In Vitro Antifungal Antibacterial Activity of Partitions from Euphorbia tirucalli L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
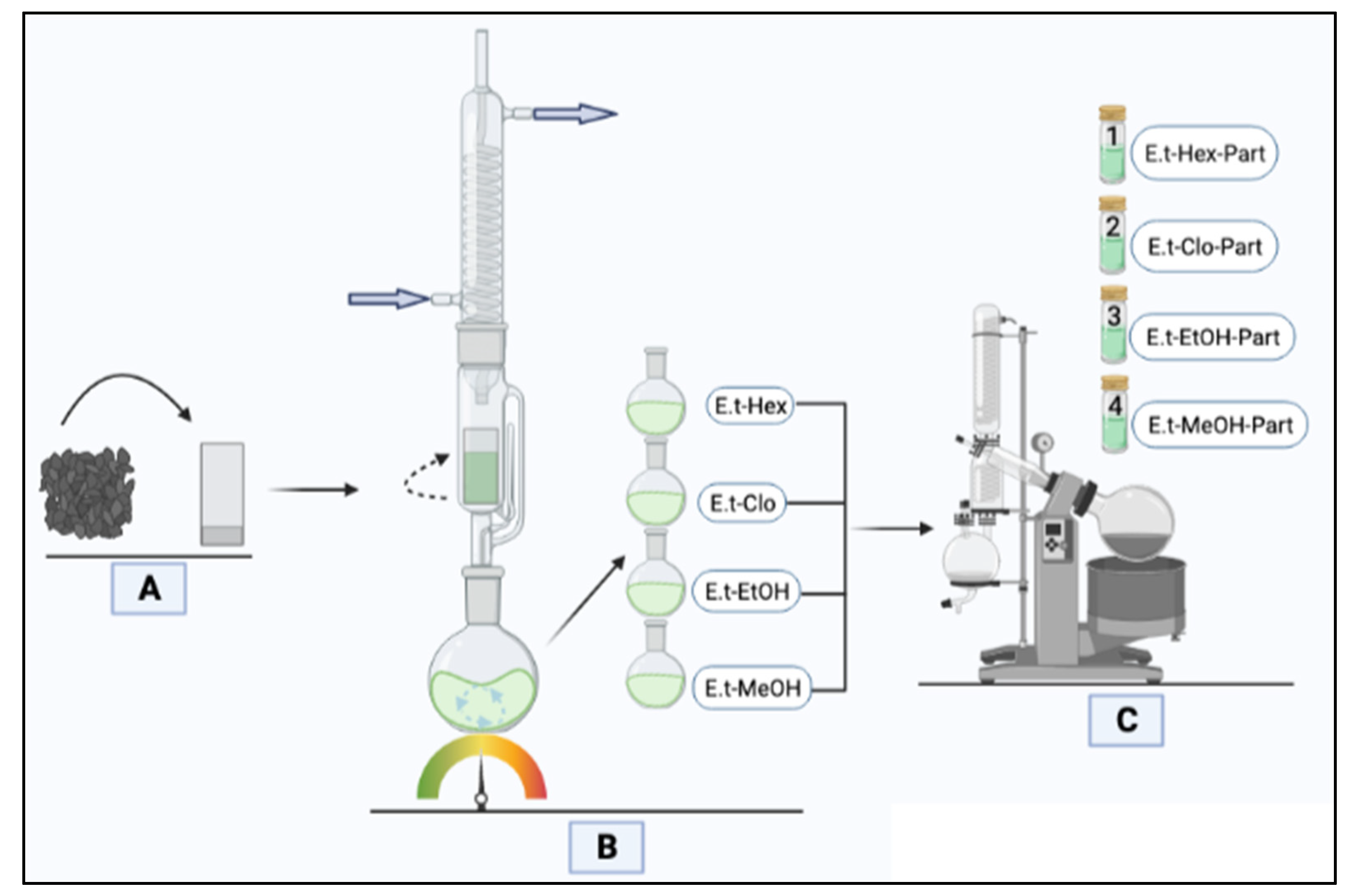
2.2. Collection of Plant Material
2.3. Preparation of Extracts
2.4. Preparation of Treatments
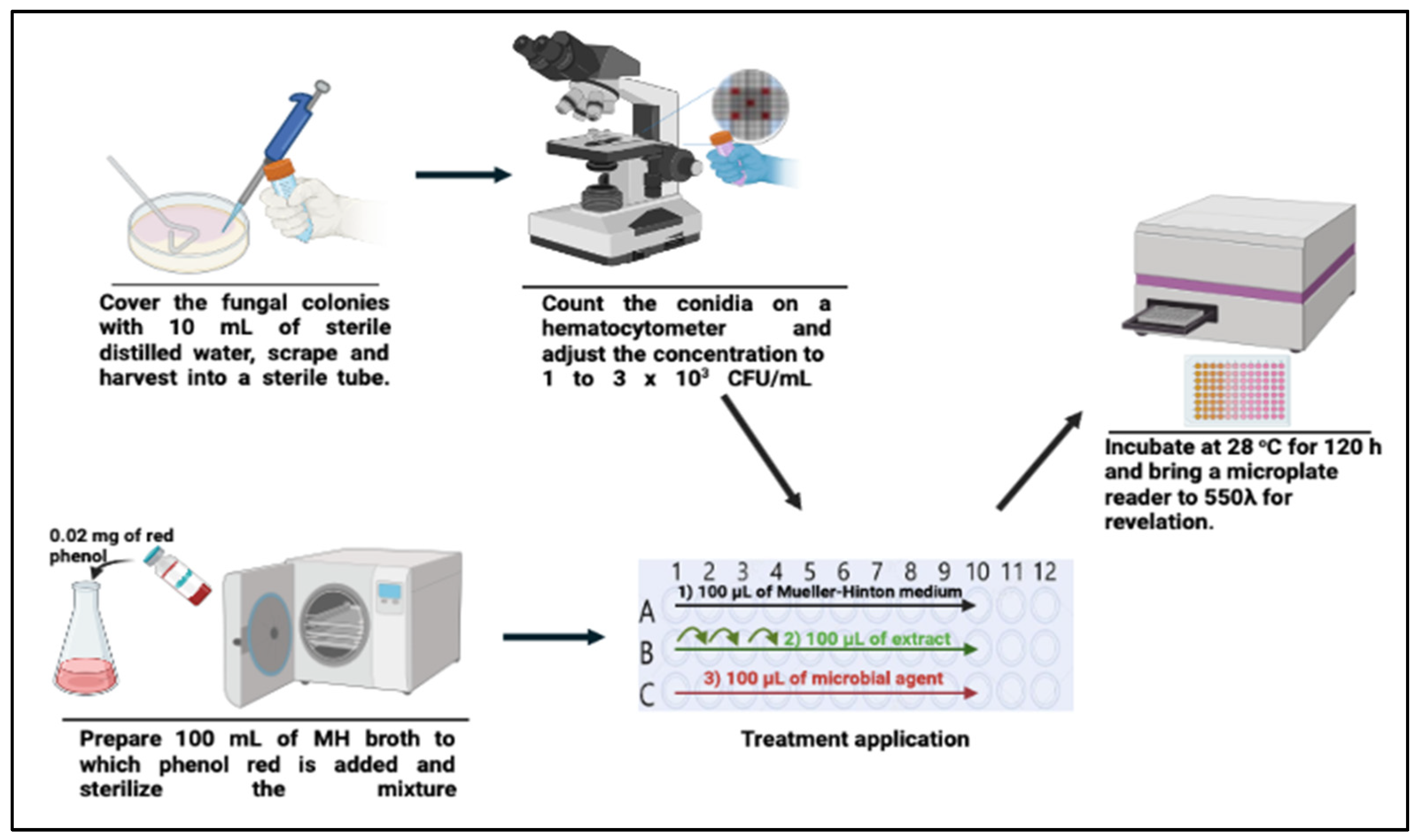
2.5. Preparation of Inoculate
2.6. Determination of the Antidermatophytic Activity of the E. tirucalli L. Partitions
2.7. Antibacterial Activity of E. tirucalli L. Partitions
3. Results
3.1. Percentage of Extraction Yield
3.2. Antifungal Activity
3.3. Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martini, M.C.; Zhang, T.; Williams, J.T.; Abramovitch, R.B.; Weathers, P.J.; Shell, S.S. Artemisia annua and Artemisia afra extracts exhibit strong bactericidal activity against Mycobacterium tuberculosis. J. Ethnopharmacol. 2020, 262, 113191. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.M.; Cuong, D.X.; Thinh, P.V.; Minh, T.N.; Manh, T.D.; Duong, T.-H.; Minh, T.T.L.; Oanh, V.T.T. Phytochemical Screening and Evaluation of Antioxidant Properties and Antimicrobial Activity against Xanthomonas axonopodis of Euphorbia tirucalli Extracts in Binh Thuan Province, Vietnam. Molecules 2021, 26, 941. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Huang, Y.; Fidock, D.A.; Polyak, S.J.; Wagoner, J.; Towler, M.J.; Weathers, P.J. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J. Ethnopharmacol. 2021, 274, 114016. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Ojo, O.A.; Amanze, J.C.; Oni, A.I.; Grant, S.; Iyobhebhe, M.; Elebiyo, T.C.; Rotimi, D.; Asogwa, N.T.; Oyinloye, B.E.; Basiru, O.A.; et al. Antidiabetic activity of avocado seeds (Persea americana Mill.) in diabetic rats via activation of PI3K/AKT signaling pathway. Sci. Rep. 2022, 12, 2919. [Google Scholar] [CrossRef]
- Butler, M.S.; Buss, A.D. Natural products—The future scaffolds for novel antibiotics? Biochem. Pharmacol. 2006, 71, 919–929. [Google Scholar] [CrossRef]
- Kirbag, S.; Erecevit, P.; Zengin, F.; Guvene, A.N. Antimicrobial activities of some Euphorbia species. Afr. J. Tradit. Complem. Altern. Med. 2013, 10, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Vishnoi, G.; Wal, A.; Wal, P. Medicinal Value of Euphorbia tirucalli. Syst. Rev. Pharm. 2013, 4, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Sultan, S.; Kimaro, C.C.; Amri, E. Antifungal Activity and Phytochemical Screening of Different Solvent Extracts of Euphorbia tirucalli Linn. J. Adv. Biol. Biotechnol. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Kumar, A.; Upadhyay, B.; Singh, K.P. Ethno-Medicinal, Phytochemical and Antimicrobial Studies of Euphorbia tirucalli L. J. Phytol. 2010, 2, 65–77. [Google Scholar]
- Martinez-Rossi, N.M.; Peres, N.T.A.; Rossi, A. Antifungal resistance mechanisms in dermatophytes. Mycopathologia 2008, 166, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Heya, M.S.; Verde-Star, M.J.; Galindo-Rodríguez, S.A.; García-Hernández, D.G.; Rivas-Morales, C.; Robledo-Leal, E. Diagnóstico de la tinea pedis y tinea unguium en la zona metropolitana de Monterrey, Nuevo León, México. Dermatol. Rev. Mex. 2002, 65, 839–849. [Google Scholar]
- Arenas, R. Dermatofitosis en México. Rev. Iberoam. Micol. 2002, 19, 63–67. [Google Scholar] [PubMed]
- Rudramurthy, S.M.; Shankarnarayan, S.A.; Dogra, S.; Shaw, D.; Mushtaq, K.; Paul, R.A.; Narang, T.; Chakrabarti, A. Mutation in the squalene epoxidase gene of trichophyton interdigitale and trichophyton rubrum associated with allylamine resistance. Antimicrob. Agents Chemother. 2018, 62, e02522-17. [Google Scholar] [CrossRef] [Green Version]
- Avershina, E.; Shapovalova, V.; Shipulin, G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Kelber, O.; Bauer, R.; Kubelka, W. Phytotherapy in Functional Gastrointestinal Disorders. Dig. Dis. 2018, 35, 36–42. [Google Scholar] [CrossRef]
- De Boer, E.; Heuvelink, A.E. Methods for the detection and isolation of Shiga toxin-producing Escherichia coli. J. Appl. Microbiol. Symp. Suppl. 2000, 88, 133S–143S. [Google Scholar] [CrossRef]
- Musser, A.W.; Beamer, P.R. Infections caused by Pseudomonas aeruginosa. J. Indiana State Med. Assoc. 1961, 54, 1627–1634. [Google Scholar]
- Melo, G.E.M.; Méndez, G.L.; Fortich, M.D.R.O. Actividad antibacteriana in vitro de diecinueve aceites esenciales frente a bacterias asociadas al acné. Rev. Cuba. Farm. 2015, 49, 103–116. [Google Scholar]
- Clinical for Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard, 2nd ed.; Document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Barry, A.L.; National Committee for Clinical Laboratory Standards. M26-A—Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline; This document provides procedures for determining the lethal activity of antimicrobial agents; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Parekh, J.; Chanda, S. In vitro antifungal activity of methanol extracts of some Indian medicinal plants against pathogenic yeast and moulds. Afr. J. Biotechnol. 2008, 7, 4349–4353. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Antimicrobial activities and phytochemical profiles of endemic medicinal plants of Mauritius. Pharm. Biol. 2005, 43, 237–242. [Google Scholar] [CrossRef]
- Cristian, N.; Sanabria, Z.G.; Consuelo, L. Actividad antimicrobiana de cuatro variedades de plantas frente a patógenos de importancia clínica en Antimicrobial activity of four varieties of plants against pathogens clinical. Nova 2017, 15, 119–129. [Google Scholar]
- Veloza Cely, W.F.; Javier, A.; Matulevich, W.F.C. Triterpenos y esteroles de Salvia leucantha (Lamiaceae) y evaluación de su capacidad antioxidante. Rev. Fac. Cienc. Básicas 2014, 10, 68–79. [Google Scholar] [CrossRef]
- Miron, D.; Battisti, F.; Silva, F.K.; Lana, A.D.; Pippi, B.; Casanova, B.; Gnoatto, S.; Fuentefria, A.; Mayorga, P.; Schapoval, E.E.S. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Braz. J. Pharmacogn. 2014, 24, 660–667. [Google Scholar] [CrossRef]
- Abdul, Q.K.; Tahir, R.; Syed, N.-ul-H.K.; Zaheer, A.; Abdul, M. Cycloeuphordenol, a New Triterpene from Euphorbza. Phytochemistry 1989, 27, 2279–2281. [Google Scholar]
- Rasool, N.; Khan, A.Q.; Malik, A. A taraxerane Type Triterpene from Euphorbia tirucalli. Phytochemistry 1989, 28, 1193–1195. [Google Scholar] [CrossRef]
- Duong, T.-H.; Beniddir, M.A.; Genta-Jouve, G.; Nguyen, H.H.; Nguyen, D.P.; Nguyen, T.A.T.; Mac, D.H.; Boustie, J.; Nguyen, K.P.P.; Chavasiri, W.; et al. Further terpenoids from Euphorbia tirucalli. Fitoterapia 2019, 135, 44–51. [Google Scholar] [CrossRef]
- Álvaro, R.; Marín, E.; Rodríguez, C.; Conde, B.C. In vitro Study of the Antifungal Activity from a new natural extract. Psychol. Lat. 2018, Especial, 399–402. [Google Scholar]
- Martinez-Florez, S.; González-Gallego, J.; Culebras, J.; Tuñón, J. Los flavonoides: Propiedades y acciones antioxidantes. Nutr. Hosp. 2002, 17, 271–278. [Google Scholar]
- Aisah, S.; Utami, P.I.; Genatrika, E. The effectiveness of ointment of Patah Tulang Stem’s (Euphorbia tirucalli) ethanol extract for Burn Wound Healing on White Rats (Rattus norvegicus). IOP Conf. Ser. Mater. Sci. Eng. 2018, 288, 12055. [Google Scholar] [CrossRef]
- Goutam, M.; Sadhan, K.; Jnanojjal, C. Euphorbia tirucalli L.: A review on its potential pharmacological use in chronic diseases. IJSR 2017, 6, 241–245. [Google Scholar]
| Solvents | IQ (g) | OQ (g) | R (%) | Efficacy (%) | Performance (%) |
|---|---|---|---|---|---|
| Hexane | 40 | 1.852 | 4.630 | 4.630 | 4.630 |
| Chloroform | 38.789 | 1.211 | 3.028 | 3.122 | 3.122 |
| Ethanol | 37.578 | 3.110 | 7.775 | 8.276 | 8.276 |
| Methanol | 34.468 | 4.969 | 12.423 | 14.416 | 14.416 |
| Dermatophytic Strains | E.t-MeOH-Part | E.t-EtOH-Part | Clotrimazole | |||
|---|---|---|---|---|---|---|
| % H | MIC (µg/mL) | % H | MIC (µg/mL) | % H | MIC (µg/mL) | |
| T. rubrum | 80.25 ± 2.06 | 125 | 81.11 ± 2 | 125 | 82.30 ± 0.94 | 0.1 |
| T. interdigitalis | 86.12 ± 4.08 | 125 | 81.99 ± 3.53 | 500 | 92.57 ± 3.11 | 0.0004 |
| Clinically Important Strain | Partition (Part.) | MIC (mg/mL) | Biological Activity |
|---|---|---|---|
| P. aeruginosa ATCC 27853 | E.t-MeOH | 1.56 ± 0.02 | Bacteriostatic |
| P. aeruginosa ATCC 27853 | E.t-EtOH | 3.13 ± 0.13 | Bactericidal |
| E. coli ATCC 25922 | E.t-MeOH | 1.56 ± 0.04 | Bacteriostatic |
| E. coli ATCC 25922 | E.t-EtOH | 3.13 ± 0.15 | Bacteriostatic |
| S. aureus BAA-44 | E.t-EtOH | 6.25 ± 0.04 | Bactericidal |
| S. aureus clinical isolate | E.t-EtOH | 1.56 ± 0.02 | Bacteriostatic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heya, M.S.; Verde-Star, M.J.; Galindo-Rodríguez, S.A.; Rivas-Morales, C.; Robledo-Leal, E.; García-Hernández, D.G. In Vitro Antifungal Antibacterial Activity of Partitions from Euphorbia tirucalli L. Analytica 2022, 3, 228-235. https://doi.org/10.3390/analytica3020016
Heya MS, Verde-Star MJ, Galindo-Rodríguez SA, Rivas-Morales C, Robledo-Leal E, García-Hernández DG. In Vitro Antifungal Antibacterial Activity of Partitions from Euphorbia tirucalli L. Analytica. 2022; 3(2):228-235. https://doi.org/10.3390/analytica3020016
Chicago/Turabian StyleHeya, Michel Stéphane, María Julia Verde-Star, Sergio Arturo Galindo-Rodríguez, Catalina Rivas-Morales, Efrén Robledo-Leal, and David Gilberto García-Hernández. 2022. "In Vitro Antifungal Antibacterial Activity of Partitions from Euphorbia tirucalli L." Analytica 3, no. 2: 228-235. https://doi.org/10.3390/analytica3020016
APA StyleHeya, M. S., Verde-Star, M. J., Galindo-Rodríguez, S. A., Rivas-Morales, C., Robledo-Leal, E., & García-Hernández, D. G. (2022). In Vitro Antifungal Antibacterial Activity of Partitions from Euphorbia tirucalli L. Analytica, 3(2), 228-235. https://doi.org/10.3390/analytica3020016






