Abstract
This work presents the first development of an analytical turbidimetric method for the determination of legal alcohols in alcohol-based hand sanitizer products. A typical iodoform reaction is exploited to form a yellow product in the form of precipitates. An iodoform test shows a positive result as yellow precipitates in the presence of ethanol and isopropanol; therefore, the test can only be used to distinguish between methanol and those legal alcohols. In the presence of molecular iodine (I2) and a strong alkaline solution, the legal alcohol is converted to the corresponding carbonyl compound (i.e., ethanol to acetaldehyde, isopropanol to acetone). The susceptibility of this intermediate towards the reaction with hydroxide ions (strong alkaline condition) results in formations of yellow precipitation of iodoform (CHI3) and a water-soluble carboxylate salt in the solution. Therefore, this change allows for the detection of legal alcohols through either naked-eye observation (as semi-quantitative analysis) or a common benchtop/portable photometer/spectrophotometer (as quantitative analysis) by means of turbidimetric analysis. In this work, turbidimetry is employed, which is a useful alternative detection method in analytical practice, especially with colored samples in hand sanitizing products. This is because they can employ wavelengths at which the colored solution does not absorb light. As a result of our developed method, the calibration plots are in the range of 30 to 100% (v/v) for both ethanol and isopropanol. The limit of detection (LOD) (3SD of y-intercept/slope) was found to be 7.4% (v/v) ethanol and 6.5% (v/v) isopropanol. Direct analysis of the non-pretreatment of the sample is achieved. The results indicate that our new proposed analytical method is fit for purpose and valid to detect the legal alcohols in alcohol-based hand sanitizing products for both international and Thai regulations (at least 70% (v/v)). Our quantitative results were also comparable to a standard analytical method, such as the use of a gas chromatography-flame ionization detector (GC-FID). Our developed method and analytical operation could potentially be developed into a practically portable analysis.
1. Introduction
During the period of the Coronavirus Disease 2019 (COVID-19) outbreaks, beginning in 2019 [1], the World Health Organization (WHO) raised people’s concerns and awareness about personal protection from the infection by wearing a mask, maintaining a physical distance, and cleaning hands properly. Thus, alcohol-based hand sanitizing products have become a common portable item for convenient hand rubbing in our daily lives. According to the WHO regulations, hand sanitizing products should only use ethanol and isopropanol, defined as legal alcohols in this work, as their main ingredient. A significant increase in the global demand for legal alcohols has led to concern about methanol adulteration in the step of production of alcohol-based hand sanitizing products. The use of methanol (also called wood alcohol) as an ingredient in hand sanitizers and disinfectants is banned in the EU, US, and most other countries.
To legalize the quality of alcohol-based hand sanitizers, some guidelines have been launched to limit the methanol contents found in the products. The United States Food and Drug Administration (U.S. FDA) limits the concentration of methanol found in hand sanitizers at 200 mg L−1 (equivalent to 0.02% (v/v) [2]. According to the regulation of Thailand, the FDA-Cosmetic Control Group declares alcohol-based hand sanitizers as cosmetic products, with an allowance level of denatured methanol ≤ 5% (v/v) and at least 70% (v/v) legal alcohol (i.e., either ethanol, isopropanol, n-propanol, or a mixture of these alcohols) [3,4]. The concentration range with the greatest germicidal efficacy is important as it is a human healthcare product. While non-healthcare groups also recommend alcohol-based hand sanitizers, they usually do not specify an appropriate concentration of alcohol. According to the global healthcare issue, the detection of adulterated methanol and legal alcohols in such samples of alcohol-based hand sanitizing products is therefore necessary prior to retail to customers.
Based on the alcohol and composition of the alcohol-based hand sanitizer products, distillation can be employed to determine the amount of alcohol in samples [5]. However, the distillation method has relative sensitivity. A simple alcoholmeter [6] and simple spectrophotometry [7], or the exploitation of the schlieren effect in liquid flow [8] based on the reaction of ethanol with a stable acidic dichromate reagent, can also be used for liquid forms of samples, although not for gel or foam formulations.
Gas chromatography (GC) is normally used to determine the alcohol contents in alcohol-based hand sanitizer products. The use of headspace-GC coupled with either a flame ionization detector (FID) or a mass spectrometry detector [9] is a common technique that provides adequate sensitivity, accuracy, and automation. GC is a typically analytical method for the determination of various types of alcohols [10]. Separation is carried out through the vaporization of alcohol from the liquid phase to the gaseous phase and through a partition to the stationary phase coated on the GC capillary column. Solid phase microextraction (SPME) [11] is used to enhance the elimination of sample matrices prior to injecting it in the GC system. Nuclear magnetic resonance [12] and a Fourier Transform infrared (FTIR) spectrometer can be employed to measure the methanol adulterated in ethanol fuel [13]. The use of a Raman spectrometer is also a powerful method for the identification of alcohol structures in alcoholic drinks [14]. With the characteristic peaks in the spectra obtained from the NMR, FTIR, and Raman spectrometers, chemometric analysis with multivariate (i.e., principal component analysis, PCA, and partial least square, PLS) is practically applied to the data set for differentiating and determining the alcohol contents in hand sanitizer products. Regarding the employment of large instrumentation, it requires the pre-treatment of the sample, the use of solvents, significant maintenance cost, and a long time to perform the analyzes. Reducing the time-consuming operation would then assist the rapid detection and quantitation of legal alcohol in sanitizing products.
To date, only a handful of chemical reactions have elicited significantly different naked-eye responses in the presence of methanol, ethanol, and isopropanol. The facile on-site quality monitoring of alcohol-based hand sanitizers through phase separation and color development with a butyl acetate–crystal violet mixture [15] is currently reported. The method utilizes the alcohol-dependent miscibility of alcohol–water binary mixtures in n-butyl acetate for analysis. The phase separations in the alcohol/water/n-butyl acetate ternary mixtures are quantitatively identified through color contrast, developed through the addition of crystal violet.
The iodoform test [16,17], a typical organic reaction, produces a yellow precipitation of triiodomethane (iodoform) after applying a molecular iodine (I2) to a solution containing ethanol under basic conditions, while other primary alcohols (e.g., methanol) fail to give the same results. The test also gives a positive result for isopropanol, a secondary alcohol commonly used in sanitizing products, and to the sanitizing products that mainly contain legal alcohols contaminated with significant amounts of methanol. Due to the non-highly specific nature of the iodoform test, its application is limited to only differentiating pure methanol from pure legal alcohols (e.g., ethanol, isopropanol). However, there is no report of an exploited iodoform reaction for the quantitation of legal alcohols in methanol-free hand sanitizer products.
Based on the literature, we aim to develop a simple method for the determination of legal alcohol (ethanol and isopropanol), as well as the detection of the adulteration of methanol. Although the iodoform reaction is commonly used to distinguish methanol from legal alcohol, the typical iodoform reaction has yet to be reported for the quantitative analysis of ethanol and isopropanol. Herein, we present the first development of a simple analytical turbidimetric method using the typical iodoform reaction for the analysis of ethanol and isopropanol in hand sanitizing products. The iodoform test shows a positive result (yellow precipitation) in the presence of ethanol and isopropanol, whereas methanol does not give the precipitate formation. Therefore, our proposed test can be used to both distinguish between methanol and those legal alcohols and to quantify the amount of legal alcohol. In the presence of molecular iodine (I2) and a base, the alcohol of interest is converted to the corresponding carbonyl compound (i.e., ethanol to acetaldehyde, isopropanol to acetone). The susceptibility of this intermediate towards the reaction with hydroxide ions (strong alkaline condition) results in formations of a yellow precipitation of iodoform (CHI3) and a water-soluble carboxylate salt in the solution. Therefore, this change allows for the detection of legal alcohols through naked-eye observation and quantitative analysis by means of turbidimetric analysis. The sequence of the addition of the reagent and the optimization were investigated. Our quantitative results were also compared with a standard analytical method, such as the use of a gas chromatography-flame ionization detector (GC-FID).
2. Materials and Methods
2.1. Standard Alcohols and Reagents
All solutions were prepared in deionized Milli-Q® water (Merck, Darmstadt, Germany). Highly purified alcohols were used for the preparation of a series of standard alcohol solutions. Methanol (CH3OH, 99.9% purity), ethanol (C2H5OH, 99.9% purity), and isopropanol ((CH3)2CHOH, 99.8% purity) were purchased from RCI Labscan, Dublin, Ireland. All standard alcohol solutions were prepared in the unit of percentage by volume (% (v/v)), depending on the types of alcohol. The two types of standard alcohols used in this work were single standard solutions. For example, a 10.00 mL single standard of 10.0% (v/v) ethanol and isopropanol was prepared by mixing 1.00 mL of pure ethanol and pure isopropanol with 9.00 mL of deionized water, respectively.
In addition, 12% (w/v) of sodium hydroxide was prepared by dissolving 12.0 g of NaOH (Merck, Darmstadt, Germany) in 100.0 mL of deionized water in a 500 mL beaker. The beaker was placed in a bowl of water to absorb the heat generated by the dissolution process. Next, 3% (w/v) of iodine solution was prepared by dissolving 3.0 g of anhydrous iodine (I2, BDH, Istanbul, Turkey) and excess of potassium iodide (KI, Merck, Germany) into 100.0 mL of deionized water. This iodine solution was kept in a brown container to avoid light and stored in the refrigerator (4 °C) for a week before usage.
2.2. Samples
Seven synthetic samples (SS1–SS4 as a single component, and SS5–SS7 as a mixed component) with nominal values were prepared by diluting alcohol(s) in deionized water. Two commercial samples (CS1 and CS2) of alcohol-based hand sanitizers in the form of spray were purchased from local drugstores in Bangkok, Thailand. Sample dilutions were not required prior to the quantitation of either ethanol or isopropanol.
2.3. Analytical Procedure of Alcohol Analysis Using Iodoform Reaction-Based Turbidimetry
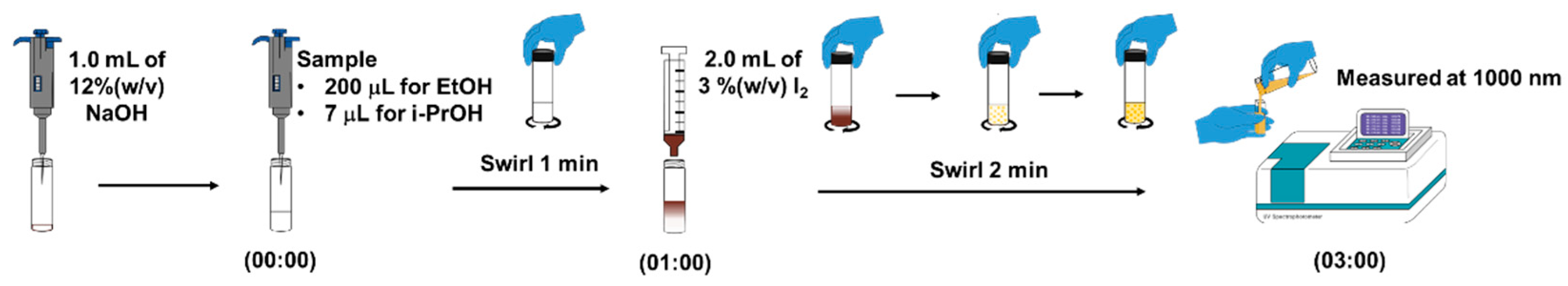
In this work, we exploited the typical iodoform reaction to develop our analytical procedure for the analysis of ethanol and isopropanol. Figure 1 shows the optimum operating procedure employed in the direct detection of either ethanol or isopropanol. A glass reaction vial was filled with 1.00 mL of 12% (w/v) of sodium hydroxide solution. A certain sample (or alcoholic standard solution) was then added into the same vial (200 μL for ethanol and 7 μL for isopropanol). The mixture in the vial was gently swirled for 1 min. To achieve the iodoform reaction, 2.00 mL of 3% (w/v) of iodine solution was finally added into the vial and, subsequently, gently swirled for 2 min. The yellow precipitates are then formed and clearly observed as visualizations of the turbidity. The mixture with precipitate was immediately transferred into a 1 cm quartz cuvette and measured the transmittance (practically in absorbance as default of Perkin Elmer Lambda 25 UV/Visible Spectrophotometer) at 1000 nm. A signal response as absorbance at 1000 nm was then employed to construct the calibration plots for the analysis of the alcohols in the hand sanitizer samples. Each experiment was a triplicate measurement in order to consider the precision, with statistical values of SD and %RSD.

Figure 1.
Analytical procedure of iodoform reaction-based turbidimetric determination of ethanol and isopropanol in alcohol-based hand sanitizer products.
2.4. Validation
The gas chromatographic (GC) technique coupled with a flame ionization detector (FID) (model 6890N, Agilent Technologies, Santa Clara, CA, USA) was employed to detect and conform the types of alcohols and their concentrations with our developed method. The GC-FID condition was carrier helium gas at 1.0 mL min−1 flow rate, 1 μL injection volume, with 1:50 split ratio. The inlet temperature was 260 °C and a flame ionization detector was set at 250 °C. The temperature program was employed with holding for 6.5 min with the initial temperature at 30 °C, and rising by 100 °C min−1 to 250 °C and holding for 3.0 min. The HP-Innowax GC column was 320 μm i.d., 0.25 μm film thickness. A series of working standard solutions of alcohols (methanol, ethanol, and isopropanol) in the range of 0.3–10.0% (v/v) were prepared from the pure alcohols.
3. Results and Discussion
3.1. Prove of Concept Applicable to Turbidimetric Determiantion of Legal Alcohol in Alcohol-Based Hand Sanitizing Products
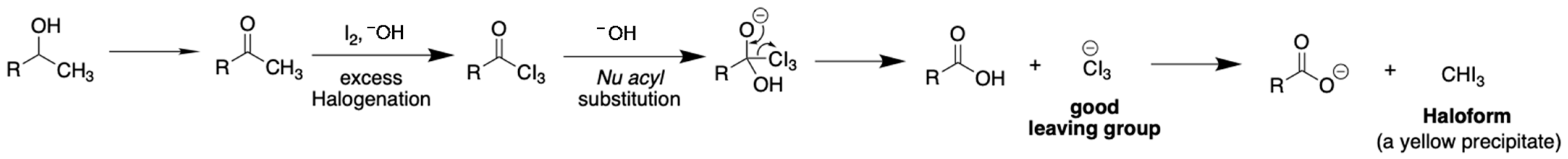
As shown in the chemical reaction in Figure 2, in the presence of molecular iodine (I2) and a base, the alcohol of interest is converted to the corresponding carbonyl compound (i.e., ethanol to acetaldehyde, isopropanol to acetone). A hydrogen atom at an α-carbonyl carbon is deprotonated by the base to generate a carbanion (or an enolate) that subsequently reacts with a molecular iodine. This step can be conducted repetitively until achieving the triiodoacetyl derivative. The susceptibility of this intermediate towards the reaction with hydroxide ions results in formations of yellow precipitation of iodoform (CHI3) and a water-soluble carboxylate salt in the solution.
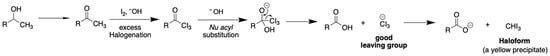
Figure 2.
Chemical reaction of iodoform for quantitation of ethanol and isopropanol in alcohol-based hand sanitizing products.
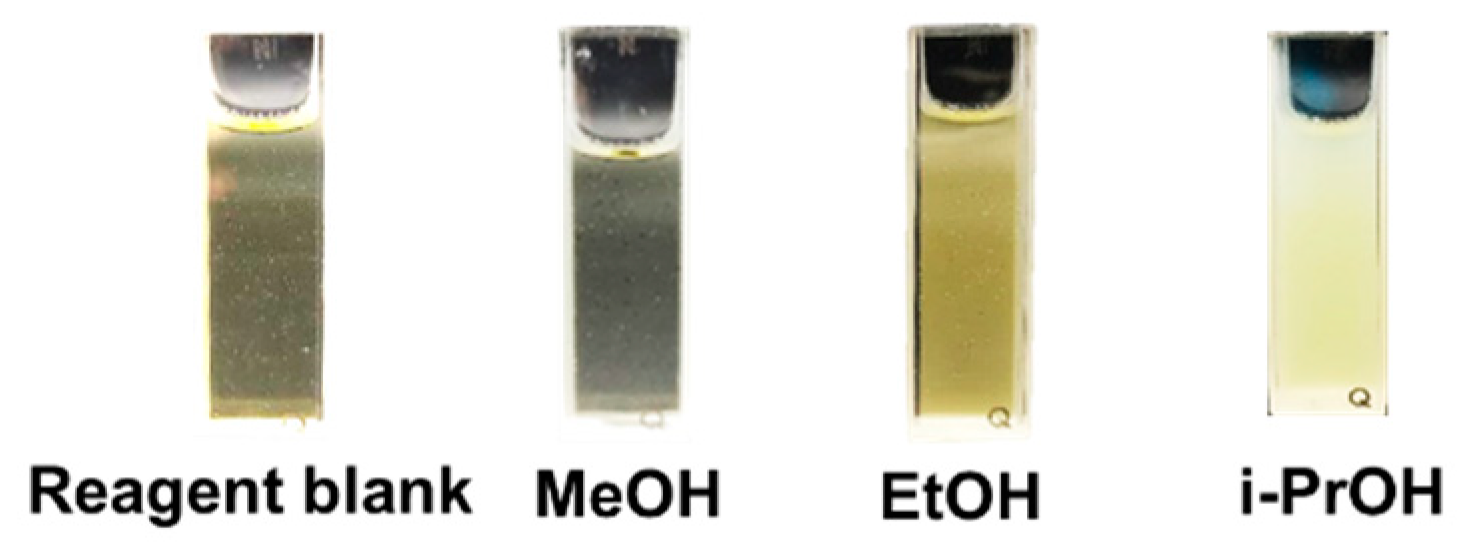
We prove the concept of the capability of an iodoform reaction with several types of alcohol. The results are shown in Figure 3. The reagent blank is clear with a yellow color due to the molecular iodine solution. With the addition of methanol in the reagent blank, we still observe the clear yellow solution, but it is a little faded due to the dilution after the addition of the methanol solution. We clearly observe the yellow precipitate and turbid in the cuvette as either ethanol or isopropanol is added to mix with the reagent blank. Therefore, this change allows for the detection of legal alcohols (ethanol and isopropanol) through naked-eye observation and quantitative analysis by means of turbidimetric analysis. The iodoform test therefore gives the positive result (yellow precipitation) in the presence of ethanol and isopropanol; thus, the test can be only used to distinguish between methanol and those legal alcohols.

Figure 3.
Comparison of appearance of product formation using iodoform reaction with reagent blank (iodine solution mixed with NaOH) mixed with methanol (MeOH), ethanol (EtOH) and isopropanol (i-PrOH).
In a borderline case, legal alcohols (ethanol and isopropanol) adulterated with methanol still obtain positive results due to the iodoform reaction of legal alcohols. In addition, a sample containing a mixture of legal alcohol, i.e., ethanol mixed with isopropanol, to be at least 70% (v/v), could not be differentiated by our developed method as both alcohols achieve positive results. However, we found that all of the samples we tested contained a single type of alcohol, either ethanol or isopropanol, which was confirmed by the results of the gas chromatography. Therefore, our developed turbidimetric method with an iodoform reaction is still applicable to the analysis of legal alcohol in alcohol-based hand sanitizer products.
3.2. Necessity of Exploitation of Turbidimetric Quantitation of Legal Alcohols Using the Iodoform Reaction
Detection based on the phenomena of light scattering, or so-called turbidimetry, is an alternative detection method that is useful in analytical practice, especially with colored samples. This is because they can employ wavelengths at which the colored solution does not absorb the light. Turbidimetry is the process of measuring the loss of intensity of transmitted light due to the scattering effect of particles suspended in the mixture (solid-phased suspended in liquid phase). Light passes through a filter, creating light of a known wavelength (with light absorption-free color sample), which is then passed through a sample cuvette. A typical benchtop/handheld type of photometer/spectrophotometer can be employed to detect the transmitted light, which is able to plot the calibration graph for quantitative analysis.
Regarding the iodoform reaction, it is effective for distinguishing methanol from ethanol and isopropanol. The reaction also shows a positive result for the alcohol-based hand sanitizing products that mainly contain legal alcohols contaminated with significant amounts of methanol. As the iodoform test does not have a highly specific nature, its application is limited to only differentiating pure methanol from pure legal alcohols (e.g., ethanol, isopropanol).
The regulation of alcohol allowance in sanitizing products—which requires methanol contents of less than 5% (v/v)—does not guarantee the quality control of hand sanitizer because the level of legal alcohol must also reach 70% (v/v) as a key assessment. Therefore, the iodoform test is necessary for the quantitation of legal alcohols —in which methanol does not interfere with the analysis—to comply with the health and safety regulation of alcohol-based hand sanitizing products.
In addition, the light scattering of colloid particles (turbidimetry) has been widely used through the formation of solid particles using suitable precipitating reagents. The benefit of the phenomenon of light scattering is useful when dealing with colored samples. This is because it can employ wavelengths at which the colored solution does not absorb the light. In this work, we employed a near infrared (NIR) region with a wavelength of 1000 nm as the detection wavelength to ensure that the colorant in the sample and the reagent color do not interfere with the analysis.
3.3. Optimization
According to the operational procedure mentioned in Section 2.3 and Figure 1, physical and chemical parameters were optimized to obtain the suitable procedure for the turbidimetric quantitation of legal alcohols using the iodoform reaction.
3.3.1. Sequence of the Reagent Addition
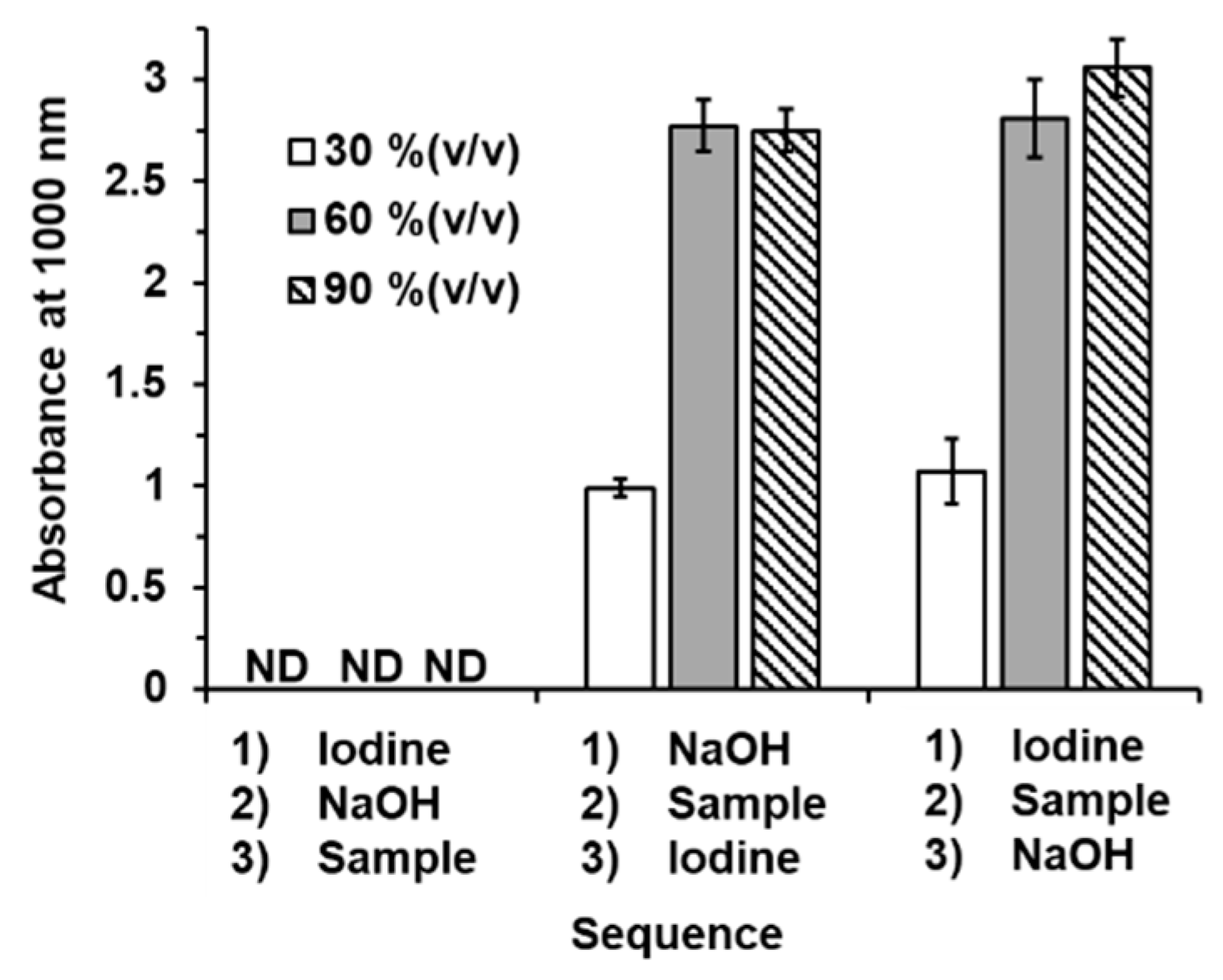
According to the mechanism of the iodoform reaction, shown in Figure 2, we designed the sequence of the addition of the solution with a stepwise operation. The aqueous standard solutions of methanol at 30, 60, and 90% (v/v) were tested with three designate sequences, as follows. Sequence 1: iodine solution + NaOH solution + sample solution; Sequence 2 NaOH solution + sample solution + iodine solution; Sequence 3: iodine solution + sample solution + NaOH solution.
The results are shown in the bar graphs in Figure 4. With sequence 1, there were no observed signals from any of the standard methanol solutions. Due to no excess halogenation reacting to the alcohol, an iodoform product was not generated. We then modified the sequence regarding the chemical reaction in Figure 2. We designed the other two sequences, allowing the sample to first react to either NaOH (sequence 2) or iodine (sequence 3). We found that both modified sequences produced the iodoform product. The signal responses from sequences 2 and 3 are comparable. However, in this work, sequence 2 was selected for further experiments as it gave more precise experimental data (RSD < 4.6%).
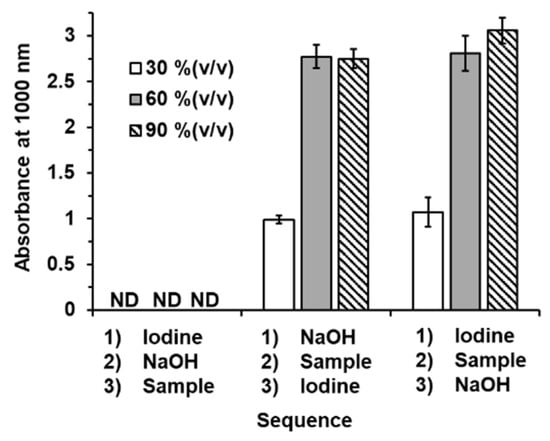
Figure 4.
Bar graphs showing the absorbances measured at 1000 nm obtained from three sequences of stepwise addition of solution with iodoform reaction for quantitation of ethanol in hand sanitizers. Error bars in calibration plot were from triplicate measurements. ND: not detected.
3.3.2. Effect of Chemical Concentration for Iodoform Reaction
The concentration of the iodine and NaOH solutions were investigated in terms of their sensitivity and linearity response. Table 1 shows the results of the investigation of the chemical concentrations for the iodoform reaction. The selection criterion is based on the compensation of the slope from the linear equation and the linear range. According to the results, iodine at 3% (w/v) and NaOH at 12% (w/v) were selected as the optimal concentrations for further experiments.

Table 1.
Investigation of concentrations of iodine and sodium hydroxide for iodoform reaction-based turbidimetric detection of ethanol.
3.4. Concentration-Dependent Turbidimetric Determination of Ethanol and Isopropanol in Alcohol-Based Hand Sanitizing Products
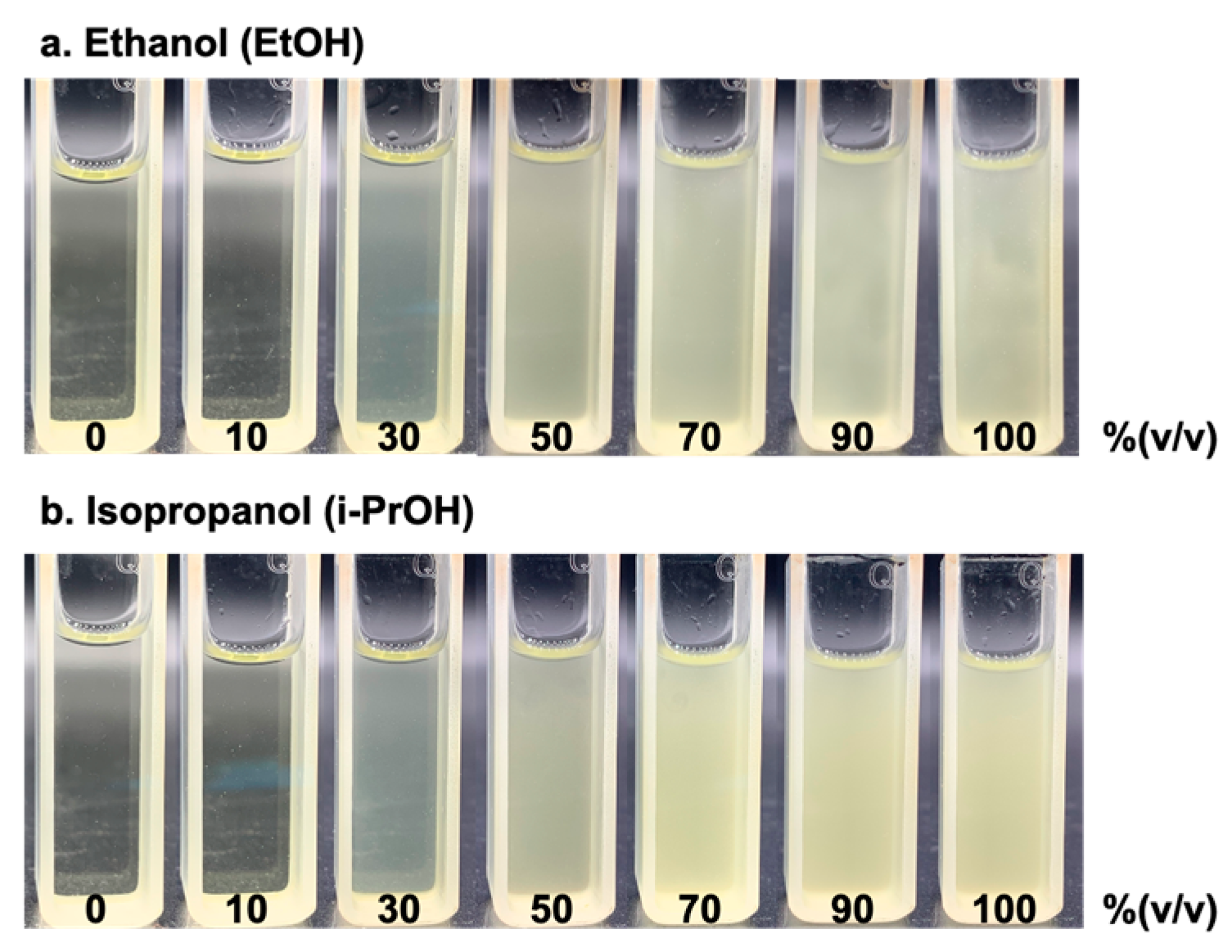
With the optimal analytical condition, as mentioned in Section 3.3, we selected sequence 2, with the use of 3% iodine (w/v) and NaOH at 12% (w/v), to complete the chemical reaction (shown in Figure 2). We then tested the concentration-dependent detection with a series of standard solutions of ethanol and isopropanol, from 10 to 100% (v/v). Figure 5 displays the images of the products of the iodoform reaction as yellow precipitates. As observed in Figure 5, the turbidity increases with the increasing concentrations of alcohol as the limiting agent in the reaction.
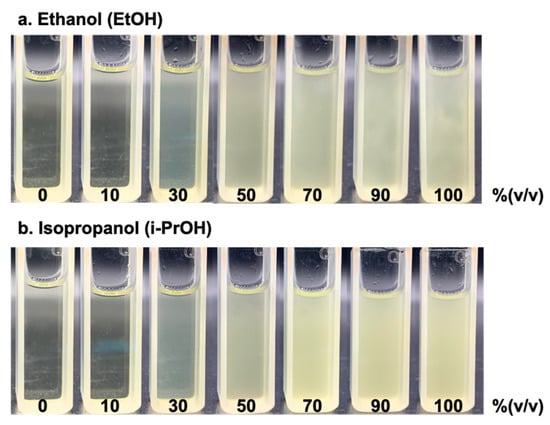
Figure 5.
Images taken from products of iodoform reaction with yellow precipitates obtained from (a) ethanol and (b) isopropanol ranges between 0 to 100% (v/v) alcohol.
As shown in Figure 5, the turbidity from the yellow precipitates obtained from the iodoform reaction with ethanol is slightly less turbid compared to those from isopropanol, even though we added a relatively low volume of isopropanol. This indicates that the kinetic reaction of ethanol is relatively slow compared to isopropanol.
3.5. Analytical Performance of Iodoform Reaction-Based Turbidimetric Determination of Legals Alcohol in Hand Sanitizing Products
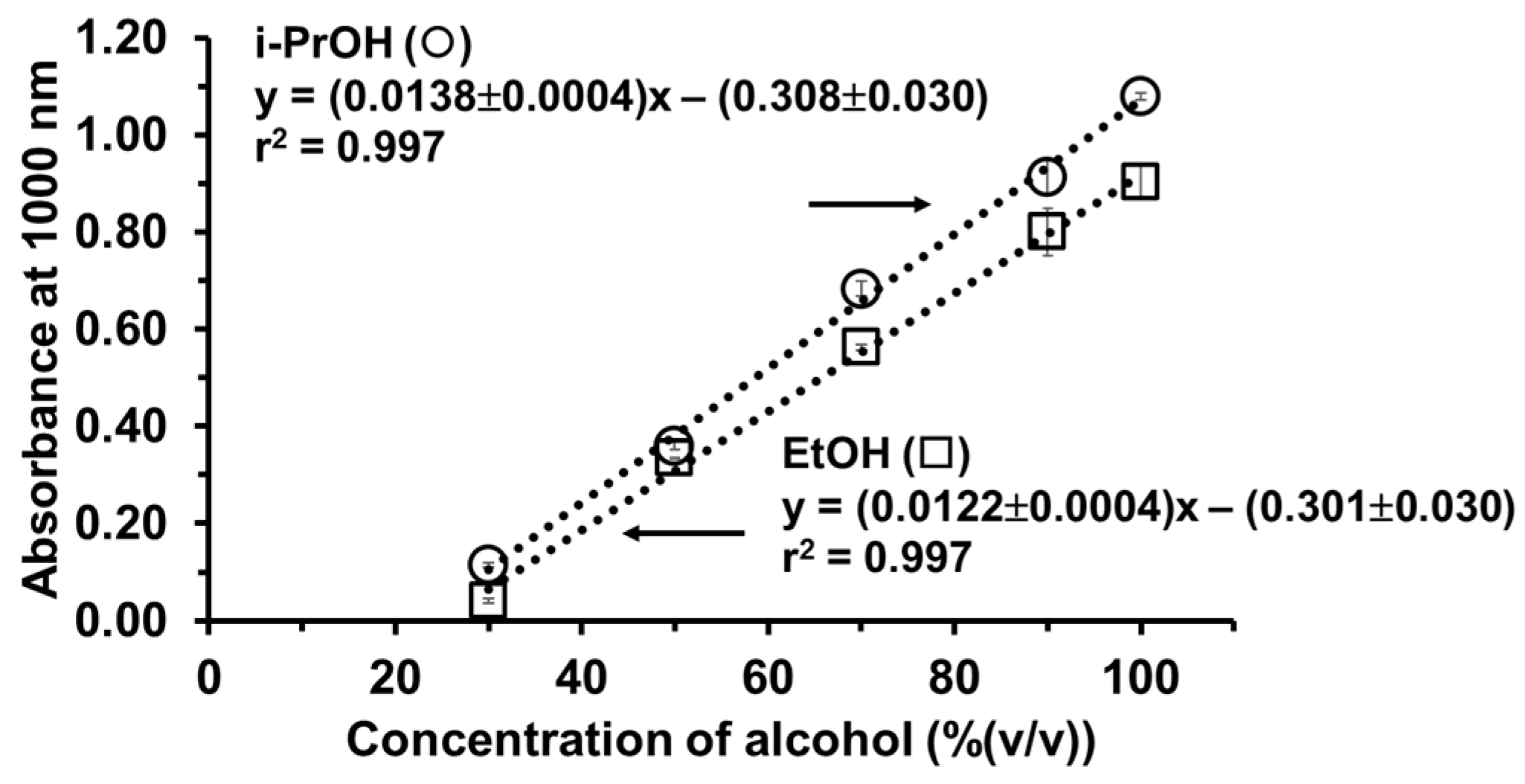
As shown in Figure 6, the concentration dependence of either ethanol or isopropanol (10–100% (v/v)) is confirmed with the linear calibration plots. The calibration graph of ethanol is Absorbance1000nm = (0.0122 ± 0.0004)% ethanol (v/v) − (0.301 ± 0.030), r2 = 0.997, whereas the calibration graph of isopropanol is Absorbance1000nm = (0.0138 ± 0.0004)% isopropanol (v/v) − (0.308 ± 0.030), r2 = 0.997). The limit of detection (LOD) (3SD of y-intercept/slope) was found to be 7.4% (v/v) ethanol and 6.5% (v/v) isopropanol. The results indicate that our calibration plots are fit for purpose and valid to detect the legal alcohols in alcohol-based hand sanitizers for both international and Thai regulations. Direct analysis of the non-pretreatment of sample can be achieved.

Figure 6.
Calibration plots of ethanol and isopropanol employing the iodoform reaction-based turbidimetric determination in alcohol-based hand sanitizer samples.
3.6. Applications to Alcohol-Based Hand Sanitizer Samples
According to the analytical procedure in Figure 1, we then demonstrated the applicability of the optimal sequence of adding the chemical solution and the concentration of the reagents in Section 3.3 for the quantitation of ethanol and isopropanol in alcohol-based hand sanitizer samples. As shown in Table 2, an aqueous solution of MeOH (70% v/v) did not produce precipitations after applying the conditions of the iodoform test (SS1), which allows for the quantitative analysis of ethanol and isopropanol contaminated with MeOH (5% v/v) (SS4 and SS5). Moreover, the test could be applied for the analysis of legal alcohols in real samples (CS1 and CS2).

Table 2.
Quantitative results for alcohol contents in alcohol-based hand sanitizing products obtained from iodoform reaction-based turbidimetric determination as compared with GC-FID method.
According to the results in Table 2, we then selected the quantitative data from samples SS2, SS3, SS4, SS5, CS1, and CS2 for a statistic comparison of our developed method and GC-FID. Our developed iodoform-based turbidimetric method gave a good agreement with those obtained from GC-FID (Paired t-test: tstat = 0.99, tcrit = 2.57, p = 0.05). In term of the statistical comparison, a Pearson’s correlation plot provides the acceptable results with the linear equation y = 0.9087x + 6.4257 (r2 = 0.9887). This indicates that there is no statistical difference between the two methods. The iodoform test could therefore be used to examine the concentration of ethanol and isopropanol in alcohol-based hand sanitizing products without a difference in the result compared to GC-FID and without any interference from methanol in the samples (SS4 and SS).
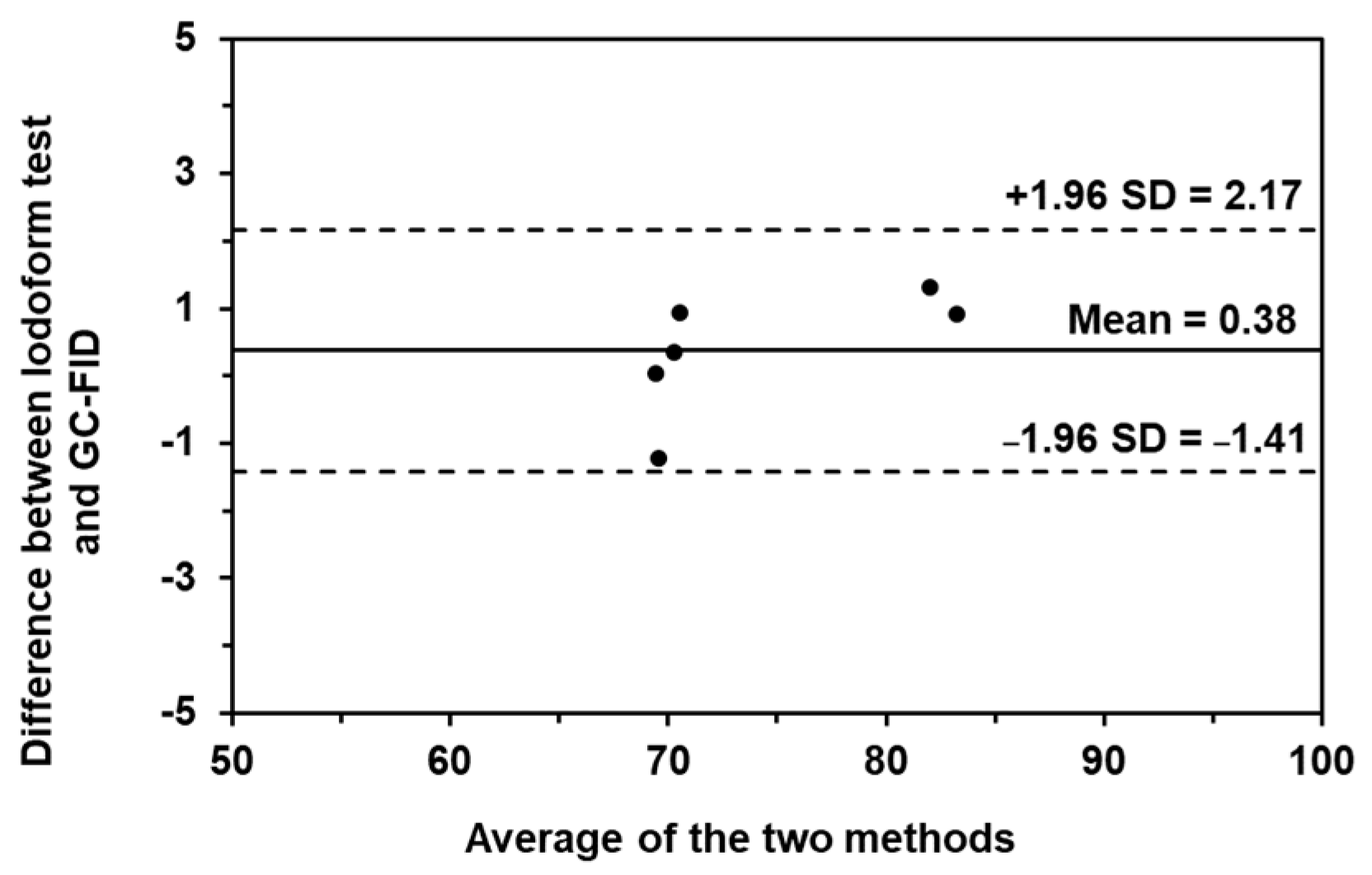
In addition, the Bland-Altman plot (as shown in Figure 7) was also plotted. The plotting is a relationship between the difference of the two methods and the average concentration of the two methods. The data were subsequently plotted in this relationship and sandwiched with two straight horizontal lines of ±1.96 SD. If the data is spread inside the boundaries of ±1.96 SD, it indicates that there is no difference between the two methods. Thus, both methods can be used to investigate such samples, with similar results. From the results, our method provided the data in the boundaries of ±1.96 SD, which means that the iodoform test could be used to examine the concentration of ethanol and isopropanol in alcohol-based hand sanitizers without a difference in the result compared to GC-FID.
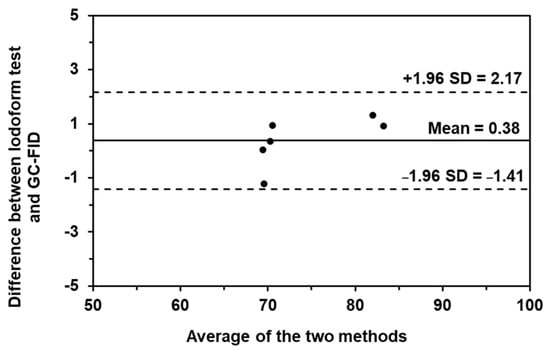
Figure 7.
The Bland–Altman plot of the iodoform-based turbidimetric detection and GC-FID for quantitation of ethanol and isopropanol in alcohol-based hand sanitizing products.
In addition, our work was compared with other reported methods for the quantitation of ethanol, isopropanol, and methanol in hand sanitizing samples. Details of the comparison are shown in Table 3.

Table 3.
Comparison of our developed turbidimetric method and previous works for analysis of alcohols in hand sanitizer products.
4. Conclusions
In this work, we exploit a typical iodoform reaction with a turbidimetric determination for legal alcohols (ethanol and isopropanol) in alcohol-based hand sanitizer products. Methanol does not give a positive result with the iodoform reaction. Yellow precipitates of CHI3 can be produced when either ethanol or isopropanol react with a molecular iodine solution in a strong alkaline condition. Turbidimetric determination is then employed to detect the yellow precipitates of CHI3 at a detection wavelength of 1000 nm. Concentration-dependent turbidimetric determination is therefore reasonable and acceptable for ethanol and isopropanol in alcohol-based hand sanitizing products. The turbidity relates to the concentration of legal alcohols in hand sanitizer, leading to an evaluation of the disinfectant efficiency of the product. Our developed method provides the calibration plots that are fit for purpose and valid to detect the legal alcohols in alcohol-based hand sanitizers for both international and Thai regulations. Direct analysis of the non-pretreatment of samples can be achieved. This is the first report of a typical iodoform reaction for an aspect of analytical chemistry in terms of quantitative analysis. Our developed method and analytical operation could potentially be developed into a practically portable analysis.
Author Contributions
Conceptualization, P.S.; methodology, C.P. and P.S.; validation, C.P.; formal analysis, C.P. and P.S.; investigation, C.P.; resources, P.S.; data curation, C.P. and P.S.; writing—original draft preparation, C.P. and P.S.; writing—review and editing, P.S.; visualization, P.S.; supervision, P.S.; project administration, P.S.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
National Research Council of Thailand (NRCT) (contract no. N41D640035). National Research Council of Thailand (N11A650144) chaired by Assoc. Prof. Dr. Duangjai Nacapricha.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research and innovation activity is funded by the National Research Council of Thailand (NRCT) (contract no. N41D640035) given to CP. This work was also supported in part by grant from the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation and the National Research Council of Thailand (N11A650144) chaired by Duangjai Nacapricha. Scholarship support from the Development and Promotion of Science and Technology Talents Project (DPST) given to CP is also acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duong, D. Alpha, Beta, Delta, Gamma: What’s important to know about SARS-CoV-2 variants of concern? Cmaj 2021, 193, E1059–E1060. [Google Scholar] [CrossRef] [PubMed]
- Güntner, A.T.; Magro, L.; van den Broek, J.; Pratsinis, S.E. Detecting methanol in hand sanitizers. iScience 2021, 24, 102050. [Google Scholar] [CrossRef] [PubMed]
- ANNEX III—List of Substances Which Cosmetic Products Must Not Contain Except Subject to Restrictions and Conditions Laid Down. Available online: https://www.fda.moph.go.th/sites/Cosmetic/Shared%20Documents/ASEAN%20Cosmetic/Annex%20III_2021_2.pdf (accessed on 31 January 2023).
- Cosmetic Control Group of Thailand. Available online: https://www.fda.moph.go.th/sites/cosmetic/Pages/Main.aspx (accessed on 31 January 2023).
- Bambach, K.; Rider, T.H. The determination of alcohol in pharmaceutical liquids. II. A new method. J. Am. Pharm. Assoc. 1936, 25, 982–985. [Google Scholar]
- Estevão, P.L.S.; Colodi, F.G.; Carmo, L.C.L.d.; Santos, M.d.F.C.; Barison, A.; D’Oca, C.R.M.; Nagata, N.; Freitas, R.A.d. Alcoholmeter as a Simple and Accessible Way for Ethanol Determination in Alcohol-Based Hand Sanitizers. J. Braz. Chem. Soc. 2021, 32, 1239–1248. [Google Scholar] [CrossRef]
- Williams, M.B.; Reese, H.D. Colorimetric Determination of Ethyl Alcohol. Anal. Chem. 1950, 22, 1556–1561. [Google Scholar] [CrossRef]
- Suwanrut, J.; Chantipmanee, N.; Kamsong, W.; Buking, S.; Mantim, T.; Saetear, P.; Nacapricha, D. Temperature-dependent schlieren effect in liquid flow for chemical analysis. Talanta 2018, 188, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Yang, H.; Shin, G.; Koo, J.-M.; Hwang, S.; Park, J.; Oh, D. Determination of Methanol in Commercialized Alcohol-based Hand Sanitizing and Other Similar Products using Headspace GC-MS. Curr. Anal. Chem. 2022, 18, 774–780. [Google Scholar] [CrossRef]
- Wang, M.-L.; Wang, J.-T.; Choong, Y.-M. A rapid and accurate method for determination of methanol in alcoholic beverage by direct injection capillary gas chromatography. J. Food Compos. Anal. 2004, 17, 187–196. [Google Scholar] [CrossRef]
- Vazquez, L.; Celeiro, M.; Castiñeira-Landeira, A.; Dagnac, T.; Llompart, M. Development of a solid phase microextraction gas chromatography tandem mass spectrometry methodology for the analysis of sixty personal care products in hydroalcoholic gels-hand sanitizers—In the context of COVID-19 pandemic. Anal. Chim. Acta 2022, 1203, 339650. [Google Scholar] [CrossRef] [PubMed]
- Isaac-Lam, M.F. Determination of Alcohol Content in Alcoholic Beverages Using 45 MHz Benchtop NMR Spectrometer. Int. J. Spectrosc. 2016, 2016, 2526946. [Google Scholar] [CrossRef]
- Carneiro, H.S.P.; Medeiros, A.R.B.; Oliveira, F.C.C.; Aguiar, G.H.M.; Rubim, J.C.; Suarez, P.A.Z. Determination of Ethanol Fuel Adulteration by Methanol Using Partial Least-Squares Models Based on Fourier Transform Techniques. Energy Fuels 2008, 22, 2767–2770. [Google Scholar] [CrossRef]
- Vaskova, H. Spectroscopic determination of methanol content in alcoholic drinks. Int. J. Biol. Biomed. Eng. 2014, 8, 27–34. [Google Scholar]
- Thangavel, S.; Durgaprasad, A.; Dash, K.; Kumar, S. Facile on-site quality monitoring of alcohol based hand sanitizers by phase separation and color development with butyl acetate-crystal violet mixture. Microchem. J. 2021, 169, 106578. [Google Scholar] [CrossRef]
- Fuson, R.C.; Bull, B.A. The Haloform Reaction. Chem. Rev. 1934, 15, 275–309. [Google Scholar] [CrossRef]
- A Test to Distinguish between Ethanol and Methanol. Available online: https://edu.rsc.org/experiments/a-test-to-distinguish-between-ethanol-and-methanol/548.article (accessed on 31 January 2023).
- Pasquini, C.; Hespanhol, M.C.; Cruz, K.A.M.L.; Pereira, A.F. Monitoring the quality of ethanol-based hand sanitizers by low-cost near-infrared spectroscopy. Microchem. J. 2020, 159, 105421. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.K.; Tay, F.H.; Wray, P.S.; Mohd Saberi, S.S.; Ken Ting, K.K.; Khor, S.M.; Chan, K.L.A. Inexpensive Portable Infrared Device to Detect and Quantify Alcohols in Hand Sanitizers for Public Health and Safety. Anal. Chem. 2021, 93, 15015–15023. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.R.B.d.; Haddad, L.P.E.; Caleffo Piva Bigão, V.L.; Martinis, B.S.D. Quantifying Ethanol in Ethanol-Based Hand Sanitizers by Headspace Gas Chromatography with Flame Ionization Detector (HS-GC/FID). J. AOAC Int. 2021, 105, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, N.; Ruzicka, C.; Faustino, P.; Stiber, N.; NguyenPho, A.; O’Connor, T.; Shakleya, D. Development and validation of a headspace GC-MS method to evaluate the interconversion of impurities and the product quality of liquid hand sanitizers. AAPS Open 2022, 8, 1. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).