, G.T.; Marriott, P.J. Application of integrated comprehensive/multidimensional gas chromatography with mass spectrometry and olfactometry for aroma analysis in wine and coffee. Food Chem. 2015, 185, 355–361. [Google Scholar] [CrossRef]
- Yan, D.; Wong, Y.F.; Tedone, L.; Shellie, R.A.; Marriott, P.J.; Whittock, S.P.; Koutoulis, A. Chemotyping of new hop (Humulus lupulus L.) genotypes using comprehensive two-dimensional gas chromatography with quadrupole accurate mass time-of-flight mass spectrometry. J. Chromatogr. A 2018, 1536, 110–121. [Google Scholar] [CrossRef]
- Carlin, S.; Vrhovsek, U.; Franceschi, P.; Lotti, C.; Bontempo, L.; Camin, F.; Toubiana, D.; Zottele, F.; Toller, G.; Fait, A.; et al. Regional features of northern Italian sparkling wines, identified using solid-phase micro extraction and comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. Food Chem. 2016, 208, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Whitener, M.E.B.; Stanstrup, J.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Effect of non- Saccharomyces yeasts on the volatile chemical profile of Shiraz wine: Shiraz wines fermented by non- Saccharomyces yeasts. Aust. J. Grape Wine Res. 2017, 23, 179–192. [Google Scholar] [CrossRef]
- Stefanuto, P.-H.; Perrault, K.A.; Dubois, L.M.; L’Homme, B.; Allen, C.; Loughnane, C.; Ochiai, N.; Focant, J.-F. Advanced method optimization for volatile aroma profiling of beer using two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2017, 1507, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Furdíková, K.; Bajnociová, L.; Malík, F.; Špánik, I. Investigation of volatile profile of varietal Gewürztraminer wines using two-dimensional gas chromatography. J. Food Nutr. Res. 2017, 56, 73–85. [Google Scholar]
- Lago, L.O.; Nicolli, K.P.; Marques, A.B.; Zini, C.A.; Welke, J.E. Influence of ripeness and maceration of the grapes on levels of furan and carbonyl compounds in wine—Simultaneous quantitative determination and assessment of the exposure risk to these compounds. Food Chem. 2017, 230, 594–603. [Google Scholar] [CrossRef]
- Nicolli, K.P.; Biasoto, A.C.T.; Souza-Silva, É.A.; Guerra, C.C.; dos Santos, H.P.; Welke, J.E.; Zini, C.A. Sensory, olfactometry and comprehensive two-dimensional gas chromatography analyses as appropriate tools to characterize the effects of vine management on wine aroma. Food Chem. 2018, 243, 103–117. [Google Scholar] [CrossRef]
- Crucello, J.; Miron, L.F.O.; Ferreira, V.H.C.; Nan, H.; Marques, M.O.M.; Ritschel, P.S.; Zanus, M.C.; Anderson, J.L.; Poppi, R.J.; Hantao, L.W. Characterization of the aroma profile of novel Brazilian wines by solid-phase microextraction using polymeric ionic liquid sorbent coatings. Anal. Bioanal. Chem. 2018, 410, 4749–4762. [Google Scholar] [CrossRef]
- Martins, C.; Brandão, T.; Almeida, A.; Rocha, S.M. Enlarging Knowledge on Lager Beer Volatile Metabolites Using Multidimensional Gas Chromatography. Foods 2020, 9, 1276. [Google Scholar] [CrossRef]
- Reichenbach, S.E.; Zini, C.A.; Nicolli, K.P.; Welke, J.E.; Cordero, C.; Tao, Q. Benchmarking machine learning methods for comprehensive chemical fingerprinting and pattern recognition. J. Chromatogr. A 2019, 1595, 158–167. [Google Scholar] [CrossRef]
- Šuklje, K.; Carlin, S.; Antalick, G.; Blackman, J.W.; Deloire, A.; Vrhovsek, U.; Schmidtke, L.M. Regional Discrimination of Australian Shiraz Wine Volatome by Two-Dimensional Gas Chromatography Coupled to Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 10273–10284. [Google Scholar] [CrossRef]
- Šuklje, K.; Carlin, S.; Stanstrup, J.; Antalick, G.; Blackman, J.W.; Meeks, C.; Deloire, A.; Schmidtke, L.M.; Vrhovsek, U. Unravelling wine volatile evolution during Shiraz grape ripening by untargeted HS-SPME-GC×GC-TOFMS. Food Chem. 2019, 277, 753–765. [Google Scholar] [CrossRef]
- Schoenauer, S.; Schieberle, P. Screening for Novel Mercaptans in 26 Fruits and 20 Wines Using a Thiol-Selective Isolation Procedure in Combination with Three Detection Methods. J. Agric. Food Chem. 2019, 67, 4553–4559. [Google Scholar] [CrossRef]
- Nicolotti, L.; Mall, V.; Schieberle, P. Characterization of Key Aroma Compounds in a Commercial Rum and an Australian Red Wine by Means of a New Sensomics-Based Expert System (SEBES)—An Approach to Use Artificial Intelligence in Determining Food Odor Codes. J. Agric. Food Chem. 2019, 67, 4011–4022. [Google Scholar] [CrossRef]
- Paiva, A.C.; Simões Oliveira, D.; Hantao, L.W. A Bottom-Up Approach for Data Mining in Bioaromatization of Beers Using Flow-Modulated Comprehensive Two-Dimensional Gas Chromatography/Mass Spectrometry. Separations 2019, 6, 46. [Google Scholar] [CrossRef]
- Furdíková, K.; Machyňáková, A.; Drtilová, T.; Špánik, I. Comparison of Different Categories of Slovak Tokaj Wines in Terms of Profiles of Volatile Organic Compounds. Molecules 2020, 25, 669. [Google Scholar] [CrossRef]
- Aith Barbará, J.; Primieri Nicolli, K.; Souza-Silva, É.A.; Camarão Telles Biasoto, A.; Welke, J.E.; Alcaraz Zini, C. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chem. 2020, 308, 125552. [Google Scholar] [CrossRef] [PubMed]
- Vyviurska, O.; Špánik, I. Assessment of Tokaj varietal wines with comprehensive two-dimensional gas chromatography coupled to high resolution mass spectrometry. Microchem. J. 2020, 152, 104385. [Google Scholar] [CrossRef]
- Zhang, P.; Carlin, S.; Lotti, C.; Mattivi, F.; Vrhovsek, U. On sample preparation methods for fermented beverage VOCs profiling by GCxGC-TOFMS. Metabolomics 2020, 16, 102. [Google Scholar] [CrossRef]
- Lukić, I.; Carlin, S.; Vrhovsek, U. Comprehensive 2D gas chromatography with TOF-MS detection confirms the matchless discriminatory power of monoterpenes and provides in-depth volatile profile information for highly efficient white wine varietal differentiation. Foods 2020, 9, 1787. [Google Scholar] [CrossRef] [PubMed]
- Machyňáková, A.; Khvalbota, L.; Špánik, I. Enantiomer distribution of major chiral volatile organic compounds in botrytized grapes and wines. Eur. Food Res. Technol. 2021, 247, 2321–2331. [Google Scholar] [CrossRef]
- Furdíková, K.; Khvalbota, L.; Machyňáková, A.; Špánik, I. Volatile composition and enantioselective analysis of chiral terpenoids in Tokaj varietal wines. J. Chromatogr. B 2021, 1167, 122565. [Google Scholar] [CrossRef] [PubMed]
- Welke, J.E.; Nicolli, K.P.; Hernandes, K.C.; Biasoto, A.C.T.; Zini, C.A. Adaptation of an olfactometric system in a GC-FID in combination with GCxGC/MS to evaluate odor-active compounds of wine. Food Chem. 2022, 370, 131004. [Google Scholar] [CrossRef]
- Sudol, P.E.; Gough, D.V.; Prebihalo, S.E.; Synovec, R.E. Impact of data bin size on the classification of diesel fuels using comprehensive two-dimensional gas chromatography with principal component analysis. Talanta 2020, 206, 120239. [Google Scholar] [CrossRef]
- Lukić, I.; Carlin, S.; Vrhovsek, U. Utility of Comprehensive GC×GC Gas Chromatography in Finding Varietal Markers among Volatile Compounds in Non-Aromatic Red Wines. Agronomy 2022, 12, 2512. [Google Scholar] [CrossRef]
- Carlin, S.; Mattivi, F.; Durantini, V.; Dalledonne, S.; Arapitsas, P. Flint glass bottles cause white wine aroma identity degradation. Proc. Natl. Acad. Sci. USA 2022, 119, e2121940119. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Iglesias, C.; Montero, O.; Sancho, D.; Blanco, C.A. New trends in beer flavour compound analysis. J. Sci. Food Agric. 2015, 95, 1571–1576. [Google Scholar] [CrossRef]
- Horák, T.; Čulík, J.; Jurková, M.; Čejka, P.; Kellner, V. Determination of free medium-chain fatty acids in beer by stir bar sorptive extraction. J. Chromatogr. A 2008, 1196–1197, 96–99. [Google Scholar] [CrossRef]
- Liu, Y.; Su, H.; Song, H.-L. Comparison of Four Extraction Methods, SPME, DHS, SAFE, Versus SDE, for the Analysis of Flavor Compounds in Natto. Food Anal. Methods 2018, 11, 343–354. [Google Scholar] [CrossRef]
- Kolb, B.; Ettre, L.S. Static Headspace-Gas Chromatography: Theory and Practice, 2nd ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-471-74944-8. [Google Scholar]
- Dunn, W.B.; Townshend, A.; Dunn, W.B.; Green, J.D. Comparison of total vaporisation and dynamic headspace techniques combined with direct mass spectrometric detection for the on-line analysis of liquid process streams. Analyst 1998, 123, 343–348. [Google Scholar] [CrossRef]
- Kolb, B.; Auer, M.; Pospisil, P. Angewandte Chromatographie–Applied Chromatography; Bodenseewerk Perkin-Elmer & Co.: Überlingen, Germany, 1981. [Google Scholar]
- Vyviurska, O.; Hanobiková, M.; Gomes, A.A.; Špánik, I. Multivariate optimization of dual-sorbent dynamic headspace extraction of volatiles in wine analysis. Food Chem. 2021, 365, 130449. [Google Scholar] [CrossRef]
- Ross, C.F. Headspace Analysis. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; pp. 27–50. ISBN 978-0-12-381374-9. [Google Scholar]
- Soria, A.C.; García-Sarrió, M.J.; Sanz, M.L. Volatile sampling by headspace techniques. TrAC Trends Anal. Chem. 2015, 71, 85–99. [Google Scholar] [CrossRef]
- Liu, J.; Toldam-Andersen, T.B.; Petersen, M.A.; Zhang, S.; Arneborg, N.; Bredie, W.L.P. Instrumental and sensory characterisation of Solaris white wines in Denmark. Food Chem. 2015, 166, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Lopez, R.; Ferreira, V. An automated gas chromatographic-mass spectrometric method for the quantitative analysis of the odor-active molecules present in the vapors emanated from wine. J. Chromatogr. A 2018, 1534, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Aberl, A.; Coelhan, M. Determination of Volatile Compounds in Different Hop Varieties by Headspace-Trap GC/MS—In Comparison with Conventional Hop Essential Oil Analysis. J. Agric. Food Chem. 2012, 60, 2785–2792. [Google Scholar] [CrossRef]
- Franchina, F.A.; Purcaro, G. Sample preparation strategies for comprehensive volatile fingerprinting. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 96, pp. 155–184. ISBN 978-0-323-98881-0. [Google Scholar]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacı, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef]
- Zhu, J.; Chai, X. Some Recent Developments in Headspace Gas Chromatography. Curr. Anal. Chem. 2005, 1, 79–83. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Alam, M.N.; Pawliszyn, J. Review of geometries and coating materials in solid phase microextraction: Opportunities, limitations, and future perspectives. Anal. Chim. Acta 2017, 984, 42–65. [Google Scholar] [CrossRef]
- Rodrigues, F.; Caldeira, M.; Câmara, J.S. Development of a dynamic headspace solid-phase microextraction procedure coupled to GC–qMSD for evaluation the chemical profile in alcoholic beverages. Anal. Chim. Acta 2008, 609, 82–104. [Google Scholar] [CrossRef]
- Lashgari, M.; Yamini, Y. An overview of the most common lab-made coating materials in solid phase microextraction. Talanta 2019, 191, 283–306. [Google Scholar] [CrossRef]
- Xu, J.; Ouyang, G. Extraction: Solid-Phase Microextraction. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; p. B9780124095472146000. ISBN 978-0-12-409547-2. [Google Scholar]
- Pawliszyn, J. Handbook of Solid Phase Microextraction; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-12-416017-0. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wang, J.-L.; Shu, Y.-Y. Purge-assisted and temperature-controlled headspace solid-phase microextraction combined with gas chromatography–mass spectrometry for determination of six common phthalate esters in aqueous samples. J. Food Meas. Charact. 2020, 14, 1833–1841. [Google Scholar] [CrossRef]
- Mihaljević Žulj, M.; Maslov, L.; Tomaz, I.; Jeromel, A. Determination of 2-aminoacetophenone in white wines using ultrasound assisted SPME coupled with GC-MS. J. Anal. Chem. 2015, 70, 814–818. [Google Scholar] [CrossRef]
- Orazbayeva, D.; Kenessov, B.; Psillakis, E.; Nassyrova, D.; Bektassov, M. Determination of transformation products of unsymmetrical dimethylhydrazine in water using vacuum-assisted headspace solid-phase microextraction. J. Chromatogr. A 2018, 1555, 30–36. [Google Scholar] [CrossRef]
- Vakinti, M.; Mela, S.-M.; Fernández, E.; Psillakis, E. Room temperature and sensitive determination of haloanisoles in wine using vacuum-assisted headspace solid-phase microextraction. J. Chromatogr. A 2019, 1602, 142–149. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, G.; Zhou, G.; Jiang, J.; Yuan, K.; Pan, Y.; Song, Z.; Li, Z.; Xia, Q.; Lu, X.; et al. Rapid determination of the volatile components in tobacco by ultrasound-microwave synergistic extraction coupled to headspace solid-phase microextraction with gas chromatography-mass spectrometry: Sample Preparation. J. Sep. Sci. 2016, 39, 1173–1181. [Google Scholar] [CrossRef]
- Psillakis, E. Vacuum-assisted headspace solid-phase microextraction: A tutorial review. Anal. Chim. Acta 2017, 986, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Burzynski-Chang, E.A.; Ryona, I.; Reisch, B.I.; Gonda, I.; Foolad, M.R.; Giovannoni, J.J.; Sacks, G.L. HS-SPME-GC-MS Analyses of Volatiles in Plant Populations—Quantitating Compound × Individual Matrix Effects. Molecules 2018, 23, 2436. [Google Scholar] [CrossRef] [PubMed]
- Piergiovanni, M.; Carlin, S.; Lotti, C.; Vrhovsek, U.; Mattivi, F. Development of a Fully Automated Method HS-SPME-GC-MS/MS for the Determination of Odor-Active Carbonyls in Wines: A “Green” Approach to Improve Robustness and Productivity in the Oenological Analytical Chemistry. J. Agric. Food Chem. 2023. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Termopoli, V. Derivatization Strategies in Flavor Analysis: An Overview over the Wine and Beer Scenario. Chemistry 2022, 4, 1679–1695. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.-R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Yiantzi, E.; Psillakis, E.; Tyrovola, K.; Kalogerakis, N. Vortex-assisted liquid–liquid microextraction of octylphenol, nonylphenol and bisphenol-A. Talanta 2010, 80, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Psillakis, E.; Kalogerakis, N. Developments in single-drop microextraction. TrAC Trends Anal. Chem. 2002, 21, 54–64. [Google Scholar] [CrossRef]
- Davies, J.T. Turbulence Phenomena; Elsevier: Amsterdam, The Netherlands, 1972; ISBN 978-0-12-206070-0. [Google Scholar]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, R.; Eftekhari, A.A.; Dafnomilis, G.; Lake, L.W.; Bruining, J. On the sustainability of CO2 storage through CO2—Enhanced oil recovery. Appl. Energy 2020, 261, 114467. [Google Scholar] [CrossRef]
- Jessop, P.G.; Heldebrant, D.J.; Li, X.; Eckert, C.A.; Liotta, C.L. Reversible nonpolar-to-polar solvent. Nature 2005, 436, 1102. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Berrou, K.; Dunyach-Remy, C.; Lavigne, J.-P.; Roig, B.; Cadiere, A. Multiple stir bar sorptive extraction combined with gas chromatography-mass spectrometry analysis for a tentative identification of bacterial volatile and/or semi-volatile metabolites. Talanta 2019, 195, 245–250. [Google Scholar] [CrossRef]
- Castro, L.F.; Ross, C.F. Determination of flavour compounds in beer using stir-bar sorptive extraction and solid-phase microextraction: Determination of flavor compounds in beer. J. Inst. Brew. 2015, 121, 197–203. [Google Scholar] [CrossRef]
- Gilart, N.; Marcé, R.M.; Borrull, F.; Fontanals, N. New coatings for stir-bar sorptive extraction of polar emerging organic contaminants. TrAC Trends Anal. Chem. 2014, 54, 11–23. [Google Scholar] [CrossRef]
- David, F.; Sandra, P. Stir bar sorptive extraction for trace analysis. J. Chromatogr. A 2007, 1152, 54–69. [Google Scholar] [CrossRef]
- Gritti, F.; Guiochon, G. Adsorption mechanism in reversed-phase liquid chromatography. J. Chromatogr. A 2006, 1115, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Martire, D.E.; Boehm, R.E. Unified theory of retention and selectivity in liquid chromatography. 2. Reversed-phase liquid chromatography with chemically bonded phases. J. Phys. Chem. 1983, 87, 1045–1062. [Google Scholar] [CrossRef]
- Pap, T.; Horváth, V.; Tolokán, A.; Horvai, G.; Sellergren, B. Effect of solvents on the selectivity of terbutylazine imprinted polymer sorbents used in solid-phase extraction. J. Chromatogr. A 2002, 973, 1–12. [Google Scholar] [CrossRef]
- Babaee, S.; Daneshfar, A.; Khezeli, T. Determination of carboxylic acids in non-alcoholic beer samples by an ultrasonic-assisted dispersive micro-solid phase extraction based on Ni/Cu-Al layered double hydroxide nanocomposites followed by gas chromatography. Ultrason. Sonochem. 2017, 34, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Hu, K.; Zhang, Y.; Zhao, W.; Wang, F.; Guo, L.; Zhang, W.; He, J.; Huang, Y.; Zhang, S. On-cartridge derivatisation using a calixarene solid-phase extraction sorbent for facile, sensitive and fast determination of formaldehyde in beer. Food Chem. 2016, 211, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-J.; Wang, Y.-L.; Liu, P.; Zhang, Z.; Yu, L.; Yuan, B.-F.; Feng, Y.-Q. Stable isotope labeling-solid phase extraction-mass spectrometry analysis for profiling of thiols and aldehydes in beer. Food Chem. 2017, 237, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Carrascon, V.; Escudero, A.; Ferreira, V.; Lopez, R. Characterisation of the key odorants in a squid broth (Illex argentinus). LWT Food Sci. Technol. 2014, 57, 656–662. [Google Scholar] [CrossRef]
- Milheiro, J.; Ferreira, L.C.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. A simple dispersive solid phase extraction clean-up/concentration method for selective and sensitive quantification of biogenic amines in wines using benzoyl chloride derivatisation. Food Chem. 2019, 274, 110–117. [Google Scholar] [CrossRef]
- Pichon, V.; Chapuis-Hugon, F. Role of Molecularly Imprinted Polymers for Selective Determination of Environmental Pollutants—A Review. Anal. Chim. Acta 2008, 622, 48–61. [Google Scholar] [CrossRef]
- Pisaniello, L.; Watson, F.; Siebert, T.; Francis, L.; Hixson, J.L. The Varietal Influence of Flavour Precursors from Grape Marc on Monoterpene and C13-Norisoprenoid Profiles in Wine as Determined by Membrane-Assisted Solvent Extraction (MASE) GC-MS. Molecules 2022, 27, 2046. [Google Scholar] [CrossRef]
- Zoccali, M.; Tranchida, P.Q.; Mondello, L. Fast gas chromatography-mass spectrometry: A review of the last decade. TrAC Trends Anal. Chem. 2019, 118, 444–452. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Reyes-De-Corcuera, J.I.; Etxeberria, E.; Danyluk, M.D.; Rodrick, G.E. Metabolomic analysis in food science: A review. Trends Food Sci. Technol. 2009, 20, 557–566. [Google Scholar] [CrossRef]
- Liu, Z.; Phillips, J.B. Comprehensive Two-Dimensional Gas Chromatography using an On-Column Thermal Modulator Interface. J. Chromatogr. Sci. 1991, 29, 227–231. [Google Scholar] [CrossRef]
- Adahchour, M.; Beens, J.; Vreuls, R.J.J.; Brinkman, U.A.T. Recent developments in comprehensive two-dimensional gas chromatography (GC×GC). TrAC Trends Anal. Chem. 2006, 25, 438–454. [Google Scholar] [CrossRef]
- Edwards, M.; Mostafa, A.; Górecki, T. Modulation in comprehensive two-dimensional gas chromatography: 20 years of innovation. Anal. Bioanal. Chem. 2011, 401, 2335–2349. [Google Scholar] [CrossRef]
- Prebihalo, S.E.; Berrier, K.L.; Freye, C.E.; Bahaghighat, H.D.; Moore, N.R.; Pinkerton, D.K.; Synovec, R.E. Multidimensional Gas Chromatography: Advances in Instrumentation, Chemometrics, and Applications. Anal. Chem. 2018, 90, 505–532. [Google Scholar] [CrossRef] [PubMed]
- Beens, J.; Boelens, H.; Tijssen, R.; Blomberg, J. Quantitative Aspects of Comprehensive Two-Dimensional Gas Chromatography (GC×GC). J. High Resolut. Chromatogr. 1998, 21, 47–54. [Google Scholar] [CrossRef]
- Bahaghighat, H.D.; Freye, C.E.; Synovec, R.E. Recent advances in modulator technology for comprehensive two dimensional gas chromatography. TrAC Trends Anal. Chem. 2019, 113, 379–391. [Google Scholar] [CrossRef]
- Sharif, K.M.; Chin, S.-T.; Kulsing, C.; Marriott, P.J. The microfluidic Deans switch: 50 years of progress, innovation and application. TrAC Trends Anal. Chem. 2016, 82, 35–54. [Google Scholar] [CrossRef]
- Seeley, J.V.; Micyus, N.J.; Bandurski, S.V.; Seeley, S.K.; McCurry, J.D. Microfluidic Deans Switch for Comprehensive Two-Dimensional Gas Chromatography. Anal. Chem. 2007, 79, 1840–1847. [Google Scholar] [CrossRef]
- Duhamel, C.; Cardinael, P.; Peulon-Agasse, V.; Firor, R.; Pascaud, L.; Semard-Jousset, G.; Giusti, P.; Livadaris, V. Comparison of cryogenic and differential flow (forward and reverse fill/flush) modulators and applications to the analysis of heavy petroleum cuts by high-temperature comprehensive gas chromatography. J. Chromatogr. A 2015, 1387, 95–103. [Google Scholar] [CrossRef]
- Boswell, H.; Chow, H.-Y.; Gorecki, T. Modulators. In Separation Science and Technology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 12, pp. 89–140. ISBN 978-0-12-813745-1. [Google Scholar]
- Giddings, J.C. Concepts and comparisons in multidimensional separation. J. High Resolut. Chromatogr. 1987, 10, 319–323. [Google Scholar] [CrossRef]
- Giddings, J.C. Sample dimensionality: A predictor of order-disorder in component peak distribution in multidimensional separation. J. Chromatogr. A 1995, 703, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Stefanuto, P.-H.; Focant, J.-F. Columns and column configurations. In Separation Science and Technology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 12, pp. 69–88. ISBN 978-0-12-813745-1. [Google Scholar]
- Zhu, S. Effect of column combinations on two-dimensional separation in comprehensive two-dimensional gas chromatography: Estimation of orthogonality and exploring of mechanism. J. Chromatogr. A 2009, 1216, 3312–3317. [Google Scholar] [CrossRef]
- Golay, M.J.E. Height Equivalent to a Theoretical Plate of Tubular Gas Chromatographic Columns lined with a Porous Layer. Nature 1963, 199, 370–371. [Google Scholar] [CrossRef]
- Peroni, D.; Janssen, H.-G. Comprehensive two-dimensional gas chromatography under high outlet pressure conditions: A new approach to correct the flow-mismatch issue in the two dimensions. J. Chromatogr. A 2014, 1332, 57–63. [Google Scholar] [CrossRef]
- Liu, Z.; Patterson, D.G.; Lee, M.L. Geometric Approach to Factor Analysis for the Estimation of Orthogonality and Practical Peak Capacity in Comprehensive Two-Dimensional Separations. Anal. Chem. 1995, 67, 3840–3845. [Google Scholar] [CrossRef]
- Camenzuli, M.; Schoenmakers, P.J. A new measure of orthogonality for multi-dimensional chromatography. Anal. Chim. Acta 2014, 838, 93–101. [Google Scholar] [CrossRef]
- Gilar, M.; Olivova, P.; Daly, A.E.; Gebler, J.C. Orthogonality of Separation in Two-Dimensional Liquid Chromatography. Anal. Chem. 2005, 77, 6426–6434. [Google Scholar] [CrossRef]
- Davis, J.M.; Stoll, D.R.; Carr, P.W. Dependence of Effective Peak Capacity in Comprehensive Two-Dimensional Separations on the Distribution of Peak Capacity between the Two Dimensions. Anal. Chem. 2008, 80, 8122–8134. [Google Scholar] [CrossRef] [PubMed]
- Semard, G.; Peulon-Agasse, V.; Bruchet, A.; Bouillon, J.-P.; Cardinaël, P. Convex hull: A new method to determine the separation space used and to optimize operating conditions for comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2010, 1217, 5449–5454. [Google Scholar] [CrossRef]
- Nowik, W.; Héron, S.; Bonose, M.; Nowik, M.; Tchapla, A. Assessment of Two-Dimensional Separative Systems Using Nearest-Neighbor Distances Approach. Part 1: Orthogonality Aspects. Anal. Chem. 2013, 85, 9449–9458. [Google Scholar] [CrossRef]
- Nowik, W.; Bonose, M.; Héron, S.; Nowik, M.; Tchapla, A. Assessment of Two-Dimensional Separative Systems Using the Nearest Neighbor Distances Approach. Part 2: Separation Quality Aspects. Anal. Chem. 2013, 85, 9459–9468. [Google Scholar] [CrossRef]
- Pérez-Jiménez, M.; Muñoz-González, C.; Pozo-Bayón, M.Á. Specificity of Saliva Esterases by Wine Carboxylic Esters and Inhibition by Wine Phenolic Compounds Under Simulated Oral Conditions. Front. Nutr. 2021, 8, 761830. [Google Scholar] [CrossRef]
- Herrero, M.; Cacciola, F.; Donato, P.; Giuffrida, D.; Dugo, G.; Dugo, P.; Mondello, L. Serial coupled columns reversed-phase separations in high-performance liquid chromatography. J. Chromatogr. A 2008, 1188, 208–215. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Gosetti, F.; Rocío-Bautista, P.; Termopoli, V. Aroma determination in alcoholic beverages: Green MS-based sample preparation approaches. Mass Spectrom. Rev. 2022, e21802. [Google Scholar] [CrossRef] [PubMed]
- Vyviurska, O.; Khvalbota, L.; Koljančić, N.; Špánik, I.; Gomes, A.A. Wine age prediction using digital images and multivariate calibration. Microchem. J. 2023, 190, 108738. [Google Scholar] [CrossRef]
- Pollo, B.J.; Teixeira, C.A.; Belinato, J.R.; Furlan, M.F.; Cunha, I.C.D.M.; Vaz, C.R.; Volpato, G.V.; Augusto, F. Chemometrics, Comprehensive Two-Dimensional gas chromatography and “omics” sciences: Basic tools and recent applications. TrAC Trends Anal. Chem. 2021, 134, 116111. [Google Scholar] [CrossRef]
- Pérez-Cova, M.; Tauler, R.; Jaumot, J. Chemometrics in comprehensive two-dimensional liquid chromatography: A study of the data structure and its multilinear behavior. Chemom. Intell. Lab. Syst. 2020, 201, 104009. [Google Scholar] [CrossRef]
- Stilo, F.; Bicchi, C.; Robbat, A.; Reichenbach, S.E.; Cordero, C. Untargeted approaches in food-omics: The potential of comprehensive two-dimensional gas chromatography/mass spectrometry. TrAC Trends Anal. Chem. 2021, 135, 116162. [Google Scholar] [CrossRef]
- De Juan, A.; Tauler, R. Comparison of three-way resolution methods for non-trilinear chemical data sets. J. Chemom. 2001, 15, 749–771. [Google Scholar] [CrossRef]
- Stilo, F.; Bicchi, C.; Jimenez-Carvelo, A.M.; Cuadros-Rodriguez, L.; Reichenbach, S.E.; Cordero, C. Chromatographic fingerprinting by comprehensive two-dimensional chromatography: Fundamentals and tools. TrAC Trends Anal. Chem. 2021, 134, 116133. [Google Scholar] [CrossRef]
- Stefanuto, P.-H.; Smolinska, A.; Focant, J.-F. Advanced chemometric and data handling tools for GC×GC-TOF-MS. TrAC Trends Anal. Chem. 2021, 139, 116251. [Google Scholar] [CrossRef]
- Wilde, M.J.; Zhao, B.; Cordell, R.L.; Ibrahim, W.; Singapuri, A.; Greening, N.J.; Brightling, C.E.; Siddiqui, S.; Monks, P.S.; Free, R.C. Automating and Extending Comprehensive Two-Dimensional Gas Chromatography Data Processing by Interfacing Open-Source and Commercial Software. Anal. Chem. 2020, 92, 13953–13960. [Google Scholar] [CrossRef]
- Wilson, J.D.; McInnes, C.A.J. The elimination of errors due to baseline drift in the measurement of peak areas in gas chromatography. J. Chromatogr. A 1965, 19, 486–494. [Google Scholar] [CrossRef]
- Eilers, P.H.C. A Perfect Smoother. Anal. Chem. 2003, 75, 3631–3636. [Google Scholar] [CrossRef]
- Lopatka, M.; Barcaru, A.; Sjerps, M.J.; Vivó-Truyols, G. Leveraging probabilistic peak detection to estimate baseline drift in complex chromatographic samples. J. Chromatogr. A 2016, 1431, 122–130. [Google Scholar] [CrossRef]
- Zhang, P.; Carlin, S.; Franceschi, P.; Mattivi, F.; Vrhovsek, U. Application of a Target-Guided Data Processing Approach in Saturated Peak Correction of GC×GC Analysis. Anal. Chem. 2022, 94, 1941–1948. [Google Scholar] [CrossRef]
- Korytár, P.; van Stee, L.L.P.; Leonards, P.E.G.; de Boer, J.; Brinkman, U.A.T. Attempt to unravel the composition of toxaphene by comprehensive two-dimensional gas chromatography with selective detection. J. Chromatogr. A 2003, 994, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Vivó-Truyols, G.; Marriott, P.J.; Schoenmakers, P.J. Development of an algorithm for peak detection in comprehensive two-dimensional chromatography. J. Chromatogr. A 2007, 1156, 14–24. [Google Scholar] [CrossRef]
- Vivó-Truyols, G. Bayesian Approach for Peak Detection in Two-Dimensional Chromatography. Anal. Chem. 2012, 84, 2622–2630. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Reichenbach, S.E.; Shi, J. Automated Unmixing of Comprehensive Two-Dimensional Chemical Separations with Mass Spectrometry. In Proceedings of the 2005 IEEE International Conference on Electro Information Technology, Lincoln, NE, USA, 22–25 May 2005; pp. 1–6. [Google Scholar]
- Reichenbach, S.E.; Ni, M.; Kottapalli, V.; Visvanathan, A. Information technologies for comprehensive two-dimensional gas chromatography. Chemom. Intell. Lab. Syst. 2004, 71, 107–120. [Google Scholar] [CrossRef]
- Kim, S.; Ouyang, M.; Jeong, J.; Shen, C.; Zhang, X. A new method of peak detection for analysis of comprehensive two-dimensional gas chromatography mass spectrometry data. Ann. Appl. Stat. 2014, 8, 1209. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, G.S.; Prebihalo, S.E.; Reaser, B.C.; Marney, L.C.; Synovec, R.E. Statistical inference of mass channel purity from Fisher ratio analysis using comprehensive two-dimensional gas chromatography with time of flight mass spectrometry data. J. Chromatogr. A 2020, 1627, 461401. [Google Scholar] [CrossRef]
- Viant, M.R.; Kurland, I.J.; Jones, M.R.; Dunn, W.B. How close are we to complete annotation of metabolomes? Curr. Opin. Chem. Biol. 2017, 36, 64–69. [Google Scholar] [CrossRef]
- Veenaas, C.; Haglund, P. A retention index system for comprehensive two-dimensional gas chromatography using polyethylene glycols. J. Chromatogr. A 2018, 1536, 67–74. [Google Scholar] [CrossRef]
- De Cripan, S.M.; Cereto-Massagué, A.; Herrero, P.; Barcaru, A.; Canela, N.; Domingo-Almenara, X. Machine Learning-Based Retention Time Prediction of Trimethylsilyl Derivatives of Metabolites. Biomedicines 2022, 10, 879. [Google Scholar] [CrossRef]
- Boegelsack, N.; Sandau, C.; McMartin, D.W.; Withey, J.M.; O’Sullivan, G. Development of retention time indices for comprehensive multidimensional gas chromatography and application to ignitable liquid residue mapping in wildfire investigations. J. Chromatogr. A 2021, 1635, 461717. [Google Scholar] [CrossRef]
- Jiang, M.; Kulsing, C.; Nolvachai, Y.; Marriott, P.J. Two-Dimensional Retention Indices Improve Component Identification in Comprehensive Two-Dimensional Gas Chromatography of Saffron. Anal. Chem. 2015, 87, 5753–5761. [Google Scholar] [CrossRef]
- Abramson, F.P. Automated identification of mass spectra by the reverse search. Anal. Chem. 1975, 47, 45–49. [Google Scholar] [CrossRef]
- Stein, S.E.; Scott, D.R. Optimization and testing of mass spectral library search algorithms for compound identification. J. Am. Soc. Mass Spectrom. 1994, 5, 859–866. [Google Scholar] [CrossRef]
- Kim, S.; Koo, I.; Jeong, J.; Wu, S.; Shi, X.; Zhang, X. Compound Identification Using Partial and Semipartial Correlations for Gas Chromatography–Mass Spectrometry Data. Anal. Chem. 2012, 84, 6477–6487. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zhang, X. Comparative Analysis of Mass Spectral Similarity Measures on Peak Alignment for Comprehensive Two-Dimensional Gas Chromatography Mass Spectrometry. Comput. Math. Methods Med. 2013, 2013, 509761. [Google Scholar] [CrossRef] [PubMed]
- Samokhin, A.; Sotnezova, K.; Lashin, V.; Revelsky, I. Evaluation of mass spectral library search algorithms implemented in commercial software: Evaluation of library search algorithms. J. Mass Spectrom. 2015, 50, 820–825. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Y.; Zheng, C.H.; Wang, B.; Zhang, X.; Chen, P. Combine multiple mass spectral similarity measures for compound identification. Int. J. Data Min. Bioinform. 2016, 15, 84. [Google Scholar] [CrossRef]
- Reichenbach, S.E.; Carr, P.W.; Stoll, D.R.; Tao, Q. Smart Templates for peak pattern matching with comprehensive two-dimensional liquid chromatography. J. Chromatogr. A 2009, 1216, 3458–3466. [Google Scholar] [CrossRef] [PubMed]
- Robbat, A.; Kfoury, N.; Baydakov, E.; Gankin, Y. Optimizing targeted/untargeted metabolomics by automating gas chromatography/mass spectrometry workflows. J. Chromatogr. A 2017, 1505, 96–105. [Google Scholar] [CrossRef]
- Marney, L.C.; Christopher Siegler, W.; Parsons, B.A.; Hoggard, J.C.; Wright, B.W.; Synovec, R.E. Tile-based Fisher-ratio software for improved feature selection analysis of comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry data. Talanta 2013, 115, 887–895. [Google Scholar] [CrossRef]
- Tao, Q.; Reichenbach, S.E.; Heble, C.; Wu, Z. New investigator tools for finding unique and common components in multiple samples with comprehensive two-dimensional chromatography. In Chromatography Today; International Labmate Limited: Hertfordshire, UK, 2018. [Google Scholar]
- Bendik, J.; Kalia, R.; Sukumaran, J.; Richardot, W.H.; Hoh, E.; Kelley, S.T. Automated high confidence compound identification of electron ionization mass spectra for nontargeted analysis. J. Chromatogr. A 2021, 1660, 462656. [Google Scholar] [CrossRef]
- Mommers, J.; Ritzen, E.; Dutriez, T.; van der Wal, S. A procedure for comprehensive two-dimensional gas chromatography retention time locked dual detection. J. Chromatogr. A 2016, 1461, 153–160. [Google Scholar] [CrossRef]
- Lee, S.-J.; Noble, A.C. Use of Partial Least Squares Regression and Multidimensional Scaling on Aroma Models of California Chardonnay Wines. Am. J. Enol. Vitic. 2006, 57, 363. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review: Humulus lupulus—A story that begs to be told. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Gawel, R. Red wine astringency: A review. Aust. J. Grape Wine Res. 1998, 4, 74–95. [Google Scholar] [CrossRef]
- Bindon, K.A.; Smith, P.A.; Kennedy, J.A. Interaction between Grape-Derived Proanthocyanidins and Cell Wall Material. 1. Effect on Proanthocyanidin Composition and Molecular Mass. J. Agric. Food Chem. 2010, 58, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Cadahía, E.; del Álamo, M.; Nevares, I. Effect of size, seasoning and toasting in the volatile compounds in toasted oak wood and in a red wine treated with them. Anal. Chim. Acta 2010, 660, 211–220. [Google Scholar] [CrossRef]
- Pons, A.; Lavigne, V.; Landais, Y.; Darriet, P.; Dubourdieu, D. Identification of a Sotolon Pathway in Dry White Wines. J. Agric. Food Chem. 2010, 58, 7273–7279. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 3920. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Brandão, T.; Vicente, A.A. Continuous beer fermentation—Diacetyl as a villain: Diacetyl as a villain. J. Inst. Brew. 2015, 121, 55–61. [Google Scholar] [CrossRef]
- Berrier, K.L.; Prebihalo, S.E.; Synovec, R.E. Advanced data handling in comprehensive two-dimensional gas chromatography. In Separation Science and Technology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 12, pp. 229–268. ISBN 978-0-12-813745-1. [Google Scholar]
- Mommers, J.; Knooren, J.; Mengerink, Y.; Wilbers, A.; Vreuls, R.; van der Wal, S. Retention time locking procedure for comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2011, 1218, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Skov, T.; Hoggard, J.C.; Bro, R.; Synovec, R.E. Handling within run retention time shifts in two-dimensional chromatography data using shift correction and modeling. J. Chromatogr. A 2009, 1216, 4020–4029. [Google Scholar] [CrossRef]
- Johnson, K.J.; Prazen, B.J.; Young, D.C.; Synovec, R.E. Quantification of naphthalenes in jet fuel with GC×GC/Tri-PLS and windowed rank minimization retention time alignment. J. Sep. Sci. 2004, 27, 410–416. [Google Scholar] [CrossRef]
- Pierce, K.M.; Wood, L.F.; Wright, B.W.; Synovec, R.E. A Comprehensive Two-Dimensional Retention Time Alignment Algorithm to Enhance Chemometric Analysis of Comprehensive Two-Dimensional Separation Data. Anal. Chem. 2005, 77, 7735–7743. [Google Scholar] [CrossRef] [PubMed]
- Weusten, J.J.A.M.; Derks, E.P.P.A.; Mommers, J.H.M.; van der Wal, S. Alignment and clustering strategies for GC×GC–MS features using a cylindrical mapping. Anal. Chim. Acta 2012, 726, 9–21. [Google Scholar] [CrossRef]
- Harvey, P.M.; Shellie, R.A. Data Reduction in Comprehensive Two-Dimensional Gas Chromatography for Rapid and Repeatable Automated Data Analysis. Anal. Chem. 2012, 84, 6501–6507. [Google Scholar] [CrossRef] [PubMed]
- Cordero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Reichenbach, S.E.; Tian, X.; Tao, Q. Targeted and Non-Targeted Approaches for Complex Natural Sample Profiling by GCxGC-qMS. J. Chromatogr. Sci. 2010, 48, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Stadler, S.; Stefanuto, P.-H.; Brokl, M.; Forbes, S.L.; Focant, J.-F. Characterization of Volatile Organic Compounds from Human Analogue Decomposition Using Thermal Desorption Coupled to Comprehensive Two-Dimensional Gas Chromatography–Time-of-Flight Mass Spectrometry. Anal. Chem. 2013, 85, 998–1005. [Google Scholar] [CrossRef]
- Bean, H.D.; Hill, J.E.; Dimandja, J.-M.D. Improving the quality of biomarker candidates in untargeted metabolomics via peak table-based alignment of comprehensive two-dimensional gas chromatography–mass spectrometry data. J. Chromatogr. A 2015, 1394, 111–117. [Google Scholar] [CrossRef]
- Pierce, K.M.; Hoggard, J.C.; Hope, J.L.; Rainey, P.M.; Hoofnagle, A.N.; Jack, R.M.; Wright, B.W.; Synovec, R.E. Fisher Ratio Method Applied to Third-Order Separation Data to Identify Significant Chemical Components of Metabolite Extracts. Anal. Chem. 2006, 78, 5068–5075. [Google Scholar] [CrossRef]
- Sudol, P.E.; Ochoa, G.S.; Synovec, R.E. Investigation of the limit of discovery using tile-based Fisher ratio analysis with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2021, 1644, 462092. [Google Scholar] [CrossRef]
- De-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Valentin, D.; Ferreira, V. Fourteen ethyl esters of wine can be replaced by simpler ester vectors without compromising quality but at the expense of increasing aroma concentration. Food Chem. 2020, 307, 125553. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Versari, A.; Laurie, V.F.; Ricci, A.; Laghi, L.; Parpinello, G.P. Progress in authentication, typification and traceability of grapes and wines by chemometric approaches. Food Res. Int. 2014, 60, 2–18. [Google Scholar] [CrossRef]
- Petrick, L.M.; Shomron, N. AI/ML-driven advances in untargeted metabolomics and exposomics for biomedical applications. Cell Rep. Phys. Sci. 2022, 3, 100978. [Google Scholar] [CrossRef] [PubMed]
- Sudol, P.E.; Schöneich, S.; Synovec, R.E. Principal component analysis of comprehensive three-dimensional gas chromatog-raphy time-of-flight mass spectrometry data. J. Chromatogr. Open 2022, 2, 100043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).