A Stretchable and Self-Healing Dual-Functional Wearable Sensor Enabled by Wet-Spun Conductive Thermoplastic Nanocomposite Fibers
Abstract
1. Introduction
2. Experimental Sections
2.1. Materials
2.2. Fabrication of the Conductive Fibers
2.3. Fabrication of the Dual-Functional Fiber Sensors
2.4. Characterizations
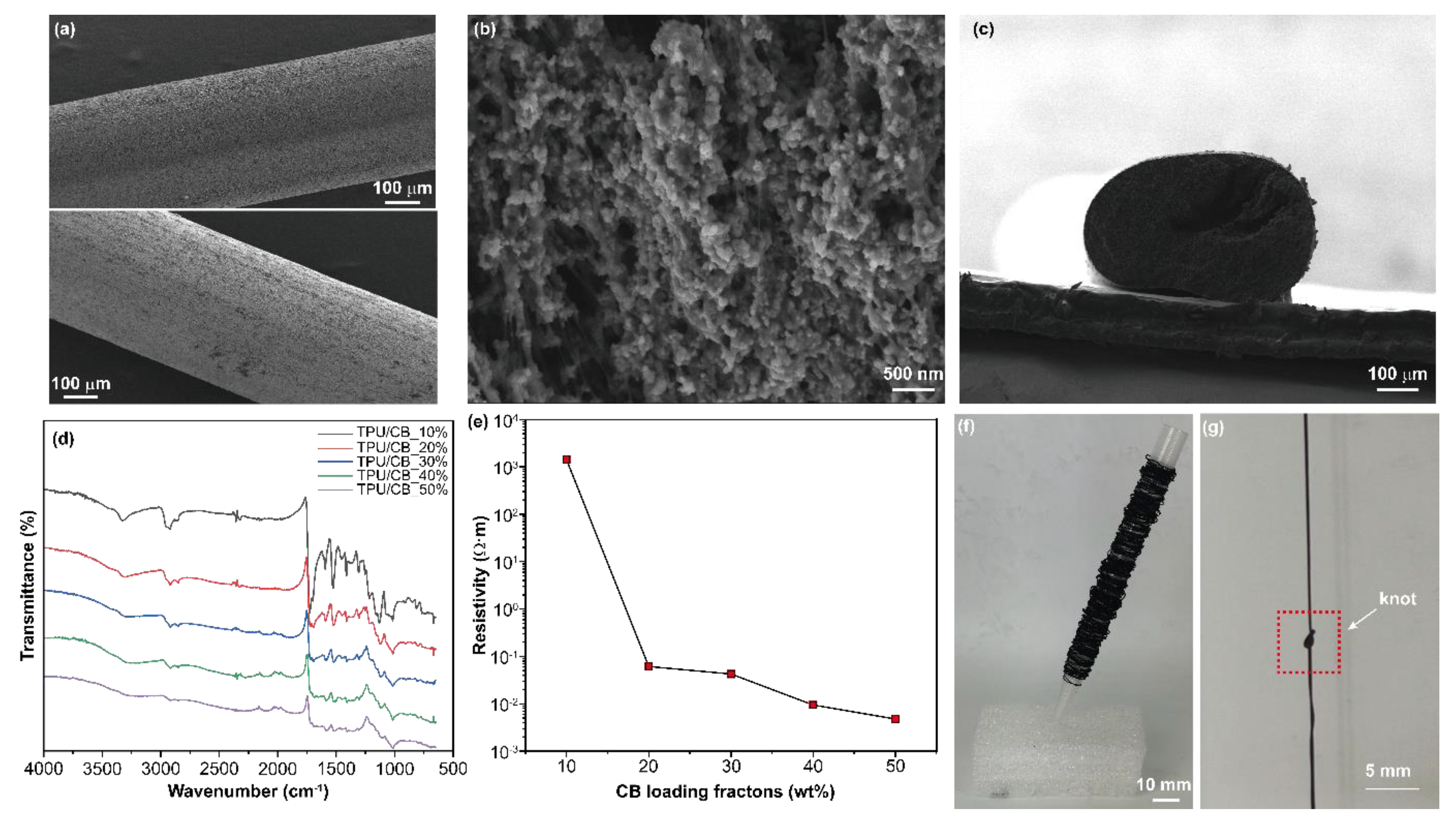
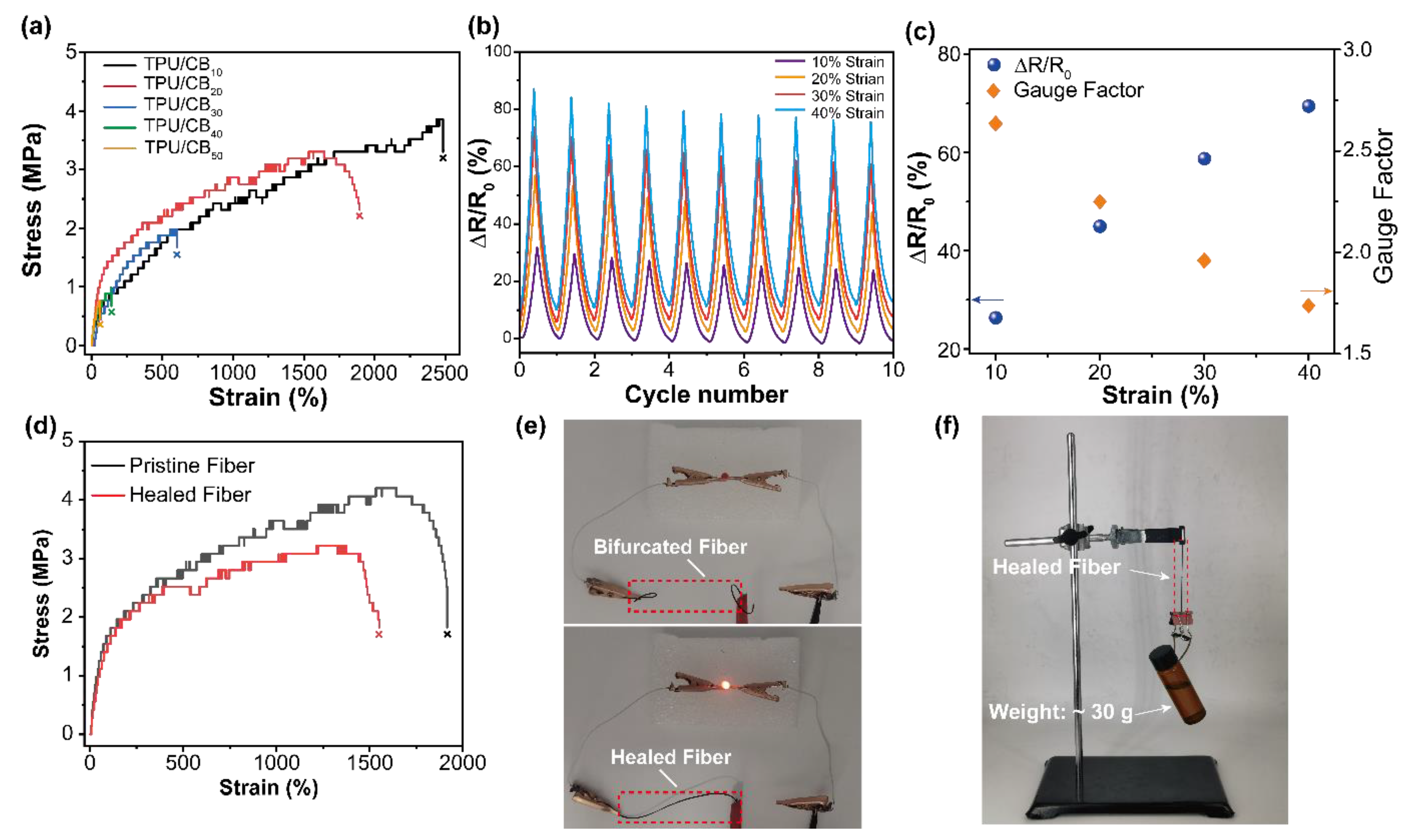
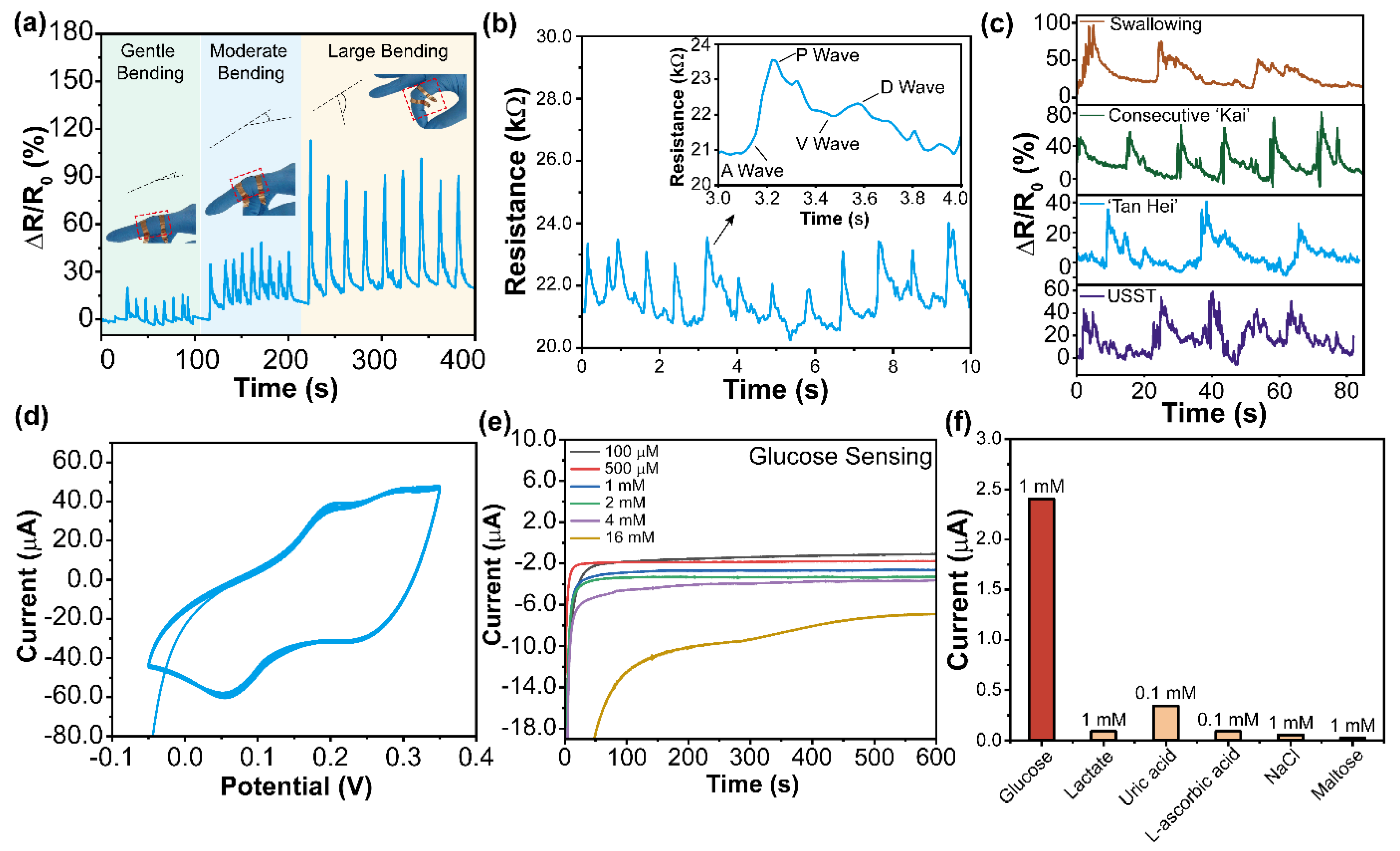
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heo, J.S.; Eom, J.; Kim, Y.H.; Park, S.K. Recent Progress of Textile-Based Wearable Electronics: A Comprehensive Review of Materials, Devices, and Applications. Small 2018, 14, 1703034. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.L.; Chen, W.Y.; Zhang, T.; Zou, J.; Chen, Z.G. Fiber-Based Thermoelectrics for Solid, Portable, and Wearable Electronics. Energy Environ. Sci. 2021, 14, 729–764. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, T.; Wang, J.; Zheng, Z.; Li, Y.; Li, J.; Lai, Y. Functionalized Fiber-Based Strain Sensors: Pathway to Next-Generation Wearable Electronics. Nano-Micro Lett. 2022, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Shao, H.; Liu, X.; Chen, F.; Li, Y.; Tang, C.; Zheng, Y. Superhydrophobic and Recyclable Cellulose-Fiber-Based Composites for High-Efficiency Passive Radiative Cooling. ACS Appl. Mater. Interfaces 2021, 13, 22521–22530. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Xie, L.; He, M.; Jaisutti, R.; Zhu, Z. Flexible Fabric Gas Sensors Based on Reduced Graphene-Polyaniline Nanocomposite for Highly Sensitive NH3 Detection at Room Temperature. Nanotechnology 2021, 32, 305501. [Google Scholar] [CrossRef] [PubMed]
- Tao, X. Study of Fiber-Based Wearable Energy Systems. Acc. Chem. Res. 2019, 52, 307–315. [Google Scholar] [CrossRef]
- Chen, X.; Rogers, J.A.; Lacour, S.P.; Hu, W.; Kim, D.H. Materials Chemistry in Flexible Electronics. Chem. Soc. Rev. 2019, 48, 1431–1433. [Google Scholar] [CrossRef]
- Lee, J.; Jeon, S.; Seo, H.; Lee, J.T.; Park, S. Fiber-Based Sensors and Energy Systems for Wearable Electronics. Appl. Sci. 2021, 11, 531. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wang, Z.; He, B.; Chen, M.; Qi, M.; Liu, Y.; Xin, J.; Wei, L. Recent Progress of Fiber-Based Transistors: Materials, Structures and Applications. Front. Optoelectron. 2022, 15, 2. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Zhao, Z.; Hu, Z.; Liu, X.; Yu, G. Flexible Sodium-Ion Based Energy Storage Devices: Recent Progress and Challenges. Energy Storage Mater. 2020, 26, 83–104. [Google Scholar] [CrossRef]
- Huang, L.; Lin, S.; Xu, Z.; Zhou, H.; Duan, J.; Hu, B.; Zhou, J. Fiber-Based Energy Conversion Devices for Human-Body Energy Harvesting. Adv. Mater. 2020, 32, 1902034. [Google Scholar] [CrossRef] [PubMed]
- Rafique, A.; Ferreira, I.; Abbas, G.; Baptista, A.C. Recent Advances and Challenges toward Application of Fibers and Textiles in Integrated Photovoltaic Energy Storage Devices. Nano-Micro Lett. 2023, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lu, C.; Jiang, H.; Han, F.; Shi, X.; Wu, J.; Wang, L.; Chen, T.; Wang, J.; Zhang, Y.; et al. Scalable Production of High-Performing Woven Lithium-Ion Fibre Batteries. Nature 2021, 597, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zuo, Y.; Zhai, P.; Shen, J.; Yang, Y.; Gao, Z.; Liao, M.; Wu, J.; Wang, J.; Xu, X.; et al. Large-Area Display Textiles Integrated with Functional Systems. Nature 2021, 591, 240–245. [Google Scholar] [CrossRef]
- Wang, T.; Meng, J.; Zhou, X.; Liu, Y.; He, Z.; Han, Q.; Li, Q.; Yu, J.; Li, Z.; Liu, Y.; et al. Reconfigurable Neuromorphic Memristor Network for Ultralow-Power Smart Textile Electronics. Nat. Commun. 2022, 13, 7432. [Google Scholar] [CrossRef]
- Li, T.; Su, Y.; Chen, F.; Zheng, H.; Meng, W.; Liu, Z.; Ai, Q.; Liu, Q.; Tan, Y.; Zhou, Z. Bioinspired Stretchable Fiber-Based Sensor toward Intelligent Human–Machine Interactions. ACS Appl. Mater. Interfaces 2022, 14, 22666–22677. [Google Scholar] [CrossRef]
- He, M.; Xie, L.; Luo, G.; Li, Z.; Wright, J.; Zhu, Z. Flexible Fabric Gas Sensors Based on Pani/WO3 p−n Heterojunction for High Performance NH3 Detection at Room Temperature. Sci. China Mater. 2020, 63, 2028–2039. [Google Scholar] [CrossRef]
- Cho, H.; Lee, H.; Lee, S.; Kim, S. Reduced Graphene Oxide-Based Wearable and Bio-Electrolyte Triggered Pressure Sensor with Tunable Sensitivity. Ceram. Int. 2021, 47, 17702–17710. [Google Scholar] [CrossRef]
- Gao, T.; Yan, G.; Yang, X.; Yan, Q.; Tian, Y.; Song, J.; Li, F.; Wang, X.; Yu, J.; Li, Y.; et al. Wet Spinning of Fiber-Shaped Flexible Zn-Ion Batteries toward Wearable Energy Storage. J. Energy Chem. 2022, 71, 192–200. [Google Scholar] [CrossRef]
- Tang, Z.; Jia, S.; Wang, F.; Bian, C.; Chen, Y.; Wang, Y.; Li, B. Highly Stretchable Core–Sheath Fibers Via Wet-Spinning for Wearable Strain Sensors. ACS Appl. Mater. Interfaces 2018, 10, 6624–6635. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, D.; Gong, S.; Brassart, L.; Wang, Y.; An, T.; Cheng, W. A Moss-Inspired Electroless Gold-Coating Strategy toward Stretchable Fiber Conductors by Dry Spinning. Adv. Electron. Mater. 2019, 5, 1800462. [Google Scholar] [CrossRef]
- Feng, L.; Chang, Y.; Zhong, J.; Jia, D.C. Dry Spin Graphene Oxide Fibers: Mechanical/Electrical Properties and Microstructure Evolution. Sci. Rep. 2018, 8, 10803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, T.; Wang, Z.; Zhang, T.; Chen, M.; Zhang, J.; Liu, L.; Qi, M.; Zhang, Q.; Yang, J.; et al. Designer Patterned Functional Fibers Via Direct Imprinting in Thermal Drawing. Nat. Commun. 2020, 11, 3842. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Z.; Zhang, Q.; Wang, Z.; Liu, W.; Chen, M.; Wei, L. Self-Powered Multifunctional Sensing Based on Super-Elastic Fibers by Soluble-Core Thermal Drawing. Nat. Commun. 2021, 12, 1416. [Google Scholar] [CrossRef]
- Azimi, B.; Milazzo, M.; Lazzeri, A.; Berrettini, S.; Uddin, M.J.; Qin, Z.; Buehler, M.J.; Danti, S. Electrospinning Piezoelectric Fibers for Biocompatible Devices. Adv. Healthc. Mater. 2020, 9, 1901287. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun Nano-Fibers for Biomedical and Tissue Engineering Applications: A Comprehensive Review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef]
- Sun, L.; Shang, L.; Xiao, L.; Zhang, M.; Li, M.; Ao, Y. Structural Changes of Polyacrylonitrile Fibers in the Process of Wet Spinning. J. Appl. Polym. Sci. 2020, 137, 48905. [Google Scholar] [CrossRef]
- Chang, L.; Wang, F.; Guo, Y.; Li, J.; Gong, Y.; Shi, Q. Green Preparation of Thermochromic Starch-Based Fibers through a Wet-Spinning Process. ACS Appl. Polym. Mater. 2021, 3, 436–444. [Google Scholar] [CrossRef]
- Tang, P.; Deng, Z.; Zhang, Y.; Liu, L.X.; Wang, Z.; Yu, Z.Z.; Zhang, H.B. Tough, Strong, and Conductive Graphene Fibers by Optimizing Surface Chemistry of Graphene Oxide Precursor. Adv. Funct. Mater. 2022, 32, 2112156. [Google Scholar] [CrossRef]
- Lee, D.; Kim, S.G.; Hong, S.; Madrona, C.; Oh, Y.; Park, M.; Komatsu, N.; Taylor, L.W.; Chung, B.; Kim, J.; et al. Ultrahigh Strength, Modulus, and Conductivity of Graphitic Fibers by Macromolecular Coalescence. Sci. Adv. 2022, 8, eabn0939. [Google Scholar] [CrossRef]
- Eom, W.; Shin, H.; Ambade, R.B.; Lee, S.H.; Lee, K.H.; Kang, D.J.; Han, T.H. Large-Scale Wet-Spinning of Highly Electroconductive Mxene Fibers. Nat. Commun. 2020, 11, 2825. [Google Scholar] [CrossRef] [PubMed]
- Seyedin, S.; Uzun, S.; Levitt, A.; Anasori, B.; Dion, G.; Gogotsi, Y.; Razal, J.M. MXene Composite and Coaxial Fibers with High Stretchability and Conductivity for Wearable Strain Sensing Textiles. Adv. Funct. Mater. 2020, 30, 1910504. [Google Scholar] [CrossRef]
- Seyedin, S.; Razal, J.M.; Innis, P.C.; Wallace, G.G. A Facile Approach to Spinning Multifunctional Conductive Elastomer Fibres with Nanocarbon Fillers. Smart Mater. Struct. 2016, 25, 35015. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, R.; Li, G.; Chen, Y.; Tang, Z.; Wang, Y.; Liu, Z.; Jiang, H.; Zhi, C. Flexible Dual-Mode Tactile Sensor Derived from Three-Dimensional Porous Carbon Architecture. ACS Appl. Mater. Interfaces 2017, 9, 22685–22693. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, C.; Sun, J.; Zhang, H.; Feng, Y.; Qin, M.; Feng, W. Highly Thermally Conductive Polymer/Graphene Composites with Rapid Room-Temperature Self-Healing Capacity. Nano-Micro Lett. 2022, 14, 135. [Google Scholar] [CrossRef]
- Zhou, X.; Rajeev, A.; Subramanian, A.; Li, Y.; Rossetti, N.; Natale, G.; Lodygensky, G.A.; Cicoira, F. Self-Healing, Stretchable, and Highly Adhesive Hydrogels for Epidermal Patch Electrodes. Acta Biomater. 2022, 139, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Adusumalli, R.B.; Venkateshan, K.C.; Kunchi, C.; Vadlamani, S.R. Tensile Testing of Single Fibres. Procedia Struct. Integr. 2019, 14, 150–157. [Google Scholar] [CrossRef]
- Ilankeeran, P.K.; Mohite, P.M.; Kamle, S. Axial Tensile Testing of Single Fibres. Mod. Mech. Eng. 2012, 2, 151–156. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, F.; Yang, L.; Wang, B.; Huang, Y.; Hu, Z. Closed-Loop Recycling of Carbon Fiber-Reinforced Composites Enabled by a Dual-Dynamic Cross-Linked Epoxy Network. ACS Sustain. Chem. Eng. 2023, 11, 1527–1539. [Google Scholar] [CrossRef]
- Shirvan, A.R.; Nouri, A.; Sutti, A. A Perspective on the Wet Spinning Process and Its Advancements in Biomedical Sciences. Eur. Polym. J. 2022, 181, 111681. [Google Scholar] [CrossRef]
- He, Z.; Cao, Q.; Jing, B.; Wang, X.; Deng, Y. Gel Electrolytes Based on Poly(Vinylidenefluoride-Co-Hexafluoropropylene)/Thermoplastic Polyurethane/Poly(Methyl Methacrylate) with in Situ SiO2 for Polymer Lithium Batteries. RSC Adv. 2017, 7, 3240–3248. [Google Scholar] [CrossRef]
- Lopes, G.H.; Junges, J.; Fiorio, R.; Zeni, M.; Zattera, A.J. Thermoplastic Polyurethane Synthesis Using Poss as a Chain Modifier. Mater. Res. 2012, 15, 698–704. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, F.; Mei, Y.; Ding, Y.; Pang, H.; Zhang, P. Gel Polymer Electrolyte Based on Hydrophilic–Lipophilic TiO2-Modified Thermoplastic Polyurethane for High-Performance Li-Ion Batteries. J. Mater. Sci. 2021, 56, 2474–2485. [Google Scholar] [CrossRef]
- Chang, S. The Meridian System and Mechanism of Acupuncture—A Comparative Review. Part 2: Mechanism of Acupuncture Analgesia. Taiwan J. Obstet. Gynecol. 2013, 52, 14–24. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, X.; Cui, J.; Shi, Z.; Yan, F.; Han, Y.; Li, Z.; Zhu, Z. A Stretchable and Self-Healing Dual-Functional Wearable Sensor Enabled by Wet-Spun Conductive Thermoplastic Nanocomposite Fibers. Analytica 2023, 4, 336-346. https://doi.org/10.3390/analytica4030025
Wang Z, Wang X, Cui J, Shi Z, Yan F, Han Y, Li Z, Zhu Z. A Stretchable and Self-Healing Dual-Functional Wearable Sensor Enabled by Wet-Spun Conductive Thermoplastic Nanocomposite Fibers. Analytica. 2023; 4(3):336-346. https://doi.org/10.3390/analytica4030025
Chicago/Turabian StyleWang, Zifeng, Xiyu Wang, Jiaming Cui, Zhuo Shi, Feng Yan, Yutong Han, Zhanhong Li, and Zhigang Zhu. 2023. "A Stretchable and Self-Healing Dual-Functional Wearable Sensor Enabled by Wet-Spun Conductive Thermoplastic Nanocomposite Fibers" Analytica 4, no. 3: 336-346. https://doi.org/10.3390/analytica4030025
APA StyleWang, Z., Wang, X., Cui, J., Shi, Z., Yan, F., Han, Y., Li, Z., & Zhu, Z. (2023). A Stretchable and Self-Healing Dual-Functional Wearable Sensor Enabled by Wet-Spun Conductive Thermoplastic Nanocomposite Fibers. Analytica, 4(3), 336-346. https://doi.org/10.3390/analytica4030025







