Thallium: A Polluting Metal of New Generation. Its Voltammetric Determination in Herbal Medicines in Presence of Metal Interferences
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Reference Solutions
2.2. Sample Preparation
2.3. Voltammetry
2.4. Spectroscopy
3. Results and Discussion
3.1. Aqueous Reference Solutions
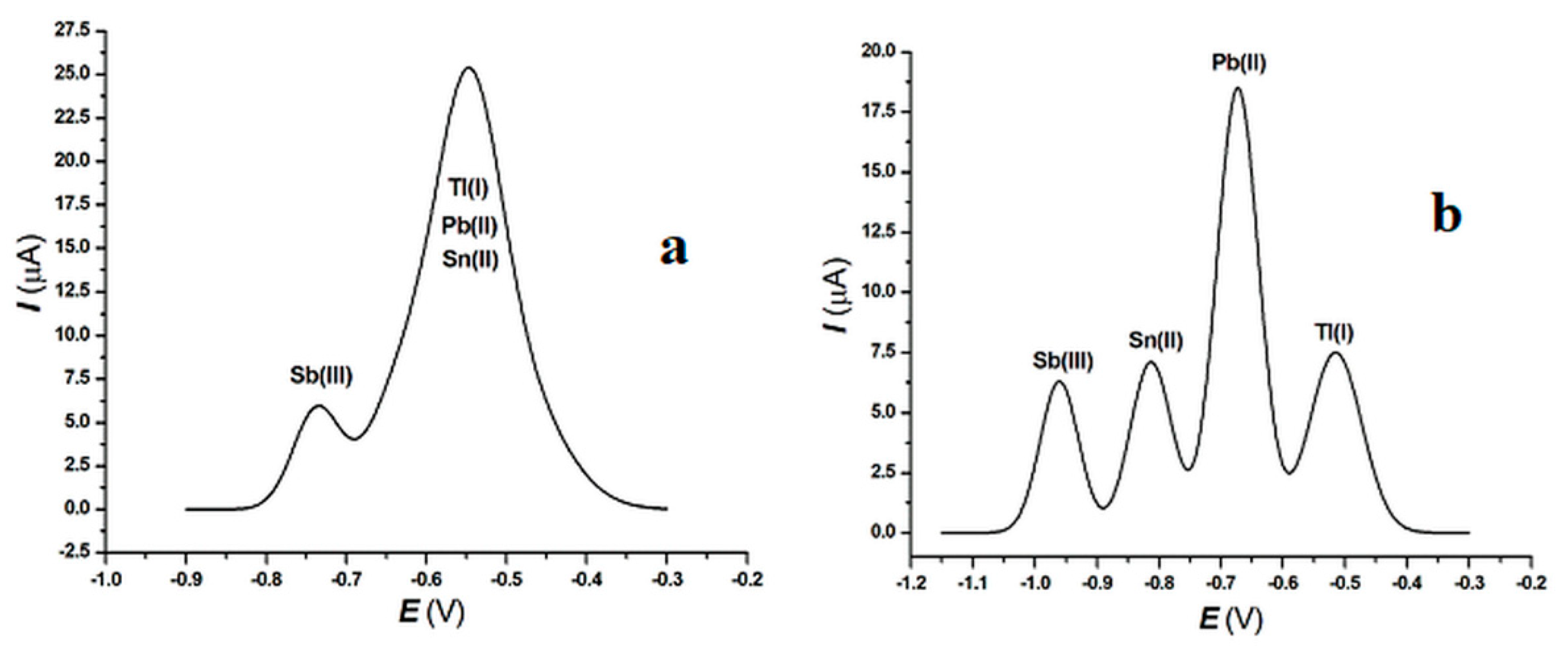
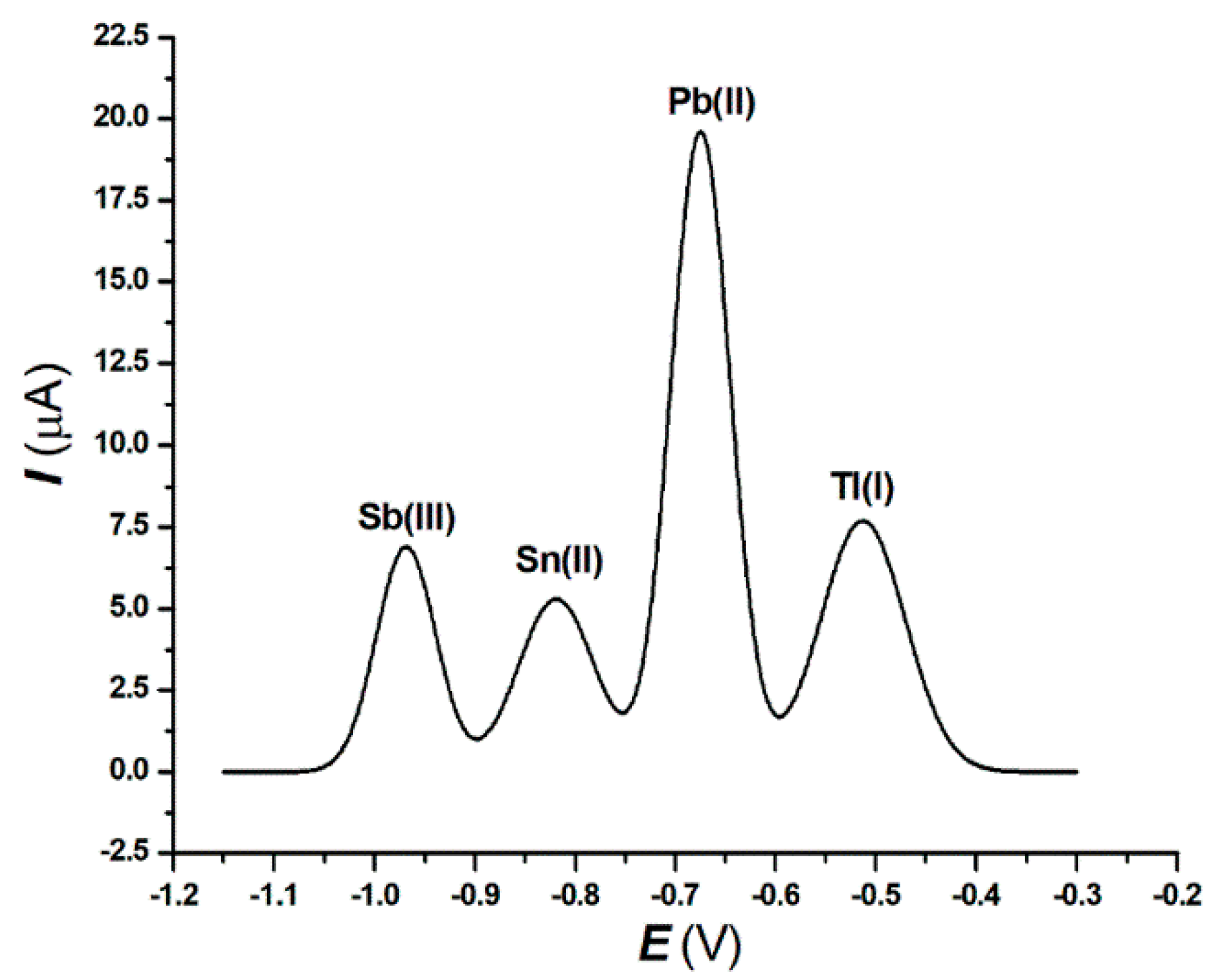
3.2. Interferences from Pb(II), Sn(II), and Sb(III) in the Tl(I) Determination and Choice of the Supporting Electrolyte
3.3. Limits of Detection
3.4. Quality Control and Quality Assessment
3.5. Practical Applications
3.6. Comparison between Spectroscopic and Voltammetric Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Locatelli, C. Metals. In Comprehensive Analytical Chemistry; Picò, Y., Ed.; Elsevier: Amsterdam, The Netherland, 2008; Volume 51, pp. 571–598. [Google Scholar]
- Ferré-Huguet, N.; Martì-Cid, R.; Schuhmacher, M.; Domingo, J.L. Risk assessment of metals from consuming vegetables, fruits and rice grown on soils irrigated with waters of the Ebro river in Catalonia, Spain. Biol. Trace Elem. Res. 2008, 123, 66–79. [Google Scholar] [CrossRef]
- Merian, E.; Anke, M.; Ihnat, M.; Stoeppler, M. Elements and Their Compounds in the Environment—Occurrence, Analysis and Biological Relevance; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Reilly, C. Metal. Contamination of Food—Its Significance for Food Quality and Human Health; Blackwell Science Ltd: Oxford, UK, 2002. [Google Scholar]
- Ebdon, L.; Pitts, L.; Cornelis, R. (Eds.) Trace Element Speciation for Environment, Food and Health; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Melucci, D.; Locatelli, M.; Locatelli, C.; Zappi, A.; De Laurentiis, F.; Carradori, S.; Campestre, C.; Leporini, L.; Zengin, G.; Picot, C.M.N.; et al. A comparative assessment of biological effects and chemical profile of Italian Asphodeline lutea extracts. Molecules 2018, 23, 461. [Google Scholar] [CrossRef] [PubMed]
- Michalski, R.; Szopa, S.; Jabłońska, M.; Łyko, A. Application of hyphenated techniques in speciation analysis of arsenic, antimony, and thallium. Sci. World J. 2012, 2012, 902464. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.S.J.; Lau, J.S.H.; El-Nezami, H. Herbal medicines. Toxicity and recent trends in assessing their potential toxic effects. Adv. Bot. Res. 2012, 62, 365–384. [Google Scholar]
- Zhang, J.; Wider, B.; Shang, H.; Li, X.; Ernst, E. Quality of herbal medicines: Challenges and solutions. Complement. Ther. Med. 2012, 20, 100–106. [Google Scholar] [CrossRef]
- Yuan, X.; Chapman, R.L.; Wu, Z. Analytical methods for heavy metals in herbal medicines. Phytochem. Anal. 2011, 22, 189–198. [Google Scholar] [CrossRef]
- Sahoo, N.; Manchikanti, P.; Dey, S. Herbal drugs: Standards and regulation. Fitoterapia 2010, 81, 462–471. [Google Scholar] [CrossRef]
- Mosihuzzaman, M.; Choudhary, M.I. Protocols on safety, efficacy, standardization and documentation of herbal medicine (IUPAC technical report). Pure Appl. Chem. 2008, 80, 2195–2230. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Supplementary Guidelines on Good Manufacturing Practices for the Manufacture of Herbal Medicine; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- European Medicines Agency (EMEA). Guideline on Specifications: Test Procedures and Acceptance Criteria for Herbal Substances, Herbal Preparations and Herbal Medicinal Products/Traditional Herbal Products; EMEA: London, UK, 2006.
- World Health Organization (WHO). Guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Locatelli, C.; Melucci, D.; Locatelli, M. Toxic metals in herbal medicines. A review. Curr. Bioact. Compd. 2014, 10, 181–188. [Google Scholar] [CrossRef]
- Rao, M.M.; Galib, A.K.M. Detection of toxic heavy metals and pesticide residue in herbal plants which are commonly used in the herbal formulations. Environ. Monit. Assess. 2011, 181, 267–271. [Google Scholar] [CrossRef]
- Kalny, P.; Fijalek, Z.; Daszczuk, A.; Ostapczuk, P. Determination of selected microelements in Polish herbs and their infusions. Sci. Total Environ. 2007, 381, 99–104. [Google Scholar] [CrossRef]
- Basgel, S.; Erdemoglu, S.B. Determination of mineral and trace elements in some medicinal herbs and their infusions consumed in Turkey. Sci. Total Environ. 2006, 359, 82–89. [Google Scholar] [CrossRef]
- Chizzola, R.; Michitsch, H.; Franz, C. Monitoring of metallic micronutriente and heavy metals in herbs, species and medicinal plants from Austria. Eur. Food Res. Technol. 2003, 216, 407–411. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Ma, X.F.; Fan, L.L.; Mao, F.Y.; Tian, H.L.; Xu, R.; Cao, Z.; Zhang, X.H.; Fu, X.Y.; Sui, H. Discrimination of geographical origin of cultivated Polygala tenuifolia based on multi-element fingerprinting by inductively coupled plasma mass spectrometry. Sci. Rep. 2017, 7, 12577. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Liu, Y.Q. Evaluation of trace and toxic element concentrations in Paris polyphylla from China with empirical and chemometric approaches. Food Chem. 2010, 121, 887–892. [Google Scholar] [CrossRef]
- Ma, X.F.; Fan, L.L.; Mao, F.Y.; Zhao, Y.S.; Yan, Y.G.; Tian, H.L.; Xu, R.; Peng, Y.Q.; Sui, H. Discrimination of three Ephedra species and their geographical origins based on multi-element fingerprinting by inductively coupled plasma mass spectrometry. Sci. Rep. 2018, 8, 10271. [Google Scholar] [CrossRef] [PubMed]
- Esteki, M.; Vander Heyden, Y.; Farajmand, B.; Kolahderazi, Y. Qualitativeand quantitative analysis of peanut adulteration in almond powder samples using multi-elemental fingerprinting combined with multivariate data analysis methods. Food Control 2017, 82, 31–41. [Google Scholar] [CrossRef]
- Melucci, D.; Locatelli, M.; De Laurentiis, F.; Zengin, G.; Locatelli, C. Herbal medicines: Application of a sequential voltammetric procedure to the determination of mercury, copper, lead, cadmium and zinc at trace level. Lett. Drug Des. Discov. 2018, 15, 270–280. [Google Scholar] [CrossRef]
- Melucci, D.; Casolari, S.; De Laurentiis, F.; Zappi, A.; Locatelli, M.; Locatelli, C. Toxic metals in Camellia sinensis: Analytical methods, human health risk and regulations. In Agricultural Research Updates; Gorawala, P., Mandhatri, S., Eds.; Nova Science Publ. Inc.: New York, NY, USA, 2019; Volume 26, pp. 99–146. [Google Scholar]
- Melucci, D.; Locatelli, M.; Locatelli, C. Trace level voltammetric determination of heavy metals and total mercury in tea matrices (Camellia sinensis). Food Chem. Toxicol. 2013, 62, 901–907. [Google Scholar] [CrossRef]
- Welz, B.; Sperling, M. Atomic Absorption Spectrometr, 3rd ed.; Wiley VCH: Weinheim, Germany, 1999. [Google Scholar]
- Currie, L.A. Detection: International update, and some emerging dilemmas involving calibration, the blank, and multiple detection decisions. Chemometr. Intell. Lab. Syst. 1997, 37, 151–181. [Google Scholar] [CrossRef]
- Locatelli, C. Simultaneous determination of osmium, ruthenium, copper and lead by electrocatalytic voltammetry. Application to superficial waters. Microchem. J. 2012, 102, 54–60. [Google Scholar] [CrossRef]
- Locatelli, C. Ultratrace osmium, ruthenium and lead in airbornr particulate matter: Peak area as instrumental datum to improve their simultaneous voltammetric determination. Electroanalysis 2012, 24, 2273–2282. [Google Scholar] [CrossRef]
- Fujinaga, T.; Isutsu, K. The Use of Electrochemical Masking for the Polarographic Analysis. Rev. Polarogr. 1961, 9, 36–40. [Google Scholar] [CrossRef][Green Version]
- Miller, J.C.; Miller, J.N. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Ltd.: London, UK, 2010. [Google Scholar]
- Ferreira, S.L.C.; Korn, M.G.A.; Ferreira, H.S.; da Silva, E.G.P.; Araujo, R.G.O.; Souza, A.S.; Macedo, S.M.; Lima, D.C.; de Jesus, R.M.; Amorim, F.A.C.; et al. Application of multivariate techniques in optimization of spectroanalytical methods. Appl. Spectrosc. Rev. 2007, 42, 475–491. [Google Scholar] [CrossRef]
| Voltammetry | Spectroscopy | |
|---|---|---|
| Supporting electrolyte | 0.070 | 0.13 |
| Solution from digestion of Spinach Leaves NIST-SRM 1570a | 2.7 | 4.1 |
| Solution from digestion of Tomato Leaves NIST-SRM 1573a | 3.0 | 4.5 |
| Solution from digestion of Taraxacum officinale weber | 2.9 | 4.3 |
| Solution from digestion of Eucalyptus globulus | 2.4 | 4.9 |
| Solution from digestion of Harpagophytum procumbens DC | 2.7 | 4.7 |
| Spinach Leaves NIST-SRM 1570a | |||||
| Voltammetry | Spectroscopy | ||||
| Results | e (%) | sr (%) | Results | e (%) | sr (%) |
| 46.7 ± 3.6 | −5.8 | 5.7 | 52.7 ± 3.5 | +6.3 | 5.9 |
| Tomato Leaves NIST-SRM 1573a | |||||
| Voltammetry | Spectroscopy | ||||
| Results | e (%) | sr (%) | Results | e (%) | sr (%) |
| 52.6b ± 3.9 | +6.0 | 5.8 | 46.4 ± 3.7 | −6.5 | 6.1 |
| Voltammetry | Spectroscopy | |
|---|---|---|
| Taraxacum officinale weber | 65.1 ± 4.7 | 69.6 ± 4.9 |
| Eucalyptus globulus | 53.9 ± 3.7 | 50.7 ± 3.9 |
| Harpagophytum procumbens DC | 40.9 ± 3.5 | 43.7 ± 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melucci, D.; Casolari, S.; Locatelli, M.; Locatelli, C. Thallium: A Polluting Metal of New Generation. Its Voltammetric Determination in Herbal Medicines in Presence of Metal Interferences. Analytica 2021, 2, 76-83. https://doi.org/10.3390/analytica2030009
Melucci D, Casolari S, Locatelli M, Locatelli C. Thallium: A Polluting Metal of New Generation. Its Voltammetric Determination in Herbal Medicines in Presence of Metal Interferences. Analytica. 2021; 2(3):76-83. https://doi.org/10.3390/analytica2030009
Chicago/Turabian StyleMelucci, Dora, Sonia Casolari, Marcello Locatelli, and Clinio Locatelli. 2021. "Thallium: A Polluting Metal of New Generation. Its Voltammetric Determination in Herbal Medicines in Presence of Metal Interferences" Analytica 2, no. 3: 76-83. https://doi.org/10.3390/analytica2030009
APA StyleMelucci, D., Casolari, S., Locatelli, M., & Locatelli, C. (2021). Thallium: A Polluting Metal of New Generation. Its Voltammetric Determination in Herbal Medicines in Presence of Metal Interferences. Analytica, 2(3), 76-83. https://doi.org/10.3390/analytica2030009










