Abstract
Fluoroquinolones (FQs) have been traditionally used for prophylaxis against bacterial infection. However, the rapid emergence of FQ-resistant Escherichia coli due to overuse and misuse have resulted in an increase in post-biopsy infections. We requested 723 patients undergoing transrectal or transrectal plus transperineal targeted prostate biopsy to provide preprocedure rectal swabs. The rectal swabs were plated onto deoxycholate hydrogen sulfate lactose agar culture and FQ resistance tests were conducted using the disc diffusion method following the guidelines of the Clinical and Laboratory Standards Institute. All patients undergoing biopsy were given a 1.0 g intravenous injection of cefmetazole (CMZ) 30 min before and 12 h after biopsy. Patients with FQ-resistant organisms received an additional 1.0 g intravenous injection of CMZ every 12 h for an additional 1.5 days, while those without FQ-resistant organisms received levofloxacin 500 mg for 4 days. We evaluated infectious symptoms during the 30 days after the biopsy. We also evaluated the incidence of acute prostatitis within 7 days after the biopsy and isolation rates of FQ-resistant strains. A total of 289 patients (40%) had FQ-resistant isolates on rectal swabs. The overall infectious complication rate was 0.69%. Two patients with FQ-resistant isolates and three patients without them experienced infectious episodes. One patient with FQ-resistant isolates and two patients without them suffered acute prostatitis. The difference in the rates of infectious complication and acute prostatitis rates between FQ-resistant and FQ-susceptible carriers were not significant (p = 1.0 and 1.0, respectively). Post-biopsy sepsis was identified in one patient (0.14%) who had FQ-resistant Escherichia coli. Targeted antimicrobial prophylaxis with cefmetazole based on presence of FQ-resistant isolates on rectal swabs may prevent post-prostate biopsy infectious complications, especially in geographic lesions with a high incidence of FQ-resistant strains in rectal flora.
1. Introduction
Although the mortality of prostate cancer (PCa) has been slightly decreasing recently, it is still the main malignancy in Japan and in Western countries [1]. Transrectal and transperineal prostate biopsies are mandatory for histological confirmation of the diagnosis. In practice, transrectal prostate biopsy is easy to perform in an outpatient setting with or without local anesthesia. However, transrectal biopsies can cause severe infections such as sepsis, prostatitis, and urinary tract infections in comparison to transperineal prostate biopsies [2,3].
Fluoroquinolones (FQs) have been traditionally used for prophylaxis against bacterial infection after transrectal prostate biopsy because of their coverage against common causative bacteria and favorable prostatic penetration [2]. However, the rapid emergence of FQ-resistant Escherichia coli (E. coli) due to overuse and misuse of FQs has increased post-biopsy infections [2,4,5]. The European Commission have prohibited the use of FQs and recommended the use of fosfomycin trometamol, cephalosporins, and aminoglycosides for antimicrobial prophylaxis [6]. On the other hand, the American Urological Association recommends applying FQs or cephalosporins (most commonly, ceftriaxone) together with an aminoglycoside [7]. The prevalence of FQ-resistant bacteria in the rectum of patients undergoing a transrectal biopsy has been reported to be approximately 20%, and the frequency of FQ-resistant bacteria in Asia is greater than that reported in Western countries [8,9,10,11]. Therefore, knowledge of geographical differences in antibiotic resistance would be required to select appropriate prophylactic antibiotics for reducing post-biopsy infectious complications [12].
To overcome this complication related to FQ-resistant Enterobacteriaceae, a candidate approach is rectal culture-based antibiotic prophylaxis. This strategy may reduce the use of therapeutic antibiotics after prostate biopsies and will prevent the development of drug-resistant strains. Taylor first reported that targeted antimicrobial prophylaxis using rectal swab cultures was associated with a notable decrease in the incidence of infectious complications after transrectal prostate biopsy caused by FQ-resistant bacteria [13]. Among the 112 men who underwent rectal swabs before transrectal biopsy, 22% harbored FQ-resistant organisms and all these men followed the targeted antimicrobial prophylaxis approach [13]. As a result, none had an infectious complication [13]. In contrast, 9 (2.6%) of the 335 men undergoing empirical prophylaxis had an infectious complication [13]. It is noteworthy that seven of these infections were due to FQ-resistant strains [13]. Although targeted antimicrobial prophylaxis is a theoretical approach for preventing post-prostate biopsy infections, there are conflicting reports regarding the impact of rectal culture-based targeted antimicrobial prophylaxis for reducing infectious complications and/or cost of care [13,14,15,16].
Augmented prophylaxis is another strategy to prevent infectious complications after prostate biopsy under the high prevalence of FQ-resistant strains. The idea is to use two or more different classes of antibiotics, broadening the antimicrobial spectrum to cover possible resistance to a single antibiotic [6]. Most randomized control studies compared augmented prophylaxis including FQ with another antibiotic and empirical monoprophylaxis [6]. However, no recommendation can be derived from the previous randomized control studies at present.
The European Association of Urology recommends selecting the transperineal approach for prostate biopsy, which is the least contaminating method, according to several meta-analyses and a systematic review [6]. Transperineal prostate biopsies were associated with significantly fewer infectious complications compared with transrectal prostate biopsies (risk ratio 0.55) [3].
We have started rectal swab cultures before transrectal prostate biopsies and isolated FQ-resistant organisms since 2013 in our institution. According to the high positivity of FQ-resistant rectal flora, we started a targeted antibiotic prophylaxis to reduce post-prostate biopsy infectious complications. In this study, we retrospectively evaluated the incidence of infectious complications after prostate biopsies and validated our antimicrobial prophylaxis approach in its ability to reduce the rate of complications.
2. Materials and Methods
2.1. Study Population, Rectal Swab Method, and Endpoints
From March 2013 to December 2019, we requested 723 patients undergoing transrectal or transrectal plus transperineal targeted prostate biopsies to provide preprocedure rectal swabs. Transrectal biopsy was performed after rectal povidone–iodine preparation without anesthesia and the transrectal plus transperineal targeted prostate biopsy was also performed after rectal povidone–iodine preparation with spinal or general anesthesia. We obtained demographic data on all patients and their history of antibiotic use within the preceding 12 months. This clinical study was approved by the Mie University Institutional Review Board (#H2020-017). Indications for performing a prostate biopsy were suspicious characteristics on a digital rectal examination or elevated prostate-specific antigen levels. In addition, systematic plus targeted prostate biopsies were recommended for patients with a Prostate Imaging-Reporting and Data System (PI-RADS) score of 3 or higher on multiparametric or biparametric magnetic resonance imaging (MRI). Patients who had prior complications with cephalosporins or fluoroquinolones were excluded.
Rectal swabs were plated onto deoxycholate hydrogen sulfate lactose (DHL) agar (Kyokuto Pharmaceutical Industrial Co., Ltd., Tokyo, Japan) and incubated overnight at 35 °C in ambient air. FQ resistance tests were conducting using the disc diffusion method following the guidelines of the Clinical and Laboratory Standards Institute using commercially available antibiotic discs.
The primary endpoint of this study was to retrospectively analyze the infectious complication rates during the 30 days following the biopsy using electronic medical records. The secondary endpoints were to examine the prevalence of FQ-resistant organisms by rectal swab tests preceding prostate biopsies at our institution and the incidence rate of acute prostatitis rates within 7 days following a prostate biopsy [15,17]. Blood culture and urine culture were carried out to diagnose an acute prostatitis.
2.2. Antibiotic Prophylaxis before and after Prostate Biopsy
All patients undergoing biopsy were given a 1.0 g intravenous injection of cefmetazole (CMZ) 30 min before and 12 h after the biopsy. After disinfection of the rectum with povidone–iodine, 12 cores of prostate tissue were collected in most cases by transrectal ultrasonography-guided biopsy with or without additional transperineal targeted biopsy. If the DHL agar with an antibiotic disc had no colonies, we used levofloxacin (LVFX) 500 mg for 4 days. If DHL agar with the disc had colonies, we carried out 1.0 g intravenous injection of CMZ every 12 h for an additional 1.5 days (Figure 1). We evaluated infectious symptoms during the 30 days after the biopsy using electronic medical records.
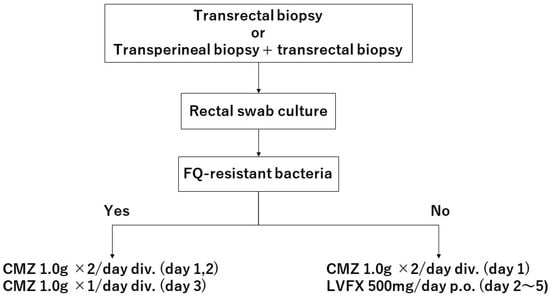
Figure 1.
A flow chart of antibiotic prophylaxis in patients undergoing prostate biopsies. FQ, fluoroquinolones; CMZ, cefmetazole; LVFX, levofloxacin.
2.3. Statistical Analysis
Differences in the demographic features between patients with FQ-resistant bacteria and without it were statistically compared using the Mann–Whitney U test or chi-square tests and p-values less than 0.05 were considered significant. All analyses were performed using EZR version 1.61 (Jichi Medical University Saitama Medical Center, Saitama, Japan) [18].
3. Results
Among the 723 cases, a total of 289 patients (40%) had FQ-resistant isolates on rectal swabs, while 433 patients did not carry them. The median age, body mass index, serum PSA level, history of prior prostate biopsy, presence or absence of diabetes, and use of antimicrobial agents within the past year were not significantly different between patients with and without FQ-resistant bacteria, but the prostate volume was significantly smaller in patients with FQ-resistant bacteria (p = 0.011) (Table 1). The overall infectious complication rate was 0.69%. Two patients with FQ-resistant isolates and three patients without them experienced infectious episodes, but the difference was not significant (p = 1.0). Among the patients with infectious complications, the diagnoses of two patients were not confirmed as a bacterial acute prostatitis, since one was suspected to be a diverticulitis of the colon and the other developed a fever 15 days after the prostate biopsy (Table 2). Therefore, the actual cases with acute prostatitis according to the definition were one patient with FQ-resistant isolates and two patients without them, and the difference was not significant (p = 1.0). Post-biopsy sepsis was identified in one patient (0.14%) who had FQ-resistant E. coli.

Table 1.
Patient’s characteristics.

Table 2.
Patients’ clinical parameters and infectious complications.
4. Discussion
In the present study, the high prevalence (40%) of FQ-resistant bacteria in the rectal swabs of men undergoing transrectal prostate biopsy was revealed in our institution. The FQ-resistant bacterial rate in rectal cultures before transrectal prostate biopsy was 9.62–48.1% in previous reports [9,11,13,19,20,21]. The prevalence of FQ-resistant bacteria varies significantly among different geographical areas [22]. A nationwide multi-center survey investigating the incidence of infections following prostate biopsies was conducted [23]. Among patients with positive culture findings, E. coli was the most frequently isolated strain. Moreover, among the E. coli strains isolated by urine culture, 66.7% of them produced extended-spectrum β-lactamase (ESBL) and 77.8% showed ofloxacin resistance [23]. Similarly, among the E. coli strains isolated by blood culture, 66.7% produced ESBL and 100% showed levofloxacin resistance [23]. As the results show, E. coli is the most commonly isolated organism from post-biopsy infections, and FQ-resistant and extended-spectrum β-lactamase (ESBL)-producing types were the two important pathogens in post-biopsy infections [24]. Infections caused by a transrectal biopsy depend upon the bacterial flora harbored in the rectum, which was introduced into the urinary tract or into the bloodstream by perforating the rectal mucosa with the biopsy needle [19,25,26]. Therefore, it is critical to select the appropriate antimicrobial prophylaxis depending on the risk factors for infectious complications after transrectal prostate biopsy which is increasingly linked with FQ-resistant strains.
Several factors associated with FQ-resistant bacteria on rectal swabs in men undergoing transrectal biopsy have been reported. Tan et al. reported that an increasing patient age, use of antimicrobials within the last 6 months, and ethnicity were associated with a higher risk of harboring FQ-resistant bacteria in the rectal vault [27]. The use of fluoroquinolones less than 6 months before biopsy was also reported as a risk factor for fecal carriage of FQ-resistant strains [28]. Kamei et al. showed that diabetes was a risk factor for antimicrobial resistance carriage before biopsy, which included carriers of FQ-resistant and ESBL-producing E. coli [29]. We also investigated several factors using our cohorts to detect any association with FQ-resistant strains carriage in the rectal swabs, but only prostate volume was significantly different between FQ-resistant strain carriers and non-carriers. The reason for the difference remains unknown.
Recently, a large randomized trial evaluated rectal culture-based prophylaxis and its effect on infection rates of transrectal prostate biopsies [15]. After rectal swab collection, the patients were randomized 1:1 to receive empirical prophylaxis with oral ciprofloxacin or culture-based prophylaxis. Among the 1288 patients with available data, infection rates within 7 days after biopsy were 4.3% and 2.5%, respectively, with no statistically significant difference. However, the presence of ciprofloxacin-resistant strains in rectal flora resulted in a 6.2-fold higher risk of early post-biopsy infection in the empirical prophylaxis cohort compared to almost identical rates in the culture-based prophylaxis cohort. In this study, ciprofloxacin-resistant bacteria were detected in 15.2% of patients [15], which is relatively low compared to our results. Therefore, the results should be interpreted carefully in geographic regions with higher rates of resistant strains in rectal flora, where the impact of culture-based prophylaxis will likely be more significant.
Although ESBL-producing Enterobacteriaceae have been increasingly identified in post-biopsy infection, we focused on FQ-resistant strains in the rectal swabs before biopsy since the rate of resistance to FQ among ESBL-producing E. coli ranges from 50% up to 100% [30,31]. Additionally, the national surveillance of the prevalence of ESBL-producing strains in Enterobacteriaceae was 3.1–6.2% at the time of planning this study [32,33]. On the other hand, our hospital belongs to an academic medical center and thus we received a lot of high-risk candidates for prostate biopsy. Therefore, we selected CMZ as the targeted antimicrobial prophylaxis for the carriers with FQ-resistant strains since the sensitivity of CMZ is also high against ESBL-producing E. coli in Japan [34,35]. As for carriers of FQ-susceptible strains, since we could not completely exclude the possibility of non-FQ-resistant, ESBL-producing Enterobacteriaceae, we selected CMZ together with LVFX as targeted antimicrobial prophylaxes according to a report by Shigemura [36].
According to our targeted antimicrobial prophylaxis strategy, the incidence of post-biopsy infectious complications was 0.69%, which was lower compared to that reported by national survey 15 years ago in Japan (1.1%) but was similar to recent reports by Sadahira (0.6%) and Hiyama (0.7%) [10,19,37]. Moreover, the incidence of post-biopsy acute prostatitis was also low (0.41%). Selecting CMZ as the targeted antimicrobial prophylaxis may have contributed to this low incidence.
Our study revealed no difference in the incidence of infectious complications and acute prostatitis between patients with FQ-susceptible strains and FQ-resistant ones. The low incidences may partially contribute to the non-statistical difference in these outcomes between the two groups. Additionally, since patients with FQ-susceptible strains had a much larger prostate than patients with FQ-resistant ones (which is a risk factor for post-biopsy prostatitis), we should also carefully interpret the results because this bias may distort the results. Despite this, our approach may be effective for preventing post-biopsy prostatitis, even if the FQ-resistant bacterial rate in the rectal culture before transrectal prostate biopsy was high as detected in our cohort or as Sadahira reported in a recent Japanese cohort [19].
While the optimal duration and regimen for reducing the risk of infectious complications for men undergoing prostate biopsy is still not standardized, a single dose of LVFX was recommended for low-risk cases and 1-day intravenous piperacillin and tazobactam was recommended to high-risk patients according to the 2015 guidelines for the prevention of preoperative infections published by the Japanese Urological Association [38]. According to a recent systematic review, a full 1-day prophylaxis antibiotic is recommended [2]. Therefore, our antimicrobial prophylaxis for transrectal prostate biopsy might be overuse and according to the results of the study, we have recently changed our protocol for antimicrobial prophylaxis. A single dose of 500 mg LVFX (before biopsy) for patients with FQ-susceptible strains and 1-day intravenous 1.0 g CMZ (30 min before and 4 h after biopsy) for patients with FQ-resistant strains are currently selected for the prophylaxis at our institution.
The reduction of infectious complications also depends upon nonantibiotics strategies, such as consideration of biopsy route (transperineal biopsy rather than transrectal biopsy), rectal preparation with povidone–iodine, and addition of natural flavonoids to antibiotics [3,39]. In Japan, magnetic resonance imaging–ultrasound fusion-guided biopsy has been covered by government health insurance since April 2022, and therefore, a transperineal approach using this method is currently commonly used at our institution.
The present study has some limitations. First, this study is a retrospective analysis. However, during the study time, most men undergoing transrectal prostate biopsy received a rectal swab examination and followed the regimens of antibiotic prophylaxis as described. Second, we only evaluated the fecal carriage of FQ-insusceptible bacteria at a single point in time and did not evaluate ESBL-producing strains. The optimal culture strategy needs to be established in consideration of local antimicrobial resistance patterns. Third, although most cases received rectal swabs within one month before transrectal prostate biopsy, the timing was not standardized. Liss et al. reported that screening rectal cultures obtained from the office visit before biopsy (approximately 2 weeks before) and the cultures performed at prostate biopsy showed similar results in 93% of the cases [40]. The optimal timing of rectal swab collection should be established. Fourth, we did not evaluate and consider the cost effectiveness of rectal culture-based antibiotic prophylaxis for transrectal prostate biopsy [14].
Despite the limitations, our data suggest that rectal swabs before transrectal prostate biopsy and targeted antibiotic prophylaxis based on the detection of FQ-resistant strains could reduce post-biopsy infectious complications even if the rate of FQ-insusceptible bacteria carriers who received the biopsy was as high as 40%. The optimal antibiotics agents and duration need to be further analyzed.
5. Conclusions
Targeted antimicrobial prophylaxis based on the presence of FQ-resistant isolates on rectal swabs with cefmetazole yields similar infection rates in FQ-resistant strain carriers compared to non-carriers, and was validated in this study, although geographic microbial heterogeneity can limit the generalizability of the results.
Author Contributions
Conceptualization, Y.Y. and H.K.; methodology, Y.Y. and H.K.; formal analysis, S.H.; investigation, S.H.; resources, S.H., Y.Y., H.K., T.N., M.K. (Momoko Kato), Y.S., T.S., M.K. (Manabu Kato), S.M., K.N. and T.I.; data curation, S.H.; writing—original draft preparation, S.H. and T.I.; writing—review and editing, Y.Y., H.K., T.N., M.K. (Momoko Kato), Y.S., T.S., M.K. (Manabu Kato), S.M., K.N. and T.I.; visualization, S.H.; supervision, T.I.; project administration, T.I.; funding acquisition, T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Grants-in-Aid for Scientific Research (21H03066) from the Japan Society for the Promotion of Science (T.I.).
Institutional Review Board Statement
This clinical study was approved by the Mie University Institutional Review Board (#H2020-017). This study was performed in accordance with the Declaration of Helsinki.
Informed Consent Statement
As the study design was retrospective and observational, the requirement for obtaining informed consent from the participants was waived.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We should like to acknowledge all the clinical staff of the Department of Nephron-Urologic Surgery and Clinical Laboratory Mie University Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Katanoda, K.; Hori, M.; Saito, E.; Shibata, A.; Ito, Y.; Minami, T.; Ikeda, S.; Suzuki, T.; Matsuda, T. Updated Trends in Cancer in Japan: Incidence in 1985–2015 and Mortality in 1958–2018—A Sign of Decrease in Cancer Incidence. J. Epidemiol. 2021, 31, 426–450. [Google Scholar] [CrossRef]
- Pilatz, A.; Dimitropoulos, K.; Veeratterapillay, R.; Yuan, Y.; Omar, M.I.; MacLennan, S.; Cai, T.; Bruyère, F.; Bartoletti, R.; Köves, B.; et al. Antibiotic Prophylaxis for the Prevention of Infectious Complications following Prostate Biopsy: A Systematic Review and Meta-Analysis. J. Urol. 2020, 204, 224–230. [Google Scholar] [CrossRef]
- Pradere, B.; Veeratterapillay, R.; Dimitropoulos, K.; Yuan, Y.; Omar, M.I.; MacLennan, S.; Cai, T.; Bruyère, F.; Bartoletti, R.; Köves, B.; et al. Nonantibiotic Strategies for the Prevention of Infectious Complications following Prostate Biopsy: A Systematic Review and Meta-Analysis. J. Urol. 2021, 205, 653–663. [Google Scholar] [CrossRef]
- Ishikawa, K.; Matsumoto, T.; Yasuda, M.; Hattori, R.; Uehara, S.; Muratani, T.; Yagisawa, M.; Sato, J.; Niki, Y.; Totsuka, K.; et al. The nationwide study of bacterial pathogens associated with urinary tract infections conducted by the Japanese Society of Chemotherapy. J. Infect. Chemother. 2011, 17, 126–138. [Google Scholar] [CrossRef]
- Carignan, A.; Roussy, J.F.; Lapointe, V.; Valiquette, L.; Sabbagh, R.; Pépin, J. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: Time to reassess antimicrobial prophylaxis? Eur. Urol. 2012, 62, 453–459. [Google Scholar] [CrossRef]
- Pilatz, A.; Veeratterapillay, R.; Dimitropoulos, K.; Omar, M.I.; Pradere, B.; Yuan, Y.; Cai, T.; Mezei, T.; Devlies, W.; Bruyère, F.; et al. European Association of Urology Position Paper on the Prevention of Infectious Complications Following Prostate Biopsy. Eur. Urol. 2021, 79, 11–15. [Google Scholar] [CrossRef]
- Lightner, D.J.; Wymer, K.; Sanchez, J.; Kavoussi, L. Best Practice Statement on Urologic Procedures and Antimicrobial Prophylaxis. J. Urol. 2020, 203, 351–356. [Google Scholar] [CrossRef]
- Togo, Y.; Yamamoto, S. Prevention of infectious complications after prostate biopsy procedure. Int. J. Urol. 2017, 24, 486–492. [Google Scholar] [CrossRef]
- Chung, H.S.; Hwang, E.C.; Yu, H.S.; Jung, S.I.; Lee, S.J.; Lim, D.H.; Cho, W.J.; Choe, H.S.; Park, S.W. Prevalence of fluoroquinolone-resistant rectal flora in patients undergoing transrectal ultrasound-guided prostate needle biopsy: A prospective multicenter study. Int. J. Urol. 2018, 25, 278–283. [Google Scholar] [CrossRef]
- Hiyama, Y.; Takahashi, S.; Uehara, T.; Ichihara, K.; Hashimoto, J.; Matsukawa, M.; Taguchi, K.; Kunishima, Y.; Hotta, H.; Yanase, M.; et al. Selective culture of Escherichia coli to prevent infective complications of transrectal ultrasound-guided prostate biopsy: Clinical efficacy and analysis of characteristics of quinolone-resistant Escherichia coli. Int. J. Urol. 2019, 26, 655–660. [Google Scholar] [CrossRef]
- Liss, M.A.; Taylor, S.; Batura, D.; Steensels, D.; Chayakulkeeree, M.; Soenens, C.; Rao, G.G.; Dash, A.; Park, S.; Patel, N.; et al. Fluoroquinolone Resistant Rectal Colonization Predicts Risk of Infectious Complications after Transrectal Prostate Biopsy. J. Urol. 2014, 192, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, R.S.; Schaeffer, E.M.; Shore, N.D. The Effect of Local Antibiogram-based Augmented Antibiotic Prophylaxis on Infection-related Complications Following Prostate Biopsy. Rev. Urol. 2019, 21, 93–101. [Google Scholar] [PubMed]
- Taylor, A.K.; Zembower, T.R.; Nadler, R.B.; Scheetz, M.H.; Cashy, J.P.; Bowen, D.; Murphy, A.B.; Dielubanza, E.; Schaeffer, A.J. Targeted Antimicrobial Prophylaxis Using Rectal Swab Cultures in Men Undergoing Transrectal Ultrasound Guided Prostate Biopsy is Associated with Reduced Incidence of Postoperative Infectious Complications and Cost of Care. J. Urol. 2012, 187, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Tops, S.C.; Kolwijck, E.; Koldewijn, E.L.; Somford, D.M.; Delaere, F.J.; van Leeuwen, M.A.; Breeuwsma, A.J.; de Vocht, T.F.; Broos, H.J.; Schipper, R.A.; et al. Cost Effectiveness of Rectal Culture-based Antibiotic Prophylaxis in Transrectal Prostate Biopsy: The Results from a Randomized, Nonblinded, Multicenter Trial. Eur. Urol. Open Sci. 2023, 50, 70–77. [Google Scholar] [CrossRef]
- Tops, S.C.; Kolwijck, E.; Koldewijn, E.L.; Somford, D.M.; Delaere, F.J.; van Leeuwen, M.A.; Breeuwsma, A.J.; de Vocht, T.F.; Broos, H.J.; Schipper, R.A.; et al. Rectal Culture-Based Versus Empirical Antibiotic Prophylaxis to Prevent Infectious Complications in Men Undergoing Transrectal Prostate Biopsy: A Randomized, Nonblinded Multicenter Trial. Clin. Infect. Dis. 2023, 76, 1188–1196. [Google Scholar] [CrossRef]
- Scott, S.; Harris, P.N.; Williamson, D.A.; Liss, M.A.; Doi, S.A.R.; Roberts, M.J. The effectiveness of targeted relative to empiric prophylaxis on infectious complications after transrectal ultrasound-guided prostate biopsy: A meta-analysis. World J. Urol. 2018, 36, 1007–1017. [Google Scholar] [CrossRef]
- Özden, E.; Bostanci, Y.; Yakupoglu, K.Y.; Akdeniz, E.; Yılmaz, A.F.; Tulek, N.; Sarıkaya, S. Incidence of Acute Prostatitis Caused by Extended-spectrum β-Lactamase-producing Escherichia coli after Transrectal Prostate Biopsy. Urology 2009, 74, 119–123. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Sadahira, T.; Wada, K.; Araki, M.; Ishii, A.; Watanabe, T.; Nasu, Y.; Tsugawa, M.; Takenaka, T.; Nasu, Y.; Kumon, H. Impact of selective media for detecting fluoroquinolone-insusceptible/extended-spectrum beta-lactamase-producing Escherichia coli before transrectal prostate biopsy. Int. J. Urol. 2017, 24, 842–847. [Google Scholar] [CrossRef]
- Sieczkowski, M.; Gibas, A.; Bronk, M.; Matuszewski, M. Fluoroquinolone-based antimicrobial prophylaxis in patients undergoing transrectal ultrasound-guided prostate biopsy. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1815–1821. [Google Scholar] [CrossRef]
- Tsu, J.H.-L.; Ma, W.-K.; Chan, W.K.-W.; Lam, B.H.-S.; To, K.-C.; To, W.-K.; Ng, T.-K.; Liu, P.-L.; Cheung, F.-K.; Yiu, M.-K. Prevalence and Predictive Factors of Harboring Fluoroquinolone-resistant and Extended-spectrum β-Lactamase–producing Rectal Flora in Hong Kong Chinese Men Undergoing Transrectal Ultrasound-guided Prostate Biopsy. Urology 2015, 85, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Nys, S.; Okeke, I.; Kariuki, S.; Dinant, G.J.; Driessen, C.; Stobberingh, E.E. Antibiotic resistance of faecal Escherichia coli from healthy volunteers from eight developing countries. J. Antimicrob. Chemother. 2004, 54, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Togo, Y.; Kubo, T.; Taoka, R.; Hiyama, Y.; Uehara, T.; Hashimoto, J.; Kurimura, Y.; Takahashi, S.; Tsukamoto, T.; Miyazaki, J.; et al. Occurrence of infection following prostate biopsy procedures in Japan. J. Infect. Chemother. 2014, 20, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.J.; Williamson, D.A.; Hadway, P.; Doi, S.A.; Gardiner, R.A.; Paterson, D.L. Baseline prevalence of antimicrobial resistance and subsequent infection following prostate biopsy using empirical or altered prophylaxis: A bias-adjusted meta-analysis. Int. J. Antimicrob. Agents 2014, 43, 301–309. [Google Scholar] [CrossRef]
- Batura, D.; Rao, G.G.; Nielsen, P.B. Prevalence of antimicrobial resistance in intestinal flora of patients undergoing prostatic biopsy: Implications for prophylaxis and treatment of infections after biopsy. BJU Int. 2010, 106, 1017–1020. [Google Scholar] [CrossRef]
- Zowawi, H.M.; Harris, P.N.; Roberts, M.J.; Tambyah, P.A.; Schembri, M.A.; Pezzani, M.D.; Williamson, D.A.; Paterson, D.L. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat. Rev. Urol. 2015, 12, 570–584. [Google Scholar] [CrossRef]
- Tan, W.P.; Papagiannopoulos, D.; Latchamsetty, K.C.; Wilson, N.; O’block, N.; Raff, L.; Lora, A.M.; Coogan, C.L.; Abern, M.R. Predictors of fluoroquinolone-resistant bacteria in the rectal vault of men undergoing prostate biopsy. Prostate Cancer Prostatic Dis. 2019, 22, 268–275. [Google Scholar] [CrossRef]
- Steensels, D.; Slabbaert, K.; De Wever, L.; Vermeersch, P.; Van Poppel, H.; Verhaegen, J. Fluoroquinolone-resistant E. coli in intestinal flora of patients undergoing transrectal ultrasound-guided prostate biopsy-should we reassess our practices for antibiotic prophylaxis? Clin. Microbiol. Infect. 2012, 18, 575–581. [Google Scholar] [CrossRef]
- Kamei, J.; Yagihara, Y.; Kume, H.; Horiuchi, T.; Sato, T.; Nakagawa, T.; Fujimura, T.; Fukuhara, H.; Moriya, K.; Homma, Y. Prevalence and characteristics of fecal antimicrobial-resistant Escherichia coli in a cohort of Japanese men undergoing prostate biopsy. Int. J. Urol. 2017, 24, 295–300. [Google Scholar] [CrossRef]
- Williamson, D.A.; Barrett, L.K.; Rogers, B.A.; Freeman, J.T.; Hadway, P.; Paterson, D.L. Infectious Complications Following Transrectal Ultrasound-Guided Prostate Biopsy: New Challenges in the Era of Multidrug-Resistant Escherichia coli. Clin. Infect. Dis. 2013, 57, 267–274. [Google Scholar] [CrossRef]
- Miyazaki, M.; Yamada, Y.; Matsuo, K.; Komiya, Y.; Uchiyama, M.; Nagata, N.; Takata, T.; Jimi, S.; Imakyure, O. Change in the Antimicrobial Resistance Profile of Extended-Spectrum β-Lactamase-Producing Escherichia coli. J. Clin. Med. Res. 2019, 11, 635–641. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ishii, Y.; Iwata, M.; Watanabe, N.; Shinagawa, M.; Yasujima, M.; Suwabe, A.; Kuroda, M.; Kaku, M.; Kitagawa, M.; et al. Nationwide surveillance of parenteral antibiotics containing meropenem activities against clinically isolated strains in 2009. Jpn. J. Antibiot. 2011, 64, 53–95. [Google Scholar] [PubMed]
- Yamaguchi, K.; Ishii, Y.; Tateda, K.; Iwata, M.; Watanabe, N.; Shinagawa, M.; Kayaba, H.; Kimura, M.; Suwabe, A.; Kaku, M.; et al. Nationwide surveillance of parenteral antibiotics containing meropenem activities against clinically isolated strains in 2012. Jpn. J. Antibiot. 2014, 67, 73–107. [Google Scholar] [PubMed]
- Namikawa, H.; Yamada, K.; Fujimoto, H.; Oinuma, K.-I.; Tochino, Y.; Takemoto, Y.; Kaneko, Y.; Shuto, T.; Kakeya, H. Clinical Characteristics of Bacteremia Caused by Extended-spectrum Beta-lactamase-producing Escherichia coli at a Tertiary Hospital. Intern. Med. 2017, 56, 1807–1815. [Google Scholar] [CrossRef]
- Nasu, Y.; Sako, S.; Yano, T.; Kosaka, N. Surveillance of Antimicrobial Resistant Escherichia coli by Rectal Swab Method—Annual Change of Prevalence of Quinolone-resistant and ESBL Producing Strains from 2009 to 2013. J. Jpn. Assoc. Infect. Dis. 2015, 89, 583–587. [Google Scholar] [CrossRef]
- Shigemura, K.; Tanaka, K.; Yamamichi, F.; Arakawa, S.; Fujisawa, M. Prophylactic efficacy of cephamycin plus fluoroquinolones in high risk patients on inhibiting infectious complications after transrectal prostate biopsy. J. Chemother. 2016, 28, 513–516. [Google Scholar] [CrossRef]
- Kakehi, Y.; Naito, S. Japanese Urological Association Complication rates of ultrasound-guided prostate biopsy: A nation-wide survey in Japan. Int. J. Urol. 2008, 15, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Shigemura, K.; Kiyota, H.; Wada, K.; Hayami, H.; Yasuda, M.; Takahashi, S.; Ishikawa, K.; Hamasuna, R.; Arakawa, S.; et al. Essential Japanese guidelines for the prevention of perioperative infections in the urological field: 2015 edition. Int. J. Urol. 2016, 23, 814–824. [Google Scholar] [CrossRef]
- Lin, R.D.; Chin, Y.P.; Lee, M.H. Antimicrobial activity of antibiotics in combination with natural flavonoids against clinical extended-spectrum beta-lactamase (ESBL)-producing Klebsiella pneumoniae. Phytother. Res. 2005, 19, 612–617. [Google Scholar] [CrossRef]
- Liss, M.A.; Nakamura, K.K.; Meuleners, R.; Kolla, S.B.; Dash, A.; Peterson, E.M. Screening Rectal Culture to Identify Fluoroquinolone-resistant Organisms Before Transrectal Prostate Biopsy: Do the Culture Results between Office Visit and Biopsy Correlate? Urology 2013, 82, 67–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).