Achievements of Mesoporous Carbon Solution and Single-Walled Carbon Nanotube Composite on the Sensitive Electrochemical Assay of Ivabradine
Abstract
:1. Introduction
2. Experimental
2.1. Apparatus
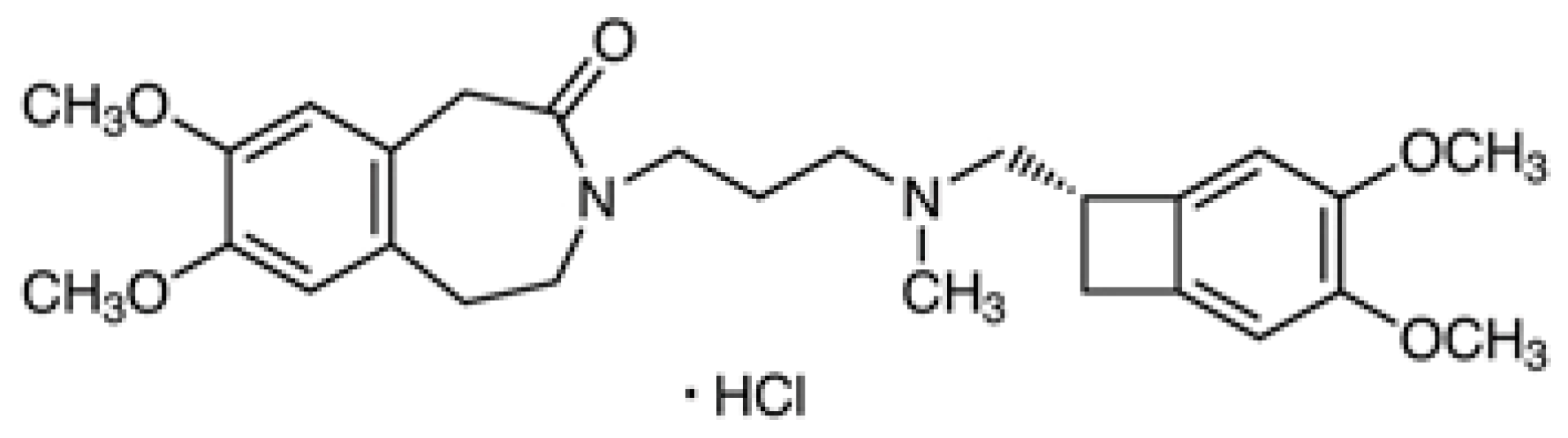
2.2. Reagents and Chemicals
2.3. Preparation of MCS/SWCNT-COOH/GCE
2.4. Preparation of Pharmaceutical and Biological Samples
3. Results and Discussion
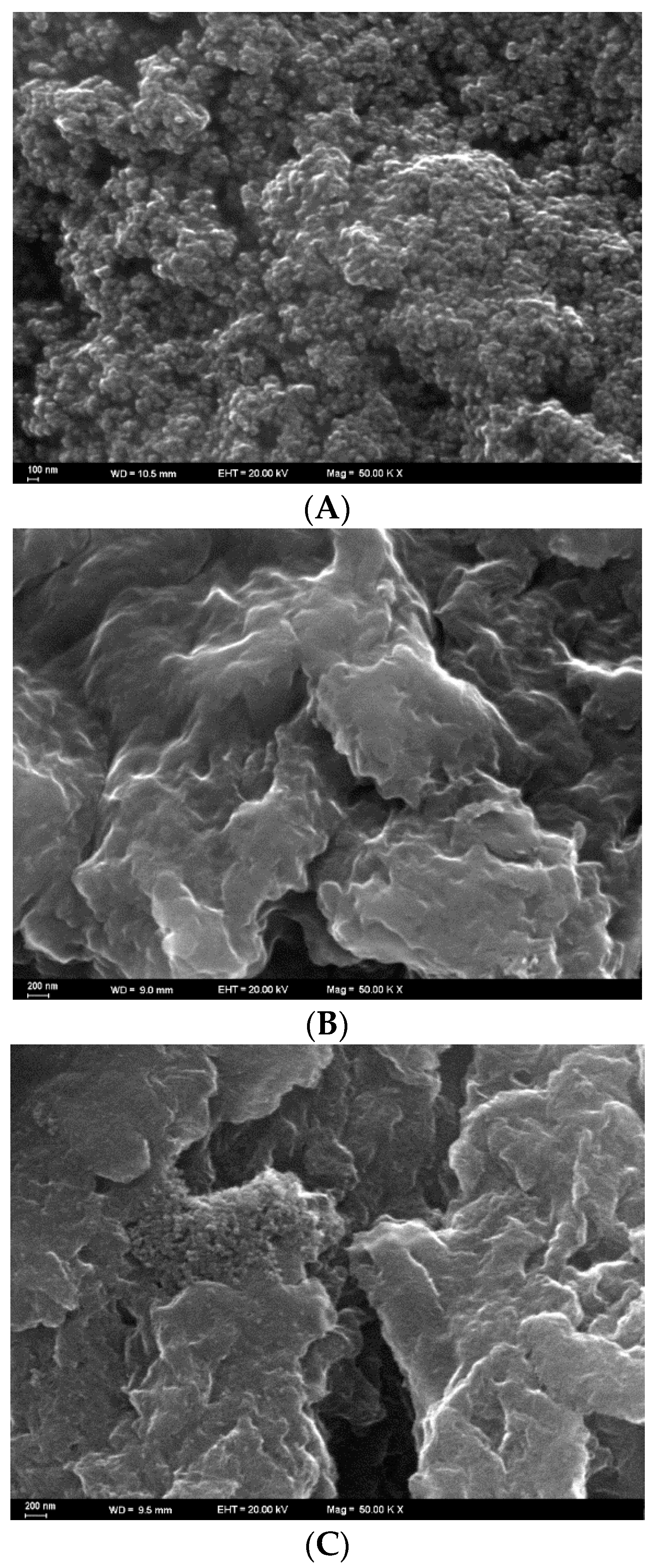
3.1. Characterization of the Modification Materials
3.2. Effect of MCS/SWCNT-COOH
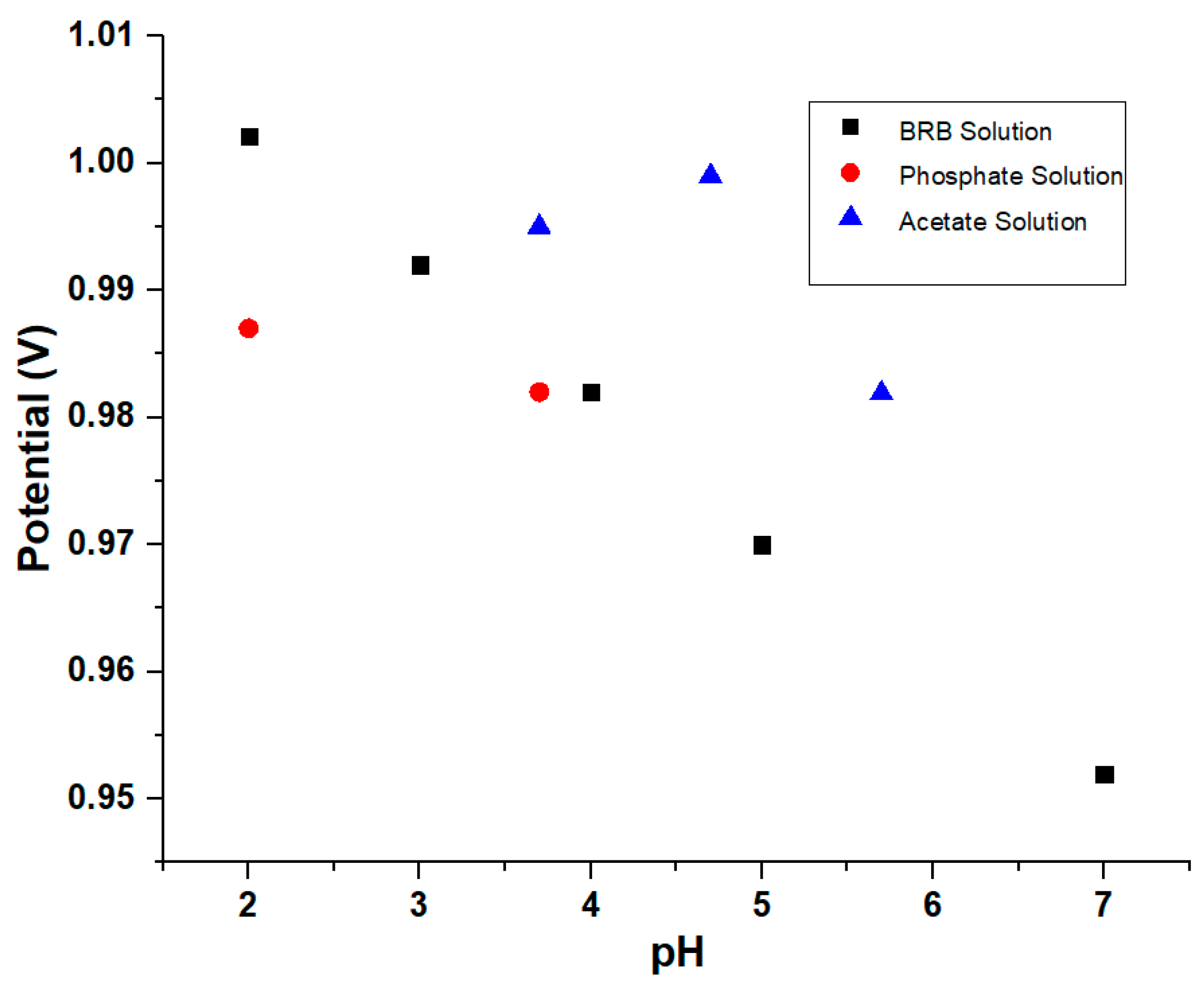
3.3. Effect of the pH
3.4. Effect of Scan Rate on MCS/SWCNT-COOH/GCE
- Ip (µA) = 0.1971 ν1/2 (mV s−1) + 0.9414; r = 0.988 (n = 5) at MCS/SWCNT-COOH/GCE
- log ip (µA) = 0.262 log ν (mV s−1) − 6.0674; r = 0.996 (n = 5) at MCS/SWCNT-COOH/GCE
- α: the transfer coefficient,
- k0: standard the heterogeneous rate constant v: scan rate,
- E0: formal potential,
- R: gas constant,
- T: temperature,
- F: Faraday constant,
- n: the number of electrons that are involved in electro-oxidation of IH.
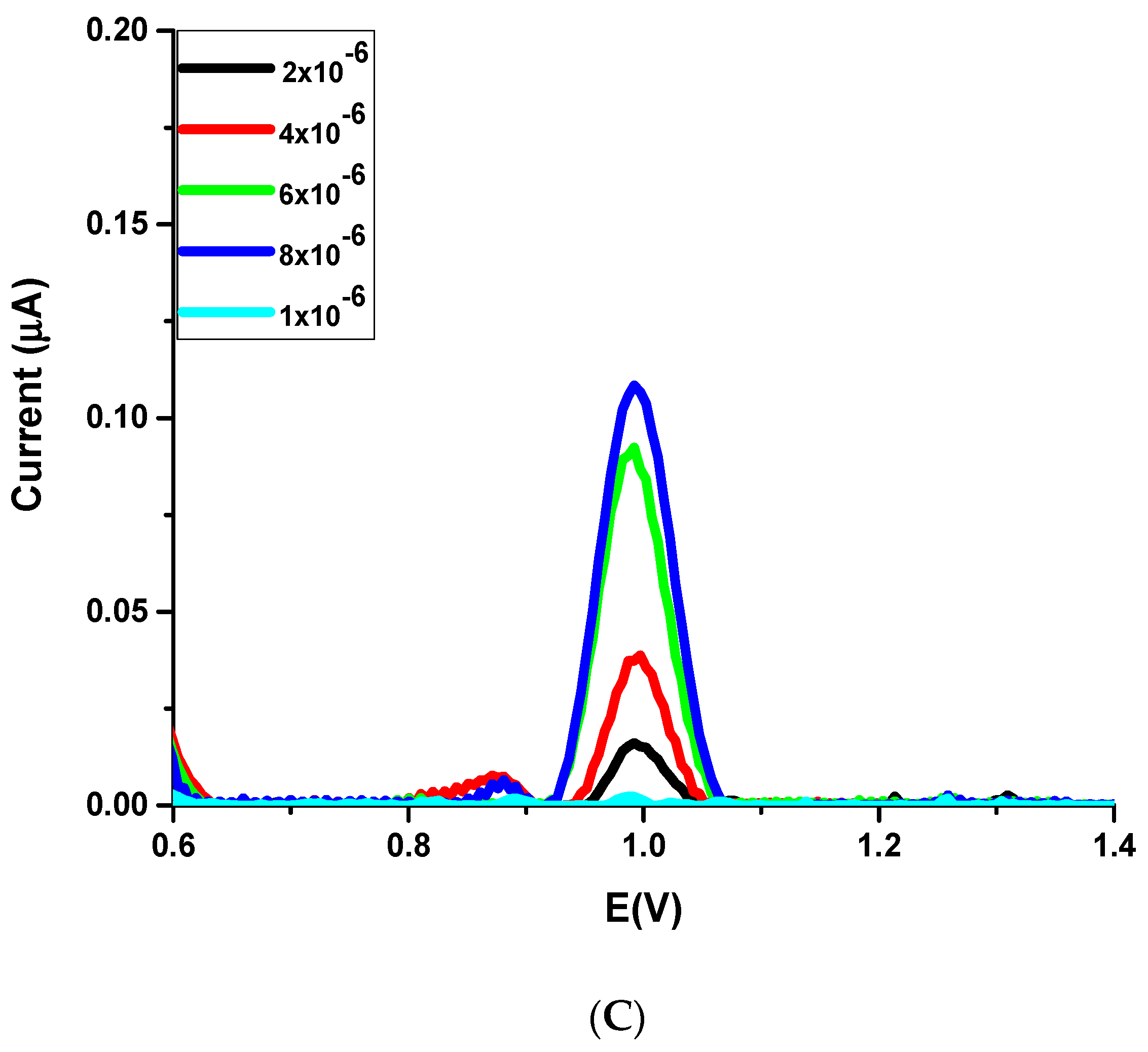
3.5. Analytical Characterization and Validation of the Method
3.6. Determination of IH in Tablet Dosage Form, Spiked Serum, and Urine Samples Using the MCS/SWCNT-COOH/GCE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tse, S.; Mazzola, N. Ivabradine (Corlanor) for heart failure: The first selective and specific if inhibitor. Pharm. Ther. 2015, 40, 810–814. [Google Scholar]
- Badu-Boateng, C.; Jennings, R.; Hammersley, D. The therapeutic role of ivabradine in heart failure. Ther. Adv. Chronic Dis. 2018, 9, 199–207. [Google Scholar] [CrossRef]
- Zhou, X.-B.; Zhu, J.-R.; Liu, J.-L.; Jin, Z.-P.; Tanga, F.-Y.; Hu, X.-R. Crystal structures and properties of two hydrated conglomerate forms of the heart-rate-lowering agent ivabradine hydro chloride. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 545–553. [Google Scholar] [CrossRef]
- Wang, F.; Hu, S. Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim. Acta 2009, 165, 1–22. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; El-Moghazy, A.Y.; Hussin, M.; Abu-Saied, M.A.; El-Shanshory, A.A.; Soliman, H.M.A. The nanomaterials and recent progress in biosensing systems: A review. Trends Environ. Anal. Chem. 2020, 26, e00087. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ara, M.G.; Alim, M.A.; Uddin, M.S.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; Mousa, S.A.; Abdel-Daim, M.M. Mesoporous carbon: A versatile material for scientific applications. Int. J. Mol. Sci. 2021, 22, 4498. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Z. Carbon nanomaterial-based electrochemical biosensors: An overview. Nanoscale 2015, 7, 6420–6431. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.R.; Oliveira, O.N. Carbon-Based Nanomaterials in: Nanostructures; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 233–249. [Google Scholar] [CrossRef]
- Ahmed, J.; Rashed, M.A.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsareii, S.A. Novel SWCNTs-mesoporous silicon nanocomposite as efficient non-enzymatic glucose biosensor. Appl. Surf. Sci. 2021, 552, 149477. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Talemi, R.P. Synergistic effect of magnetite and gold nanoparticles onto the response of a label-free impedimetric hepatitis B virus DNA biosensor. Mater. Sci. Eng. C 2016, 59, 773–781. [Google Scholar] [CrossRef]
- Aftab, S.; Kurbanoglu, S.; Ozcelikay, G.; Shah, A.; Ozkan, S.A. NH2-Functionalized Multi Walled Carbon Nanotubes Decorated with ZnO Nanoparticles and Graphene Quantum Dots for Sensitive Assay of Pimozide. Electroanalysis 2019, 31, 1083–1094. [Google Scholar] [CrossRef]
- Gandi, S.; Manikandan, A.; Rao, S.V. Novel stability indicating rp-uplc method for simultaneous determination of ivabradine and metoprolol drug materials in bulk and their pharmaceutical dosage forms. Res. J. Pharm. Technol. 2020, 13, 250–254. [Google Scholar] [CrossRef]
- Tomić, J.; Ivković, B.; Oljačić, S.; Nikolić, K.; Maljuric, N.; Dani, A.P.; Agbaba, C. Chemometrically Assisted RP-HPLC Method Development for Efficient Separation of Ivabradine and its Eleven Impurities. Acta Chromatogr. 2020, 32, 53–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, M.; Xie, S.; Ye, X.; Xu, X. Simultaneous determination of amiodarone, dronedarone, and their principal metabolites in SD rat plasma by UPLC-MS/MS and its application in pharmacokinetics. Arab. J. Chem. 2021, 14, 103300. [Google Scholar] [CrossRef]
- Smirnova, L.A.; Tolkachev, B.E.; Ryabukha, A.F.; Suchkov, E.A.; Kuznetsov, K.A.; Magnitskaya, O.V.; Petrov, V.I. Quantitative Determination of Ivabradine and Its N-Demethylated Metabolite in Volunteer Blood and Urine. Pharm. Chem. J. 2019, 53, 275–278. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Majeed, A.; Hussain, I.; Rasool, M.F. Quantitative Determination and Validation of Ivabradine-HCl in Pharmaceutical Formulation and Rabbit Plasma by High Performance Liquid Chromatography Method. Curr. Pharm. Anal. 2016, 13, 446–451. [Google Scholar] [CrossRef]
- Nowakowska, J.; Pikul, P.; Marszałł, M.; Ciura, K. Application and Validation of Simple Isocratic HPLC-UV-DAD Method with Dual Wavelength Detection for Ivabradine Determination and Its Application in the Study of Stress Degradation. J. Chem. 2017, 2017, 2069571. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, S.A. Analytical method validation: The importance for pharmaceutical analysis. Pharm. Sci. 2018, 24, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, S.A.; Uslu, B. From mercury to nanosensors: Past, present and the future perspective of electrochemistry in pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2016, 130, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; Madbouly, A.; el Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.G.; Barhoum, A. Molecularly Imprinted Electrochemical Sensor-Based Fe2O3@MWCNTs for Ivabradine Drug Determination in Pharmaceutical Formulation, Serum, and Urine Samples. Front. Bioeng. Biotechnol. 2021, 9, 648704. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; el Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.G.; Barhoum, A. t-Butyl calixarene/Fe2O3@MWCNTs composite-based potentiometric sensor for determination of ivabradine hydrochloride in pharmaceutical formulations. Mater. Sci. Eng. C 2020, 116, 111110. [Google Scholar] [CrossRef]
- Attia, A.K.; Abo-Talib, N.F.; Tammam, M.H. Voltammetric determination of ivabradine hydrochloride using multiwalled carbon nanotubes modified electrode in presence of sodium dodecyl sulfate. Adv. Pharm. Bull. 2017, 7, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.A.; Kauffmann, J.-M.; Zuman, P. Solid Electrodes in Drug Analysis; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Guidance for Industry Q2B Validation of Analytical Procedures: Methodology. 1996. Available online: https://scholar.google.com/scholar?cluster=10623296102212878913&hl=tr&as_sdt=0,5 (accessed on 14 November 2021).
- Bilge, S.; Bakirhan, N.K.; Donar, Y.O.; Sinag, A.; Ozkan, S.A.; Okudan, E.Ş. Green synthesis of carbon based biosensor materials from algal biomass for the sensitive detection of vardenafil. J. Electroanal. Chem. 2020, 871, 114286. [Google Scholar] [CrossRef]
- Bilge, S.; Dogan-Topal, B.; Atici, E.B.; Sınağ, A.; Ozkan, S.A. Rod-like CuO nanoparticles/waste masks carbon modified glassy carbon electrode as a voltammetric nanosensor for the sensitive determination of anti-cancer drug pazopanib in biological and pharmaceutical samples. Sens. Actuators B Chem. 2021, 343, 130109. [Google Scholar] [CrossRef]
- Gosser, D.K., Jr. Simulation and Analysis of Reaction Mechanisms; Wiley-VCH Publishers: Hoboken, NJ, USA, 1993. [Google Scholar]
- Laviron, E.J. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Greef, R.; Peat, R.; Peter, L.M.; Pletcher, D.; Robinson, J. Instrumental Methods in Electrochemistry; Ellis Horwood: Chichester, UK, 1990. [Google Scholar]
- Ermer, J.; Nethercote, P.W. Method Validation in Pharmaceutical Analysis: A Guide to Best Practice, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Riley, C.M.; Rosanske, T.W. Development and Validation of Analytical Methods; Elsevier: Hoboken, NJ, USA, 1996. [Google Scholar]



| Standard Solution | Serum | Urine | |
|---|---|---|---|
| Measured Potential (V) | 0.97 | 0.98 | 0.99 |
| Calibration range (M) | 1 × 10−6–1 × 10−5 | 1 × 10−6–1 × 10−5 | 1 × 10−6–8 × 10−6 |
| Slope (A M−1) | 0.0738 | 0.0367 | 0.0152 |
| Intercept (A) | −4 × 10−8 | 4 × 10−8 | −10−8 |
| Correlation coefficient | 0.99 | 0.99 | 0.99 |
| LOD (M) | 1.47 × 10−7 | 2.31 × 10−7 | 1.66 × 10−7 |
| LOQ (M) | 4.92 × 10−7 | 7.70 × 10−7 | 5.58 × 10−7 |
| Precision (RSD %) | 1.18 | 1.34 | 1.44 |
| Coralan® Tablet | Serum | Urine | |
|---|---|---|---|
| Labeled claim (mg) | 5.00 | – | - |
| Amount found (mg) a | 4.98 | – | - |
| RSD% | 0.95 | – | - |
| Bias% | 0.39 | – | - |
| Added (mg) | 5.00 | 5.00 | 5.00 |
| Found (mg) a | 4.92 | 4.90 | 5.01 |
| Average recovery % | 98.4 | 98 | 100.2 |
| RSD% of recovery | 0.76 | 0.87 | 1.35 |
| Bias% | 1.4 | 0.42 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yence, M.; Karadurmus, L.; Ozcelikay, G.; K. Bakirhan, N.; Ozkan, S.A. Achievements of Mesoporous Carbon Solution and Single-Walled Carbon Nanotube Composite on the Sensitive Electrochemical Assay of Ivabradine. Analytica 2021, 2, 195-205. https://doi.org/10.3390/analytica2040017
Yence M, Karadurmus L, Ozcelikay G, K. Bakirhan N, Ozkan SA. Achievements of Mesoporous Carbon Solution and Single-Walled Carbon Nanotube Composite on the Sensitive Electrochemical Assay of Ivabradine. Analytica. 2021; 2(4):195-205. https://doi.org/10.3390/analytica2040017
Chicago/Turabian StyleYence, Merve, Leyla Karadurmus, Goksu Ozcelikay, Nurgul K. Bakirhan, and Sibel A. Ozkan. 2021. "Achievements of Mesoporous Carbon Solution and Single-Walled Carbon Nanotube Composite on the Sensitive Electrochemical Assay of Ivabradine" Analytica 2, no. 4: 195-205. https://doi.org/10.3390/analytica2040017
APA StyleYence, M., Karadurmus, L., Ozcelikay, G., K. Bakirhan, N., & Ozkan, S. A. (2021). Achievements of Mesoporous Carbon Solution and Single-Walled Carbon Nanotube Composite on the Sensitive Electrochemical Assay of Ivabradine. Analytica, 2(4), 195-205. https://doi.org/10.3390/analytica2040017







