Socioeconomic Disparities Along the Cancer Continuum for Hepatocellular Carcinoma: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
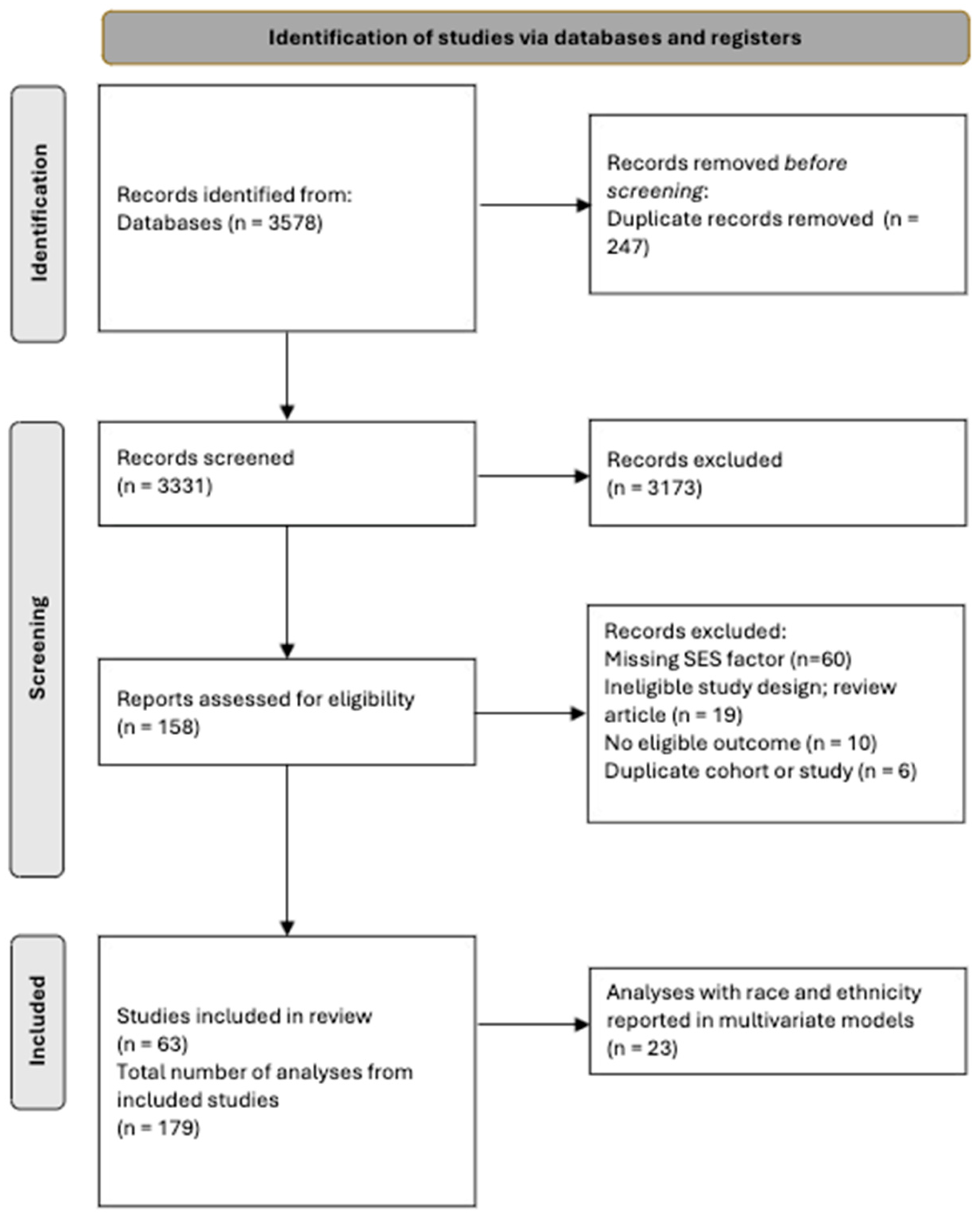
2.2. Study Selection
2.3. Data Extraction
2.4. Analytic Plan and Quality Assessment
3. Results
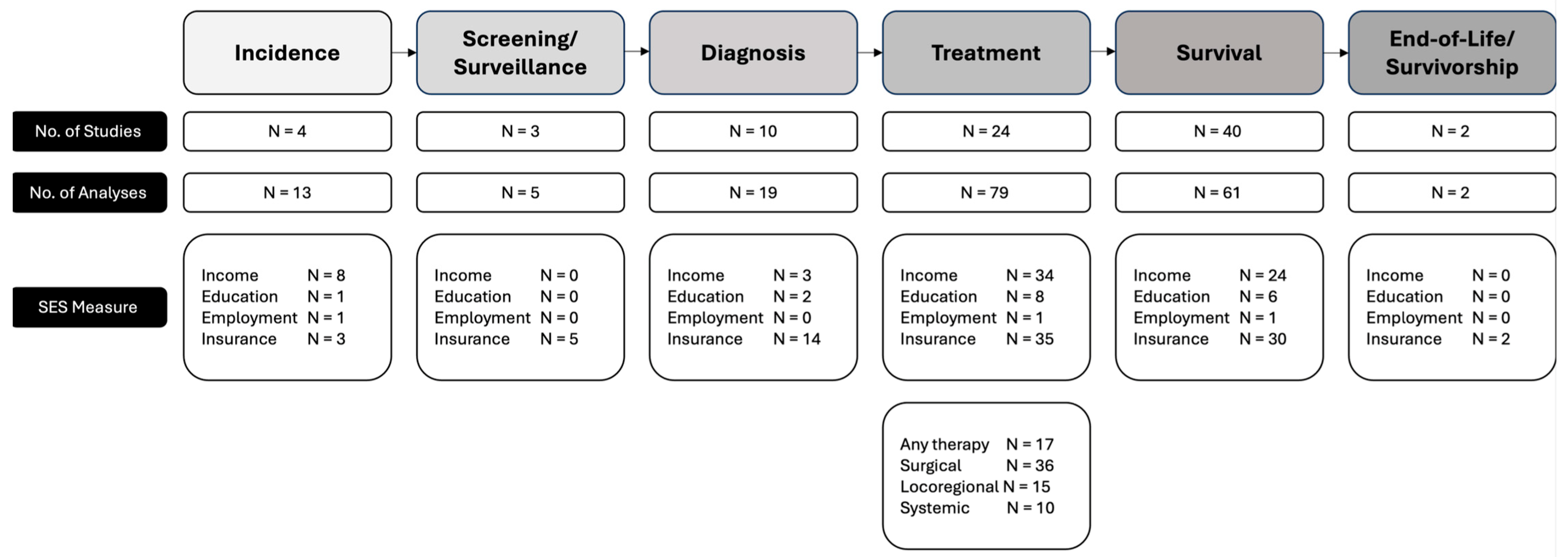
3.1. Study Characteristics and Thematic Design
3.2. Incidence
3.3. Screening and Surveillance
3.4. Diagnosis
3.5. Treatment
3.5.1. Any Treatment
3.5.2. Locoregional Therapies
3.5.3. Surgical Therapies Including Liver Resection and Transplantation
3.5.4. Systemic Therapies
3.6. Survival
3.6.1. Survival and Income
3.6.2. Survival and Education
3.6.3. Survival and Insurance
3.7. End-of-Life and Survivorship
3.8. Impact of SES Adjustment on Racial and Ethnic Disparities
3.9. Quality Assessment of Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| LT | Liver transplant |
| NCDB | National Cancer Database |
| NIS | National Inpatient Sample |
| OS | Overall survival |
| SDOH | Social determinants of health |
| SEER | Surveillance, Epidemiology and End Results |
| SES | Socioeconomic status |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- American Cancer Society. Key Statistics About Liver Cancer. 2025. Available online: https://www.cancer.org/cancer/types/liver-cancer/about/what-is-key-statistics.html (accessed on 22 April 2025).
- Rich, N.E.; Carr, C.; Yopp, A.C.; Marrero, J.A.; Singal, A.G. Racial and Ethnic Disparities in Survival Among Patients With Hepatocellular Carcinoma in the United States: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, e267–e288. [Google Scholar] [CrossRef]
- Wagle, N.S.; Park, S.; Washburn, D.; Ohsfeldt, R.L.; Rich, N.E.; Singal, A.G.; Kum, H.C. Racial, Ethnic, and Socioeconomic Disparities in Treatment Delay Among Patients With Hepatocellular Carcinoma in the United States. Clin. Gastroenterol. Hepatol. 2023, 21, 1281–1292.e10. [Google Scholar] [CrossRef]
- Lee, R.M.; Gamboa, A.C.; Turgeon, M.K.; Yopp, A.; Ryon, E.L.; Kronenfeld, J.P.; Goel, N.; Wang, A.; Lee, A.Y.; Luu, S.; et al. Dissecting Disease, Race, Ethnicity, And Socioeconomic Factors For Hepatocellular Carcinoma: An Analysis From The United States Safety Net Collaborative. Surg. Oncol. 2020, 35, 120–125. [Google Scholar] [CrossRef]
- Rich, N.E.; Jones, P.D.; Zhu, H.; Prasad, T.; Hughes, A.; Pruitt, S.; Murphy, C.C.; Seif-El-Dahan, K.; Daher, D.; Figueroa, G.; et al. Impact of racial, ethnic, and socioeconomic disparities on presentation and survival of HCC: A multicenter study. Hepatol. Commun. 2024, 8, e0477. [Google Scholar] [CrossRef]
- Lu, W.; Zheng, F.; Li, Z.; Zhou, R.; Deng, L.; Xiao, W.; Chen, W.; Zhao, R.; Chen, Y.; Tan, Y.; et al. Association Between Environmental and Socioeconomic Risk Factors and Hepatocellular Carcinoma: A Meta-Analysis. Front. Public Health 2022, 10, 741490. [Google Scholar] [CrossRef] [PubMed]
- Rich, N.E.; Yopp, A.C.; Marrero, J.A.; Kalva, S.; Odewole, M.; Canales, A.; Singal, A.G. Healthcare delivery system is associated with curative treatment receipt and overall survival in patients with hepatocellular carcinoma. Hepatology 2017, 66, 727A–728A. [Google Scholar]
- Rezaee-Zavareh, M.S.; Liang, J.; Yang, J.D. Ethnic disparities in the epidemiology, treatment, and outcome of patients with hepatocellular carcinoma in the United States. Hepatoma Res. 2023, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 22 April 2025).
- Pham Hang, T.V.; Sellers, M.T.; Lalonde, C.; Wedd, J.P.; Ross, K. Socioeconomic factors predicting access to treatment in cirrhotic patients with non-metastatic hepatocellular carcinoma. Hepatology 2018, 68, 549A. [Google Scholar]
- Sodagari, F.; Golnari, P.; Chapiro, J.; Jahromi, B.S.; Yaghmai, V. Percutaneous ablative interventions vs. surgery or transplant for hepatocellular carcinoma: A national analysis of hospitalizations, 1993-2015. Abdom. Radiol. 2019, 44, 3211–3212. [Google Scholar]
- Abara, W.E.; Spradling, P.; Zhong, Y.; Moorman, A.; Teshale, E.H.; Rupp, L.; Gordon, S.C.; Schmidt, M.; Boscarino, J.A.; Daida, Y.G.; et al. Hepatocellular Carcinoma Surveillance in a Cohort of Chronic Hepatitis C Virus-Infected Patients with Cirrhosis. J. Gastrointest. Cancer 2020, 51, 461–468. [Google Scholar] [CrossRef]
- Adler Jaffe, S.; Myers, O.; Meisner, A.L.W.; Wiggins, C.L.; Hill, D.A.; McDougall, J.A. Relationship between Insurance Type at Diagnosis and Hepatocellular Carcinoma Survival. Cancer Epidemiol. Biomark. Prev. 2020, 29, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Alawadi, Z.M.; Phatak, U.R.; Kao, L.S.; Ko, T.C.; Wray, C.J. Race not rural residency is predictive of surgical treatment for hepatocellular carcinoma: Analysis of the Texas Cancer Registry. J. Surg. Oncol. 2016, 113, 84–88. [Google Scholar] [CrossRef]
- Artinyan, A.; Mailey, B.; Sanchez-Luege, N.; Khalili, J.; Sun, C.L.; Bhatia, S.; Wagman, L.D.; Nissen, N.; Colquhoun, S.D.; Kim, J. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer 2010, 116, 1367–1377. [Google Scholar] [CrossRef]
- Aru, M.; Seals, S.; Ingram, B.; Seawright, A.; Anderson, C.; Earl, T. African Americans have greater odds of late stage diagnosis of hepatocellular carcinoma in Mississippi. Am. J. Transplant. 2016, 16, 516–517. [Google Scholar]
- Bateni, S.B.; Maquire, F.; Stewart, S.L.; Gangi, A.; Morris, C.; Colquhoun, S.; Gholami, S. Regional variation in the surgical treatment and survival of early stage hepatocellular carcinoma in California. HPB 2020, 22, S70–S71. [Google Scholar] [CrossRef]
- Bemanian, A.; Cassidy, L.; Beyer, K.M.M.; Saeian, K. Investigating biomedical pathways mediating racial and socioeconomic disparities of liver cancer: Retrospective analysis of chronic liver disease patients. Hepatology 2019, 70, 535A–536A. [Google Scholar]
- Beutler, B.D.; Ulanja, M.B.; Aluru, V.; Gullapalli, N. Sociodemographic characteristics as predictors of outcomes in hepatocellular carcinoma: A retrospective cohort study. J. Clin. Oncol. 2020, 38, 503. [Google Scholar] [CrossRef]
- Bodek, D.D.; Okoronkwo, N.O.; Patel, P.A.; Pyrsopoulos, N. Disparities in Liver Transplantation Between Ethnic Groups with Hepatocellular Carcinoma from 2007 to 2014. Gastroenterology 2018, 154, S-1099. [Google Scholar] [CrossRef]
- Cheng, D.; Calfee, G.; St. Hill, C.R.; Williams, S.; Baynosa, J.L.; Kirgan, D.M. Comparison of hepatocellular carcinoma outcome disparities between the mountain region and the nation. Ann. Surg. Oncol. 2019, 26, S174. [Google Scholar]
- Chidi, A.P.; Bryce, C.L.; Myaskovsky, L.; Fine, M.J.; Geller, D.A.; Landsittel, D.P.; Tsung, A. Differences in Physician Referral Drive Disparities in Surgical Intervention for Hepatocellular Carcinoma: A Retrospective Cohort Study. Ann Surg. 2016, 263, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Danos, D.M.; Ferguson, T.F.; Simonsen, N.; Leonardi, C.; Yu, Q.; Wu, X.C.; Scribner, R. Social determinants of hepatocellular carcinoma in Louisiana. Cancer Epidemiol. Biomark. Prev. 2018, 27, A41. [Google Scholar] [CrossRef]
- Estevez, J.; Yang, J.D.; Leong, J.; Nguyen, P.; Giama, N.H.; Schwartz, M.; Roberts, L.R.; Nguyen, M.H. Black patients with hepatocellular carcinoma (HCC) diagnosed after 2010 have worse long-term survival than white patients. Gastroenterology 2017, 152, S1173–S1174. [Google Scholar] [CrossRef]
- Flores, Y.N.; Datta, G.D.; Glenn, B.A.; Bastani, R.; May, F.P. The intersection of race/ethnicity and SES in HCC incidence, stage, survival and mortality: Results from seer (2000–2015). Hepatology 2019, 70, 374A–375A. [Google Scholar]
- Ford, M.M.; Ivanina, E.; Desai, P.; Highfield, L.; Qiao, B.; Schymura, M.J.; Laraque, F. Geographic epidemiology of hepatocellular carcinoma, viral hepatitis, and socioeconomic position in New York City. Cancer Causes Control 2017, 28, 779–789. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Lewis, J.D. Hepatocellular carcinoma surveillance rates in non-cirrhotic patients with chronic hepatitis B in the United States. Hepatology 2014, 60, 991A. [Google Scholar]
- Ha, J.; Yan, M.; Wang, J.; Tana, M.M.; Cheung, R.; Bhuket, T.; Liu, B.; Wong, R.J. Education level and household income is significantly associated with disparities in hepatocellular carcinoma stage at diagnosis and treatment received. Hepatology 2016, 64, 675A–676A. [Google Scholar]
- Harlan, L.C.; Parsons, H.M.; Wiggins, C.L.; Stevens, J.L.; Patt, Y.Z. Treatment of hepatocellular carcinoma in the community: Disparities in standard therapy. Liver Cancer 2015, 4, 70–83. [Google Scholar] [CrossRef]
- Hoehn, R.S.; Hanseman, D.J.; Jernigan, P.L.; Wima, K.; Ertel, A.E.; Abbott, D.E.; Shah, S.A. Disparities in care for patients with curable hepatocellular carcinoma. HPB 2015, 17, 747–752. [Google Scholar] [CrossRef]
- Hoehn, R.S.; Hanseman, D.J.; Dhar, V.K.; Go, D.E.; Edwards, M.J.; Shah, S.A. Opportunities to Improve Care of Hepatocellular Carcinoma in Vulnerable Patient Populations. J. Am. Coll. Surg. 2017, 224, 697–704. [Google Scholar] [CrossRef]
- Hood, R.B.; Felix, A. Neighborhood disadvantage is associated with liver cancer treatment and survival. Cancer Epidemiol. Biomark. Prev. 2020, 29, C057. [Google Scholar] [CrossRef]
- Hyder, O.; Dodson, R.M.; Nathan, H.; Herman, J.M.; Cosgrove, D.; Kamel, I.; Geschwind, J.F.; Pawlik, T.M. Referral patterns and treatment choices for patients with hepatocellular carcinoma: A United States population-based study. J. Am. Coll. Surg. 2013, 217, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Jan, T.; Medvedev, S.; Cannon, R.M.; Saggi, B.; McGee, J.; Paramesh, A.; Killackey, M.; Shores, N.J.; Slakey, D.P.; Balart, L.; et al. Racial disparity and their impact on hepatocellular cancer outcomes in inner-city New Orleans. Surgery 2012, 152, 661–666; discussion 666. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.D.; Gonzalez-Diaz, J.; Martin, P.M.; Jones, P.D.; Martin, P.M. Socioeconomic and survival differences among minorities with hepatocellular carcinoma in Florida. J. Hepatocell. Carcinoma 2019, 6, 167–181. [Google Scholar] [CrossRef]
- Kangas-Dick, A.; Gall, V.; Hilden, P.; Turner, A.; Greenbaum, A.; Sesti, J.; Paul, S.; Carpizo, D.; Kennedy, T.; Sadaria Grandhi, M.; et al. Disparities in utilization of services for racial and ethnic minorities with hepatocellular carcinoma associated with hepatitis C. Surgery 2020, 168, 49–55. [Google Scholar] [CrossRef]
- Kokabi, N.; Xing, M.; Duszak, R., Jr.; Howard, D.H.; Applegate, K.E.; Camacho, J.C.; Kim, H.S. Sociodemographic impact on survival in unresectable hepatocellular carcinoma: A survival epidemiology and end results study. Future Oncol. 2016, 12, 183–198. [Google Scholar] [CrossRef]
- Kokabi, N.; Duszak, R., Jr.; Xing, M.; Howard, D.H.; Applegate, K.E.; Camacho, J.C.; Kim, H.S. Cancer-directed therapy and potential impact on survivals in nonresected hepatocellular carcinoma: SEER-Medicare population study. Future Oncol. 2017, 13, 2021–2033. [Google Scholar] [CrossRef]
- Kronenfeld, J.P.; Ryon, E.L.; Goel, N.; Goldberg, D. Disparities in presentation at time of hepatocellular carcinoma diagnosis: A united states safety-net collaborative analysis. Ann. Surg. Oncol. 2020, 27, S94. [Google Scholar] [CrossRef]
- Kronenfeld, J.P.; Ryon, E.L.; Lee, R.M.; Charles Yopp, A.; Yeelin Lee, A.; Hsu, C.; Jay Silberfein, E.; Citarella Russell, M.; Merchant, N.B.; Goel, N. Insurance Matters! Disparities in Treatment and Outcomes Based on Insurance Status of Patients with Early-Stage Hepatocellular Carcinoma: A US Safety-Net Collaborative Analysis. J. Am. Coll. Surg. 2020, 231, e152–e153. [Google Scholar] [CrossRef]
- Kwong, S.L.; Stewart, S.L.; Aoki, C.A.; Chen, M.S., Jr. Disparities in hepatocellular carcinoma survival among Californians of Asian ancestry, 1988 to 2007. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.M.; Gamboa, A.C.; Turgeon, M.K.; Yopp, A.; Ryon, E.L.; Kronenfeld, J.P.; Goel, N.; Wang, A.; Lee, A.Y.; Luu, S.; et al. The Evolving Landscape of Hepatocellular Carcinoma: A US Safety Net Collaborative Analysis of Etiology of Cirrhosis. Am. Surg. 2020, 86, 865–872. [Google Scholar] [CrossRef]
- Lee, R.T.; Cioffi, G.; Opneja, A.; Alahmadi, A.; Patil, N.; Jones, N.; Bajor, D.L.; Saltzman, J.N.; Mohamed, A.; Mangla, A.; et al. Impact of facility type, insurance status, and income on use of single agent chemotherapy (SACT) for advanced hepatocellular carcinoma (AHCC): Analysis of national cancer database (NCDB). J. Clin. Oncol. 2020, 38, 504. [Google Scholar] [CrossRef]
- Major, J.M.; Sargent, J.D.; Graubard, B.I.; Carlos, H.A.; Hollenbeck, A.R.; Altekruse, S.F.; Freedman, N.D.; McGlynn, K.A. Local geographic variation in chronic liver disease and hepatocellular carcinoma: Contributions of socioeconomic deprivation, alcohol retail outlets, and lifestyle. Ann. Epidemiol. 2014, 24, 104–110. [Google Scholar] [CrossRef]
- Mazumder, N.; Gabra, L.; Atiemo, K.; Kho, A.; Daud, A.; Levitsky, J.; Ladner, D.; Simpson, D. Outcomes for black patients with cirrhosis: Results from a large cohort study. Am. J. Transplant. 2020, 20, 59. [Google Scholar]
- Mokdad, A.A.; Murphy, C.C.; Pruitt, S.L.; Mansour, J.C.; Marrero, J.A.; Singal, A.G.; Yopp, A.C. Effect of hospital safety net designation on treatment use and survival in hepatocellular carcinoma. Cancer 2018, 124, 743–751. [Google Scholar] [CrossRef]
- Muzaffar, M.; Naqash, A.R. Impact of race and socioeconomic factors on outcome in hepatocellular carcinoma. J. Clin. Oncol. 2020, 38, e16657. [Google Scholar] [CrossRef]
- Peters, N.A.; Javed, A.A.; He, J.; Wolfgang, C.L.; Weiss, M.J. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J. Surg. Res. 2017, 210, 253–260. [Google Scholar] [CrossRef]
- Rho, Y.S.; Acoba, J.D. Factors associated with biopsy diagnosis of hepatocellular carcinoma. J. Clin. Oncol. 2019, 37, 235. [Google Scholar] [CrossRef]
- Rich, N.E.; Hester, C.; Odewole, M.; Murphy, C.C.; Parikh, N.D.; Marrero, J.A.; Yopp, A.C.; Singal, A.G. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2019, 17, 551–559.e1. [Google Scholar] [CrossRef]
- Robbins, A.S.; Cox, D.D.; Johnson, L.B.; Ward, E.M. Persistent disparities in liver transplantation for patients with hepatocellular carcinoma in the United States, 1998 through 2007. Cancer 2011, 117, 4531–4539. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Tavakoli, H.; Liu, B.; Bhuket, T.; Wong, R.J. Less than half of hepatocellular carcinoma patients among the 1945-1965 birth cohort in the U.S. were within milan criteria at time of diagnosis. Hepatology 2017, 66, 123A–124A. [Google Scholar]
- Robinson, A.; Tavakoli, H.; Liu, B.; Bhuket, T.; Wong, R.J. Advanced Hepatocellular Carcinoma Tumor Stage at Diagnosis in the 1945-1965 Birth Cohort Reflects Poor Use of Hepatocellular Carcinoma Screening. Hepatol. Commun. 2018, 2, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Sarpel, U.; Suprun, M.; Sofianou, A.; Berger, Y.; Tedjasukmana, A.; Sekendiz, Z.; Bagiella, E.; Schwartz, M.E. Disentangling the effects of race and socioeconomic factors on liver transplantation rates for hepatocellular carcinoma. Clin. Transpl. 2016, 30, 714–721. [Google Scholar] [CrossRef]
- Sarpel, U.; Heskel, M.; Spivack, J.H.; Feferman, Y.; Ang, C.; Gany, F. Disparities in Access to Sorafenib in Communities with Low Socioeconomic Status. J. Health Care Poor Underserved 2018, 29, 1123–1134. [Google Scholar] [CrossRef]
- Scaglione, S.; Adams, W.; Caines, A.; Devlin, P.; Mittal, S.; Singal, A.G.; Parikh, N.D. Association Between Race/Ethnicity and Insurance Status with Outcomes in Patients with Hepatocellular Carcinoma. Dig. Dis. Sci. 2020, 65, 1669–1678. [Google Scholar] [CrossRef]
- Sellers, C.M.; Uhlig, J.; Ludwig, J.M.; Taddei, T.; Stein, S.M.; Lim, J.K.; Kim, H.S. The impact of socioeconomic status on outcomes in hepatocellular carcinoma: Inferences from primary insurance. Cancer Med. 2019, 8, 5948–5958. [Google Scholar] [CrossRef]
- Singal, A.G.; Li, X.; Tiro, J.; Kandunoori, P.; Adams-Huet, B.; Nehra, M.S.; Yopp, A. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am. J. Med. 2015, 128, 90.e1–90.e7. [Google Scholar] [CrossRef]
- Sobotka, L.; Hinton, A.; Conteh, L. Insurance status impacts treatment for hepatocellular carcinoma. Am. J. Transplant. 2019, 19, 1099. [Google Scholar] [CrossRef]
- Turse, E.; Charlie, E.L.; Aboona, M.; Chuang, K.Y.; Bhattarai, B.; Bowie, T.; Srinivasan, I.; Kadkhodayan, K.; Forlemu, A.; Nadir, A. Factors related to survival and mortality for hepatocellular carcinoma (HCC) at a safety net hospital in Arizona. Hepatology 2020, 72 (Suppl. S1), 639A. [Google Scholar]
- Uhlig, J.; Sellers, C.; Khan, S.A.; Cha, C.; Kim, H.S.K. Impact of hospital volume and type on survival in hepatocellular carcinoma: Results from the National Cancer Database. J. Clin. Oncol. 2019, 37, 417. [Google Scholar] [CrossRef]
- Wang, J.; Ha, J.; Tana, M.; Bhuket, T.; Liu, B.; Younossi, Z.; Wong, R. Insured patients with hepatocellular carcinoma in the united states are more likely to have hepatocellular carcinoma within Milan criteria, are more likely to receive treatment, and have higher survival compared to uninsured and medicaid patients. J. Hepatol. 2016, 64, S328–S329. [Google Scholar] [CrossRef]
- Wang, J.; Ha, J.; Lopez, A.; Bhuket, T.; Liu, B.; Wong, R.J. Medicaid and Uninsured Hepatocellular Carcinoma Patients Have More Advanced Tumor Stage and Are Less Likely to Receive Treatment. J. Clin. Gastroenterol. 2018, 52, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Lee, R.M.; Citarella Russell, M.; Charles Yopp, A.; Leah Ryon, E.; Goel, N.; Luu, S.V.; Hsu, C.; Jay Silberfein, E.; Yeelin Lee, A. Disparities in Hepatocellular Carcinoma Outcomes at Safety Net Hospitals Are Greatest in Patients with Child B Cirrhosis Who Have Not Undergone Screening. J. Am. Coll. Surg. 2020, 231, S266. [Google Scholar] [CrossRef]
- Wasif, N.; Etzioni, D.; Habermann, E.B.; Mathur, A.; Pockaj, B.A.; Gray, R.J.; Chang, Y.H. Racial and Socioeconomic Differences in the Use of High-Volume Commission on Cancer-Accredited Hospitals for Cancer Surgery in the United States. Ann. Surg. Oncol. 2018, 25, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Woodrell, C.; Moreno, J.R.; Garrido, M.M.; Goldstein, N.E. Characteristics of hospitalized patients with hepatocellular carcinoma that are associated with receipt of inpatient palliative care. Hepatology 2018, 68, 305A–306A. [Google Scholar]
- Yan, M.; Ha, J.; Lopez, A.; Wang, J.; Liu, B.; Bhuket, T.; Wong, R.J. Among adults with hepatocellular carcinoma in the United States, low income patients have significantly lower overall survival independent of tumor stage and treatment received. Hepatology 2016, 64, 638A. [Google Scholar]
- Yang, J.D.; Luu, M.; Noureddin, M.; Kuo, A.; Ayoub, W.S.; Sundaram, V.; Kotler, H.; Kim, I.; Todo, T.; Kosari, K.; et al. Surgical treatment is associated with improved outcome in patients with single less than 2cm hepatocellular carcinoma. Hepatology 2019, 70, 145A–146A. [Google Scholar]
- Yu, J.C.; Neugut, A.I.; Wang, S.; Jacobson, J.S.; Ferrante, L.; Khungar, V.; Lim, E.; Hershman, D.L.; Brown, R.S., Jr.; Siegel, A.B. Racial and insurance disparities in the receipt of transplant among patients with hepatocellular carcinoma. Cancer 2010, 116, 1801–1809. [Google Scholar] [CrossRef]
- Zaydfudim, V.; Whiteside, M.A.; Griffin, M.R.; Feurer, I.D.; Wright, J.K.; Pinson, C.W. Health insurance status affects staging and influences treatment strategies in patients with hepatocellular carcinoma. Ann. Surg. Oncol. 2010, 17, 3104–3111. [Google Scholar] [CrossRef]
- Zou, W.Y.; El-Serag, H.B.; Sada, Y.H.; Temple, S.L.; Sansgiry, S.; Kanwal, F.; Davila, J.A. Determinants and Outcomes of Hospice Utilization Among Patients with Advance-Staged Hepatocellular Carcinoma in a Veteran Affairs Population. Dig. Dis. Sci. 2018, 63, 1173–1181. [Google Scholar] [CrossRef]
- Wong, R.J.; Kim, D.; Ahmed, A.; Singal, A.K. Patients with hepatocellular carcinoma from more rural and lower-income households have more advanced tumor stage at diagnosis and significantly higher mortality. Cancer 2020, 127, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Rho, Y.S.; Pagano, I.; Acoba, J.D. Racial and socioeconomic disparities in the treatment of resectable hepatocellular carcinoma. J. Clin. Oncol. 2020, 38, e19026. [Google Scholar] [CrossRef]
- Cheng, H.; Kamarck, T.; Gianaros, P.; Roecklein, K.; Tsung, A.; Geller, D.; Marsh, J.; Wang, Y.; Jones, R.; Ell, K.; et al. Predicting survival of hepatocellular carcinoma: The role of symptom severity and socioeconomic status. Psycho-Oncology 2018, 27, 81. [Google Scholar]
- Chidi, A.P.; Bryce, C.L.; Han, K.; Dong, Z.M.; Geller, D.A.; Tsung, A. Predictors of delay of surgical intervention in patients with hepatocellular carcinoma. J. Surg. Res. 2014, 186, 610–611. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Bhuket, T.; Wong, R. Disparities in health insurance coverage do not fully explain race/ethnicity-specific disparities in hepatocellular carcinoma survival in the United States. Am. J. Gastroenterol. 2016, 111, S386–S387. [Google Scholar] [CrossRef]
- Lee, R.M.; Gamboa, A.C.; Turgeon, M.K.; Yopp, A.; Ryon, E.L.; Kronenfeld, J.P.; Goel, N.; Wang, A.; Lee, A.Y.; Luu, S.; et al. Increased risk of hepatocellular carcinoma associated with neighborhood concentrated disadvantage. Front. Oncol. 2018, 8, 375. [Google Scholar] [CrossRef]
- Wang, J.; Ha, J.; Yan, M.; Aguilar, M.; Cheung, R.; Wong, R. African americans with hepatocellular carcinoma in the U.S. have higher rates of being uninsured at time of diagnosis, are less likely to have HCC within Milan criteria, and are less likely to receive any hepatocellular carcinoma treatment. J. Hepatol. 2016, 64, S328. [Google Scholar] [CrossRef]
- Wagle, N.S.; Park, S.; Washburn, D.; Ohsfeldt, R.L.; Rich, N.E.; Singal, A.G.; Kum, H.C. Racial, Ethnic, and Socioeconomic Disparities in Curative Treatment Receipt and Survival in Hepatocellular Carcinoma. Hepatol. Commun. 2022, 6, 1186–1197. [Google Scholar] [CrossRef]
- Wagle, N.S.; Park, S.; Washburn, D.; Ohsfeldt, R.; Kum, H.C.; Singal, A.G. Racial and Ethnic Disparities in Hepatocellular Carcinoma Treatment Receipt in the United States: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2024, 33, 463–470. [Google Scholar] [CrossRef]
- Zhou, K.; Song, Z.; Rostomian, N.; Dodge, J.L.; Stern, M.C.; Setiawan, V.W.; Terrault, N.A.; Cockburn, M.G.; Liu, L. Association of nativity with survival among adults with hepatocellular carcinoma. J. Natl. Cancer Inst. 2023, 115, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Nephew, L.D.; Rawl, S.M.; Carter, A.; Garcia, N.; Monahan, P.O.; Holden, J.; Ghabril, M.; Montalvan-Sanchez, E.; Patidar, K.; Desai, A.P.; et al. Health literacy and cumulative social disadvantage are associated with survival and transplant in patients with hepatocellular carcinoma: A prospective study. BMJ Open Gastroenterol. 2024, 11, e001537. [Google Scholar] [CrossRef] [PubMed]
- Poulson, M.R.; Blanco, B.A.; Geary, A.D.; Kenzik, K.M.; McAneny, D.B.; Tseng, J.F.; Sachs, T.E. The role of racial segregation in treatment and outcomes among patients with hepatocellular carcinoma. HPB 2021, 23, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Tiro, J.A.; Murphy, C.C.; Blackwell, J.M.; Kramer, J.R.; Khan, A.; Liu, Y.; Zhang, S.; Phillips, J.L.; Hernaez, R. Patient-Reported Barriers Are Associated With Receipt of Hepatocellular Carcinoma Surveillance in a Multicenter Cohort of Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2021, 19, 987–995.e1. [Google Scholar] [CrossRef]
- Wong, R.J.; Jones, P.D.; Niu, B.; Therapondos, G.; Thamer, M.; Kshirsagar, O.; Zhang, Y.; Pinheiro, P.; Kyalwazi, B.; Fass, R.; et al. Clinician-Level Knowledge and Barriers to Hepatocellular Carcinoma Surveillance. JAMA Netw. Open 2024, 7, e2411076. [Google Scholar] [CrossRef]
- Simmons, O.L.; Feng, Y.; Parikh, N.D.; Singal, A.G. Primary Care Provider Practice Patterns and Barriers to Hepatocellular Carcinoma Surveillance. Clin. Gastroenterol. Hepatol. 2019, 17, 766–773. [Google Scholar] [CrossRef]
- Parikh, N.D.; Tayob, N.; Al-Jarrah, T.; Kramer, J.; Melcher, J.; Smith, D.; Marquardt, P.; Liu, P.H.; Tang, R.; Kanwal, F.; et al. Barriers to Surveillance for Hepatocellular Carcinoma in a Multicenter Cohort. JAMA Netw. Open 2022, 5, e2223504. [Google Scholar] [CrossRef]
- Woodrell, C.D.; Hansen, L.; Schiano, T.D.; Goldstein, N.E. Palliative Care for People With Hepatocellular Carcinoma, and Specific Benefits for Older Adults. Clin. Ther. 2018, 40, 512–525. [Google Scholar] [CrossRef]
- Wachterman, M.W.; Sommers, B.D. Dying Poor in the US-Disparities in End-of-Life Care. JAMA 2021, 325, 423–424. [Google Scholar] [CrossRef]
- Tilhou, A.S.; Huguet, N.; DeVoe, J.; Angier, H. The Affordable Care Act Medicaid Expansion Positively Impacted Community Health Centers and Their Patients. J. Gen. Intern. Med. 2020, 35, 1292–1295. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Valderrama, A.; Kamalakar, R.; Sansgiry, S.S.; Babajanyan, S.; Lewis, J.D. Hepatocellular Carcinoma Surveillance Among Cirrhotic Patients With Commercial Health Insurance. J Clin. Gastroenterol. 2016, 50, 258–265. [Google Scholar] [CrossRef]
- Bensken, W.P.; McGrath, B.M.; Gold, R.; Cottrell, E.K. Area-level social determinants of health and individual-level social risks: Assessing predictive ability and biases in social risk screening. J. Clin. Transl. Sci. 2023, 7, e257. [Google Scholar] [CrossRef]
| Study Author (Year) | Database Type | Geographic Setting | Study Population | Staging System | Date Range | Sample Size |
|---|---|---|---|---|---|---|
| Abara et al. (2020) [14] | Multi-Institution | Danville, PA; Detroit, MI; Honolulu, HI; Portland, OR | Adults with hepatitis C and cirrhosis | N/A | 2011–2016 | 2933 |
| Adler Jaffe et al. (2020) [15] | SEER | National | All adults with HCC | SEER Historic Stage | 2010–2015 | 14,655 |
| Alawadi et al. (2016) [16] | Texas Cancer Registry | State (Texas) | All adults with HCC | AJCC 6th Edition | 2000–2008 | 5037 |
| Artinyan et al. (2010) [17] | SEER | National | All adults with HCC | Metastatic vs. non-metastatic | 1973–2004 | 14,906 |
| Aru et al. (2016) [18] | Mississippi Cancer Registry | State (Mississippi) | Black and white adults with HCC | AJCC 6th edition | 1995–2016 | 421 |
| Bateni et al. (2020) [19] | California Cancer Registry | State (California) | Adults with HCC | AJCC 6th edition | 2005–2017 | 19,555 |
| Bemanian et al. (2019) [20] | Not specified | Milwaukee/Racine metropolitan areas (Wisconsin) | Adults with chronic liver disease | N/A | N/A | 18,143 |
| Beutler et al. (2020) [21] | SEER | National | Adults with resectable HCC | AJCC 6th edition | 2007–2015 | 28,518 |
| Bodek et al. (2018) [22] | NIS | National | Adults hospitalized with HCC | N/A | 2007–2014 | 7244 |
| Cheng et al. (2019) [23] | NCDB | Regional (Mountain West region) | Adults with HCC | AJCC pathologic stage | 2004–2015 | 6500 |
| Chidi et al. (2016) [24] | Pennsylvania Cancer Registry | State (Pennsylvania) | Adults with HCC | SEER summary stage | 2006–2011 | 3576 |
| Danos et al. (2018) [25] | Louisiana Tumor Registry and US Census | State (Louisiana) | Black and white adults age > 35 | N/A | 2008–2012 | 1418 |
| Estevez et al. (2017) [26] | Multi-institution | Stanford U, Mt. Sinai NY, Mayo MN | Black and white adults with HCC | BCLC Staging | 1991–2016 | 1156 |
| Flores et al. (2019) [27] | SEER | National | All adults with HCC | SEER Historic Stage A variable | 2000–2015 | 45,789 |
| Ford et al. (2017) [28] | New York State Cancer Registry | City (New York City) | All adults | N/A | 2001–2012 | 8827 |
| Goldberg et al. (2014) [29] | Truven Health Analytics Database | National | Adults with non-cirrhotic chronic HBV | N/A | 2006–2009 | 4576 |
| Ha et al. (2016) [30] | SEER | National | All adults with HCC | SEER Historic Stage | 2003–2013 | 61,594 |
| Harlan et al. (2015) [31] | NCI Patterns of Care Study | National | All adults with HCC | BCLC staging | 2007 | 946 |
| Hoehn et al. (2015) [32] | NCDB | National | All adults with AJCC Stage I/II HCC | AJCC 6th edition | 1998–2011 | 43,859 |
| Hoehn et al. (2017) [33] | NCDB | National | All adults with HCC | AJCC clinical stage | 1998–2007 | 143,692 |
| Hood et al. (2020) [34] | Ohio Cancer Incidence Surveillance System | State (Ohio) | All adults with HCC | N/A | 2003–2016 | 8208 |
| Hyder et al. (2013) [35] | SEER-Medicare | National | All adults with HCC | SEER Historic Staging A | 1998–2007 | 6752 |
| Jan et al. (2012) [36] | Single institution | Tulane University Medical Center (Louisiana) | All adults with HCC | N/A; tumor size, # of tumors, vascular invasion | 2003–2011 | 206 |
| Jones et al. (2019) [37] | Florida Cancer Data System | State (Florida) | All adults with HCC | SEER Stage 2000 | 2004–2013 | 10,852 |
| Kangas-Dick et al. (2020) [38] | NIS | National | All adults hospitalized with HCC and HCV | N/A | 2005–2013 | 200,163 |
| Kokabi et al. (2016) [39] | SEER | National | All adults with HCC | N/A | 2000–2011 | 23,464 |
| Kokabi et al. (2017) [40] | SEER | National | All adults with HCC | AJCC 6th edition | 2000–2010 | 9368 |
| Kronenfeld et al. (2020, A) [41] | USSNC—Multi-institution | Metro regions of Texas, Florida, Georgia, New York | All adults with HCC | AJCC 6th edition | 2012–2014 | 1620 |
| Kronenfeld et al. (2020, B) [42] | USSNC—Multi-institution | Metro regions of Texas, Florida, Georgia, New York | Adults with HCC, AJCC Stage I/II | AJCC 6th edition | 2012–2014 | 1087 |
| Kwong et al. (2010) [43] | California Cancer Registry (CCR) | State (California) | Adults with HCC from nine Asian ethnic groups | SEER classification | 1988–2007 | 6068 |
| Lee et al. (2020, A) [5] | USSNC—Multi-institution | Metro regions of Texas, Florida, Georgia, New York | All adults with HCC | AJCC 8th edition | 2012–2014 | 1832 |
| Lee et al. (2020, B) [44] | USSNC—Multi-institution | Metro regions of Texas, Florida, Georgia, New York | All adults with HCC | AJCC 8th edition | 2004–2014 | 31,107 |
| Lee et al. (2020, C) [45] | USSNC—Multi-institution | Metro regions of Texas, Florida, Georgia, New York | Adults with HCC treated with only chemotherapy | AJCC 8th edition | 2004–2014 | 1479 |
| Major et al. (2014) [46] | SEER, NIH-AARP Diet Study | National (CA, FL, LA, NC, NJ, PA, Detroit, Atlanta) | Retired adults | N/A | 1995–2006 | 494,988 |
| Mazumder et al. (2021) [47] | HealthLNK Data Repository | Multi-institution (greater metropolitan Chicago area) | Adults with HCC | N/A | 2006–2012 | 11,277 |
| Mokdad et al. (2018) [48] | Texas Cancer Registry | State (Texas) | All adults with HCC | SEER classification | 2001–2012 | 15,932 |
| Muzaffar et al. (2020) [49] | SEER | National | All adults with HCC | SEER classification | 1990–2016 | 87,047 |
| Peters et al. (2017) [50] | SEER | National | Adults with early-stage HCC (AJCC T1/T2) | AJCC 6th edition | 2004–2012 | 13,694 |
| Pham Hang et al. (2018) [12] | Single institution | Emory Medical Center (Georgia) | Adults with non-metastatic HCC | N/A; focused on non-metastatic disease | 2013–2016 | 156 |
| Rho et al. (2019) [51] | NCDB | National | All adults with HCC | N/A; metastatic vs. non metastatic | 2004–2015 | 160,517 |
| Rich et al. (2019) [52] | Multi-institution | Parkland and UTSW (Texas) | All adults with HCC | BCLC staging | 2008–2017 | 1117 |
| Robbins et al. (2011) [53] | NCDB | National | All adults with HCC | AJCC 5th/6th editions | 1998–2007 | 7707 |
| Robinson et al. (2017) [54] | SEER | National | Adults born 1945–1965 with HCC | SEER Historic Staging | 2004–2014 | 38,045 |
| Robinson et al. (2018) [55] | SEER | National | Adults born 1945–1965 with HCC | SEER Historic Staging | 2004–2014 | 38,045 |
| Sarpel et al. (2016) [56] | Single institution | The Mount Sinai Hospital (New York) | All adults with HCC | N/A; within vs. beyond Milan criteria | 2003–2013 | 742 |
| Sarpel et al. (2018) [57] | Single institution | The Mount Sinai Hospital (New York) | Adults with BCLC-C HCC | BCLC staging | 2007–2013 | 742 |
| Scaglione et al. (2020) [58] | Multi-institution | U Mich, Loyola, Parkland, Ben-Taub | All adults with HCC | N/A; within vs. beyond Milan criteria | 2012–2013 | 379 |
| Sellers et al. (2019) [59] | Single institution | Yale Hospital Cancer Registry (Connecticut) | All adults with HCC | BCLC staging | 2005–2016 | 769 |
| Singal et al. (2015) [60] | Single institution | Parkland Health and Hospital System | All adults with cirrhosis | N/A; intra hepatic vs. extra hepatic disease | 2008–2011 | 904 |
| Sobotka et al. (2019) [61] | NIS | National | All adults hospitalized with HCC | N/A; non metastatic vs. single met vs. multiple mets | 2010–2013 | 62,368 |
| Sodagari et al. (2019) [13] | NIS | National | All adults hospitalized with HCC | N/A | 1993–2015 | 701,368 |
| Turse et al. (2022) [62] | Single institution | Valleywise Health Center (Arizona) | All adults with HCC | N/A; within or outside of Milan criteria | 2010–2020 | 161 |
| Uhlig et al. (2019) [63] | NCDB | National | All adults with HCC | AJCC 7th edition | 2004–2015 | 63,877 |
| Wang et al. (2016) [64] | SEER | National | All adults with HCC | SEER Historic Staging system | 1983–1992 | 26,535 |
| Wang et al. (2018) [65] | SEER | National | All adults with HCC | SEER Historic Staging system | 2007–2012 | 32,388 |
| Wang et al. (2020) [66] | SEER | National | All adults with HCC | AJCC 6th edition | 2004–2017 | 83,237 |
| Wasif et al. (2018) [67] | NCDB | National | Adults with HCC undergoing surgery | N/A | 2003–2012 | 3814 |
| Woodrell et al. (2021) [68] | Single institution | The Mount Sinai Hospital (New York) | All adults with HCC-related hospitalization | N/A | 2012–2016 | 842 |
| Yan et al. (2016) [69] | SEER | National | All adults with HCC | SEER Historic Staging System | 2003–2013 | 61,594 |
| Yang et al. (2019) [70] | NCDB | National | All adults with single HCC tumor < 2 cm in diameter | N/A; single HCC tumor < 2cm | 2004–2014 | 6261 |
| Yu et al. (2010) [71] | Single institution | Columbia University Medical Center (New York) | All adults with HCC | AJCC staging | 2002–2008 | 462 |
| Zaydfudim et al. (2010) [72] | Tennessee Cancer Registry | State (Tennessee) | All adults with HCC | AJCC/TNM staging | 2004–2006 | 680 |
| Zou et al. (2018) [73] | Veteran Affairs (VA) database | National | Veterans with BCLC-C/D HCC | BCLC staging | 2004–2011 | 397 |
| Study Author (Year) | SES Predictor(s) | HCC Outcome(s) | No. of Included Analyses |
|---|---|---|---|
| Abara et al. (2020) [14] | Insurance | Surveillance | 1 |
| Adler Jaffe et al. (2020) [15] | Insurance | Survival (by stage) | 3 |
| Alawadi et al. (2016) [16] | Income (area-level % poverty, income type) | Treatment (locoregional, surgical, 6) Survival (1) | 7 |
| Artinyan et al. (2010) [17] | Income (area-level median income) | Survival | 1 |
| Aru et al. (2016) [18] | Income (area-level median household income) | Diagnosis (late stage) | 1 |
| Bateni et al. (2020) [19] | Income (SES tertiles), Insurance | Survival | 2 |
| Bemanian et al. (2019) [20] | Income (neighborhood SES disadvantage index) | Incidence | 1 |
| Beutler et al. (2020) [21] | Insurance | Survival | 1 |
| Bodek et al. (2018) [22] | Income (census-tract-level income quartile), Insurance | Treatment (surgical) | 2 |
| Cheng et al. (2019) [23] | Insurance | Treatment (all treatment, locoregional, systemic, 4), Survival (1) | 5 |
| Chidi et al. (2016) [24] | Income (area-level median household income), Insurance | Treatment (surgical) | 4 |
| Danos et al. (2018) [25] | Income (neighborhood concentrated disadvantage index, CDI) | Incidence | 1 |
| Estevez et al. (2017) [26] | Insurance | Survival | 1 |
| Flores et al. (2019) [27] | Income (area-level median income) | Survival | 1 |
| Ford et al. (2017) [28] | Income (neighborhood poverty index), Insurance | Incidence | 2 |
| Goldberg et al. (2014) [29] | Insurance | Surveillance | 2 |
| Ha et al. (2016) [30] | Education (area-level % without HS degree), Income (area-level quartile) | Diagnosis (2), Treatment (all treatment, 2) | 4 |
| Harlan et al. (2015) [31] | Income (area-level median income), Insurance | Treatment (all treatment, 3, locoregional, 5, surgical, 4, systemic, 4), Survival (2) | 18 |
| Hoehn et al. (2015) [32] | Income (area-level median income), Education (area-level % without HS degree), Insurance | Treatment (surgical, 3), Survival (3) | 6 |
| Hoehn et al. (2017) [33] | Income (area-level median income), Education (area-level % without HS degree) | Treatment (surgical, 2, systemic, 1), Survival (2) | 5 |
| Hood et al. (2020) [34] | Income (neighborhood disadvantage index) | Treatment (all treatment, 1), survival (1) | 2 |
| Hyder et al. (2013) [35] | Income (area-level income quartiles) | Treatment (lociregional, 3, surgical, 3, systemic, 3), Survival (2) | 11 |
| Jan et al. (2012) [36] | Insurance | Survival | 1 |
| Jones et al. (2019) [37] | Insurance | Survival | 1 |
| Kangas-Dick et al. (2020) [38] | Insurance | Treatment (all treatment) | 1 |
| Kokabi et al. (2016) [39] | Income (area-level mean family income), Education (area-level % of adults with less than HS or bachelor’s), Employment (area-level unemployment rate), Insurance | Survival (5) | 5 |
| Kokabi et al. (2017) [40] | Income (area-level mean family income), Education (area-level % of adults with HS degree), Insurance | Treatment (all treatment, 4) | 4 |
| Kronenfeld et al. (2020, A) [41] | Income (area-level % below poverty line), Insurance | Diagnosis (2) | 2 |
| Kronenfeld et al. (2020, B) [42] | Income (area-level % poverty) | Survival | 2 |
| Kwong et al. (2010) [43] | Income (area-level SES quintile) | Survival | 2 |
| Lee et al. (2020, A) [5] | Income (area-level poverty), Education (area-level % adults with HS degree), Insurance | Treatment (all treatment, 3), Survival (5) | 8 |
| Lee et al. (2020, B) [44] | Income (area-level mean household income), Insurance | Survival (2) | 2 |
| Lee et al. (2020, C) [45] | Insurance | Survival (1) | 1 |
| Major et al. (2014) [46] | Income (area-level % families below poverty line, SES deprivation), Education (% without HS diploma), Employment, Insurance | Incidence (6) | 6 |
| Mazumder et al. (2021) [47] | Insurance | Survival (1) | 1 |
| Mokdad et al. (2018) [48] | Income (area-level poverty index) | Survival (1) | 1 |
| Muzaffar et al. (2020) [49] | Insurance | Survival (1) | 1 |
| Peters et al. (2017) [50] | Insurance | Treatment (locoregional, 1, surgical, 2), Survival (1) | 4 |
| Pham Hang et al. (2018) [12] | Employment (area-level unemployment rate) | Treatment (any treatment, 1) | 1 |
| Rho et al. (2019) [51] | Insurance | Diagnosis (2) | 2 |
| Rich et al. (2019) [52] | Insurance | Survival (1) | 1 |
| Robbins et al. (2011) [53] | Income (mean area-level household income), Education (area-level % adults without HS degree), Insurance | Treatment (surgical, 3), Survival (3) | 6 |
| Robinson et al. (2017) [54] | Insurance | Diagnosis (4) | 4 |
| Robinson et al. (2018) [55] | Insurance | Diagnosis (2) | 2 |
| Sarpel et al. (2016) [56] | Insurance | Treatment (surgical, 1) | 1 |
| Sarpel et al. (2018) [57] | Income (area-level SES), Insurance | Treatment (systemic, 2) | 2 |
| Scaglione et al. (2020) [58] | Insurance | Diagnosis (1), Treatment (any treatment, 1), Survival (1) | 3 |
| Sellers et al. (2019) [59] | Insurance | Survival (1) | 1 |
| Singal et al. (2015) [60] | Insurance | Surveillance (2) | 2 |
| Sobotka et al. (2019) [61] | Insurance | Diagnosis (2), Treatment (locoregional, 2, surgical, 2) | 6 |
| Sodagari et al. (2019) [13] | Insurance | Treatment (locoregional) | 1 |
| Turse et al. (2022) [62] | Insurance | Survival | 1 |
| Uhlig et al. (2019) [63] | Income (area-level mean household income), Insurance | Survival (2) | 2 |
| Wang et al. (2016) [64] | Income (area-level SES) | Survival (2) | 2 |
| Wang et al. (2018) [65] | Insurance | Diagnosis (2), Treatment (any treatment, 1, surgical, 1), Survival (2) | 6 |
| Wang et al. (2020) [66] | Income (area-level mean household income) | Diagnosis (1), Survival (1) | 2 |
| Wasif et al. (2018) [67] | Education (area-level % adults without HS degree), Insurance | Treatment (surgical, 2) | 2 |
| Woodrell et al. (2021) [68] | Insurance | End-of-life care (1) | 1 |
| Yan et al. (2016) [69] | Income (area-level income quartile) | Survival (1) | 1 |
| Yang et al. (2019) [70] | Insurance | Survival (1) | 1 |
| Yu et al. (2010) [71] | Insurance | Treatment (surgical, 1), Survival (1) | 2 |
| Zaydfudim et al. (2010) [72] | Insurance | Treatment (surgical, 1), Survival (1) | 2 |
| Zou et al. (2018) [73] | Insurance | End-of-life care (1) | 1 |
| Study | Outcome | SES Variable | Race and Ethnic Variable | UV Results | MV Results | Change in Significance | Change in Direction |
|---|---|---|---|---|---|---|---|
| Bemanian et al. (2019) [20] | Incidence with liver disease diagnosis | Income | Black | 1.459 (p < 0.001) | 1.099 (p = 0.435) | Partial | No |
| Other | 1.392 (p < 0.005) | 1.602 (<0.001) | |||||
| Multiracial | 0.978 (0.975) | 0.899 (0.886) | |||||
| White | Ref | Ref | |||||
| Chidi et al. 2016 [24] | Receipt of surgical intervention | Income | White | Ref | Ref | Partial | No |
| African Am | 0.79 (0.66–0.94) | 0.89 (0.73–1.10) | |||||
| Hispanic | 0.71 (0.48–1.04) | 0.72 (0.47–1.09) | |||||
| Asian | 1.66 (1.20–2.27) | 1.48 (1.05–2.11) | |||||
| Other/Unknown | 1.61 (1.02–2.55) | 1.44 (0.87–2.37) | |||||
| Hyder et al. 2013 [35] | Receipt of surgery | Income | White | Ref | Ref | No | |
| Black | 1.05 (0.93–1.18) | 1.00 (0.90–1.10) | |||||
| Asian | 0.87 (0.81–0.94) | 0.90 (0.83–0.96) | |||||
| Hispanic | 1.05 (0.95–1.15) | 0.97 (0.89–1.06) | |||||
| Other/Unknown | 1.07 (0.77–1.48) | 0.94 (0.73–1.23) | |||||
| Kokabi et al. 2016 [39] | Median overall survival | Mean family income | Caucasian | OS 6.0 (5.8–6.3) | 0.95 (0.93–0.97) | No | |
| African Amer | OS 5.0 (4.6–5.5) | ||||||
| AAPI | OS 8.0 (7.4–8.7) | ||||||
| Others | OS 5.4 (4.7–5.9) | ||||||
| Kwong et al. 2010 [43] | Cause-specific survival | Income (SES quintile) | Chinese | 0.83 (0.77–0.90) | 0.89 (0.82–0.96) | No | |
| Vietnamese | 0.83 (0.76–0.90) | 0.86 (0.79–0.94) | |||||
| Filipino | 0.91 (0.83–0.99) | 0.89 (0.81–0.98) | |||||
| Korean | 0.80 (0.73–0.89) | 0.90 (0.82–1.00) | |||||
| Japanese | 0.89 (0.80–1.00) | 0.99 (0.87–1.11) | |||||
| Laotian/Hmong | 2.08 (1.78–2.44) | 1.51 (1.28–1.79) | |||||
| Cambodian | 1.26 (1.06–1.51) | 1.24 (1.03–1.48) | |||||
| South Asian | 0.72 (0.57–0.92) | 0.81 (0.64–1.03) | |||||
| Thai | 1.17 (0.87–1.57) | 1.09 (0.81–1.50) | |||||
| Kwong et al. 2010 [43] | All-cause overall survival | Income (SES quintile) | Chinese | 0.80 (0.75–0.85) | 0.85 (0.79–0.91) | No | |
| Vietnamese | 0.81 (0.75–0.87) | 0.84 (0.78–0.91) | |||||
| Filipino | 0.97 (0.90–1.05) | 0.94 (0.87–1.02) | |||||
| Korean | 0.77 (0.71–0.84) | 0.86 (0.79–0.94) | |||||
| Japanese | 0.85 (0.77–0.95) | 0.93 (0.83–1.03) | |||||
| Laotian/Hmong | 1.90 (1.64–2.19) | 1.43 (1.23–1.66) | |||||
| Cambodian | 1.25 (1.07–1.46) | 1.23 (1.05–1.44) | |||||
| South Asian | 0.84 (0.69–1.02) | 0.92 (0.76–1.11) | |||||
| Thai | 1.21 (0.94–1.56) | 1.15 (0.89–1.48) | |||||
| Robbins et al. 2011 [53] | Overall survival | Income | White | Ref | Ref | No | |
| African Amer | 0.64 (0.54–0.76) | 0.62 (0.52–0.74) | |||||
| Hispanic | 0.86 (0.75–0.99) | 0.88 (0.76–1.02) | |||||
| Asian | 0.58 (0.49–0.69) | 0.67 (0.56–0.81) | |||||
| Wang et al. 2016 [78] | Overall survival | Income | Race | 1.15 (1.12–1.18) | 1.20 (1.17–1.24) | No | |
| Wang et al. 2016 [78] | Overall survival | Income | Race | 1.16 (1.12–1.19) | 1.20 (1.16–1.24) | No | |
| Wong et al. 2020 [74] | Distant (vs. localized) stage of HCC at time of diagnosis | Income | White | Ref | Ref | No | |
| African Amer | 1.30 (1.23–1.39) | 1.32 (1.24–1.40) | |||||
| Amer Indian/AK | 0.99 (0.82–1.20) | 0.99 (0.81–1.23) | |||||
| AAPI | 0.91 (0.86–0.97) | 0.96 (0.90–1.02) | |||||
| Hispanic | 0.92 (0.87–0.97) | 0.93 (0.88–0.98) | |||||
| Wong et al. 2020 [74] | Overall survival | Income | White | Ref | Ref | No | |
| African Amer | 1.13 (1.10–1.16) | 1.07 (1.04–1.10) | |||||
| Amer Indian/AK | 1.02 (0.94–1.10) | 0.98 (0.90–1.07) | |||||
| AAPI | 0.80 (0.78–0.83) | 0.83 (0.81–0.85) | |||||
| Hispanic | 0.99 (0.96–1.01) | 0.96 (0.94–0.98) |
| Study | Outcome | SES Variable | Race and Ethnic Variable | UV Results | MV Results | Change in Significance | Change in Direction |
|---|---|---|---|---|---|---|---|
| Adler Jaffe et al. (2020) [15] | Survival of localized tumors | Insurance | NH White | Ref | Ref | Partial | |
| NH Black | 1.27 (1.16–1.40) | 1.11 (1.01–1.22) | |||||
| NH A/PI | 0.72 (0.64–0.80) | 0.79 (0.71–0.89) | |||||
| NH AI/AN | 0.92 (0.68–1.25) | 0.83 (0.60–1.14) | |||||
| Hispanic | 1.11 (1.03–1.21) | 0.94 (0.86–1.02) | Yes | ||||
| Adler Jaffe et al. (2020) [15] | Survival of regional tumors | Insurance | NH White | Ref | Ref | Partial | |
| NH Black | 1.20 (1.10–1.32) | 1.08 (0.98–1.19) | No | ||||
| NH A/PI | 0.95 (0.85–1.06) | 1.01 (0.90–1.12) | |||||
| NH AI/AN | 0.94 (0.71–1.24) | 1.00 (0.75–1.35) | |||||
| Hispanic | 0.95 (0.87–1.05) | 0.91 (0.83–1.00) | |||||
| Chidi et al. (2016) [24] | Receipt of surgical intervention | Insurance | White | Ref | Ref | No | |
| African Am | 0.97 (0.64–1.47) | 1.02 (0.64–1.61) | |||||
| Hispanic | 0.70 (0.64–1.47) | 0.64 (0.27–1.50) | |||||
| Asian | 2.45 (0.98–6.16) | 2.29 (0.90–5.79) | |||||
| Other/Unknown | 0.44 (0.21–0.93) | 0.41 (0.19–0.86) | |||||
| Jones et al. (2019) [37] | Overall survival | Insurance | Hispanic | Ref | Ref | No | |
| White | 1.07 (1.00–1.14) | 1.09 (1.02–1.17) | |||||
| Black | 1.29 (1.18–1.40) | 1.17 (1.07–1.29) | |||||
| Asian | 0.94 (0.80–1.10) | 1.01 (0.85–1.21) | |||||
| Unknown | 1.02 (0.75–1.39) | 1.29 (0.97–1.72) | |||||
| Kangas-Dick et al. (2020) [38] | Utilization of liver-directed services and procedures | Insurance | White | Ref | Ref | No | |
| Black | 0.62 (0.56–0.69) | 0.60 (0.54–0.66) | |||||
| Hispanic | 0.79 (0.70–0.89) | 0.83 (0.74–0.93) | |||||
| AAPI | 1.31 (1.12–1.52) | 1.26 (1.08–1.48) | |||||
| Native Amer | 0.79 (0.51–1.21) | 0.80 (0.51–1.23) | |||||
| Other | 1.04 (0.84–1.28) | 0.98 (0.79–1.22) | |||||
| Kangas-Dick et al. (2020) [38] | Inpatient mortality | Insurance | White | Ref | Ref | No | |
| Black | 1.18 (1.07–1.30) | 1.23 (1.11–1.36) | |||||
| Hispanic | 0.99 (0.89–1.10) | 0.98 (0.88–1.09) | |||||
| AAPI | 1.06 (0.90–1.24) | 1.13 (0.96–1.33) | |||||
| Native Amer | 1.09 (0.74–1.60) | 1.08 (0.72–1.60) | |||||
| Other | 1.12 (0.91–1.37) | 1.19 (0.98–1.45) | |||||
| Lee et al. (2020) [45] | Overall survival | Insurance | White | Ref | Ref | Partial | |
| Black | 1.20 (1.02–1.41) | 0.78 (0.62–0.97) | Yes | ||||
| Asian | 0.72 (0.53–0.97) | 0.65 (0.43–0.99) | |||||
| Amer Indian/AK | 2.17 (0.30–15.4) | 0.17 (0.02–1.29) | |||||
| Other | 0.79 (0.30–2.12) | 0.21 (0.03–1.50 | |||||
| Unknown | 1.33 (0.66–2.68) | 2.06 (0.64–6.67) | |||||
| Mazumder et al. (2021) [47] | All-cause mortality (with just baseline covariates) | Insurance | Black vs. White | 1.24 (1.14–1.26) | 1.27 (1.14–1.40) | No | |
| Peters et al. (2017) [50] | Receipt of resection (treatment) | Insurance | Caucasian | Ref | Ref | No | |
| African Amer | 1.27 (1.07–1.52) | 1.67 (1.13–2.48) | |||||
| Amer Indian | 0.63 (0.31–1.3) | 1.62 (0.46–5.71) | |||||
| AAPI | 2.82 (2.47–3.22) | 2.34 (1.70–3.21) | |||||
| Non-Hispanic | Ref | Ref | |||||
| Hispanic | 0.45 (0.38–0.53) | 0.47 (0.30–0.74) | |||||
| Peters et al. (2017) [50] | Receipt of transplantation (treatment) | Insurance | Caucasian | Ref | Ref | No | |
| African Amer | 0.64 (0.54–0.75) | 0.54 (0.36–0.79) | |||||
| Amer Indian | 0.45 (0.25–0.83) | 0.97 (0.35–2.69) | |||||
| AAPI | 0.76 (0.66–0.88) | 0.71 (0.52–0.98) | |||||
| Non-Hispanic | Ref | Ref | |||||
| Hispanic | 0.74 (0.66–0.84) | 0.76 (0.57–1.01) | |||||
| Peters et al. (2017) [50] | Overall survival | Insurance | Caucasian | Ref | Ref | Partial | |
| African Amer | 0.88 (0.76–1.02) | 1.24 (0.92–1.66) | |||||
| Amer Indian | 1.39 (0.95–2.03) | 1.35 (0.60–3.03) | |||||
| AAPI | 1.49 (1.33–1.68) | 0.96 (0.74–1.25) | Yes | ||||
| Non-Hispanic | Ref | Ref | |||||
| Hispanic | 0.71 (0.64–0.80) | 0.63 (0.48–0.82) | |||||
| Rich et al. (2019) [52] | Overall survival | Insurance | White | Ref | Ref | No | |
| Hispanic | 1.07 (0.78–1.50) | 0.83 (0.74–0.94) | |||||
| Black | 1.34 (1.11–1.61) | 1.12 (1.10–1.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, J.; LeTran, V.H.; Wong, C.; Tchan, J.; Zhou, S.; Chen, A.; Zhou, K. Socioeconomic Disparities Along the Cancer Continuum for Hepatocellular Carcinoma: A Systematic Review. Livers 2025, 5, 59. https://doi.org/10.3390/livers5040059
Ong J, LeTran VH, Wong C, Tchan J, Zhou S, Chen A, Zhou K. Socioeconomic Disparities Along the Cancer Continuum for Hepatocellular Carcinoma: A Systematic Review. Livers. 2025; 5(4):59. https://doi.org/10.3390/livers5040059
Chicago/Turabian StyleOng, Justin, Vivian H. LeTran, Christopher Wong, Jonathan Tchan, Selena Zhou, Ariana Chen, and Kali Zhou. 2025. "Socioeconomic Disparities Along the Cancer Continuum for Hepatocellular Carcinoma: A Systematic Review" Livers 5, no. 4: 59. https://doi.org/10.3390/livers5040059
APA StyleOng, J., LeTran, V. H., Wong, C., Tchan, J., Zhou, S., Chen, A., & Zhou, K. (2025). Socioeconomic Disparities Along the Cancer Continuum for Hepatocellular Carcinoma: A Systematic Review. Livers, 5(4), 59. https://doi.org/10.3390/livers5040059






