Concomitant Radical Cystectomy and Infrarenal Aortic Aneurysm Repair with Cryopreserved Aortic Allograft: A Case Report
Abstract
1. Introduction
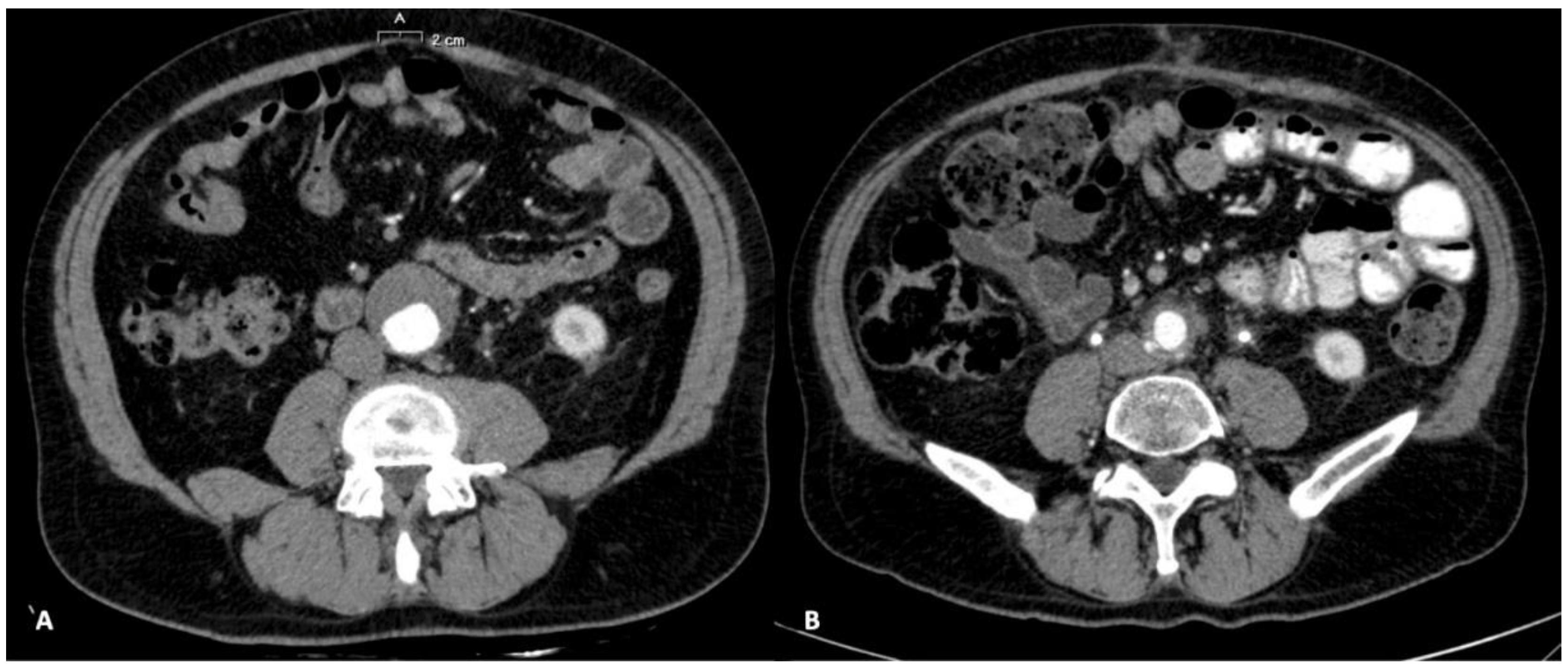
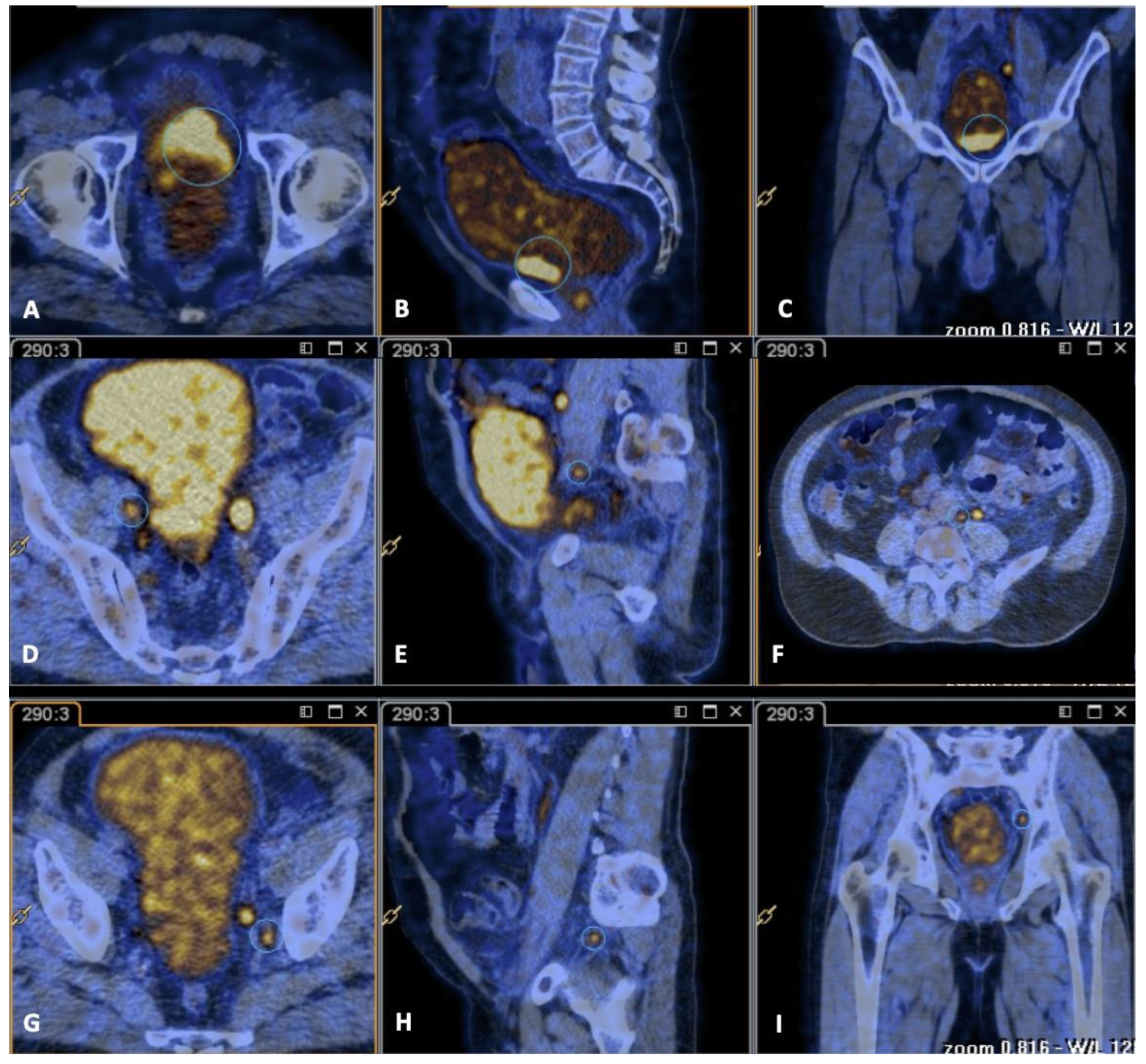
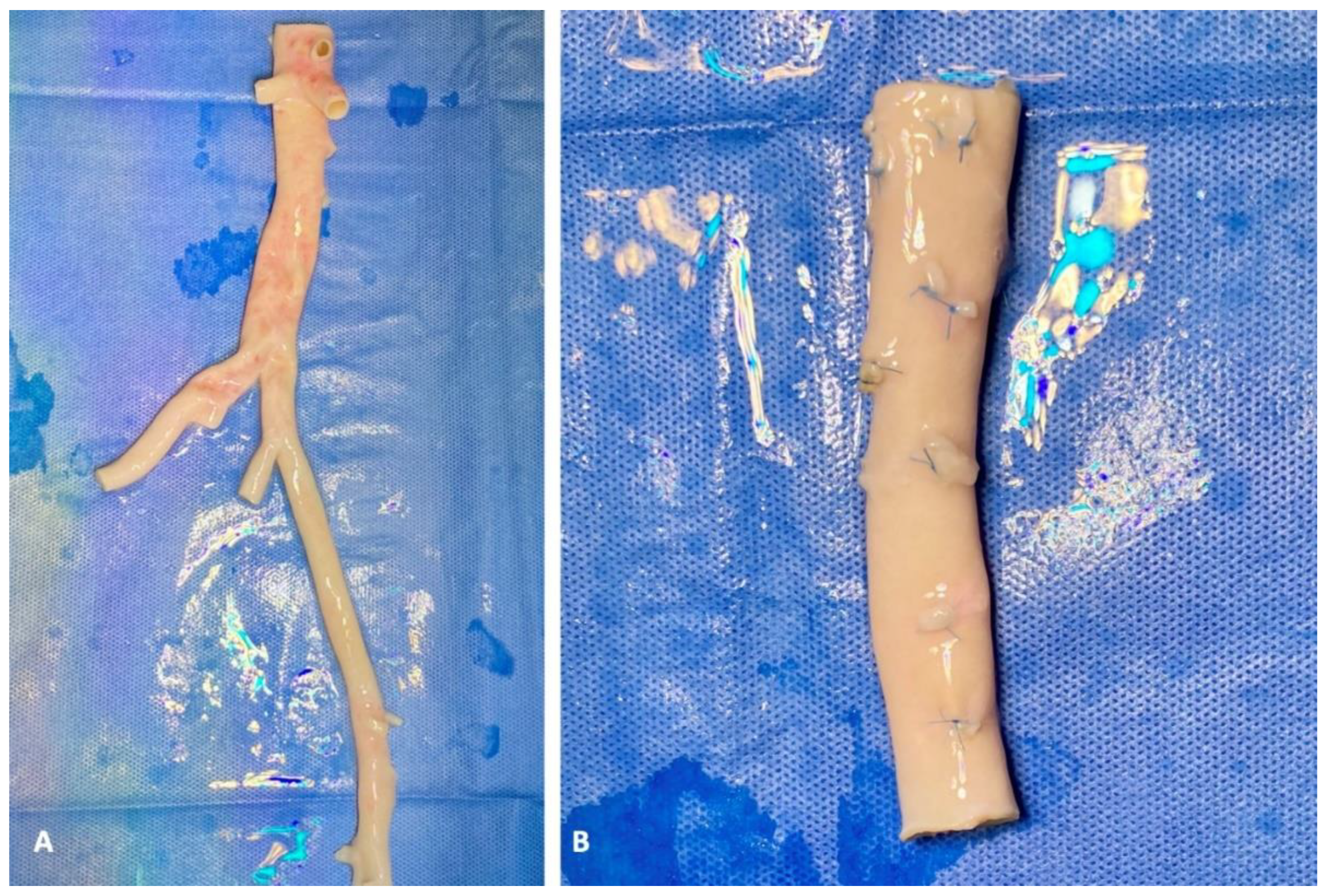
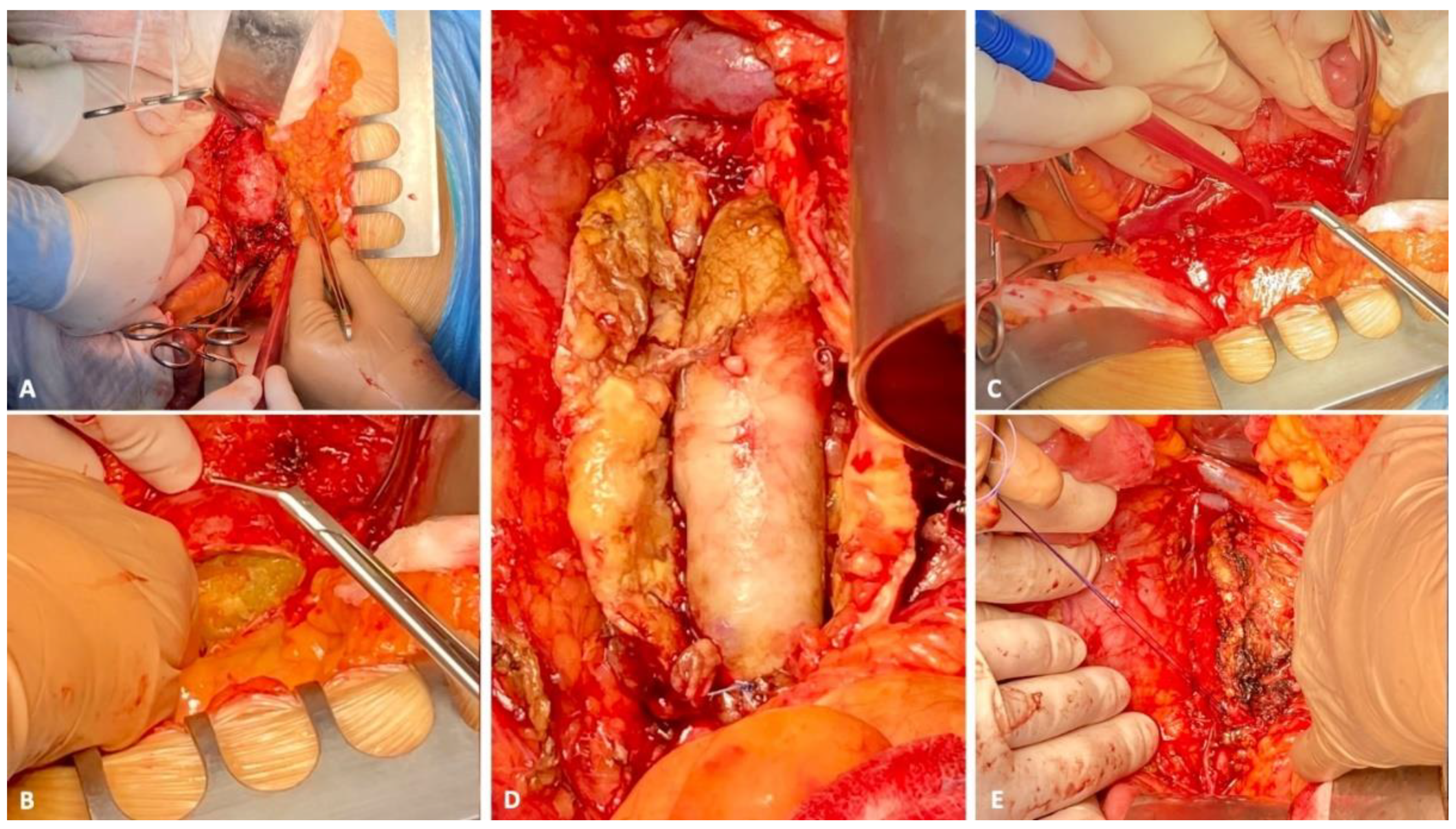
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- Harlander-Locke, M.P.; Harmon, L.K.; Lawrence, P.F.; Oderich, G.S.; McCready, R.A.; Morasch, M.D.; Feezor, R.J. The use of cryopreserved aortoiliac allograft for aortic reconstruction in the United States. J. Vasc. Surg. 2014, 59, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Diao, X.; Diao, F.; Fan, X.; Zheng, J.; Yan, D.; Huang, J.; Qin, H.; Lin, T. Causes of death in long-term bladder cancer survivors: A population-based study. Asia-Pacific J. Clin. Oncol. 2019, 15, e167–e174. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, K.M.; Deng, L.; Bluethmann, S.M.; Zhou, S.; Trifiletti, D.M.; Jiang, C.; Kelly, S.; Zaorsky, N.G. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur. Heart J. 2019, 40, 3889–3897. [Google Scholar] [CrossRef] [PubMed]
- Barbalias, G.A.; Liatsikos, E.N.; Yarmenitis, S.; Maroulis, I.; Tsolakis, I. Simultaneously occurring abdominal aortic aneurysm and invasive transitional cell carcinoma of the bladder and their synchronous management. Urol. Int. 1998, 60, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Grego, F.; Lepidi, S.; Bassi, P.; Tavolini, I.M.; Noventa, F.; Pagano, F.; Deriu, G.P. Simultaneous surgical treatment of abdominal aortic aneurysm and carcinoma of the bladder. J. Vasc. Surg. 2003, 37, 607–614. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ito, S.; Nakamura, Y.; Noumi, T.; Sasaki, Y. Acute aortic thrombosis during cisplatin based chemotherapy for gastric cancer. Intern. Med. 2013, 52, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Madden, G.W.; Ishaq, M.K.; Gupta, R. Acute aortic dissection in a patient receiving gemcitabine and cisplatin. Am. J. Ther. 2014, 21, e21–e25. [Google Scholar] [CrossRef] [PubMed]
- Lierz, M.F.; Davis, B.E.; Noble, M.J.; Wattenhofer, S.P.; Thomas, J.H. Management of Abdominal Aortic Aneurysm and Invasive Transitional Cell Carcinoma of Bladder. J. Urol. 1993, 149, 476–479. [Google Scholar] [CrossRef]
- Mearini, L.; Zucchi, A.; Pizzirusso, G.; Vivacqua, C.; Mearini, E. Incidence and evolution of aortic aneurysm in patients with bladder cancer. Arch. Ital. di Urol. e Androl. 2004, 76, 80–82. [Google Scholar]
- Members, T.F.; Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; di Bartolomeo, R.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur. Hear. J. 2014, 35, 2873–2926. [Google Scholar]
- Hautmann, R.E.; de Petriconi, R.C.; Volkmer, B.G. Lessons Learned from 1000 Neobladders: The 90-Day Complication Rate. J. Urol. 2010, 184, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Vogt, P.R. Arterial Allografts in Treating Aortic Graft Infections: Something Old, Something New. Semin. Vasc. Surg. 2011, 24, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-H.; Kim, Y.-W.; Woo, S.-Y.; Park, Y.-J.; Kim, D.-K.; Chung, D.-R. Recent Results of In Situ Abdominal Aortic Reconstruction with Cryopreserved Arterial Allograft. Eur. J. Vasc. Endovasc. Surg. 2016, 53, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Lejay, A.; Delay, C.; Girsowicz, E.; Chenesseau, B.; Bonnin, E.; Ghariani, M.-Z.; Thaveau, F.; Georg, Y.; Geny, B.; Chakfe, N. Cryopreserved Cadaveric Arterial Allograft for Arterial Reconstruction in Patients with Prosthetic Infection. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, D.A.; Esrig, D.; Grossfeld, G.D.; Stein, J.P.; Freeman, J.A.; Yellin, A.E.; Lieskovsky, G.; Weaver, F.A.; Skinner, D.G. Technique of radical cystectomy and simultaneous repair of an abdominal aortic aneurysm. Urology 1996, 47, 120–122. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianflone, F.; Bianchi, A.; Novella, G.; Tafuri, A.; Cerruto, M.A.; Zivi, A.; Veraldi, G.F.; Antonelli, A. Concomitant Radical Cystectomy and Infrarenal Aortic Aneurysm Repair with Cryopreserved Aortic Allograft: A Case Report. Uro 2022, 2, 6-12. https://doi.org/10.3390/uro2010002
Cianflone F, Bianchi A, Novella G, Tafuri A, Cerruto MA, Zivi A, Veraldi GF, Antonelli A. Concomitant Radical Cystectomy and Infrarenal Aortic Aneurysm Repair with Cryopreserved Aortic Allograft: A Case Report. Uro. 2022; 2(1):6-12. https://doi.org/10.3390/uro2010002
Chicago/Turabian StyleCianflone, Francesco, Alberto Bianchi, Giovanni Novella, Alessandro Tafuri, Maria Angela Cerruto, Andrea Zivi, Gian Franco Veraldi, and Alessandro Antonelli. 2022. "Concomitant Radical Cystectomy and Infrarenal Aortic Aneurysm Repair with Cryopreserved Aortic Allograft: A Case Report" Uro 2, no. 1: 6-12. https://doi.org/10.3390/uro2010002
APA StyleCianflone, F., Bianchi, A., Novella, G., Tafuri, A., Cerruto, M. A., Zivi, A., Veraldi, G. F., & Antonelli, A. (2022). Concomitant Radical Cystectomy and Infrarenal Aortic Aneurysm Repair with Cryopreserved Aortic Allograft: A Case Report. Uro, 2(1), 6-12. https://doi.org/10.3390/uro2010002






