Secondary Perfusion to Model Viability of Livers Declined for Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Overview
2.2. Conclusion of Primary Perfusion
- Identify when a liver undergoing NMP does not meet viability criteria and is declined for transplantation. Confirm the research consent of the donor and that the declined organ will not be accepted for transplant by another center.
- Stop perfusion. Remove the liver from the perfusion device and transfer the organ to a basin containing surgical-grade ice.
- Connect a flush line to the portal vein (PV) and hepatic artery (HA) cannulae. It is important that the inferior vena cava is open to allow for drainage. Administer cold flush solution as per center standard (options include University of Wisconsin solution (UW-Madison Department of Surgery, Madison, WI, USA) and Custodial histidine-tryptophan-ketoglutarate (HTK) solution (Essential Pharmaceuticals, LLC, Raleigh, NC, USA)). Use 2–4 L of flush solution as necessary for the effluent to become clear and the liver to show a homogenous appearance.
- Weigh the liver to obtain a post-perfusion weight. Capture post-perfusion photographs, tissue biopsies, and/or perfusate and bile samples as per center protocol.
- Enclose the liver within a sterile organ bag and store the liver using SCS. At our center, 0.5–1 h is the typical timeframe from the removal of the organ from the perfusion platform until implantation commences, so our secondary perfusion protocol allows the liver to incur 1–1.5 h of cold ischemic time (CIT). Subjecting the organ to cold ischemia in the secondary perfusion model is crucial to mimic properly the insult that would have occurred during transplantation. By prolonging the CIT somewhat, we aim to inflict the upper limit of injury reasonably expected for a liver undergoing transplantation post-NMP. This timeframe will also enable researchers to complete necessary preparations for secondary NMP. However, each center will likely vary in typical CIT during the liver offboarding process. We recommend adapting the experimental protocol to fit individual circumstances.
2.3. Cold Storage Phase
- Assemble the designated perfusion device as per the manufacturer’s instructions. For the secondary perfusion model to properly mimic reperfusion, it is vital that the perfusion be normothermic (36–37 °C). Beyond this physiologic requirement, any NMP device is acceptable for the secondary perfusion model, including the Liver Assist (XVIVO, Gothenburg, Sweden), the metra (OrganOx, Oxford, UK), the Organ Care System (TransMedics, Andover, MA, USA), and homemade platforms. Our center utilizes the metra for clinical perfusions. Centers that utilize other devices will likely have different institutional viability criteria. Secondary perfusion may be performed regardless, with the understanding that any graft declined for transplantation after NMP has failed to meet viability criteria specific to the perfusion device used. For secondary perfusions, our center utilizes the Liver Assist for logistical reasons. Switching devices may affect the interpretation of results, as different devices have different target thresholds for values such as temperatures, flow rates, and pressures, and therefore represent a limitation to this work. If possible, we recommend that centers utilize the same device type for secondary perfusion as was used for primary perfusion to control variability and better enable comparison of perfusion data. If there are logistical constraints and a different device type must be used, we strongly recommend utilizing a consistent device type for all secondary perfusions to better enable a comparative study.
- After assembling the device, prepare the perfusate and prime the circuit. The only perfusate requirement for the secondary perfusion model is that the perfusate has a sufficient oxygen-carrying capacity; specific composition will vary with each center’s preferred device and available resources. Our center utilizes a base perfusate composition of 3 units of packed red blood cells (pRBCs) and 500 mL of 5% human albumin (Grifols, Raleigh, NC, USA). Although a high hematocrit is ideal to maximize circulating oxygen levels, a lower level is tolerated for secondary perfusion because of the scarcity of blood supplies for research [18,19]. Our center aims to maintain a hematocrit of >18%. We utilize expired human pRBCs for research perfusions. To conserve valuable resources, centers may also choose to collect blood used during the primary perfusion, wash the blood during the secondary cold storage phase, and re-use this blood for the secondary perfusion.
- Administer baseline boluses as per center protocol. We utilize the following:
- A total of 10 mL of 10% calcium gluconate (Fresenius Kabi, IL, USA);
- In total, 10,000 units of heparin (Hospira, Lake Forest, IL, USA) to prevent clotting;
- A total of 750 mg of cefuroxime (SAGENT Pharmaceuticals, Schaumburg, IL, USA), reconstituted with 10 mL of 0.9% sodium chloride solution (B. Braun Medical Inc., Bethlehem, PA, USA), to prevent infection.
- Prepare infusions as per the center’s protocol. We utilize the following infusions at a rate of 1 mL per hour:
- In total, 5.6 g of sodium taurocholate (OrganOx Ltd., Oxford, UK), reconstituted in 30 mL of 0.9% sodium chloride (B. Braun Medical Inc., Bethlehem, PA, USA);
- A total of 2 mL of 100 units/mL short-acting insulin (Eli Lilly & Co., Indianapolis, IN, USA), diluted in 28 mL of 0.9% sodium chloride (B. Braun Medical Inc., Bethlehem, PA, USA);
- In total, 0.5 mg of epoprostenol (Sun Pharmaceutical Industries, Inc., Billerica, MA, USA), reconstituted in 10 mL of sterile water (Hospira, Inc., Lake Forest, IL, USA), diluted in 25 mL of 0.9% sodium chloride (B. Braun Medical Inc., Bethlehem, PA, USA);
- A total of 5 mL of 5000 units/mL heparin (Hospira, Lake Forest, IL, USA), diluted in 25 mL of 0.9% sodium chloride (B. Braun Medical Inc., Bethlehem, PA, USA).
- Once the device is primed and perfusate is circulating, allow 10–15 min for the device to reach a normothermic temperature before conducting a baseline arterial blood gas (ABG). Confirm that the perfusate pH is in the desired range. If the pH is acidotic, add sodium bicarbonate to achieve the target pH (our center’s target range is 7.25–7.35) and allow 5–15 min to circulate.
- With the surgical team, open the sterile organ bag on the back table. Maintain the organ on surgical ice. Cannulate the organ’s vasculature as necessary for the selected perfusion device.
- Connect cannulae to a flush line. Fill the organ with room temperature 5% human albumin (500–1000 mL as needed, given organ size). This step is necessary to remove the preservation solution, which is hyperkalemic.
- Confirm that the target CIT has been achieved. In an ideal scenario, the target CIT should be long enough to stress the liver to traditional preservation stress (i.e., the second round of cooling after NMP).
2.4. Initiation of Secondary Perfusion
- Onboard the organ to the perfusion device as per the manufacturer’s instructions.
- Start perfusion. Closely monitor flow rates and pressures to ensure proper onboarding. Ligate any major bleeds to maintain hemostasis and ensure accurate interpretation of flow rate and pressure readings.
- Cannulate the bile duct and assemble the bile collection chamber as per the manufacturer’s instructions.
- Aim to maintain perfusion for a minimum of 4–6 h. A longer duration may allow for a more nuanced understanding of the organ’s trajectory post-reperfusion.
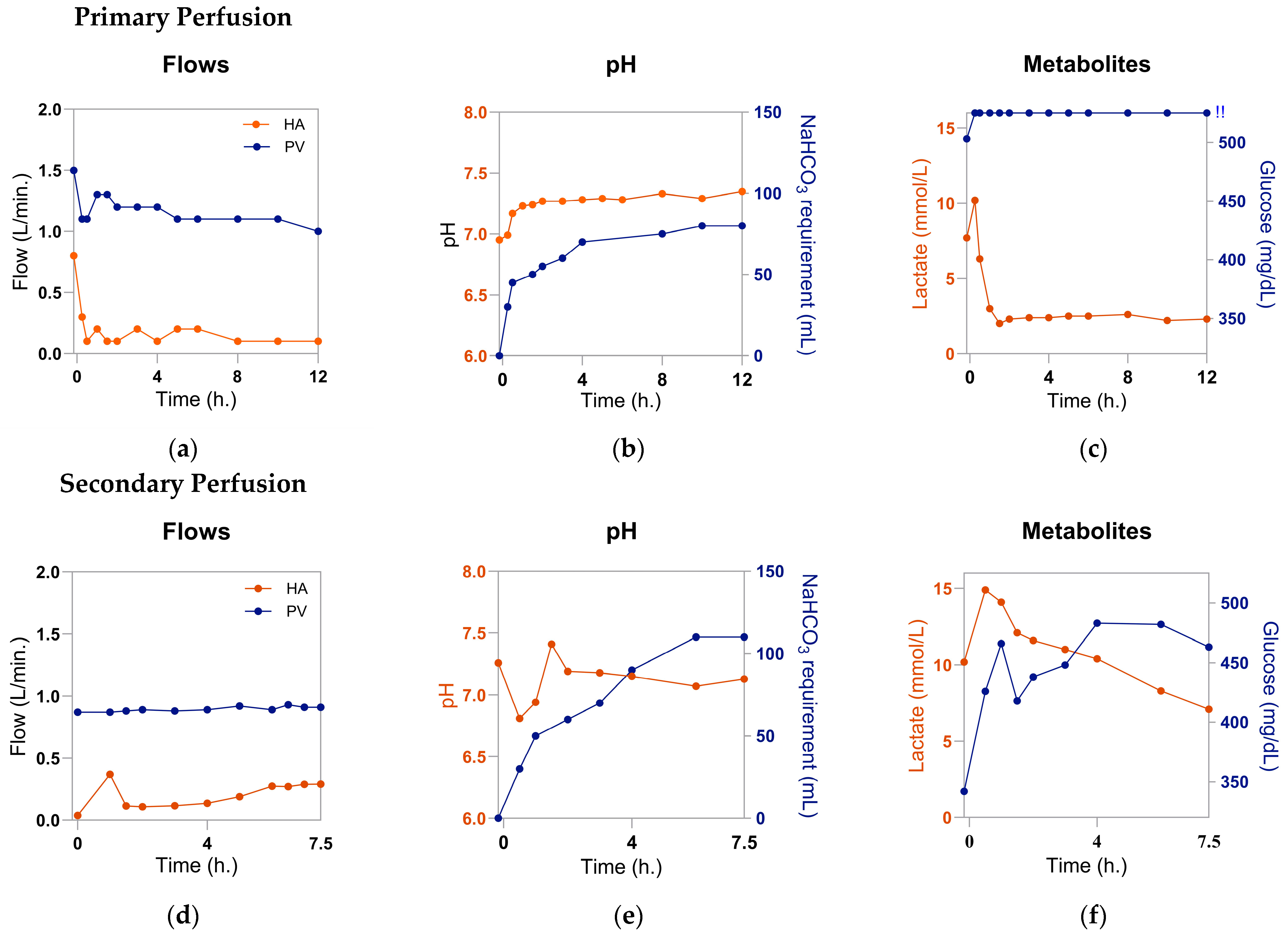
- Throughout the duration of perfusion, perform routine ABGs as per the center’s protocol. Administer medications as necessary to maintain target values of designated parameters. Our center commonly responds to the following parameters.
- pH: If persistently acidotic, bolus bicarbonate.
- Glucose: Titrate total parenteral nutrition (TPN) based on the center’s protocol and TPN composition. Our center starts with a baseline infusion of 20% dextrose TPN (Baxter Healthcare Corp., Deerfield, IL, USA) at 15 mL/h. If glucose drops below 100 mg/dL, we administer a bolus of 50% dextrose (Hospira, Inc., Lake Forest, IL, USA) for a quicker response.
- pO2: Adjust the oxygen flow rate and/or the sweep gas flow as necessary.
- Calcium: Administer calcium gluconate to achieve a target calcium level of 1.05–1.30 mmol/L.
- If bile is present, record the volume. Perform a point-of-care bile gas analysis to assess the same parameters as measured by the perfusate ABG. Bile gas results will not typically inform medication administration but will be useful for viability analysis once the experiment is concluded.
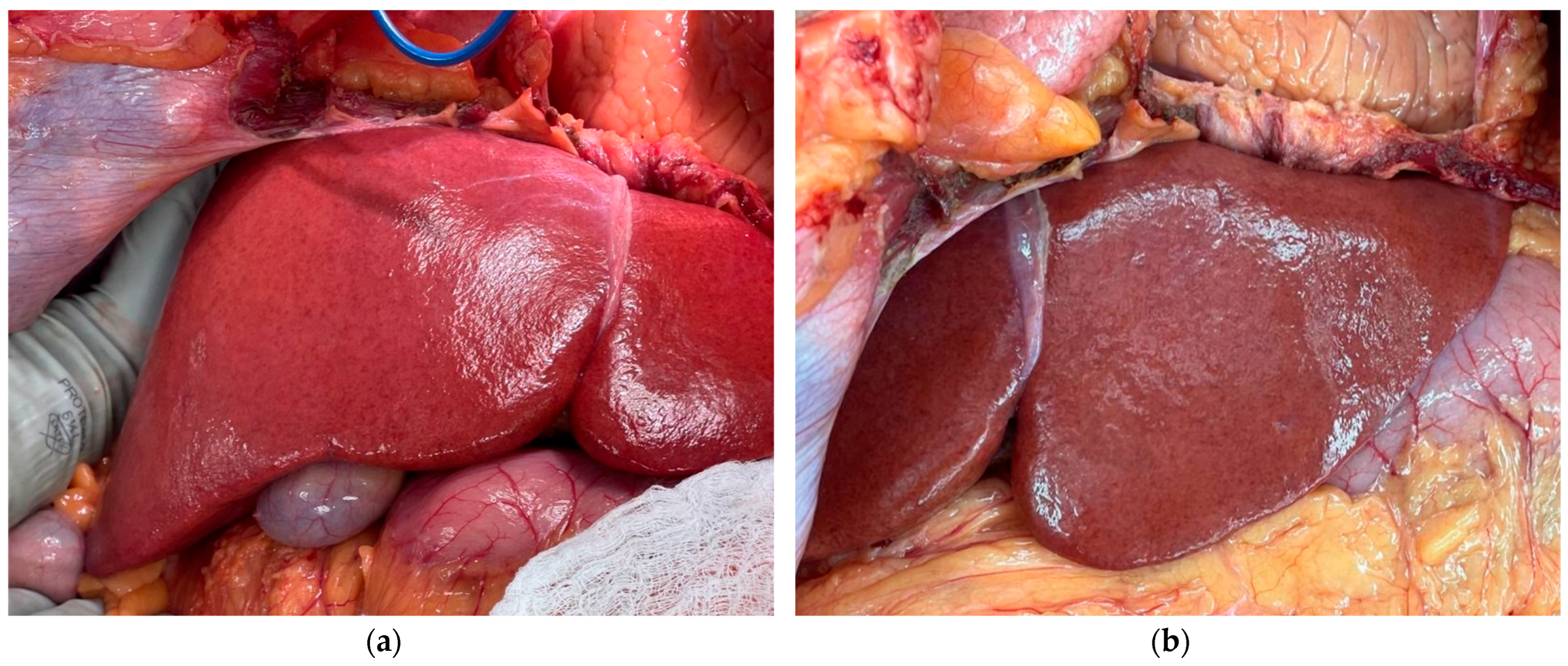
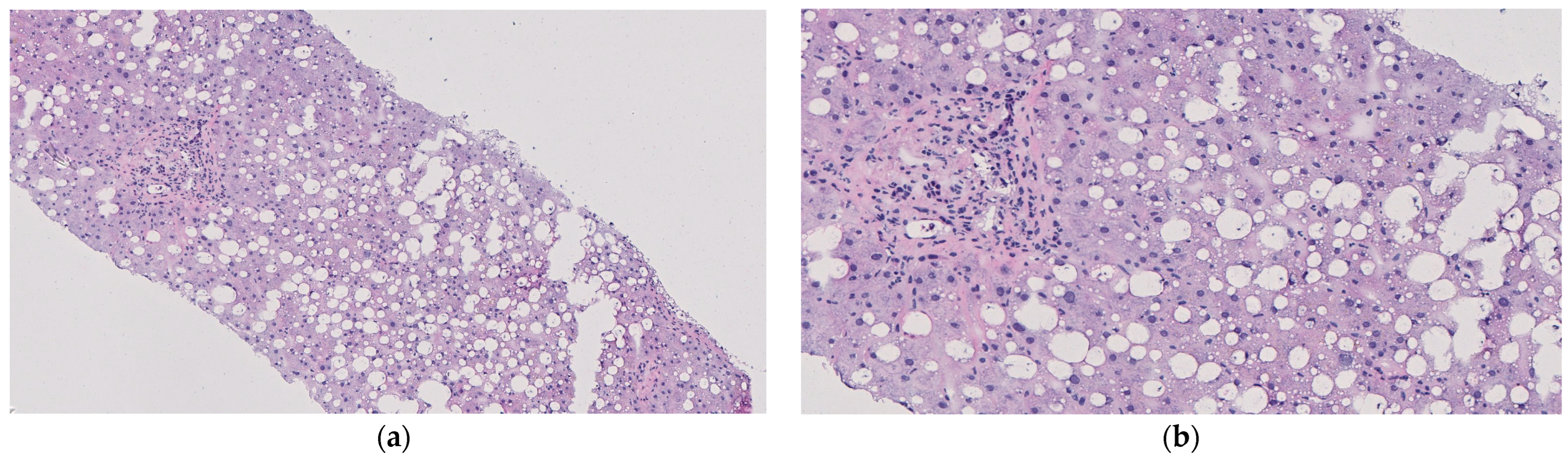
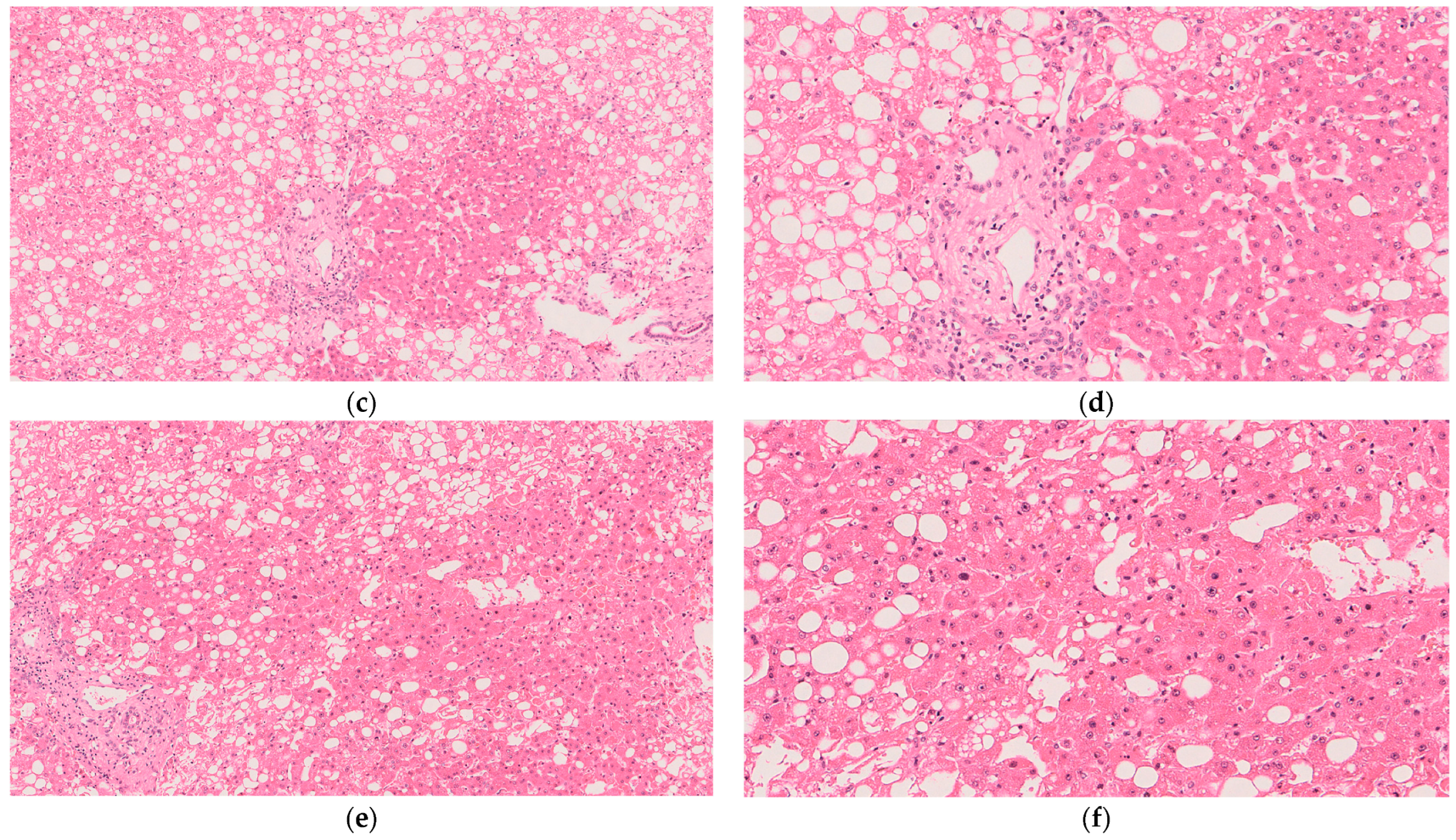
- If resources and ethical guidelines allow, we recommend performing tissue biopsies of the liver parenchyma every 2–4 h. Rotate the location of the biopsies to ensure relatively well-perfused tissue is sampled at each timepoint. Properly stored tissue samples will enable histological and metabolomics analysis following the conclusion of the experiment. In addition, capture a photograph of the graft. Gross imaging will be helpful in assessing the macroscopic appearance of the graft.
- Perform laboratory testing as per each center’s protocol. Our center performs the following tests: aspartate aminotransferase (AST) levels, alanine transaminase (ALT) levels, and D-dimer levels. AST and ALT can be used to interpret the level of hepatic injury. D-dimer level can provide context for microthrombi burden and risk of biliary injury [20].
- At every timepoint any diagnostic testing is performed, record the hemodynamic parameters measured by the perfusion device. Our center records the following parameters: HA flow, HA pressure, HA temperature, PV flow, PV pressure, and PV temperature. Because the liver will be of poor quality and has been subjected to a secondary insult, the graft resistance may be high. If flow rates are below target levels despite administering the infusion of diluted epoprostenol as described previously, we recommend administering a bolus of any available, non-diluted vasodilator, such as epoprostenol or verapamil.
- Terminate the experiment once the desired duration is reached or the maintenance of the perfusion has become futile. Signs that the perfusion has reached its limit include: perfusate lactate > 10 mmol/L for >2 h; pH persistently acidotic with >20 mL 8.4% sodium bicarbonate added every 30–45 min; persistently unstable hemodynamics despite the use of vasodilators; discolored and/or darkened parenchyma; or foul odor. At this time, stop perfusion, remove all cannulae from the organ, and obtain a post-perfusion weight, ABG, bile gas, biopsy, gross imaging, and laboratory results as desired. Properly return the organ to the center’s pathology department.
3. Results
3.1. Procurement
3.2. Primary Perfusion
3.3. Secondary Perfusion
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECD | Expanded criteria donors |
| BMI | Body mass index |
| DCD | Donation after circulatory death |
| NMP | Normothermic machine perfusion |
| AI | Artificial intelligence |
| ABG | Arterial blood gas |
| SCS | Static cold storage |
| HTK | Histidine-tryptophan-ketoglutarate |
| CIT | Cold ischemic time |
| pRBCs | Packed red blood cells |
| HA | Hepatic artery |
| PV | Portal vein |
| TPN | Total parenteral nutrition |
| OPO | Organ procurement organization |
| MELD | Model for end-stage liver disease |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
References
- Feng, S.; Lai, J.C. Expanded Criteria Donors. Clin. Liver Dis. 2014, 18, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Organ Donation Statistics. Available online: https://www.organdonor.gov/learn/organ-donation-statistics (accessed on 23 June 2025).
- Kwong, A.J.; Kim, W.R.; Lake, J.R.; Schladt, D.P.; Schnellinger, E.M.; Gauntt, K.; McDermott, M.; Weiss, S.; Handarova, D.K.; Snyder, J.J.; et al. OPTN/SRTR 2022 Annual Data Report: Liver. Am. J. Transplant. 2024, 24, S176–S265. [Google Scholar] [CrossRef]
- Mastrovangelis, C.; Frost, C.; Hort, A.; Laurence, J.; Pang, T.; Pleass, H. Normothermic Regional Perfusion in Controlled Donation after Circulatory Death Liver Transplantation: A Systematic Review and Meta-Analysis. Transpl. Int. 2024, 37, 13263. [Google Scholar] [CrossRef]
- Vodkin, I.; Kuo, A. Extended Criteria Donors in Liver Transplantation. Clin. Liver Dis. 2017, 21, 289–301. [Google Scholar] [CrossRef]
- Torabi, J.; Todd, R.; van Leeuwen, L.L.; Bekki, Y.; Holzner, M.; Moon, J.; Schiano, T.; Florman, S.S.; Akhtar, M.Z. A Decade of Liver Transplantation in the United States: Drivers of Discard and Underutilization. Transplant. Direct 2024, 10, e1605. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Hong, H.; Gross, A.; Liu, Q.; Ali, K.; Cazzaniga, B.; Miyazaki, Y.; Tuul, M.; Modaresi Esfeh, J.; Khalil, M.; et al. The impact of normothermic machine perfusion and acuity circles on waitlist time, mortality, and cost in liver transplantation: A multicenter experience. Liver Transplant. 2025, 31, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.C.; Zhang, C.; Chang, Y.H.; Li, X.; Ohara, S.Y.; Kumm, K.R.; Cosentino, C.P.; Aqel, B.A.; Lizaola-Mayo, B.C.; Frasco, P.E.; et al. Improved Outcomes and Resource Use with Normothermic Machine Perfusion in Liver Transplantation. JAMA Surg. 2025, 160, 322–330. [Google Scholar] [CrossRef]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Clarke, G.; Boteon, Y.L.; Barton, D.; Neil, D.A.H.; Isaac, J.R.; Roberts, K.J.; Abradelo, M.; et al. Discarded livers tested by normothermic machine perfusion in the VITTAL trial: Secondary end points and 5-year outcomes. Liver Transplant. 2024, 30, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, L.L.; Irizar, H.; Kim-Schluger, L.; Florman, S.; Akhtar, M.Z. The potential of machine learning to predict early allograft dysfunction after normothermic machine perfusion in liver transplantation. J. Hepatol. 2024, 81, e298–e300. [Google Scholar] [CrossRef]
- Thorne, A.M.; Wolters, J.C.; Lascaris, B.; Bodewes, S.B.; Lantinga, V.A.; Van Leeuwen, O.B.; De Jong, I.E.M.; Ustyantsev, K.; Berezikov, E.; Lisman, T.; et al. Bile proteome reveals biliary regeneration during normothermic preservation of human donor livers. Nat. Commun. 2023, 14, 7880. [Google Scholar] [CrossRef]
- Groen, P.C.; Van Leeuwen, O.B.; De Jonge, J.; Porte, R.J. Viability assessment of the liver during ex-situ machine perfusion prior to transplantation. Curr. Opin. Organ Transplant. 2024, 29, 239–247. [Google Scholar] [CrossRef]
- Orman, E.S.; Barritt, S.A.; Wheeler, S.B.; Hayashi, P.H. Declining liver utilization for transplantation in the United States and the impact of donation after cardiac death. Liver Transplant. 2013, 19, 59–68. [Google Scholar] [CrossRef]
- Raigani, S.; De Vries, R.J.; Carroll, C.; Chen, Y.; Chang, D.C.; Shroff, S.G.; Uygun, K.; Yeh, H. Viability testing of discarded livers with normothermic machine perfusion: Alleviating the organ shortage outweighs the cost. Clin. Transplant. 2020, 34, e14069. [Google Scholar] [CrossRef] [PubMed]
- Olumba, F.C.; Zhou, F.; Park, Y.; Chapman, W.C.; the RESTORE Investigators Group. Normothermic Machine Perfusion for Declined Livers: A Strategy to Rescue Marginal Livers for Transplantation. J. Am. Coll. Surg. 2023, 236, 614–625. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, O.B.; de Vries, Y.; Fujiyoshi, M.; Nijsten, M.W.N.; Ubbink, R.; Pelgrim, G.J.; Werner, M.J.M.; Reyntjens, K.M.E.M.; van den Berg, A.P.; de Boer, M.T.; et al. Transplantation of High-risk Donor Livers after Ex Situ Resuscitation and Assessment Using Combined Hypo- and Normothermic Machine Perfusion: A Prospective Clinical Trial. Ann. Surg. 2019, 270, 906–914. [Google Scholar] [CrossRef]
- Avramidou, E.; Todorov, D.; Katsanos, G.; Antoniadis, N.; Kofinas, A.; Vasileiadou, S.; Karakasi, K.-E.; Tsoulfas, G. AI Innovations in Liver Transplantation: From Big Data to Better Outcomes. Livers 2025, 5, 14. [Google Scholar] [CrossRef]
- Maeda, A.; Starkey, G.; Spano, S.; Chaba, A.; Eastwood, G.; Yoshino, O.; Perini, M.V.; Fink, M.; Bellomo, R.; Jones, R. Perfusate hemoglobin during normothermic liver machine perfusion as biomarker of early allograft dysfunction: A pilot study. Artif. Organs 2025, 49, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Mergental, H.; Stephenson, B.T.F.; Laing, R.W.; Kirkham, A.J.; Neil, D.A.H.; Wallace, L.L.; Boteon, Y.L.; Widmer, J.; Bhogal, R.H.; Perera, M.T.P.R.; et al. Development of Clinical Criteria for Functional Assessment to Predict Primary Nonfunction of High-Risk Livers Using Normothermic Machine Perfusion. Liver Transplant. 2018, 24, 1453–1469. [Google Scholar] [CrossRef]
- Watson, C.J.E.; MacDonald, S.; Bridgeman, C.; Brais, R.; Upponi, S.S.; Foukaneli, T.; Swift, L.; Fear, C.; Selves, L.; Kosmoliaptsis, V.; et al. D-dimer Release from Livers During Ex Situ Normothermic Perfusion and after In Situ Normothermic Regional Perfusion: Evidence for Occult Fibrin Burden Associated with Adverse Transplant Outcomes and Cholangiopathy. Transplantation 2023, 107, 1311–1321. [Google Scholar] [CrossRef]
- Piella, G.; Farré, N.; Esono, D.; Cordobés, M.Á.; Vázquez-Corral, J.; Bilbao, I.; Gómez-Gavara, C. LiverColor: An Artificial Intelligence Platform for Liver Graft Assessment. Diagnostics 2024, 14, 1654. [Google Scholar] [CrossRef]
- Kourounis, G.; Elmahmudi, A.; Thomson, B.; Nandi, R.; Tingle, S.; Glover, E.; Thompson, E.; Mahendran, B.; Connelly, C.; Gibson, B.; et al. Deep learning for automated boundary detection and segmentation in organ donation photography. Innov. Surg. Sci. 2024, 10, 131–141. [Google Scholar] [CrossRef]
- Hann, A.; Nutu, A.; Clarke, G.; Patel, I.; Sneiders, D.; Oo, Y.H.; Hartog, H.; Perera, M.T.P.R. Normothermic Machine Perfusion-Improving the Supply of Transplantable Livers for High-Risk Recipients. Transplant. Int. 2022, 35, 10460. [Google Scholar] [CrossRef]
- Abbas, S.H.; Ceresa, C.D.L.; Hodson, L.; Nasralla, D.; Watson, C.J.E.; Mergental, H.; Coussios, C.; Kaloyirou, F.; Brusby, K.; Mora, A.; et al. Defatting of donor transplant livers during normothermic perfusion—A randomised clinical trial: Study protocol for the DeFat study. Trials 2024, 25, 386. [Google Scholar] [CrossRef] [PubMed]
- Dengu, F.; Abbas, S.H.; Ebeling, G.; Nasralla, D. Normothermic Machine Perfusion (NMP) of the Liver as a Platform for Therapeutic Interventions during Ex-Vivo Liver Preservation: A Review. J. Clin. Med. 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Nassar, A.; Buccini, L.; Iuppa, G.; Soliman, B.; Pezzati, D.; Hassan, A.; Blum, M.; Baldwin, W.; Bennett, A.; et al. Lipid metabolism and functional assessment of discarded human livers with steatosis undergoing 24 hours of normothermic machine perfusion. Liver Transplant. 2018, 24, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Robertson, F.P.; Fuller, B.J.; Davidson, B.R. An Evaluation of Ischaemic Preconditioning as a Method of Reducing Ischaemia Reperfusion Injury in Liver Surgery and Transplantation. J. Clin. Med. 2017, 6, 69. [Google Scholar] [CrossRef]
- Fortier, A.K.; Feeney, K.; van Leeuwen, L.; Holzner, M.; DiNorcia, J.; Florman, S.; Akhtar, M. Secondary Perfusion: Modeling Viability of Organs Declined for Transplant. Am. J. Transplant. 2025, 25, S94. [Google Scholar] [CrossRef]
- Akhtar, Z.; Irizar, A.; Feeney, K.; Van Leeuwen, L.; Weissenbacher, A.; Chang, H.; Holzner, M.; Bagiella, E.; Hashimoto, K.; Schneeberger, S.; et al. Visualizing Graft Performance: Developing an Interactive Registry for Machine Perfusion. In Proceedings of the European Society for Organ Transplantation, London, UK, 29 June–2 July 2025. [Google Scholar]

| Flow Rate | |
| Hepatic artery | ≥0.2 L/min |
| Portal vein | ≥0.8 L/min |
| Hepatic function | |
| Perfusate lactate | <2.2 mmol/L |
| Perfusate pH | >7.25 with bicarbonate use <70 mL |
| Perfusate glucose | Decreasing or <180 mg/dL |
| Liver enzymes | |
| Alanine aminotransferase | <7000 u/L |
| Aspartate aminotransferase | <10,000 u/L |
| Biliary function | |
| Bile pH | >7.6 |
| Bile glucose | Differential between bile and perfusate glucose > 10 mmol/L |
| Macroscopic appearance | |
| Homogenously perfused appearance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortier, A.K.; Feeney, K.M.; Holzner, M.L.; DiNorcia, J.; Shapiro, R.; Kim-Schluger, L.; Florman, S.S.; van Leeuwen, L.L.; Akhtar, M.Z. Secondary Perfusion to Model Viability of Livers Declined for Transplantation. Livers 2025, 5, 66. https://doi.org/10.3390/livers5040066
Fortier AK, Feeney KM, Holzner ML, DiNorcia J, Shapiro R, Kim-Schluger L, Florman SS, van Leeuwen LL, Akhtar MZ. Secondary Perfusion to Model Viability of Livers Declined for Transplantation. Livers. 2025; 5(4):66. https://doi.org/10.3390/livers5040066
Chicago/Turabian StyleFortier, Avery K., Kimberly M. Feeney, Matthew L. Holzner, Joseph DiNorcia, Ron Shapiro, Leona Kim-Schluger, Sander S. Florman, L. Leonie van Leeuwen, and M. Zeeshan Akhtar. 2025. "Secondary Perfusion to Model Viability of Livers Declined for Transplantation" Livers 5, no. 4: 66. https://doi.org/10.3390/livers5040066
APA StyleFortier, A. K., Feeney, K. M., Holzner, M. L., DiNorcia, J., Shapiro, R., Kim-Schluger, L., Florman, S. S., van Leeuwen, L. L., & Akhtar, M. Z. (2025). Secondary Perfusion to Model Viability of Livers Declined for Transplantation. Livers, 5(4), 66. https://doi.org/10.3390/livers5040066






