Electrochemical Monitoring of Sulfadiazine via La@CeO Incorporated with Reduced Graphene Oxide
Abstract
1. Introduction
2. Experimental Methodology
2.1. Chemicals and Reagents
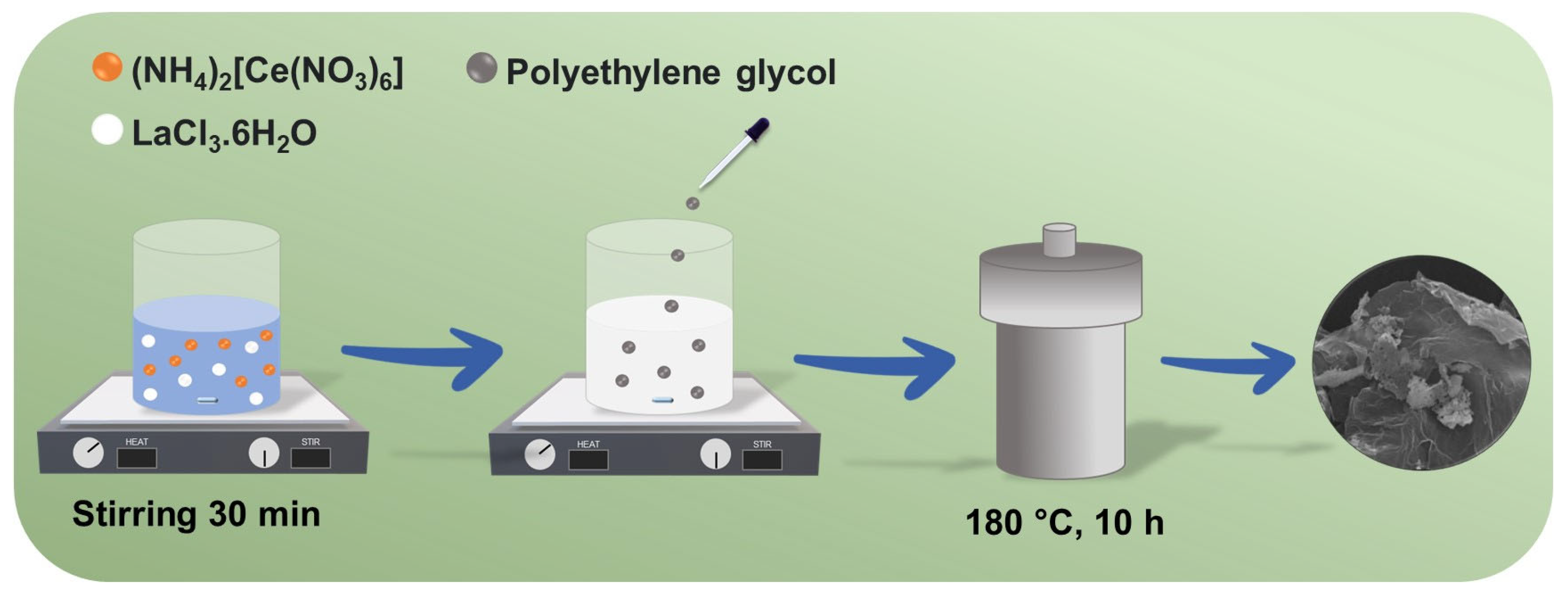
2.2. Synthesis of LCO@RGO
3. Results and Discussion
3.1. Crystalline Nature and Vibrational Spectroscopy Investigation
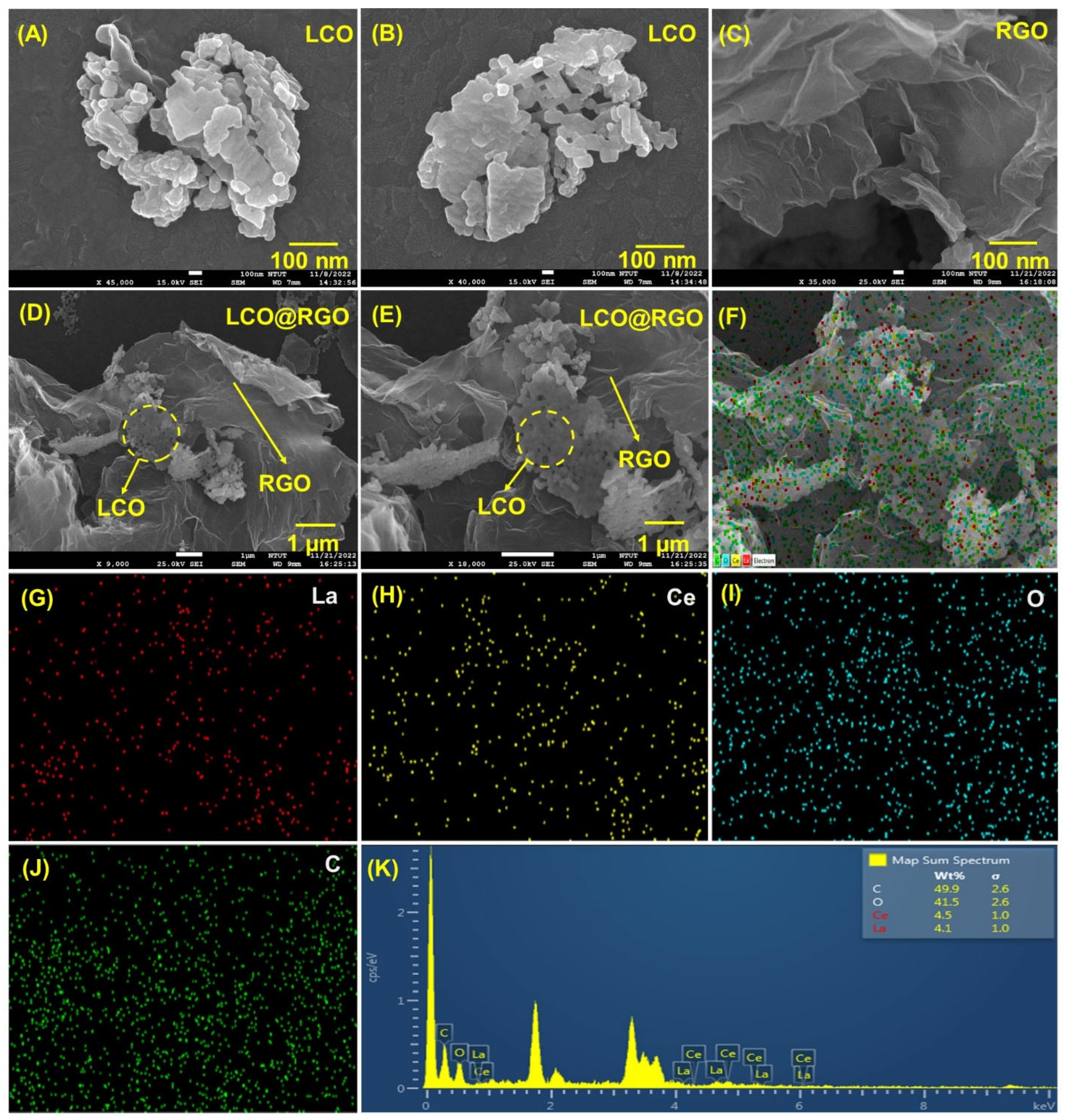
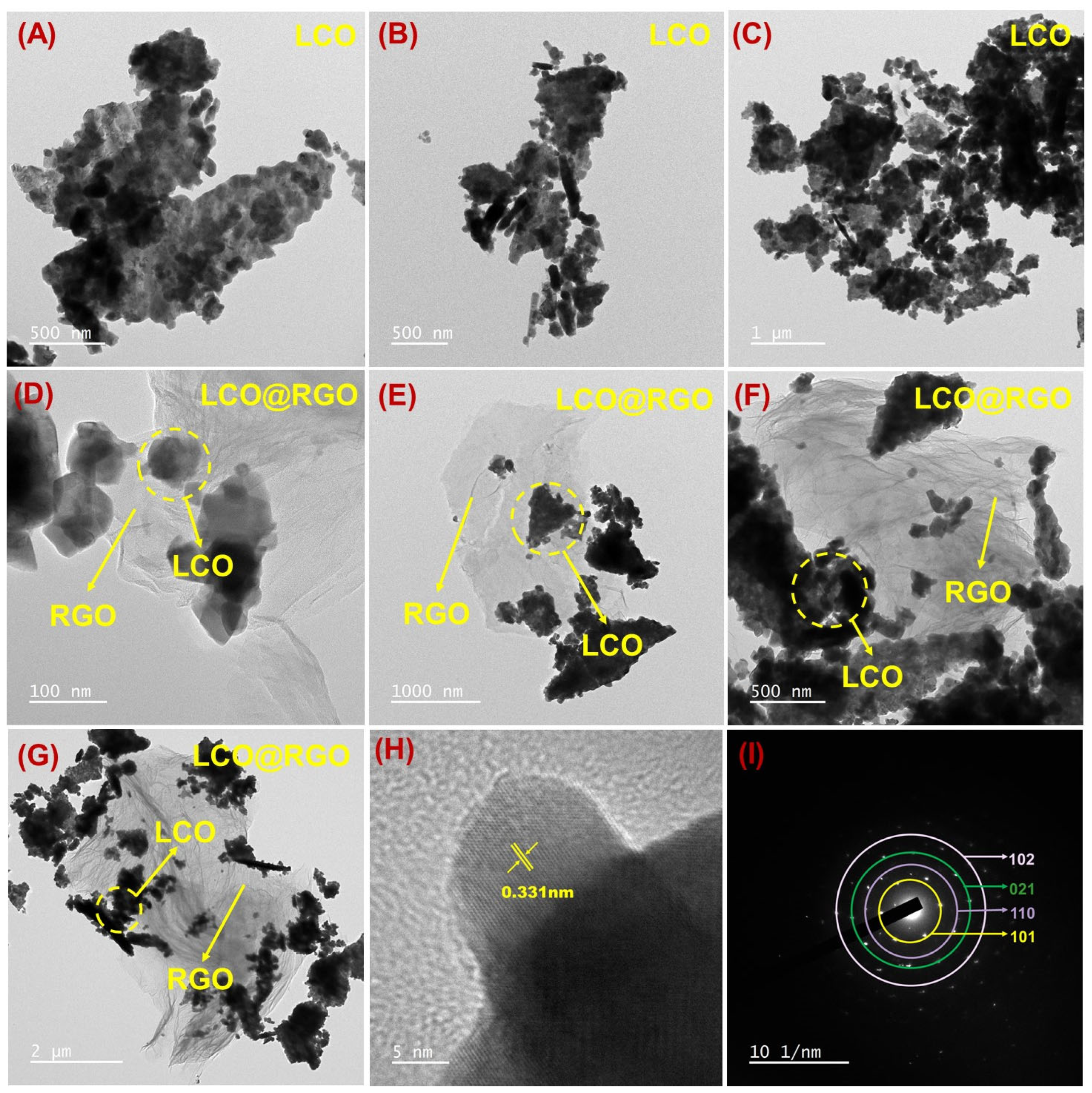
3.2. Morphological Identification
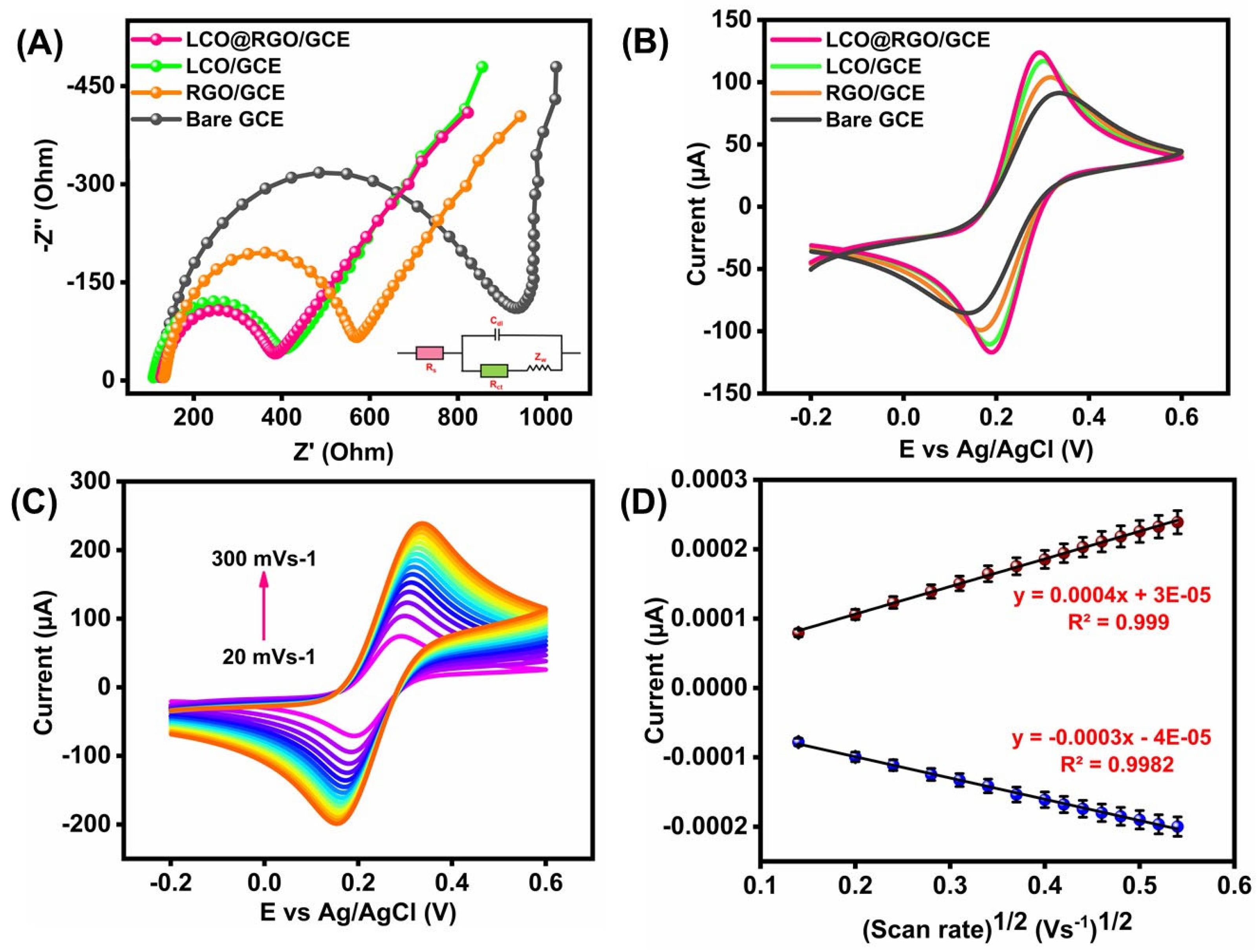
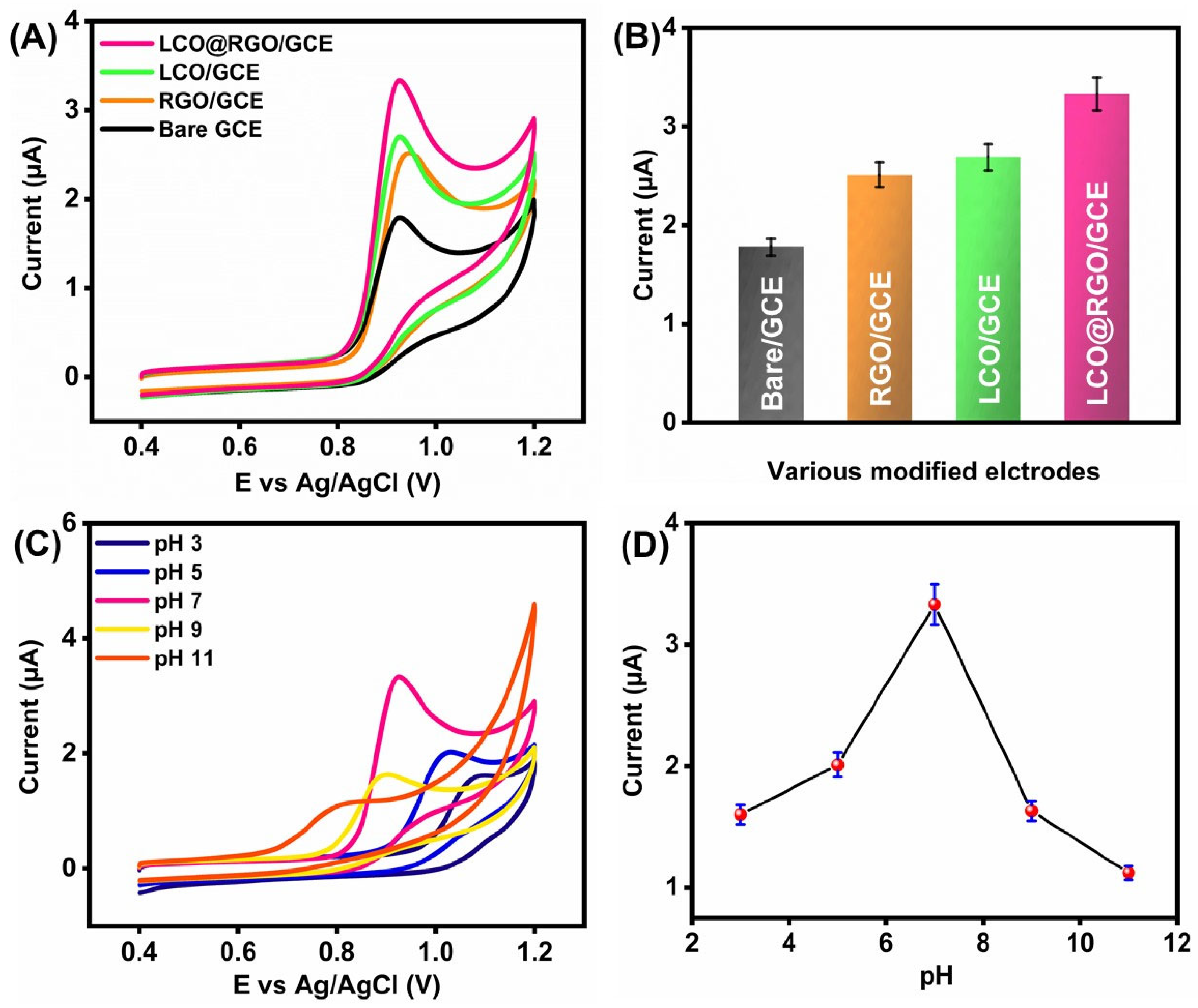
3.3. Electrochemical Characterization of LCO@RGO
3.4. Influence of SZ on Electrochemical Detection Using LCO@RGO/GCE
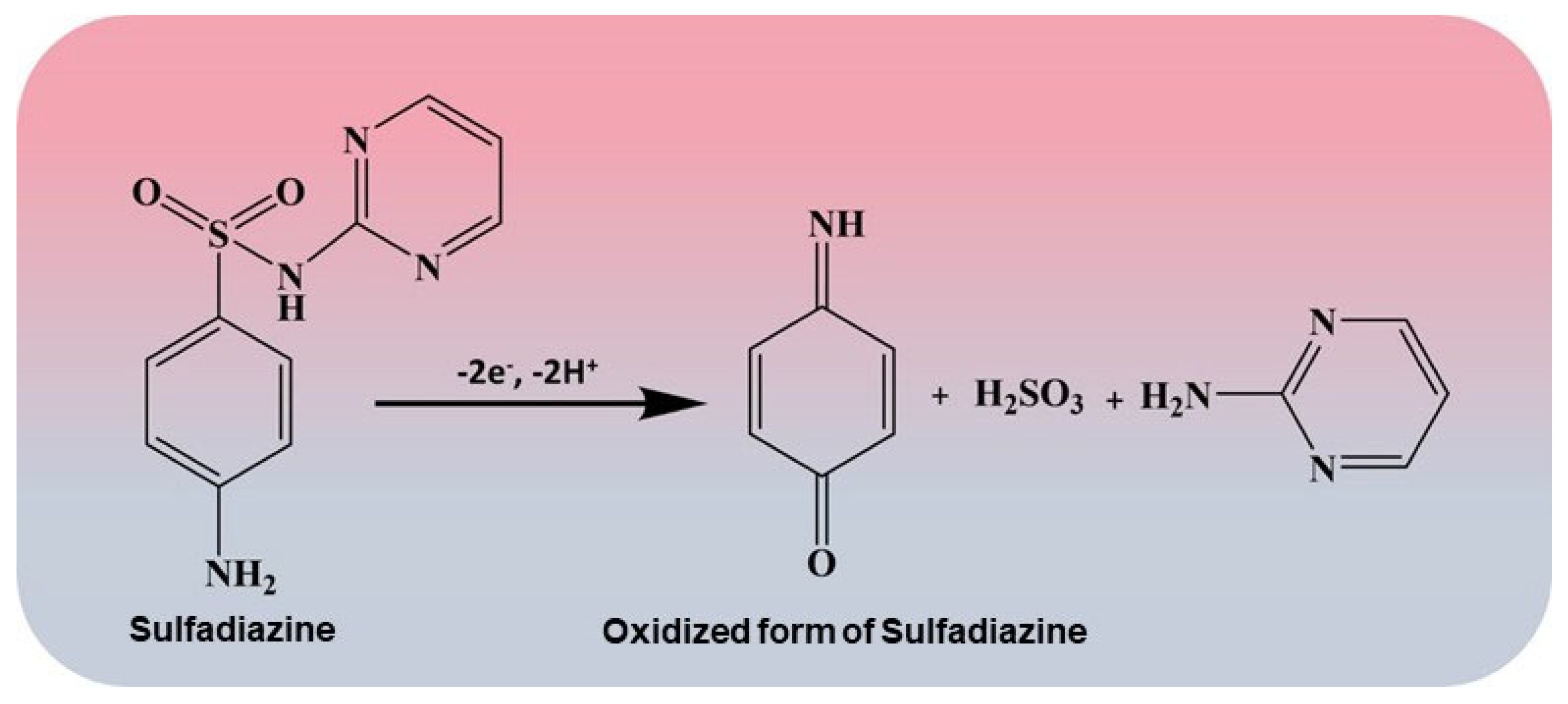
3.5. Detection of SZ Using DPV Analysis
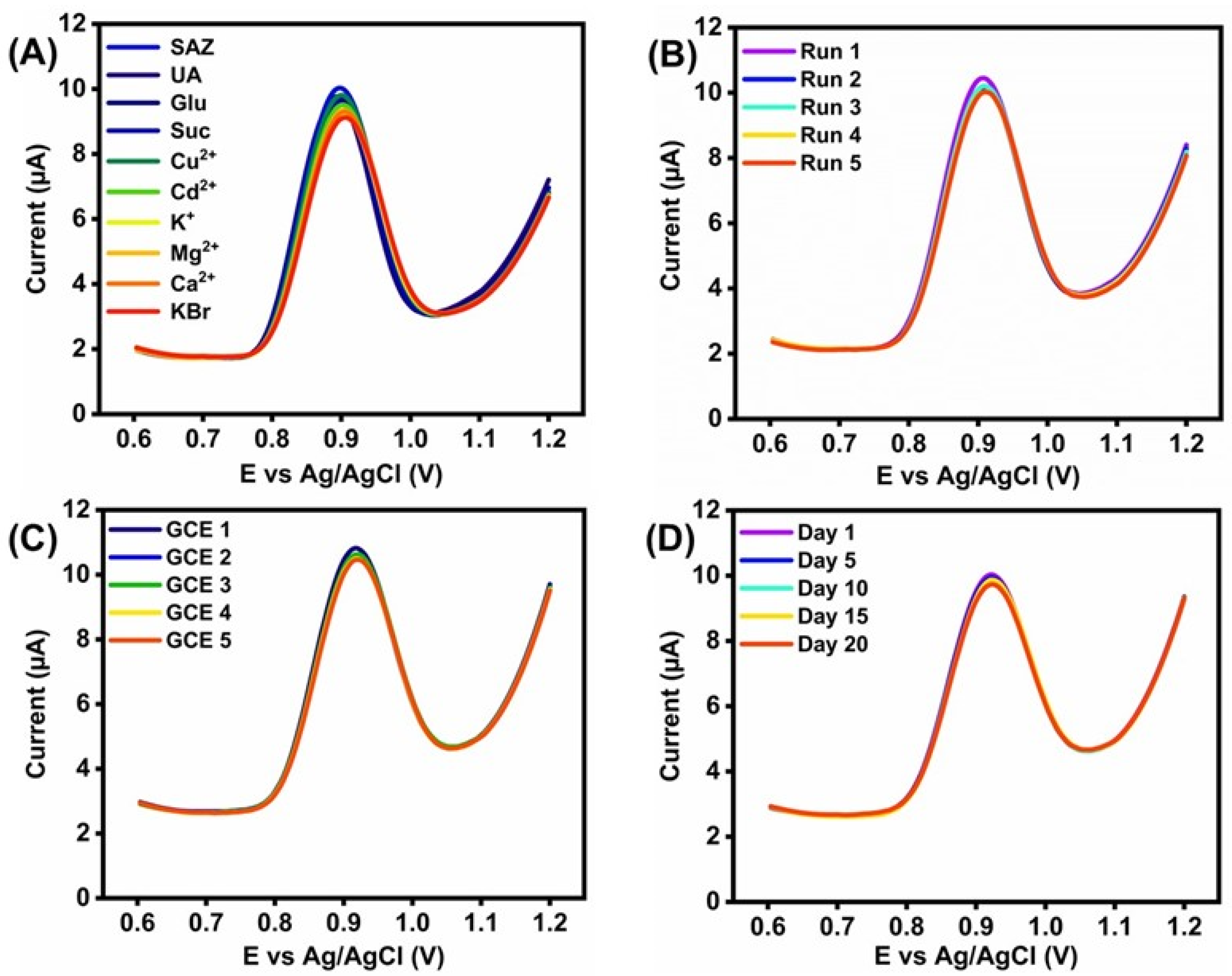
3.6. Interference, Stability, and Reproducibility of LCO@RGO in SZ
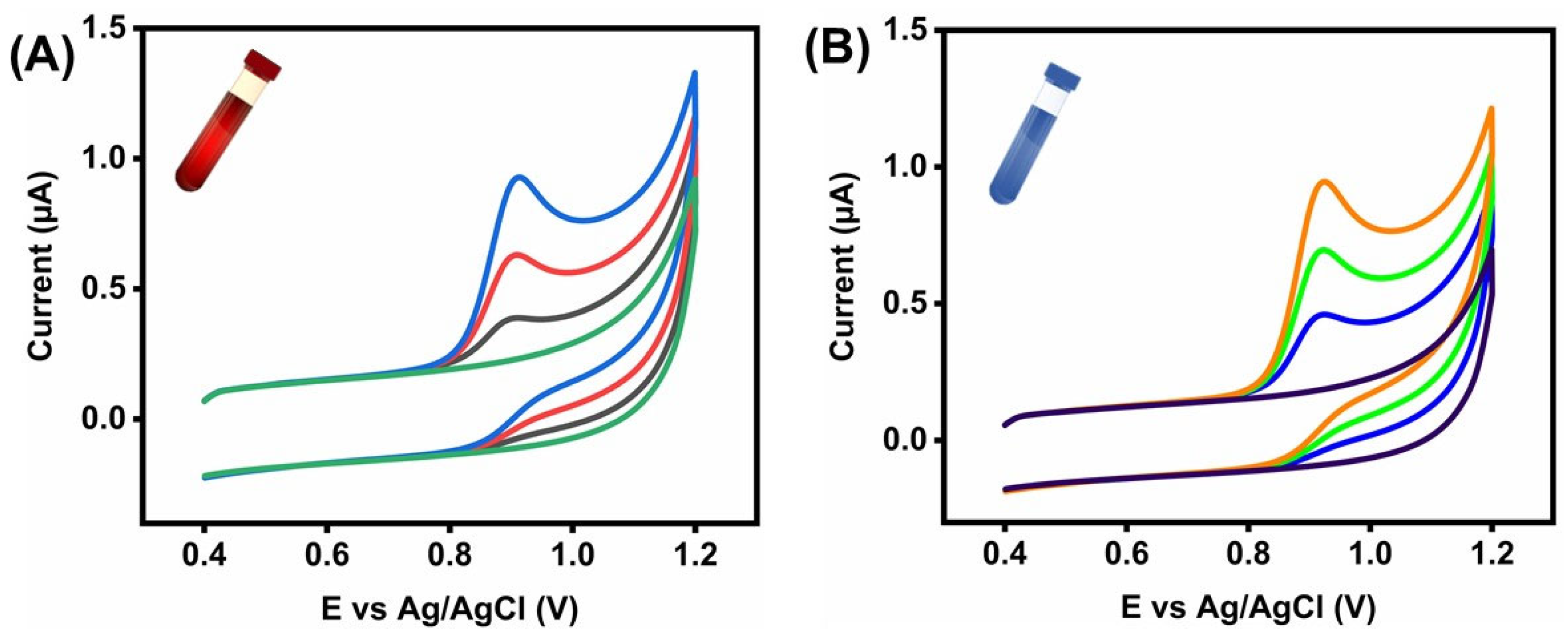
3.7. Human Blood Serum and River Water Detection in Front of SZ
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alsaiari, N.S.; Katubi, K.M.M.; Alzahrani, F.M.; Siddeeg, S.M.; Tahoon, M.A. The application of nanomaterials for the electrochemical detection of antibiotics: A review. Micromachines 2021, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.E.; Hwang, S.-K.; Lee, M.J.; Park, B.; Huh, Y.S.; Han, Y.-K. Gold nanoparticle decorated patronite on rGO for the quantification of sulfadiazine at nanomolar levels in contaminated water. Chem. Eng. J. 2022, 439, 135782. [Google Scholar] [CrossRef]
- Yao, J.; Dong, Z.; Ye, X.; Yang, J.; Jia, Y.; Zhang, Y.; Liu, H. Electrochemically activated peroxymonosulfate with mixed metal oxide electrodes for sulfadiazine degradation: Mechanism, DFT study and toxicity evaluation. Chemosphere 2022, 309, 136695. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, J.; Waterhouse, G.I.; Xu, L.; Zhang, H.; Qiao, X.; Xu, Z. A selective molecularly imprinted electrochemical sensor with GO@ COF signal amplification for the simultaneous determination of sulfadiazine and acetaminophen. Sens. Actuators B Chem. 2019, 300, 126993. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Z.; Yifeng, E.; Jiang, Y.; Wei, P.; Chen, P.; Li, L.; Qian, K. Selective and Quantitative Recovery of Sulfadiazine from Seawater by Sb Doped LTA Zeolite Electrochemical Sensor. Surf. Interfaces 2023, 37, 102666. [Google Scholar] [CrossRef]
- Yang, L.; Chen, X.; Wen, X.; Tang, J.; Zheng, X.; Li, J.; Chen, L.; Jiang, S.; Le, T. A label-free dual-modal aptasensor for colorimetric and fluorescent detection of sulfadiazine. J. Mater. Chem. B 2022, 10, 6187–6193. [Google Scholar] [CrossRef]
- Sharma, T.S.K.; Jana, J.; Bhamu, K.; Song, J.; Sivaselvam, S.; Van Tam, T.; Kang, S.G.; Chung, J.S.; Hur, S.H.; Choi, W.M. Rational synthesis of alkaline earth metal vanadates: Structural origin of MgVO3 honeycomb lattice system and its electrochemical analysis for the detection of sulfadiazine. Chem. Eng. J. 2023, 464, 142673. [Google Scholar] [CrossRef]
- Gamba, V.; Terzano, C.; Fioroni, L.; Moretti, S.; Dusi, G.; Galarini, R. Development and validation of a confirmatory method for the determination of sulphonamides in milk by liquid chromatography with diode array detection. Anal. Chim. Acta 2009, 637, 18–23. [Google Scholar] [CrossRef]
- Velmurugan, S.; Yang, T.C.-K.; Chen, S.-W.; Chen, J.-N. Metal-organic frameworks derived ZnO-Co3O4 pn heterojunction photocatalyst for the photoelectrochemical detection of sulfadiazine. J. Environ. Chem. Eng. 2021, 9, 106169. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Hu, H.; Qu, K.; Cui, Z. A Low-Cost Electrochemical Method for the Determination of Sulfadiazine in Aquaculture Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 16945. [Google Scholar] [CrossRef]
- Wang, X.; Li, K.; Shi, D.; Jin, X.; Xiong, N.; Peng, F.; Peng, D.; Bi, D. Development and validation of an immunochromatographic assay for rapid detection of sulfadiazine in eggs and chickens. J. Chromatogr. B 2007, 847, 289–295. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, Z.; Zuo, S.; Li, X.; Chen, Y. Identification of antibiotic residues in aquatic products with surface-enhanced Raman scattering powered by 1-D convolutional neural networks. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 289, 122195. [Google Scholar] [CrossRef]
- Fotouhi, L.; Hashkavayi, A.B.; Heravi, M.M. Electrochemical behaviour and voltammetric determination of sulphadiazine using a multi-walled carbon nanotube composite film-glassy carbon electrode. J. Exp. Nanosci. 2013, 8, 947–956. [Google Scholar] [CrossRef]
- Mallikarjunaiah Vinay, M.; Virupakshappa Basavarajappa, K.; Manjunatha, P.; Thimmappa Purushothama, H.; Onkarappa Yathisha, R.; Arthoba Nayaka, Y. Development of single walled carbon nanotube-molybdenum disulfide nanocomposite/poly-ethylene glycol modified carbon paste electrode as an electrochemical sensor for the investigation of sulfadiazine in biological samples. Anal. Bioanal. Electrochem. 2020, 12, 155–167. [Google Scholar]
- Sun, Y.; Xu, L.; Waterhouse, G.I.; Wang, M.; Qiao, X.; Xu, Z. Novel three-dimensional electrochemical sensor with dual signal amplification based on MoS2 nanosheets and high-conductive NH2-MWCNT@ COF for sulfamerazine determination. Sens. Actuators B Chem. 2019, 281, 107–114. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, J.; Xu, L.; Zhang, W.; Chen, Y. Controlled synthesis of Nd (OH)3 and Nd2O3 nanoparticles by microemulsion method. Mater. Chem. Phys. 2010, 122, 362–367. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, B.; Xu, F.; Zhu, F.; Yan, L.; Zhang, F.; Huang, A. Energy transfer among rare earth ions induced by annealing process of TmEr codoped aluminum oxide thin films. Phys. Lett. A 2009, 373, 890–893. [Google Scholar] [CrossRef]
- Sundaresan, R.; Mariyappan, V.; Chen, S.-M.; Ramachandran, B.; Paulsamy, R.; Rasu, R. Construction of an electrochemical sensor towards environmental hazardous 4-nitrophenol based on Nd (OH)3-embedded VSe2 nanocomposite. Environ. Sci. Pollut. Res. 2023, 1–14. [Google Scholar] [CrossRef]
- Rashmi, B.; Harlapur, S.F.; Gurushantha, K.; Ravikumar, C.; Kumar, M.A.; Santosh, M.; Kumar, V.D.; Kumar, A.N.; Azad, A.K.; Murthy, H.A. Facile green synthesis of lanthanum oxide nanoparticles using Centella asiatica and Tridax plants: Photocatalytic, electrochemical sensor and antimicrobial studies. Appl. Surf. Sci. Adv. 2022, 7, 100210. [Google Scholar] [CrossRef]
- Deng, W.; Carpenter, C.; Yi, N.; Flytzani-Stephanopoulos, M. Comparison of the activity of Au/CeO2 and Au/Fe2O3 catalysts for the CO oxidation and the water-gas shift reactions. Top. Catal. 2007, 44, 199–208. [Google Scholar] [CrossRef]
- Keating, P.R.; Scanlon, D.O.; Watson, G.W. The nature of oxygen states on the surfaces of CeO2 and La-doped CeO2. Chem. Phys. Lett. 2014, 608, 239–243. [Google Scholar] [CrossRef]
- Aneggi, E.; De Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Promotional effect of rare earths and transition metals in the combustion of diesel soot over CeO2 and CeO2–ZrO2. Catal. Today 2006, 114, 40–47. [Google Scholar] [CrossRef]
- Kırkgeçit, R.; Torun, H.Ö.; Dokan, F.K.; Öztürk, E. Optical and electrical conductivity properties of rare earth elements (Sm, Y, La, Er) co-doped CeO2. J. Rare Earths 2022, 40, 1619–1627. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, A.; Sagdeo, A.; Sagdeo, P.R. Doping-induced combined fano and phonon confinement effect in La-doped CeO2: Raman spectroscopy analysis. J. Phys. Chem. C 2021, 125, 2648–2658. [Google Scholar] [CrossRef]
- Goel, S.; Sinha, N.; Yadav, H.; Joseph, A.J.; Kumar, B. Experimental investigation on the structural, dielectric, ferroelectric and piezoelectric properties of La doped ZnO nanoparticles and their application in dye-sensitized solar cells. Phys. E Low-Dimens. Syst. Nanostructures 2017, 91, 72–81. [Google Scholar] [CrossRef]
- Zhang, Z.; Gong, Y.; Xu, J.; Zhang, Y.; Xiao, Q.; Xi, R.; Xu, X.; Fang, X.; Wang, X. Dissecting La2Ce2O7 catalyst to unravel the origin of the surface active sites devoting to its performance for oxidative coupling of methane (OCM). Catal. Today 2022, 400, 73–81. [Google Scholar] [CrossRef]
- Atacan, K. CuFe2O4/reduced graphene oxide nanocomposite decorated with gold nanoparticles as a new electrochemical sensor material for l-cysteine detection. J. Alloys Compd. 2019, 791, 391–401. [Google Scholar] [CrossRef]
- Wang, C.; Du, J.; Wang, H.; Zou, C.E.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef]
- Santhan, A.; Hwa, K.-Y.; Ganguly, A. Self-assembled nanorods with reduced graphene oxide as efficient nano-catalyst for dual modality sensing of hazardous phenolic compound. Chemosphere 2022, 307, 135715. [Google Scholar] [CrossRef]
- Sundaresan, R.; Mariyappan, V.; Chen, S.-M.; Keerthi, M.; Ramachandran, R. Electrochemical sensor for detection of tryptophan in the milk sample based on MnWO4 nanoplates encapsulated RGO nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126889. [Google Scholar] [CrossRef]
- Akilarasan, M.; Kogularasu, S.; Chen, S.-M.; Chen, T.-W.; Lin, S.-H. One-step synthesis of reduced graphene oxide sheathed zinc oxide nanoclusters for the trace level detection of bisphenol A in tissue papers. Ecotoxicol. Environ. Saf. 2018, 161, 699–705. [Google Scholar] [CrossRef]
- Vivekanandan, A.K.; Muthukutty, B.; Chen, S.-M.; Sivakumar, M.; Chen, S.-H. Intermetallic compound Cu2Sb nanoparticles for effective electrocatalytic oxidation of an antibiotic drug: Sulphadiazine. ACS Sustain. Chem. Eng. 2020, 8, 17718–17726. [Google Scholar] [CrossRef]
- Balaji, R.; Renganathan, V.; Chu, C.-P.; Liao, Y.-C.; Kao, C.; Chen, S.-M. Periodic copper microbead array on silver layer for dual mode detection of glyphosate. OpenNano 2022, 8, 100105. [Google Scholar] [CrossRef]
- Sundaresan, R.; Mariyappan, V.; Chen, T.-W.; Chen, S.-M.; Akilarasan, M.; Liu, X.; Yu, J. One-dimensional rare-earth tungstate nanostructure encapsulated reduced graphene oxide electrocatalyst-based electrochemical sensor for the detection of organophosphorus pesticide. J. Nanostructure Chem. 2023, 1–14. [Google Scholar] [CrossRef]
- Liu, B.; Ma, Y.; Zhou, F.; Wang, Q.; Liu, G. Voltammetric determination of sulfadiazine based on molecular imprinted electrochemical sensor. Int. J. Electrochem. Sci. 2020, 15, 9590–9596. [Google Scholar] [CrossRef]
- Elamin, M.B.; Ali, S.M.A.; Essousi, H.; Chrouda, A.; Alhaidari, L.M.; Jaffrezic-Renault, N.; Barhoumi, H. An Electrochemical Sensor for Sulfadiazine Determination Based on a Copper Nanoparticles/Molecularly Imprinted Overoxidized Polypyrrole Composite. Sensors 2023, 23, 1270. [Google Scholar] [CrossRef]
- Devi, R.K.; Ganesan, M.; Chen, T.-W.; Chen, S.-M.; Al-onazi, W.A.; Al-Mohaimeed, A.M.; Elshikh, M.S.; Yu, Y.-Y. 3D-nanocubes of N-doped carbon quantum dots adorned manganese oxide: A functional electrocatalyst for the sensitive detection of sulfadiazine. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129141. [Google Scholar] [CrossRef]
- Campestrini, I.; de Braga, O.C.; Vieira, I.C.; Spinelli, A. Application of bismuth-film electrode for cathodic electroanalytical determination of sulfadiazine. Electrochim. Acta 2010, 55, 4970–4975. [Google Scholar] [CrossRef]



| Materials | Method | Linear Range (µM) | Limit of Detection (µM) | Reference |
|---|---|---|---|---|
| MWCNT-MIP/GCE | DPV | 4–50 | 0.68 | [37] |
| CuNPs/MIP-OPPy/GCE | DPV | 10−9 M–10−5 | 3.1 × 10−10 | [38] |
| N-CQD/Mn3O4/SPCE | DPV | 0.5–663 | 0.014 | [39] |
| GCE | DPV | 20–300 | 6.14 | [10] |
| AuNPs/VS2-rGO/SPCE | SWV | 0.01–0.345 | 0.44 | [2] |
| Bi electrode | DPV | 3.2–97 | 2.10 | [40] |
| LCO@RGO/GCE | DPV | 0.01–265 | 0.005 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Disouza, F.P.D.; Sundaresan, R.; Chen, S.-M.; Ramachandran, B.; Chandrasekar, N. Electrochemical Monitoring of Sulfadiazine via La@CeO Incorporated with Reduced Graphene Oxide. Analytica 2023, 4, 300-312. https://doi.org/10.3390/analytica4030023
Disouza FPD, Sundaresan R, Chen S-M, Ramachandran B, Chandrasekar N. Electrochemical Monitoring of Sulfadiazine via La@CeO Incorporated with Reduced Graphene Oxide. Analytica. 2023; 4(3):300-312. https://doi.org/10.3390/analytica4030023
Chicago/Turabian StyleDisouza, Francis Packiaraj Don, Ruspika Sundaresan, Shen-Ming Chen, Balaji Ramachandran, and Narendhar Chandrasekar. 2023. "Electrochemical Monitoring of Sulfadiazine via La@CeO Incorporated with Reduced Graphene Oxide" Analytica 4, no. 3: 300-312. https://doi.org/10.3390/analytica4030023
APA StyleDisouza, F. P. D., Sundaresan, R., Chen, S.-M., Ramachandran, B., & Chandrasekar, N. (2023). Electrochemical Monitoring of Sulfadiazine via La@CeO Incorporated with Reduced Graphene Oxide. Analytica, 4(3), 300-312. https://doi.org/10.3390/analytica4030023








