Development of an Inexpensive and Comparable Microplastic Detection Method Using Fluorescent Staining with Novel Nile Red Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modification of Microscope for Fluorescent Imaging
2.2. Investigation of Fluorescent Dyes for Microplastics Detection
2.3. Recovery Rates—Comparison of Automated and Manual Particle Detection
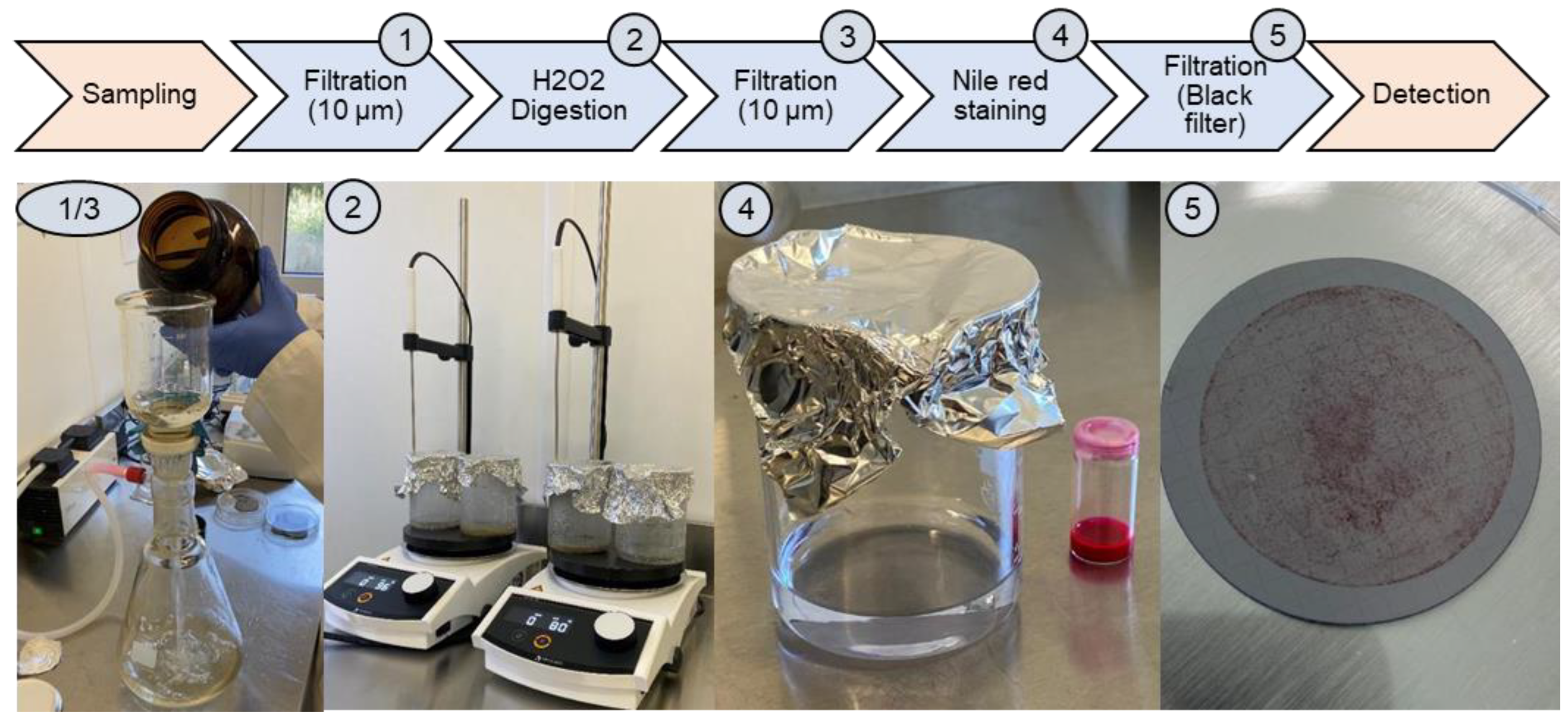
2.4. Sampling
2.5. Sample Preparation
2.6. Laboratory Requirements and Contamination Control
3. Results
3.1. Low Budget Modification of the Fluorescent Microscope
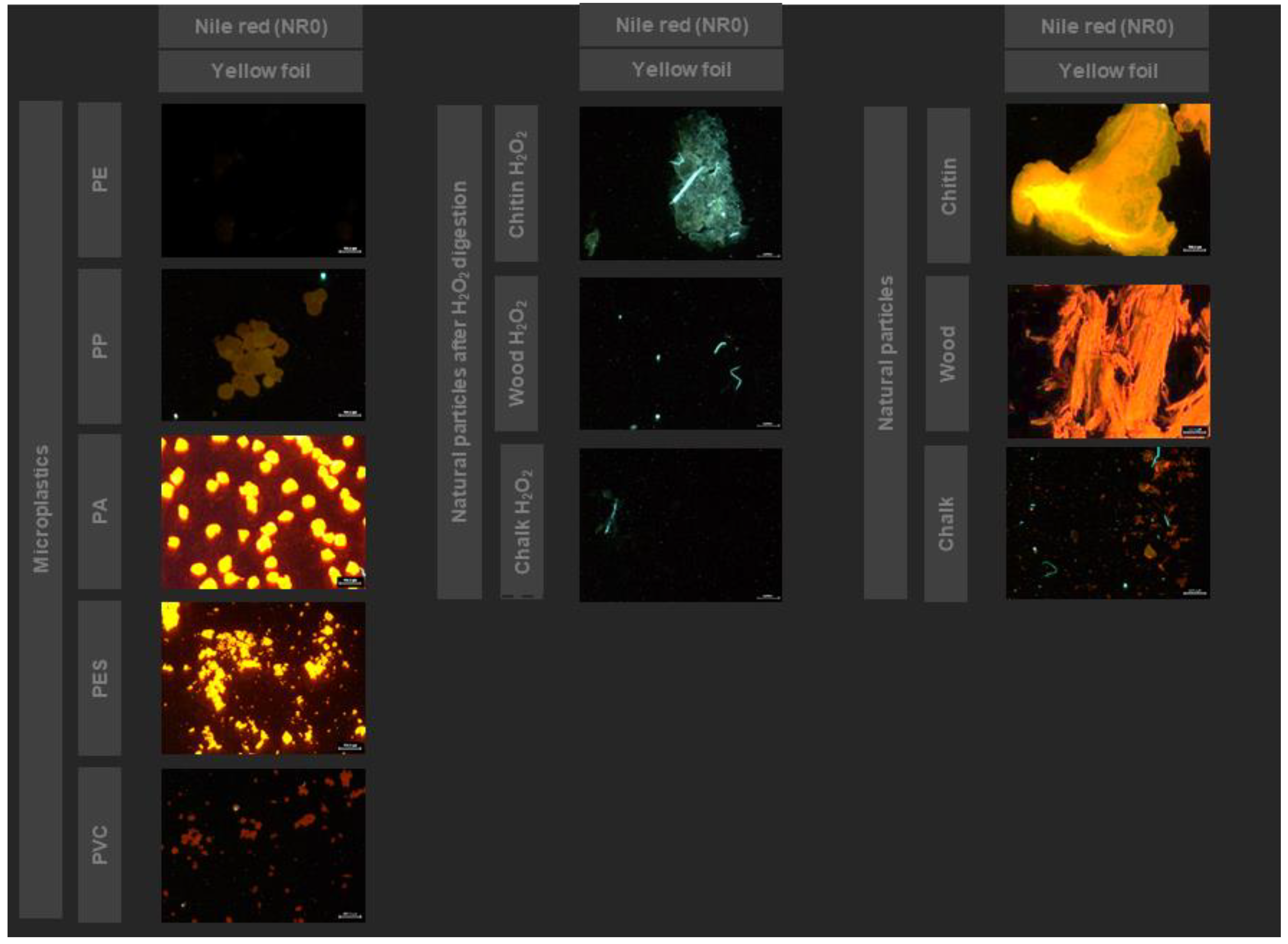
3.2. Sampling, Sample Preparation and Fluorescent Staining
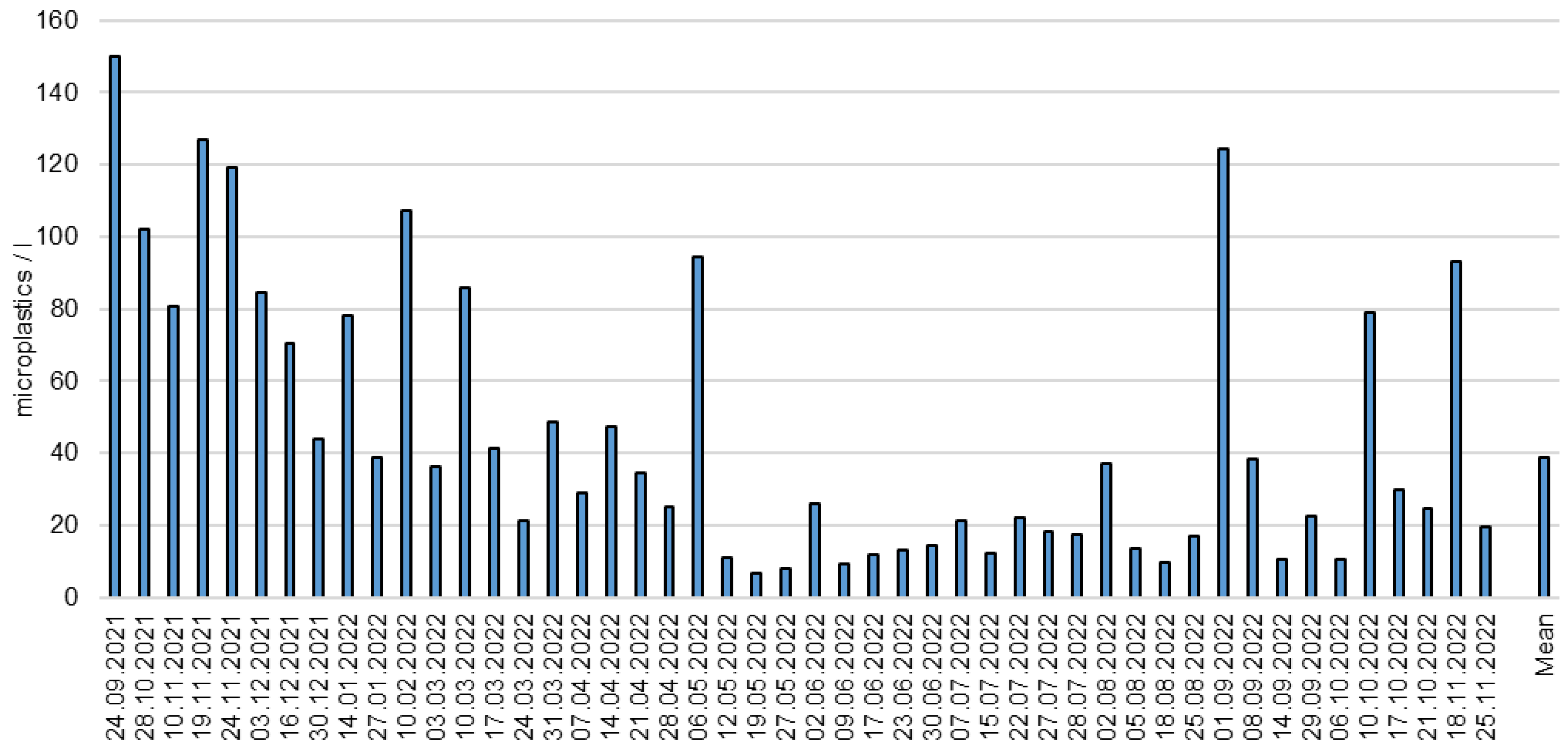
3.3. Long Time Monitoring of Microplastics in the Wastewater Treatment Plant Effluent
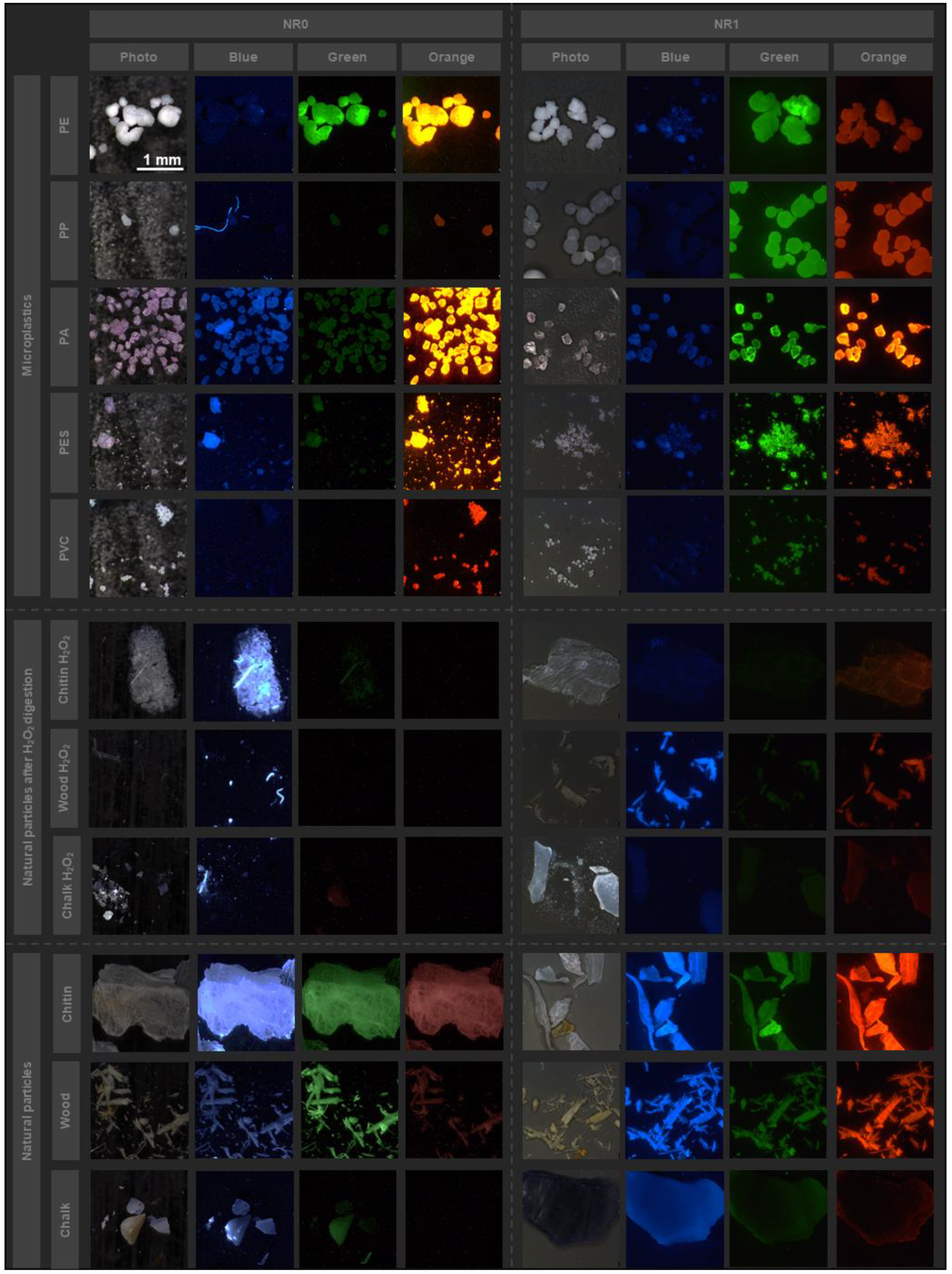
3.4. Investigation of the New Nile Red Derivatives for Microplastics Detection
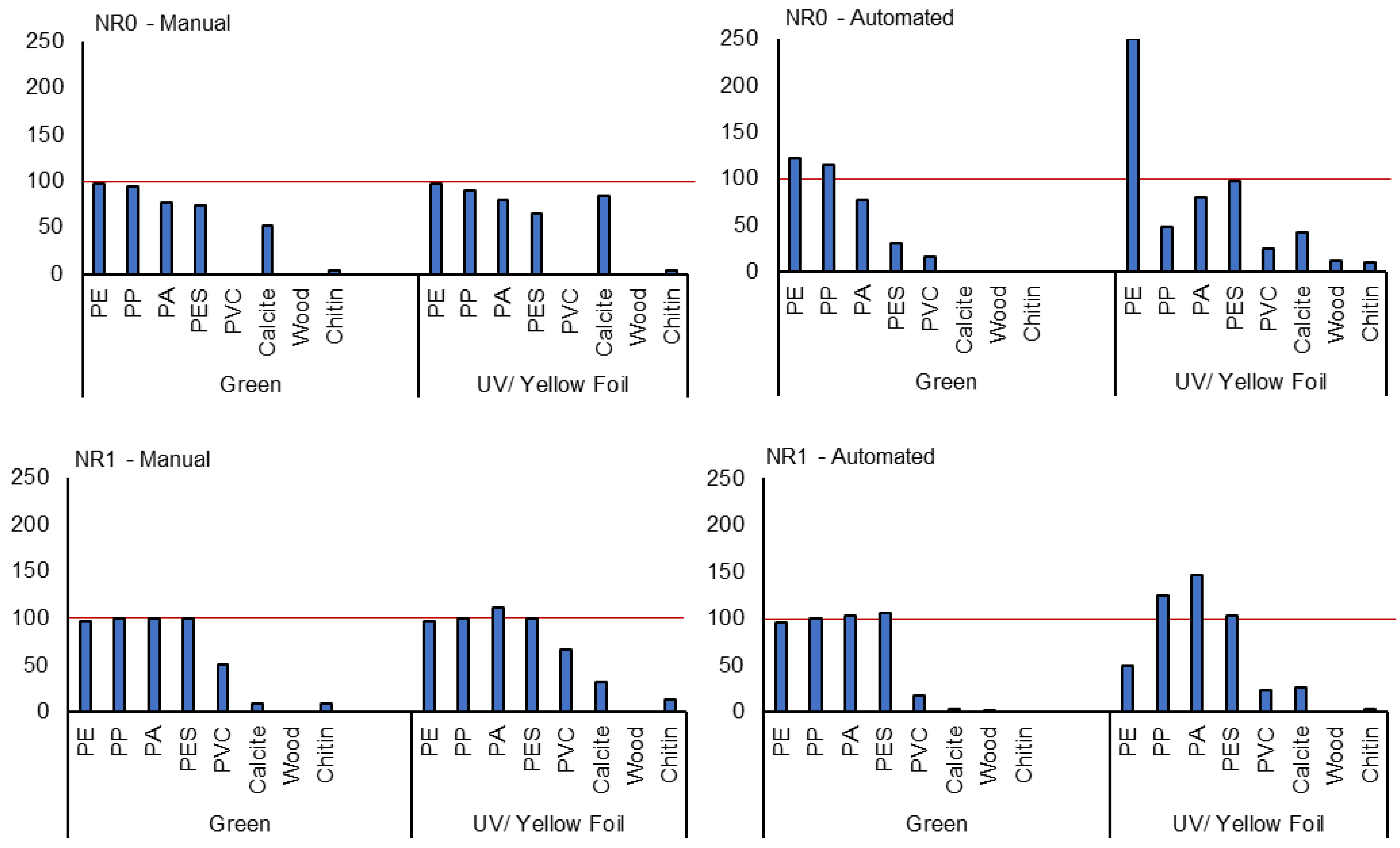
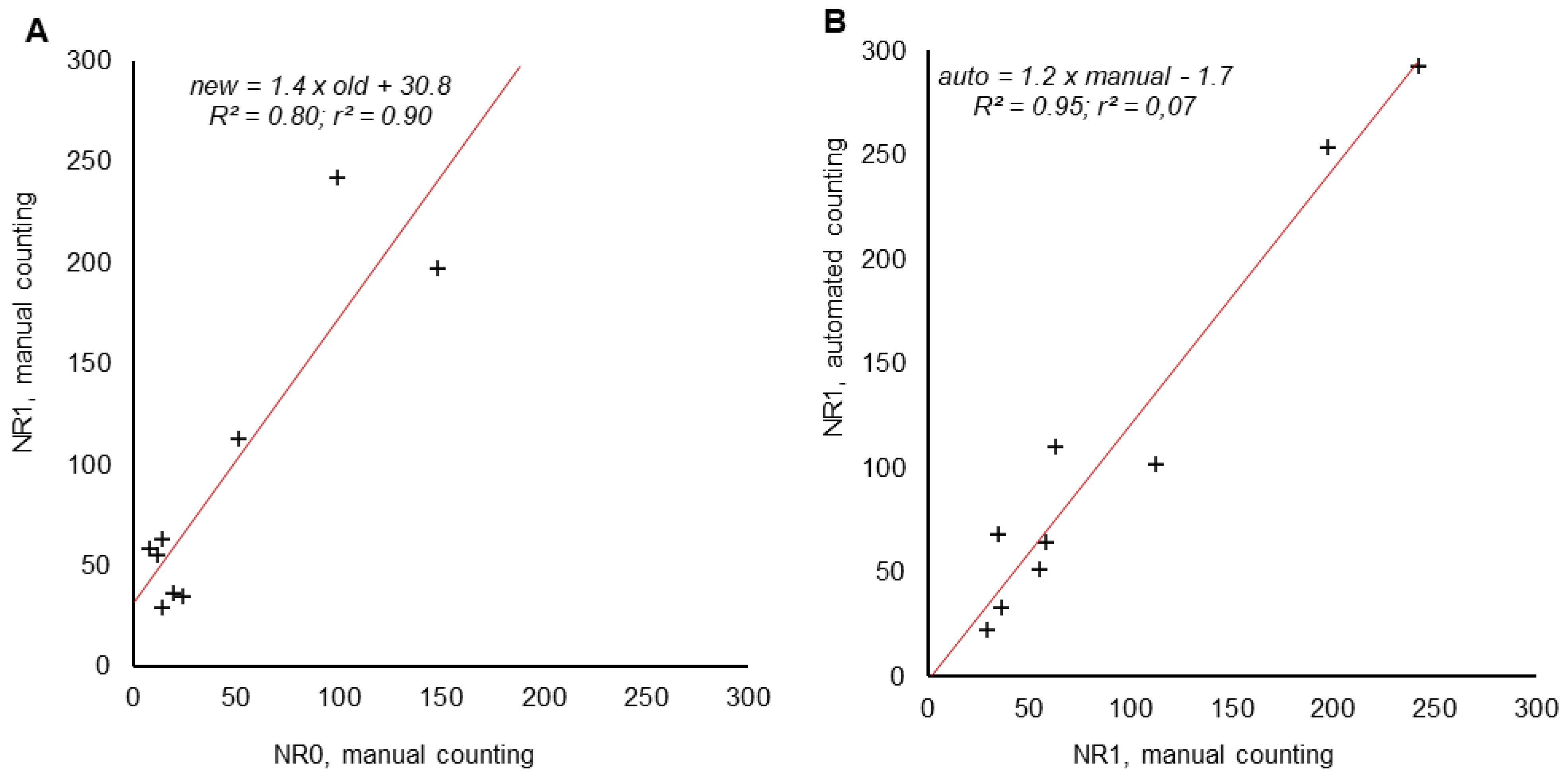
3.5. Recovery Rates and Comparison of Automated and Manual Particle Detection
3.6. Comparison of Nile Red with the New Derivatives on Wastewater Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yusuf, A.; Sodiq, A.; Giwa, A.; Eke, J.; Pikuda, O.; Eniola, J.O.; Ajiwokewu, B.; Sambudi, N.S.; Bilad, M.R. Updated review on microplastics in water, their occurrence, detection, measurement, environmental pollution, and the need for regulatory standards. Environ. Pollut. 2022, 292, 118421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, H.; Xu, L.; Liu, H.; An, L. Promising indicators for monitoring microplastic pollution. Mar. Pollut. Bull. 2022, 182, 113952. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Kelkar, V.; Kumar, R.; Halden, R.U. Methods and challenges in the detection of microplastics and nanoplastics: A mini-review. Polym. Int. 2022, 71, 543–551. [Google Scholar] [CrossRef]
- Sangeetha, D.; Shivani, A.; Anusha, J.; Ranjitha, J.; Narayanan, V. A Critical Review on Separation, Identification, Quantification and Removal of Microplastics in Environmental Samples: Developments and Challenges. In Microplastics Pollution in Aquatic Media; Sillanpää, M., Khadir, A., Muthu, S.S., Eds.; Springer: Singapore, 2022; pp. 115–135. ISBN 978-981-16-8439-5. [Google Scholar]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef] [PubMed]
- Vivekanand, A.C.; Mohapatra, S.; Tyagi, V.K. Microplastics in aquatic environment: Challenges and perspectives. Chemosphere 2021, 282, 131151. [Google Scholar] [CrossRef]
- van Mourik, L.M.; Crum, S.; Martinez-Frances, E.; van Bavel, B.; Leslie, H.A.; de Boer, J.; Cofino, W.P. Results of WEPAL-QUASIMEME/NORMANs first global interlaboratory study on microplastics reveal urgent need for harmonization. Sci. Total Environ. 2021, 772, 145071. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Imhof, H.K.; Ladehoff, M.; Löschel, L.A.; Lorenz, C.; Mintenig, S.; Piehl, S.; Primpke, S.; Schrank, I.; Laforsch, C.; et al. Enzymatic Purification of Microplastics in Environmental Samples. Environ. Sci. Technol. 2017, 51, 14283–14292. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Girão, A.V.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Identifying a quick and efficient method of removing organic matter without damaging microplastic samples. Sci. Total Environ. 2019, 686, 131–139. [Google Scholar] [CrossRef]
- Imhof, H.K.; Schmid, J.; Niessner, R.; Ivleva, N.P.; Laforsch, C. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol. Oceanogr. Methods 2012, 10, 524–537. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, G. Analyzing microplastics with Nile Red: Emerging trends, challenges, and prospects. J. Hazard. Mater. 2021, 423, 127171. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.D.; Greenspan, P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: Comparison with oil red O. J. Histochem. Cytochem. 1985, 33, 833–836. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [PubMed]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Nel, H.A.; Chetwynd, A.J.; Kelleher, L.; Lynch, I.; Mansfield, I.; Margenat, H.; Onoja, S.; Goldberg Oppenheimer, P.; Sambrook Smith, G.H.; Krause, S. Detection limits are central to improve reporting standards when using Nile red for microplastic quantification. Chemosphere 2021, 263, 127953. [Google Scholar] [CrossRef]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Patchaiyappan, A.; Dowarah, K.; Zaki Ahmed, S.; Prabakaran, M.; Jayakumar, S.; Thirunavukkarasu, C.; Devipriya, S.P. Prevalence and characteristics of microplastics present in the street dust collected from Chennai metropolitan city, India. Chemosphere 2021, 269, 128757. [Google Scholar] [CrossRef]
- de Guzman, M.K.; Andjelković, M.; Jovanović, V.; Jung, J.; Kim, J.; Dailey, L.A.; Rajković, A.; de Meulenaer, B.; Ćirković Veličković, T. Comparative profiling and exposure assessment of microplastics in differently sized Manila clams from South Korea by μFTIR and Nile Red staining. Mar. Pollut. Bull. 2022, 181, 113846. [Google Scholar] [CrossRef]
- Maxwell, S.H.; Melinda, K.F.; Matthew, G. Counterstaining to Separate Nile Red-Stained Microplastic Particles from Terrestrial Invertebrate Biomass. Environ. Sci. Technol. 2020, 54, 5580–5588. [Google Scholar] [CrossRef]
- Prata, J.C.; Godoy, V.; da Costa, J.P.; Calero, M.; Martín-Lara, M.A.; Duarte, A.C.; Rocha-Santos, T. Microplastics and fibers from three areas under different anthropogenic pressures in Douro river. Sci. Total Environ. 2021, 776, 145999. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Mawhorter, C.; Legoski, S. Quantification of microplastics by count, size and morphology in beverage containers using Nile Red and ImageJ. J. Water Health 2021, 19, 79–88. [Google Scholar] [CrossRef]
- Dowarah, K.; Patchaiyappan, A.; Thirunavukkarasu, C.; Jayakumar, S.; Devipriya, S.P. Quantification of microplastics using Nile Red in two bivalve species Perna viridis and Meretrix meretrix from three estuaries in Pondicherry, India and microplastic uptake by local communities through bivalve diet. Mar. Pollut. Bull. 2020, 153, 110982. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Peters, R.J.B.; Janssen, H.-G.; Undas, A.K.; Munniks, S.; Ruggeri, F.S.; Nielen, M.W.F. Quantitative Image Analysis of Microplastics in Bottled Water Following Nile Red Staining and Fluorescence Microscopy. ChemRvix 2022. [Google Scholar] [CrossRef]
- Sancataldo, G.; Avellone, G.; Vetri, V. Nile Red lifetime reveals microplastic identity. Environ. Sci. Process. Impacts 2020, 22, 2266–2275. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Fan, Y. Assessment of microplastics using microfluidic approach. Environ. Geochem. Health 2022. [Google Scholar] [CrossRef]
- Mesquita, P.; Gong, L.; Lin, Y. A Low-Cost Microfluidic Method for Microplastics Identification: Towards Continuous Recognition. Micromachines 2022, 13, 499. [Google Scholar] [CrossRef]
- Labbe, A.B.; Bagshaw, C.R.; Uttal, L. Inexpensive Adaptations of Basic Microscopes for the Identification of Microplastic Contamination Using Polarization and Nile Red Fluorescence Detection. J. Chem. Educ. 2020, 97, 4026–4032. [Google Scholar] [CrossRef]
- Bogdanowicz, A.; Zubrowska-Sudol, M.; Krasinski, A.; Sudol, M. Cross-Contamination as a Problem in Collection and Analysis of Environmental Samples Containing Microplastics—A Review. Sustainability 2021, 13, 12123. [Google Scholar] [CrossRef]
- Cyvin, J.B. Can We to a Greater Extent Involve Citizens in Environmental Research? Front. Environ. Sci. 2022, 10, 142422. [Google Scholar] [CrossRef]
- Shah, K.G.; Yager, P. Wavelengths and Lifetimes of Paper Autofluorescence: A Simple Substrate Screening Process to Enhance the Sensitivity of Fluorescence-Based Assays in Paper. Anal. Chem. 2017, 89, 12023–12029. [Google Scholar] [CrossRef] [PubMed]
- Kalita, E.; Nath, B.K.; Agan, F.; More, V.; Deb, P. Isolation and characterization of crystalline, autofluorescent, cellulose nanocrystals from saw dust wastes. Ind. Crops Prod. 2015, 65, 550–555. [Google Scholar] [CrossRef]
- Reinhardt, K.; Breunig, H.G.; König, K. Autofluorescence lifetime variation in the cuticle of the bedbug Cimex lectularius. Arthropod Struct. Dev. 2017, 46, 56–62. [Google Scholar] [CrossRef]
- Razeghi, N.; Hamidian, A.H.; Mirzajani, A.; Abbasi, S.; Wu, C.; Zhang, Y.; Yang, M. Sample preparation methods for the analysis of microplastics in freshwater ecosystems: A review. Environ. Chem Lett 2022, 20, 417–443. [Google Scholar] [CrossRef]
- Maw, M.M.; Boontanon, N.; Fujii, S.; Boontanon, S.K. Rapid and efficient removal of organic matter from sewage sludge for extraction of microplastics. Sci. Total Environ. 2022, 853, 158642. [Google Scholar] [CrossRef] [PubMed]
- Sturm, M.T.; Horn, H.; Schuhen, K. The potential of fluorescent dyes-comparative study of Nile red and three derivatives for the detection of microplastics. Anal. Bioanal. Chem. 2021, 413, 1059–1071. [Google Scholar] [CrossRef]
- Gao, Z.; Wontor, K.; Cizdziel, J.V. Labeling Microplastics with Fluorescent Dyes for Detection, Recovery, and Degradation Experiments. Molecules 2022, 27, 7415. [Google Scholar] [CrossRef]
- Liu, S.; Shang, E.; Liu, J.; Wang, Y.; Bolan, N.; Kirkham, M.B.; Li, Y. What have we known so far for fluorescence staining and quantification of microplastics: A tutorial review. Front. Environ. Sci. Eng. 2022, 16, 1704. [Google Scholar] [CrossRef]
- Azizi, N.; Nasseri, S.; Nodehi, R.N.; Jaafarzadeh, N.; Pirsaheb, M. Evaluation of conventional wastewater treatment plants efficiency to remove microplastics in terms of abundance, size, shape, and type: A systematic review and Meta-analysis. Mar. Pollut. Bull. 2022, 177, 113462. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, M.; Ding, J.; Cao, W.; Sun, C. Occurrence and Seasonal Variation of Microplastics in the Effluent from Wastewater Treatment Plants in Qingdao, China. JMSE 2022, 10, 58. [Google Scholar] [CrossRef]
- Kittipongvises, S.; Phetrak, A.; Hongprasith, N.; Lohwacharin, J. Unravelling capability of municipal wastewater treatment plant in Thailand for microplastics: Effects of seasonality on detection, fate and transport. J. Environ. Manag. 2022, 302, 113990. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, E.A.; Habibi, M.; Haddad, E.; Hasanin, M.; Angel, D.L.; Booth, A.M.; Sabbah, I. Microplastic distributions in a domestic wastewater treatment plant: Removal efficiency, seasonal variation and influence of sampling technique. Sci. Total Environ. 2021, 752, 141880. [Google Scholar] [CrossRef]
- Roscher, L.; Halbach, M.; Nguyen, M.T.; Hebeler, M.; Luschtinetz, F.; Scholz-Böttcher, B.M.; Primpke, S.; Gerdts, G. Microplastics in two German wastewater treatment plants: Year-long effluent analysis with FTIR and Py-GC/MS. Sci. Total Environ. 2022, 817, 152619. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef] [PubMed]
- Munno, K.; Helm, P.A.; Jackson, D.A.; Rochman, C.; Sims, A. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem. 2018, 37, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wiggin, K.J.; Holland, E.B. Validation and application of cost and time effective methods for the detection of 3-500 μm sized microplastics in the urban marine and estuarine environments surrounding Long Beach, California. Mar. Pollut. Bull. 2019, 143, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Jee, A.-Y.; Park, S.; Kwon, H.; Lee, M. Excited state dynamics of Nile Red in polymers. Chem. Phys. Lett. 2009, 477, 112–115. [Google Scholar] [CrossRef]
- Monteleone, A.; Schary, W.; Wenzel, F.; Langhals, H.; Dietrich, D.R. Label-free identification and differentiation of different microplastics using phasor analysis of fluorescence lifetime imaging microscopy (FLIM)-generated data. Chem. Biol. Interact. 2021, 342, 109466. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, X.; Wang, G.; Zuo, Y. A Rapid Method for Detecting Microplastics Based on Fluorescence Lifetime Imaging Technology (FLIM). Toxics 2022, 10, 118. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, Q.; Zhong, X.; Hu, X. Rhodamine B dye staining for visualizing microplastics in laboratory-based studies. Environ. Sci. Pollut. Res. 2021, 28, 4209–4215. [Google Scholar] [CrossRef]
- Sancataldo, G.; Ferrara, V.; Bonomo, F.P.; Chillura Martino, D.F.; Licciardi, M.; Pignataro, B.G.; Vetri, V. Identification of microplastics using 4-dimethylamino-4’-nitrostilbene solvatochromic fluorescence. Microsc. Res. Tech. 2021, 84, 2820–2831. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.; Johnson, M.; Nathanail, P.; Gomes, R.L.; Needham, T.; Burson, A. Exploring the Efficacy of Nile Red in Microplastic Quantification: A Costaining Approach. Environ. Sci. Technol. Lett. 2019, 6, 606–611. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; Matos, J.T.V.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci. Total Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Alves, J.R.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Major factors influencing the quantification of Nile Red stained microplastics and improved automatic quantification (MP-VAT 2.0). Sci. Total Environ. 2020, 719, 137498. [Google Scholar] [CrossRef] [PubMed]
- Meyers, N.; Catarino, A.I.; Declercq, A.M.; Brenan, A.; Devriese, L.; Vandegehuchte, M.; de Witte, B.; Janssen, C.; Everaert, G. Microplastic detection and identification by Nile red staining: Towards a semi-automated, cost- and time-effective technique. Sci. Total Environ. 2022, 823, 153441. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Int-Veen, I.; Loder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2016, 108, 365–372. [Google Scholar] [CrossRef]
- Lu, H.-C.; Ziajahromi, S.; Neale, P.A.; Leusch, F.D.L. A systematic review of freshwater microplastics in water and sediments: Recommendations for harmonisation to enhance future study comparisons. Sci. Total Environ. 2021, 781, 146693. [Google Scholar] [CrossRef]



| Advantage | Disadvantage |
|---|---|
|
|
| Flashlight | Peak Wavelength | Supplier |
|---|---|---|
| UV | 365 nm | TATTU U3S, Tattu, unknown, Germany |
| Blue | 470 nm | ULTRAFIRE H-B3, Hakka Trade US Inc Suwanee, GA 30024, USA |
| Green | 520–535 nm | Ultrafire H-G3, Hakka Trade US Inc Suwanee, GA 30024, USA |
| Filter | Transmissive Area | Supplier |
|---|---|---|
| Yellow-colored foil | See Transmission Spectra Figure S1 | Shenzhen Neewer Technology Co., Ltd., Shenzhen, China |
| Blue bandpass filter | 420–470 nm | Edmund Optics GmbH, Mainz, Germany |
| Green bandpass filter | 500–570 nm | |
| Orange bandpass filter | 570–540 nm | |
| Red bandpass filter | 660–710 nm |
| Combination | Flashlight | Excitation Filter | Emission Filter | Microscope Setting |
|---|---|---|---|---|
| 1—Low Budget modification | UV | None | Yellow-colored foil | Exposure time 0.5 s Gain: 4 |
| 2—Blue fluorescence | UV | None | Blue bandpass | |
| 3—Green fluorescence | Blue | Blue bandpass | Green bandpass | |
| 4—Orange fluorescence | Green | Green bandpass | Orange bandpass | |
| 5—Red fluorescence | Green | Orange bandpass | Red bandpass |
| Name | AB Number | Purchased From |
|---|---|---|
| NR 0 (Nile red) | AB 139346 | abcr GmbH |
| NR 1 (abcr eco Wasser 3.0 detect mix MP-1) | AB 930015 | abcr GmbH |
| Hydrogen peroxide (35%) | AB 171423 | abcr GmbH |
| Acetone | AB 178997 | abcr GmbH |
| Polymer | Abbreviation | Mean Size [µm] | Supplier/Preparation |
|---|---|---|---|
| Polyethylene | PE | 303 ± 249 | LyondellBasell, Basell Polyolefine GmbH, Frankfurt, Germany |
| Polypropylene | PP | 337 ± 301 | LyondellBasell, Basell Polyolefine GmbH, Frankfurt, Germany |
| Copolyamide | PA | 357 ± 60 | EMS-Grilltech, Switzerland |
| Copolyester | PES | 54 ± 87 | EMS-Grilltech, Switzerland |
| Polyvinylchloride | PVC | 110 ± 25 | Sigma-Aldrich, Germany |
| Wood | - | Fine shavings of Quercus spec. | |
| Calcite | - | Ground shell of Mytilidae | |
| Chitin | - | Ground exoskeleton of Pandalus borealis |
| Average Discharge | NR0 Low Budged Modification, Manual Counting | NR1 Green Fluorescence, Manual Counting | NR1 Green Fluorescence, Automated Counting |
|---|---|---|---|
| per liter | 41 | 87 | 103 |
| per day | 572 million | 1269 million | 1497 million |
| per year | 209 billion | 351 billion | 586 billion |
| Per day and inhabitant | 10,409 | 23,067 | 27,211 |
| Per year and inhabitant | 3.9 million | 8.4 million | 9.9 million |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sturm, M.T.; Myers, E.; Schober, D.; Korzin, A.; Schuhen, K. Development of an Inexpensive and Comparable Microplastic Detection Method Using Fluorescent Staining with Novel Nile Red Derivatives. Analytica 2023, 4, 27-44. https://doi.org/10.3390/analytica4010004
Sturm MT, Myers E, Schober D, Korzin A, Schuhen K. Development of an Inexpensive and Comparable Microplastic Detection Method Using Fluorescent Staining with Novel Nile Red Derivatives. Analytica. 2023; 4(1):27-44. https://doi.org/10.3390/analytica4010004
Chicago/Turabian StyleSturm, Michael Toni, Erika Myers, Dennis Schober, Anika Korzin, and Katrin Schuhen. 2023. "Development of an Inexpensive and Comparable Microplastic Detection Method Using Fluorescent Staining with Novel Nile Red Derivatives" Analytica 4, no. 1: 27-44. https://doi.org/10.3390/analytica4010004
APA StyleSturm, M. T., Myers, E., Schober, D., Korzin, A., & Schuhen, K. (2023). Development of an Inexpensive and Comparable Microplastic Detection Method Using Fluorescent Staining with Novel Nile Red Derivatives. Analytica, 4(1), 27-44. https://doi.org/10.3390/analytica4010004










