Abstract
Prostate cancer is the second most common form of cancer in men and the fifth leading cause of death among men worldwide. Men with metastatic castration-resistant prostate cancer (mCRPC) often have BRCA-1 or BRCA-2 gene mutations which can make them sensitive to poly-(ADP-ribose) polymerase inhibitors or PARP inhibitors (PARPi), such as Olaparib, Rucaparib, and Niraparib. Although significant advances have been made with PARPi and the prognosis of patients with mCRPC has improved dramatically, resistance often constitutes a challenge that frequently results in tumor escape. This present communication paper explores the role of PARPi in BRCA-positive prostate cancer and sheds light on numerous published and ongoing clinical trials that will determine the future of PARPi at various tumor stages as a monotherapy or polytherapy regime.
1. Introduction
Prostate cancer is the second most common form of cancer in men and the fifth leading cause of death among men worldwide. In 2020 alone, about 1.4 million new cases of prostate cancer were reported globally, accounting for 7.3% of all malignancies in males. The incidence of prostate cancer varies greatly from country to country, especially amongst countries with a high HDI (human development index) and those with a low HDI (37.5 and 11.3 per 100,000, respectively). Geographically, Europe accounts for more than a third of all registered prostate cancer cases, followed by Asia (24%), Northern America (19%), Latin America and the Caribbean (14%) and Africa (4%) [1]. It is predicted that for every 14 years of life, the prevalence of cancer doubles with age, and this proves to be one of the most significant prognostic factors for determining the prevalence of prostate cancer [2]. Furthermore, patients over 65 years old experience an independently higher predictive risk of mortality from prostate cancer [3]. In terms of mortality, the Caribbean has the highest death rate (27.9 per 100,000), while South-Central Asia has the lowest death rate (3.1 per 100,000) [1].
Despite the high incidence rates of prostate cancer worldwide, most cases are identified at an early stage, thus drastically affecting the overall survival rate. Men with a diagnosis of prostate cancer in the US are predicted to have a 5-year survival rate of 98% (all SEER—Surveillance, Epidemiology and End Result stages combined), which rises to almost 100% when the disease is diagnosed at an early stage (localized and regional stages) [4]. The data from the EUROCARE-5 study revealed a life expectancy of fatal cases for patients with prostate cancer aged 65–74 years of 7.7 years, with an overall 5-year survival rate in Europe of 83% [5].
Carcinogenesis is a complex multistage and multistep molecular cascade triggered either by the activation of oncogenes or by the suppression of tumor suppression genes. These genes are responsible for controlling genome stability, cellular proliferation, and apoptosis. Among those controlling genomic stability, BRCA-1 and BRCA-2 genes are of considerable importance. These tumor suppressor genes are involved in the homologous repair of the double-stranded breaks. Mutations in these BRCA genes can cause genomic instability that leads to the transformation of non-cancerous cells into cancer cells [6].
The type of BRCA mutation that an individual has can play an important role in choosing a personalized and efficient treatment [6]. BRCA-1 is an 1863 amino-acids-long protein with approximately 300 mutations that have been described to date [7]. These mutations predispose an individual to breast, ovarian, and prostate cancer, as well as cancer of the GI tract. The BRCA-2 protein on the other hand, is made of 3418 amino acids, with more than 1800 mutations that have been detected so far. As with BRCA-1 mutations, BRCA-2 mutations are mainly linked to breast, ovarian, and prostate cancer. However other malignancies, such as melanoma, pancreatic, gallbladder, bile duct, and stomach cancers, should also be taken into consideration [6].
2. Methods
For the purposes of this narrative review, we carried out a MEDLINE search for all articles published in the English language using the following terms: “BRCA” and “prostate cancer” and “therapy” or “PARP inhibitor“ from January 2014 through November 2022. Relevant articles were searched in the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) annual meetings. We also used relevant data from the American Cancer Society (ACS) website. A total of 144 articles were retrieved and checked for inclusion. From these 144 articles, we deemed 22 articles to be fit for inclusion in the present study (the exclusion of papers was based on insufficient data, papers describing prostate cancer with negative BRCA gene mutation, or lack of clarity about the status of BRCA gene mutation, and other review papers). Trial data was obtained from the U.S. National Library of Medicine’s clinicaltrial.gov website (https://clinicaltrials.gov/; accessed 10 January 2023).
3. PARP Inhibitors (PARPi)
Back in 1995, the spread of prostate cancer to the regional lymph nodes was considered end-stage, and radiation was administered, since the function of the PSA (prostate specific antigen) was not yet well understood. With few systemic treatment options available, mitoxantrone was mostly used, a chemotherapy drug for the treatment of androgen independent (hormone refractory) metastatic prostate cancer. The drug gave patients a median survival of fewer than six months [8]. As a result, there have been significant changes in the way that localized prostate cancer is treated, with advancements having taken place in all aspects of therapeutic care. More recently, poly-ADP-ribose polymerase (PARP) inhibitors have dramatically improved the prognosis of patients with metastatic prostate cancer carrying BRCA genes abnormalities. The accumulation of DNA lesions has also been observed to result in a considerable rise in PARP levels in the cells, thereby providing evidence for a crucial role for PARP in DNA repair. When single-stranded DNA breaks occur, base excision repair (BER) is performed by the PARP [9]. The most well-known member of this family of enzymes, PARP-1, is essential for identifying and repairing DNA breaks. When single strand DNA damage is localized, PARP-1 produces poly-ADP-ribose (PAR) and transfers it to acceptor proteins, after which it brings in additional crucial repair enzymes to the damaged DNA spot [10].
Numerous studies have revealed a high correlation between advanced prostate cancer and common deleterious germline mutations in DNA damage repair (DDR) genes, which established the rationale for using PARP inhibitors to treat this condition. Inhibiting PARP should increase the susceptibility of malignant cells to chemotherapy and other therapeutics, since tumor cells with DDR gene mutations depend largely on PARP to repair DNA breaks and mismatches [10]. PARPi act by blocking the enzymes’ ability to catalyze reactions and by binding the PARP on DNA at the locations of single-strand breaks [11]. Due to their synthetic lethality, PARP inhibitors are the first-line treatment indicated for the treatment of mCRPC (metastatic castration-resistant prostate cancer) [11].
4. PARPi Clinical Trials
Men with mCRPC often have BRCA-1 or BRCA-2 mutations, which can make them sensitive to poly-(ADP-ribose) polymerase inhibitors or PARP inhibitors (PARPi) [12]. PARP inhibitors are, hence, being investigated in numerous clinical trials for prostate cancer, either as a monotherapy or as a component of combination therapy (Table 1).

Table 1.
Clinical trials investigating PARP inhibitors for prostate cancer.
The first PARP inhibitor authorization took place on 15 May 2020 (Figure 1). Rubraca® had been granted FDA approval. Its effectiveness was examined by the TRITON 2 (NCT02952534) clinical trial, which included 115 patients with BRCA-mutated (germline and/or somatic) mCRPC who had received androgen receptor-directed treatment and taxane-based chemotherapy. Rucaparib showed potential benefit in individuals with mCRPC and a germline or somatic BRCA or other DDR gene mutation; 43.9% of these patients experienced an objective response, and 52% reported a documented PSA response. Rucaparib’s safety profile was comparable with earlier reports in cases of ovarian and prostate cancer [13].
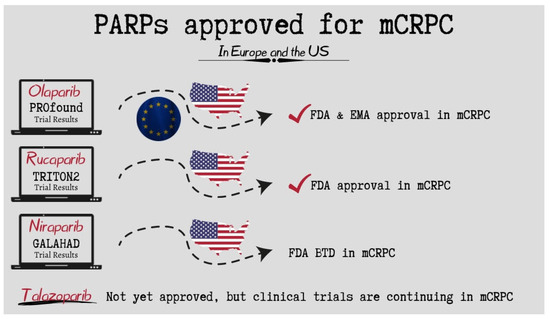
Figure 1.
PARP inhibitors approved for the treatment of mCRPC across the globe.
In the ProFOUND study, men with mCRPC who had disease progression despite hormonal treatment were included. This phase III randomized, open-label clinical trial included a total of 387 patients who were divided into two cohorts of 245 patients (cohort A) with BRCA-1, BRCA-2, or ATM mutations and 142 patients (cohort B) having all other DDR gene alterations. Subjects were divided in a 2:1 ratio to take either Olaparib or prednisolone and an AR signaling inhibitor (enzalutamide or abiraterone) as part of the control group. The primary endpoint was progression-free survival that was proven radiologically. Cohort A had a median duration of overall survival of 19.1 months with Olaparib compared to the control group, which had a median overall survival of 14.7 months (hazard ratio for death, 0.69; 95% confidence interval [CI], 0.50 to 0.97; p = 0.02). In cohort B, the median survival was 14.1 months with Olaparib and 11.5 months with the control therapy. In the overall population (cohorts A and B), the corresponding durations were 17.3 months and 14.0 months, respectively. Overall, 66% of the subjects in the control group crossed over to receive Olaparib (56 of 83 patients [67%] in cohort A). A sensitivity analysis that adjusted for crossover to Olaparib showed hazard ratios for death of 0.42 (95% CI, 0.19 to 0.91) in cohort A, 0.83 (95% CI, 0.11 to 5.98) in cohort B, and 0.55 (95% CI, 0.29 to 1.06) in the overall population. Based on the initial findings, the Food and Drug Administration (FDA), in May of 2020, approved Olaparib for mCRPC patients with deleterious HR gene mutations with disease progression following therapy with androgen receptor-signaling inhibitors [14].
A phase II trial called GALAHAD is currently being conducted on 165 patients with mCRPC, 81 of whom had germline mutations (46 BRCA and 35 non-BRCA), and 47% of whom had organ metastases. A total of 300 mg of Niraparib was administered once a day to patients who were included in this trial and whose mCRPC cancer has progressed despite receiving taxane-based chemotherapy and an AR signaling inhibitor as first-line therapy. When compared to patients without BRCA mutations, the findings showed that patients with BRCA-1 or BRCA-2 mutations had a composite rate of 63% whereas those without BRCA mutations had a CRR of 17% [15]. Trials including GALAHAD, MAGNITUDE, and QUEST continue to assess the effectiveness and safety of niraparib in patients with mCRPC and DDR mutations [16]. It is significant to highlight that, whereas the TRITON-1 and ProFOUND studies investigated patients with mono- and bi-allelic mutations, respectively, the GALAHAD study validated patient eligibility with bi-allelic mutations [17].
TALAPRO-1 was an open-label, phase II trial that assessed the efficiency of Talazoparib against mCRPC cancer. Eligibility criteria included men at the age of 18 years old or above, with progressive mCRPC and mono- or bi-allelic alterations of the DDR-HR genes, who received chemotherapy (taxane) and one or more NHT (enzalutamide, abiraterone or both). Eligible individuals received oral Talazoparib (1 mg daily). From October 18, 2017, to March 20, 2020, 128 individuals were recruited, from whom 127 got at least one dose of Talazoparib, and 104 exhibited soft-tissue disease. After 16.4 months, the odds ratio was 29.8% (31/104 patients). The most frequent side effects were anemia (31%), thrombocytopenia (9%) and neutropenia (8%). These results are very convincing and demonstrate a high anti-tumor efficacy for men with mCRPC with DDR-HR gene mutations [18].
The safety profile of PARP inhibitors in patients with mCRPC is of insignificant difference to that in patients with other solid tumors. The most often reported adverse events include fatigue, gastrointestinal side effects, and myelosuppression. The most frequent adverse effects, according to the ProFOUND trial, were nausea (41%), anemia (46%), and fatigue (41%). The drug dosage was reduced in 22% of the patients because of the side effects. Anemia, which occurs approximately in 22% of the cases, is the most frequent adverse effect, according to the GALAHAD and TRITON2 trials [15]. Myelodysplastic syndrome may be linked to PARP inhibitors in combination therapy, according to Nitecki et al., however, this complication is rare. There was no related hepatic or renal impairment, despite frequent elevations in alanine transaminase (ALT), aspartate transaminase (AST), and creatinine [19].
5. Resistance to PARPi
Although PARPi are very effective in everyday clinical practice, the increasing application of these medications in clinical settings has brought up the problem of PARPi resistance [20]. Multiple mechanisms for resistance have been proposed. Firstly, the restoration of homologous recombination (HR) may occur through a variety of different events, including intragenic mutations or the reversion of the epigenome that activates the open reading frame, thereby restoring the functionality of BRCA-1 or BRCA-2 proteins. Additionally, HR could also be recovered by other DNA repair-related proteins, such as p53-binding protein 1 (53BP1). This protein collaborates with BRCA-1 in balancing the HR and blocks the CtIP-mediated DNA end resection, which promotes DNA repair towards non-homologous end joining (NHEJ). When this protein is under-expressed in the cells that lack BRCA, it draws RAD51 and restores HR. The RAD51 protein is crucial to HR restoration, since it is placed on single and double-stranded DNA with the help of BRCA-2 to create a nuclease-resistant filament that, thus, encourages HR and PARPi resistance [21].
Secondly, an upregulation of ATP-binding cassette (ABC) transporters, which can be caused by the overexpression of the respective genes, is shown to increase the drug efflux and thus reduce the amount of the drug available intracellularly [22]. Thirdly, the accumulation of unrepaired single stranded breaks and the slowed progression of replication forks are significant causes of cell death, and PARPi capacity for PARP trapping exemplifies this. Recent research has shown that the emergence of PARPi resistance is functionally related to the suppression of PARP trapping activity [23]. In tumor samples that were resistant to PARPi, a mutation in PARP-1 (R591C) was frequently noted, which was connected to a decreased PARP-1 trapping action on DNA. In addition, the PAR glycohydrolase (PARG) enzyme participates in PARP-1 trapping action by reverting PARylation to avoid poly-ADP-ribose (PAR) buildup. Loss of the PARG causes cells that have been exposed to PARP to accumulate PAR, which is then used to restore PARP-1-dependent DNA damage signaling. PARP-1 trapping activity is hence reduced, which ultimately leads to PARPi resistance [20].
Fourthly, in halted replication fork protection, BRCA-1 and BRCA-2 genes play a crucial role. The nucleases MRE1164 and MUS8165 can target replication forks that have stalled in tumor cells with BRCA1 or BRCA-2 deficiency, thus leading to fork collapse and chromosomal abnormalities as a result. Some nucleases that can stabilize the replication fork act as a mechanism to prevent DNA replication fork disintegration when PARPi resistance develops. Particularly, the activity of EZH2 (enhancer of zeste 2 polycomb repressive complex 2 subunit) and PTIP (PAX transcription activation domain interacting protein 1-like) at the fork are suppressed in the BRCA-1 or BRCA-2 deficient cells, thereby reducing the recruitment of nucleases and promoting fork protection. Since it is known that PARPi causes unprotected replication forks to degrade, enhanced replication fork stabilization creates resistance to PARPi. Resistance to PARPi can also be mediated by several molecular signaling mechanisms controlling cell division. By being able to stimulate the PARP-1 enzymatic activity (by phosphorylation), the proto-oncogene mesenchymal-epithelial transition tyrosine kinase lowers the binding capacity of the PARPi. A considerable increase in the PI3K/AKT pathway has also been seen after using PARPi, which has the added benefit of promoting cell growth and proliferation. Lastly, the activation of the ATM/ATR pathway is also related to PARPi resistance. It serves as a crucial step in the DNA damage response pathway because it may attract DNA repair complexes by phosphorylating histone H2A. Inhibiting this route might be a future tactic to combat PARPi resistance, because it results in HR restoration [20].
6. PARPi and Immunotherapy
There is mounting evidence that immune checkpoint inhibitors and PARP inhibitors work synergistically. The programed death ligand-1 (PD-L1) plays a role in tumor immunosuppression and is activated in prostate cancer. Thus, suppression of the PD-L1 may enable efficient T-cell activation against cancerous cells. Additionally, PARP inhibition leads to the higher expression of PD-L1 in cells that express BRCA-2 in low amounts. Many ongoing clinical trials combine PARP inhibitors with PD1 blockers, such as pembrolizumab and nivolumab, and PD-L1 blockers, such as durvalumab. According to Karzai et al., mCRPC patients who received a combination of Olaparib and Durvalumab experienced a PSA drop of over 50% in most of the cases, with the median radiographic progression-free survival being 16.1 months as opposed to 4.8 months for those who did not have DDR abnormalities [24].
7. Conclusions
The PARP inhibitors are emerging as very useful treatment modalities in the management of prostate tumors caused by mutations in the HR system. Their use is associated with positive effects, including prolonged survival rates in patients with BRCA-1 or BRCA-2 gene mutations. However, the growing usage of PARP inhibitors in clinical practice sheds light on a rising clinical problem characterized by increasing resistance. There is a need for future studies investigating biomarkers other than BRCA to predict the efficacy of PARP inhibitors, given that the current clinical trials assess the utility and applicability of combination therapy in circumventing PARPi resistance.
Author Contributions
I.K., I.-P.T. and P.K. conceptualized the present paper, while all authors were involved in data collection and the preparation of the manuscript. Supervision was done by I.K. and N.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Del Mar, C.; Wright, G.; Dickinson, J.; Glasziou, P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int. J. Cancer. 2015, 137, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Knipper, S.; Pecoraro, A.; Palumbo, C.; Rosiello, G.; Luzzago, S.; Deuker, M.; Tian, Z.; Shariat, S.F.; Saad, F.; Tilki, D.; et al. The effect of age on cancer-specific mortality in patients with prostate cancer: A population-based study across all stages. Cancer Causes Control 2020, 31, 283–290. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Survival Rates for Prostate Cancer [Online]. Available online: https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 20 November 2022).
- Dal Maso, L.; Panato, C.; Tavilla, A.; Guzzinati, S.; Serraino, D.; Mallone, S.; Botta, L.; Boussari, O.; Capocaccia, R.; Colonna, M.; et al. Cancer cure for 32 cancer types: Results from the EUROCARE-5 study. Int. J. Epidemiol. 2020, 49, 1517–1525. [Google Scholar] [CrossRef]
- Gorodetska, I.; Kozeretska, I.; Dubrovska, A. BRCA Genes: The Role in Genome Stability, Cancer Stemness and Therapy Resistance. J. Cancer 2019, 10, 2109–2127. [Google Scholar] [CrossRef] [PubMed]
- Mehrgou, A.; Akouchekian, M. The importance of BRCA1 and BRCA2 genes mutations in breast cancer development. Med. J. Islam. Repub. Iran 2016, 30, 369. [Google Scholar]
- Dorff, T.B.; O’Neil, B.; Hoffman, K.E.; Lin, D.W.; Loughlin, K.R.; Dall’Era, M. 25-year perspective on prostate cancer: Conquering frontiers and understanding tumor biology. Urol. Oncol. 2021, 39, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit. Rev.™ Eukaryot. Gene Expr. 2014, 24, 15–28. [Google Scholar] [CrossRef]
- Grewal, K.; Grewal, K.; Tabbara, I.A. PARP Inhibitors in Prostate Cancer. Anticancer. Res. 2021, 41, 551–556. [Google Scholar] [CrossRef]
- Murai, J.; Zhang, Y.; Morris, J.; Ji, J.; Takeda, S.; Doroshow, J.H.; Pommier, Y. Rationale for poly(ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or temozolomide based on PARP trapping versus catalytic inhibition. J. Pharmacol. Exp. Ther. 2014, 349, 408–416. [Google Scholar] [CrossRef]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Campbell, D.; Patnaik, A.; Sautois, B.; Shapiro, J.; Vogelzang, N.; Bryce, A.; Mcdermott, R.; Ricci, F.; Rowe, J.; et al. ESMO 2019: Preliminary Results from the TRITON2 Study of Rucaparib in Patients with DNA Damage Repair-Deficient mCRPC: Updated Analyses. UroToday.com and 2019 European Society for Medical Oncology Annual Meeting, ESMO 2019 #ESMO19, 27 Sept–1 Oct 2019 in Barcelona, Spain. Available online: https://www.urotoday.com/conference-highlights/esmo-2019/esmo-2019-prostate-cancer/115264-esmo-2019-preliminary-results-from-the-triton2-study-of-rucaparib-in-patients-with-dna-damage-repair-deficient-mcrpc-updated-analyses.html (accessed on 20 November 2022).
- Hussain, M.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 383, 2345–2357. [Google Scholar] [CrossRef]
- Shah, S.; Rachmat, R.; Enyioma, S.; Ghose, A.; Revythis, A.; Boussios, S. BRCA Mutations in Prostate Cancer: Assessment, Implications and Treatment Considerations. Int. J. Mol. Sci. 2021, 22, 12628. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R. ESMO 2019: GALAHAD—A Phase 2 Study of Niraparib in Patients with mCRPC and Biallelic DNA-Repair Gene Defects, A Pre-Specified Interim Analysis. UroToday.com and 2019 European Society for Medical Oncology Annual Meeting, ESMO 2019 #ESMO19, 27 Sept–1 Oct 2019 in Barcelona, Spain. Available online: https://www.urotoday.com/conference-highlights/esmo-2019/esmo-2019-prostate-cancer/115258-esmo-2019-galaha-a-phase-2-study-of-niraparib-in-patients-with-mcrpc-and-biallelic-dna-repair-gene-defects-a-pre-specified-interim-analysis.html (accessed on 20 November 2022).
- Messina, C.; Cattrini, C.; Soldato, D.; Vallome, G.; Caffo, O.; Castro, E.; Olmos, D.; Boccardo, F.; Zanardi, E. BRCA Mutations in Prostate Cancer: Prognostic and Predictive Implications. J. Oncol. 2020, 2020, 4986365. [Google Scholar] [CrossRef]
- de Bono, J.S.; Mehra, N.; Scagliotti, G.V.; Castro, E.; Dorff, T.; Stirling, A.; Stenzl, A.; Fleming, M.T.; Higano, C.S.; Saad, F.; et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): An open-label, phase 2 trial. Lancet Oncol. 2021, 22, 1250–1264, Erratum in Lancet Oncol. 2022, 23, e207. Erratum in Lancet Oncol. 2022, 23, e249. [Google Scholar] [CrossRef] [PubMed]
- Nitecki, R.; Melamed, A.; Gockley, A.A.; Floyd, J.; Krause, K.J.; Coleman, R.L.; Matulonis, U.A.; Giordano, S.H.; Lu, K.H.; Rauh-Hain, J.A. Incidence of myelodysplastic syndrome and acute myeloid leukemia in patients receiving poly-ADP ribose polymerase inhibitors for the treatment of solid tumors: A meta-analysis of randomized trials. Gynecol. Oncol. 2021, 161, 653–659. [Google Scholar] [CrossRef]
- Giudice, E.; Gentile, M.; Salutari, V.; Ricci, C.; Musacchio, L.; Carbone, M.V.; Ghizzoni, V.; Camarda, F.; Tronconi, F.; Nero, C.; et al. PARP Inhibitors Resistance: Mechanisms and Perspectives. Cancers 2022, 14, 1420. [Google Scholar] [CrossRef]
- Mweempwa, A.; Wilson, M.K. Mechanisms of resistance to PARP inhibitors—An evolving challenge in oncology. Cancer Drug Resist. 2019, 2, 608–617. [Google Scholar] [CrossRef]
- Noordermeer, S.M.; van Attikum, H. PARP Inhibitor Resistance: A Tug-of-War in BRCA-Mutated Cells. Trends Cell Biol. 2019, 29, 820–834. [Google Scholar] [CrossRef]
- Gogola, E.; Duarte, A.A.; de Ruiter, J.R.; Wiegant, W.W.; Schmid, J.A.; de Bruijn, R.; James, D.I.; Llobet, S.G.; Vis, D.J.; Annunziato, S.; et al. Selective Loss of PARG Restores PARylation and Counteracts PARP Inhibitor-Mediated Synthetic Lethality. Cancer Cell 2019, 35, 950–952, Erratum in Cancer Cell 2018, 33, 1078–1093.e12. [Google Scholar] [CrossRef]
- Karzai, F.; VanderWeele, D.; Madan, R.A.; Owens, H.; Cordes, L.M.; Hankin, A.; Couvillon, A.; Nichols, E.; Bilusic, M.; Beshiri, M.L.; et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 2018, 6, 141. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).