1. Introduction
Hepatocellular carcinoma (HCC) is the most common liver cancer type. Although the global cancer mortality rate has been declining since 2022, incidence and mortality from liver cancer continue to rise. In 2025, liver cancer-related deaths were 809.259 compared to 758.725 in 2022 [
1]. Despite advancements in therapeutic strategies, mortality rates for liver cancer have remained stable due to a lack of prevention strategies, tumor heterogeneity, and frequent resistance to chemotherapy treatments [
2], resulting in a poor clinical prognosis related to HCC [
3]. Hepatocarcinogenesis involves a complex process of chronic inflammation leading to liver fibrosis and cirrhosis, accompanied by aberrant activation of cellular signaling pathways [
4]. The development of HCC is linked to genetic and epigenetic alterations in various genes such as c-Myc, AXIN1, and TP53, which deregulate critical cellular pathways related to proliferation, differentiation, and cell survival [
5]. The Wnt/β-catenin pathway, in particular, plays a central role in this process, as its aberrant activation in many cases of HCC contributes to the uncontrolled proliferation of hepatocytes and resistance to treatments [
6]. This pathway has been extensively studied, and although some drugs targeting this signaling pathway are in the research phase, a full understanding of their preventive and therapeutic implications remains a significant challenge [
7]. Considering the key role of the Wnt/β-catenin signaling pathway in HCC tumorigenesis, progression, and therapy resistance, targeting this pathway may be a new potential therapeutic approach for HCC patients [
8,
9].
Currently, there is an increasing interest in chemopreventive and chemotherapeutic effects of natural products-derived compounds from dietary sources [
10,
11]. Coffee consumption is inversely associated with the development of metabolic diseases and chronic liver diseases [
12], and recent evidence also indicates that coffee consumption decreases the risk of adverse clinical outcomes in chronic liver disease [
13]. Studies have also shown that coffee consumers are less likely to develop liver fibrosis, a precursor to cirrhosis and liver cancer [
14]. Preventive effects of coffee in liver cancer have also been explored, showing that regular coffee consumption is associated with a significant reduction in HCC risk [
15]. Mechanisms associated with the protective effects of coffee against liver cancer include coffee’s ability to mitigate liver damage, reduce inflammation, and even lower cancer cell proliferation [
16]. Coffee includes thousands of bioactive compounds, of which caffeine, diterpenes (cafestol and kahweol), and chlorogenic acids (CGA) have been linked with liver-protective effects [
17,
18].
Likewise, it must be recognized that coffee and its bioactive compounds—notably CGA—showed chemopreventive benefits through multiple pathways. These include activation of the Nrf2 antioxidant response pathway and inhibition of inflammatory signaling (e.g., NF-κB), thereby reducing oxidative stress and mutagenesis, upregulation of phase I/II detoxification enzymes (like glutathione S-transferases, UDP-glucuronosyltransferases), and enhanced DNA repair capacity. Epidemiological data and meta-analyses support a lower incidence of liver, colorectal, prostate, and endometrial cancers in regular coffee consumers [
19]. As well, experimental evidence demonstrated that coffee-derived components can reduce liver fibrosis and modulate key signaling pathways associated with carcinogenesis, such as the Wnt/β-catenin pathway [
20,
21]. Moreover, CGAs have demonstrated toxicity to HepG2 cells and reduce their proliferation. CGA treatment was also related to the reduction in tumor size in mouse models [
16]. Nevertheless, the molecular mechanisms related to the protective effect of coffee against HCC are not fully elucidated yet. Likewise, experimental models exploring the biological effects of coffee extracts in hepatoma cell lines are still limited, and the regulation of key signaling pathways such as Wnt/β-catenin in this context is still unclear. Although some compounds derived from natural products have shown inhibitory effects on this pathway in cancer models, research on coffee compounds in this context remains limited. This study uniquely evaluates the antiproliferative effects of Colombian coffee extracts, and compares those effects with chlorogenic acid (CGA), on HepG2 cells. We chose the HepG2 hepatocellular carcinoma (HCC) cell line due to its constitutive activation of the Wnt/β-catenin signaling pathway, allowing us to investigate the modulatory potential of these compounds on this pathway. While the antiproliferative activities of coffee components have been explored, mechanistic studies on the Wnt/β-catenin pathway in HCC models, particularly using Colombian coffee extracts and in comparison to CGA, remain scarce, emphasizing the need for this study.
2. Materials and Methods
2.1. Cells and Treatments
Experiments were performed in the HepG2 cell line (ATCC HB8065). Cells were cultured in DMEM medium (D6429 GIBCO, Life Technologies, New York, NY, USA) supplemented with 10% FBS (Ref. 10438026 GIBCO), 1% penicillin-streptomycin (Ref. 15140122 GIBCO), 1% sodium pyruvate (Ref. 11360070 GIBCO), and 1% MEM non-essential amino acids (Ref. 11140050 GIBCO). Cultures were maintained at 37 °C in a humidified atmosphere with 5% CO
2. For treatment with coffee extracts, coffee beans (
Coffea arabica) were provided by Natucafé, which were harvested from the La Arboleda farm, located in the Andes municipality, Antioquia, Colombia. Coffee extracts were prepared from both roasted and green (almond) beans and were characterized for total phenolic content and in vitro antioxidant activity by DPPH and FRAP assays. Finally, a characterization of both coffee extracts was carried out through HPLC. These results are available in the publications of Villota et al., 2021 [
22]. Chlorogenic (3-(3,4-Dihydroxycinnamoyl) quinic acid, ≥95% (C3878-1G, Sigma Aldrich, Saint Louis, MO, USA) was prepared at 50 mM, aliquoted, and stored at −20 °C. All treatments were diluted in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin, without sodium pyruvate or non-essential amino acids. Cells were seeded and cultured for 24 h, after which they were treated once with different concentrations of roasted coffee extracts (RC), green coffee extracts (GC) (125–3000 µg/mL) or Chlorogenic acids (CGA) (100–1600 µM), for 16 h, 24 h, or 48 h.
2.2. Cell Viability/Cytotoxicity Measurements
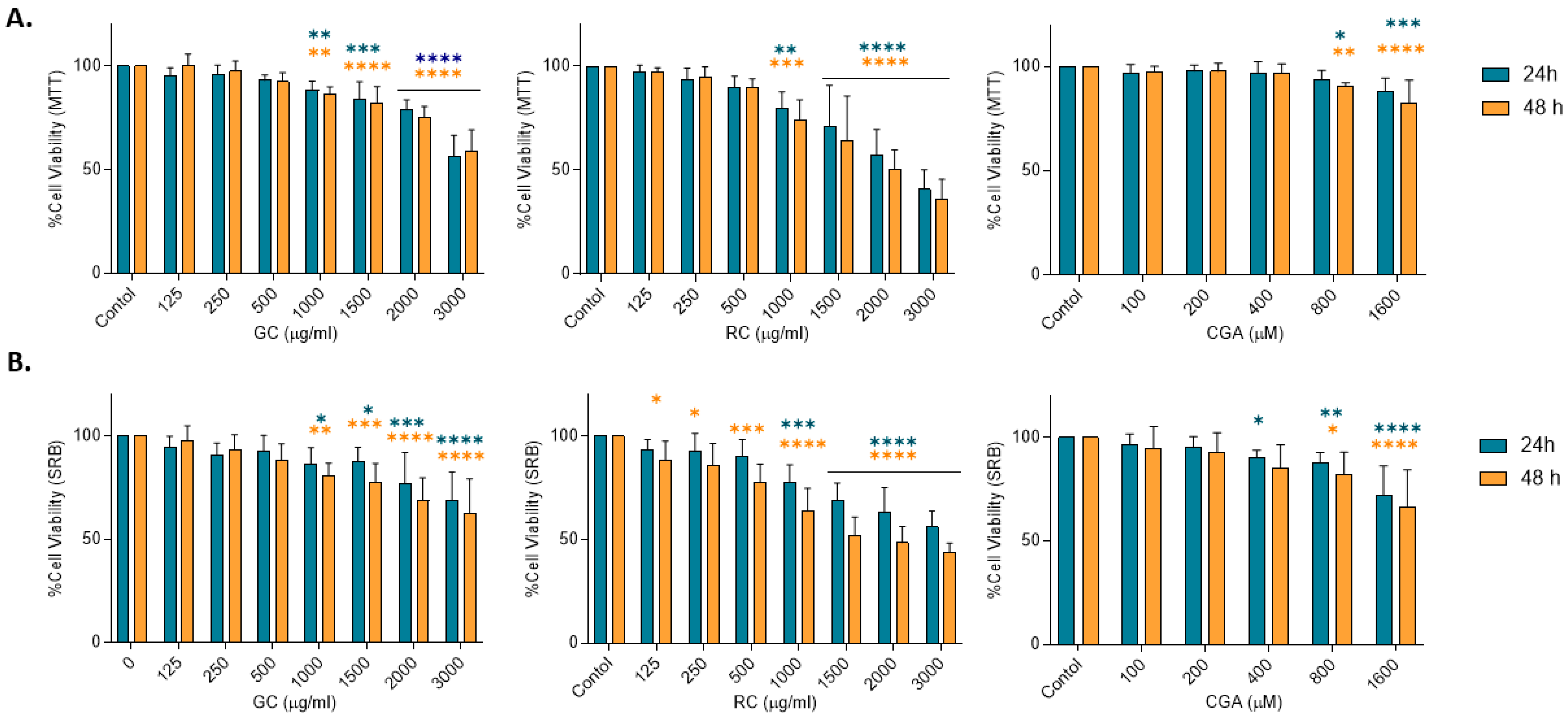
Cell viability was determined by two different assays: MTT assay (Sigma Aldrich M5556) and sulforhodamine B assay (SRB) (Sigma Aldrich 3520-421), which evaluate metabolic activity of viable cells. HepG2 cells were seeded in 96-well plates at a density of 104 cells/well and incubated overnight for treatment with green coffee and roasted coffee extracts (125–3000 µg/mL), and with Chlorogenic acid (100–1600 µM), for 24 h and 48 h. For the MTT assay, 10 µL of MTT (5 mg/mL) was added to each well, and cells were incubated for 4 h. The medium was then removed, and 100 µL of DMSO was added to solubilize the formazan crystals. For the SRB assay, cells were fixed after treatment with 50% trichloroacetic acid for one hour at 4 °C. The following day, 100 µL of SRB 0.4% was added to each well, and fixed cells were incubated for 30 min at room temperature. Cells were washed with 1% acetic acid. Absorbance was measured using a spectrophotometer at 570 nm and 490 nm for the MTT and SRB assays, respectively. Results were calculated as a percentage of cell viability relative to non-treated control cells. Percentage of cell viability: (absorbance of treated cells−absorbance of background controls)/(absorbance of nontreated cells−absorbance of background controls).
2.3. Cell Death Assessment
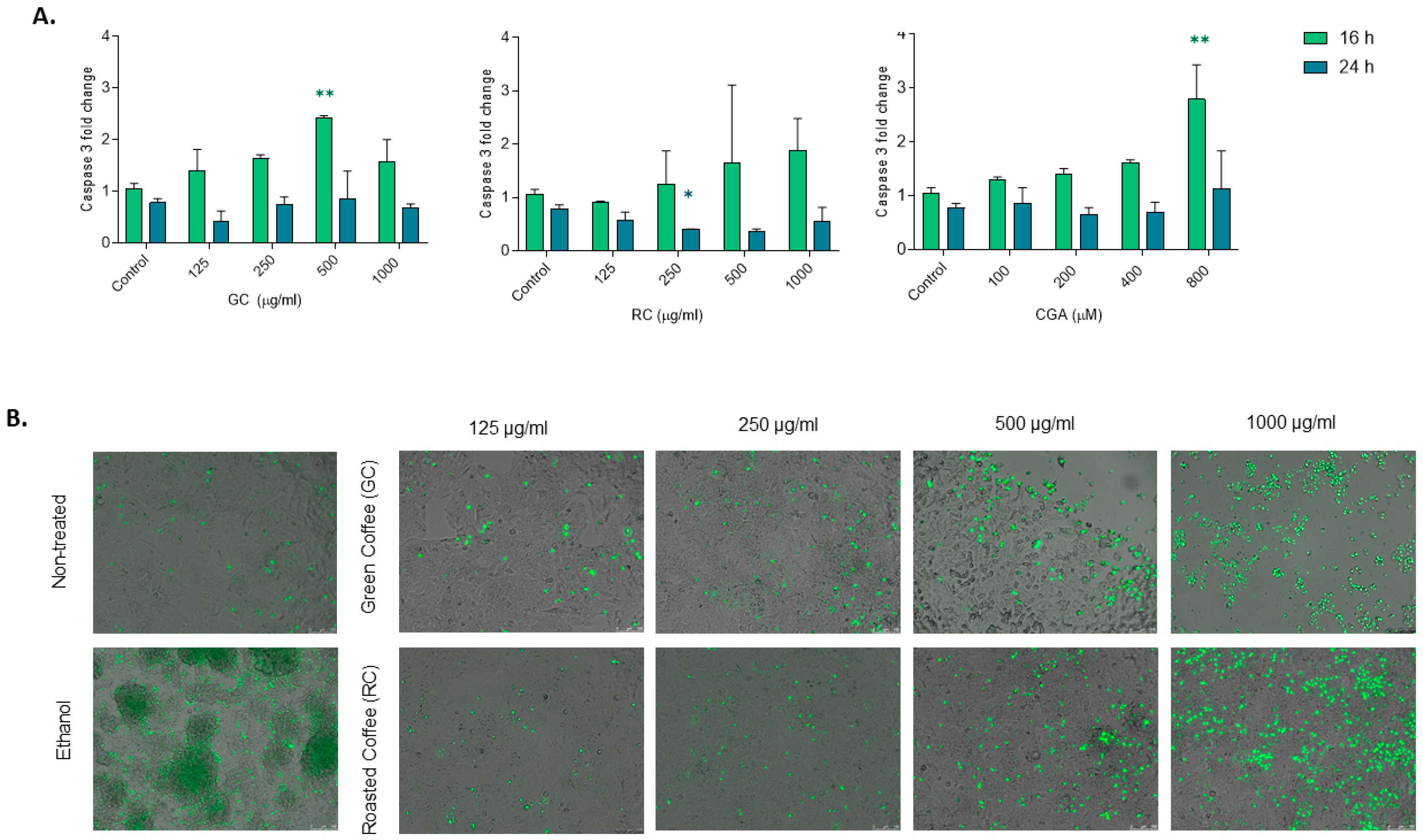
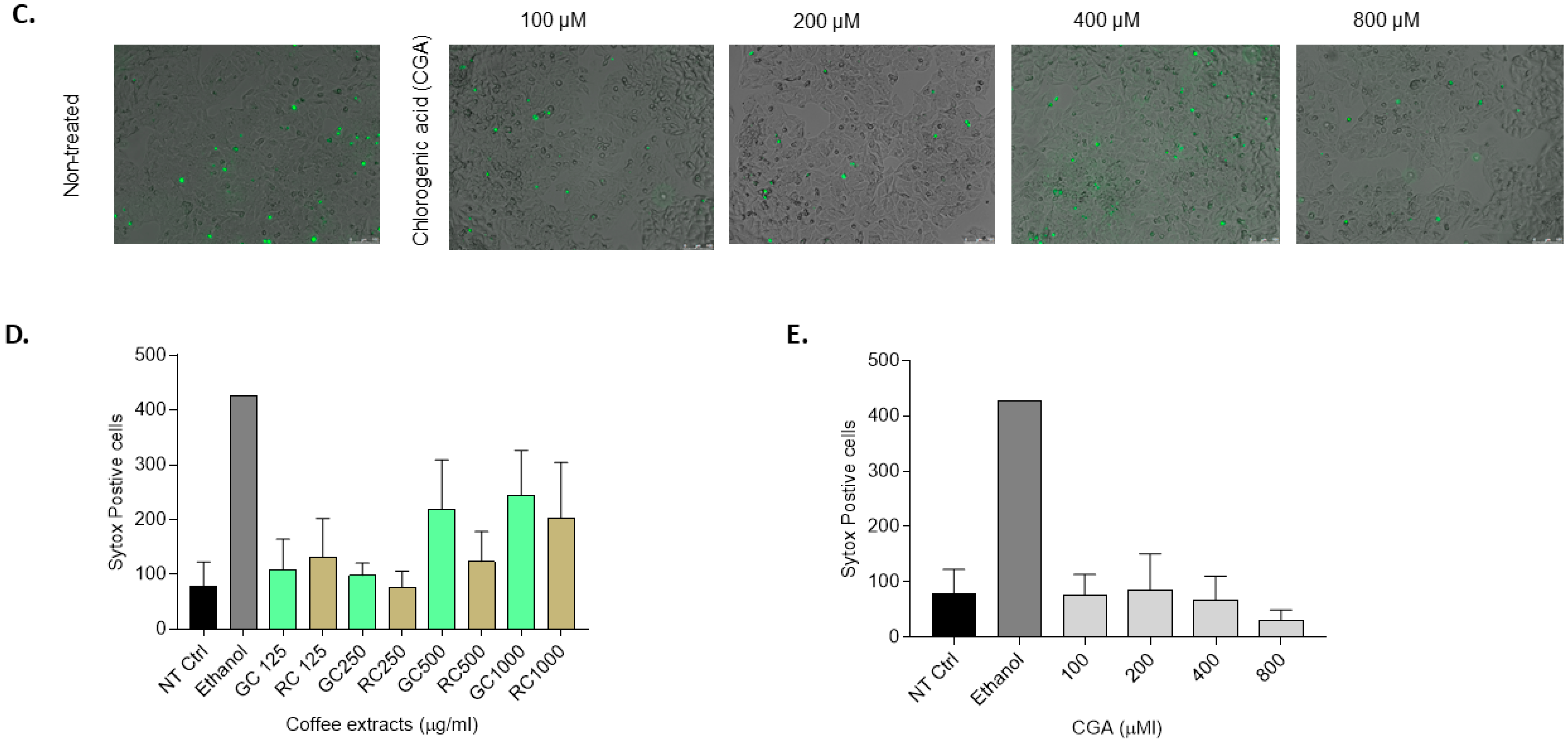
To determine apoptosis, caspase-3 enzyme activity was assayed as described previously. Fluorescence was quantified using a spectrofluorometer using an excitation wavelength of 380 nm and an emission wavelength of 460 nm. Arbitrary fluorescence units were normalized to the control condition and expressed as a fold increase compared to non-treated control cells. Additionally, SYTOX® green nucleic acid stain (S7020 Invitrogen, Carlsbad, CA, USA) was used as previously reported for staining of necrotic cells. HepG2 cells were treated with different concentrations of green coffee and roasted coffee extracts (125–1000 µg/mL) and chlorogenic acids (100–1000 µM) compounds for 48 h. SYTOX green solution (125 nmol/L) was added to the cells after the treatment for 15 min at 37 °C in a 5% (v/v) CO2-containing atmosphere. Nuclei of necrotic cells with disrupted membrane integrity were stained green and visualized using a Leica DMI6000 fluorescence microscope (Leica, Amsterdam, The Netherlands) at 512–542 nm. SYTOX green-positive cells were quantified using ImageJ J.S v.0.6.0 (Powered by ImJoy). The total number of SYTOX-positive cells (green nuclei) was counted per image, and three different images were analyzed per condition. To avoid bias because of high cell death percentage in some conditions, normalization was performed for each image by calculating cell area percentage (cell confluency), and the relative number of SYTOX-positive cells was calculated compared to the non-treated cells.
2.4. Cell Proliferation Assays
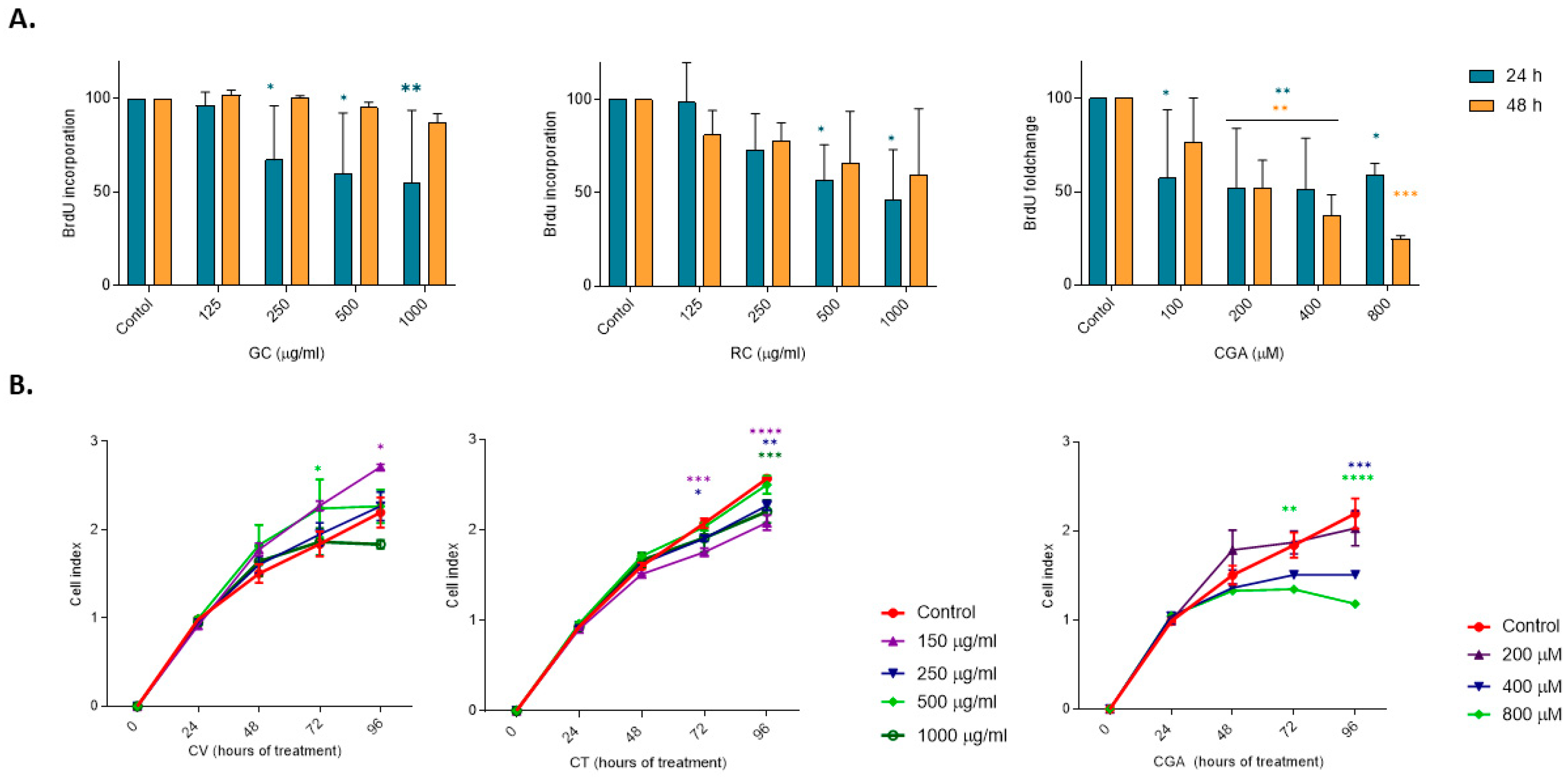
Cell proliferation was determined by the BrdU Cell Proliferation Assay (11669 915001, Roche, Mannheim, Germany). HepG2 cells were cultured in black 96-well plates and treated with green coffee, roasted coffee extracts (100–1000 µg/mL), or CGA (100–800 µM) and incubated for 24 h and 48 h, and then incubated with BrDU for 3 h. After that, cells were fixed, and an anti-BrdU-POD conjugated antibody was added and incubated for 90 min at room temperature. Chemoluminescence was measured using a luminometer reader. Results are expressed as a percentage of cell proliferation, taking as 100% untreated HepG2 cells. HepG2 cell proliferation was also assessed using a real-time cell analyzer (XCelligence RTCA DP Instrument, Agilent Technologies, Santa Clara, CA, USA), which measures electron flow across gold microelectrodes at the bottom of culture plates. Adherent cells block the electric current, and this impedance is expressed as arbitrary units (cell index). Cells were cultured in XCelligence plates (Agilent Technologies, Santa Clara, CA, USA) (96-well plates, 104 cells/well) and treated with coffee extracts (125–1000 µg/mL) and CGA (200–800 µM). Cell index was monitored from 0 h until 96 h of culture.
2.5. TOP/FOP Luciferase Reporter Assay
Wnt/β-catenin signaling activation was first evaluated using the TOP/FOP luciferase reporter system. HepG2 cells were seeded in 12-well plates (0.5 × 106 cells/well) and were transiently transfected with 1 µg of each TOP flash (wild-type TCF binding sites) or FOP flash (mutated TCF binding sites) plasmids (Merck Millipore, Burlington, MA, USA.) using Lipofectamine 3000 (L3000015, Invitrogen, Carlsbad, CA, USA). Transfected cells were incubated with green coffee, roasted coffee extracts, and CGA for 24 h. Luciferase activity was determined using a Luciferase Assay System (Promega, Madison, WI, USA) according to the manufacturer’s protocol.
2.6. Wnt/β-Catenin Related Genes RNA and Protein Expression
For RNA analysis, cells were harvested 16 h and 24 h after treatment. To evaluate the effect of green and roasted coffee extracts (250–1000 µg/mL) and chlorogenic acid (200–800 µM) on the modulation of Wnt/β-catenin signaling, mRNA levels of Wnt/β-catenin related genes: Axin2, c-Myc, β-catenin, and E-cadherin were determined. Total RNA was isolated using TRIzol (Sigma-Aldrich) according to the manufacturer’s instructions First-strand cDNA synthesis was performed using a reverse transcription kit (Ref. EP0442, Thermo Fisher, Waltham, MA, USA), using 2.0 μg of total RNA, 1X RT buffer (500 mmol/L Tris-HCl [pH 8.3]; 500 mmol/L KCl; 30 mmol/L MgCl2; 50 mmol/L DTT), 1 mmol/L deoxynucleotides triphosphate (dNTPs, R0191), 10 ng/μL random nanomers (Sigma-Aldrich), 0.6 U/μL RNaseOUT™ (Invitrogen, Carlsbad, CA, USA) and 4 U/μL M-MLV reverse transcriptase (Invitrogen) at a final volume of 20 μL. The cDNA synthesis program was 25 °C/6 min, 42 °C/60 min, and 70 °C/6 min. Complementary DNA (cDNA) was diluted 20× in nuclease-free water. Real-time qPCR was carried out in a CFX96 thermal cycler (Bio-Rad, Hercules, CA, USA) using Maxima SYBR Green qPCR Master Mix (2X) kit (Ref. K0252, Thermo Fisher). For qPCR, 2× reaction buffer, 10 μmol/L of sense and antisense primers were used (
Table S1). The PCR program comprised a 5 min enzyme activation and denaturation phase at 95 °C, followed by 40 cycles of 15 s at 95 °C and one minute at 60 °C. mRNA levels were normalized to the GAPDH housekeeping gene and further normalized to the mean expression level of the control. Primer sequences are listed in
Supplementary Table S1. For Western Blot analysis, HepG2 cells were resuspended using lysis buffer containing 25 mM HEPES, 150 mM KAc, 2 mM EDTA, and 0.1% NP-40 and protease inhibitors (Sigma-Aldrich). Protein concentrations were quantified using the Bio-Rad Protein Assay (Bio-Rad). Proteins were separated by 7.5–10% gradient sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Semi-dry blotting was performed using a Trans-Blot Turbo Midi Nitrocellulose Membrane with Trans-Blot Turbo System Transfer (BioRad). Ponceau S 0.1%
w/
v (Sigma-Aldrich) staining was used to confirm protein transfer. Specific antibodies were used to detect β-catenin and E-cadherin and GAPDH (Calbiochem #CB1001) was used as a loading control. Blots were analyzed with a ChemiDoc XRS system (Bio-Rad). Protein band intensities were quantified by ImageLab 6.1 software (BioRad).
2.7. β-Catenin and E-Cadherin Immunofluorescent Staining
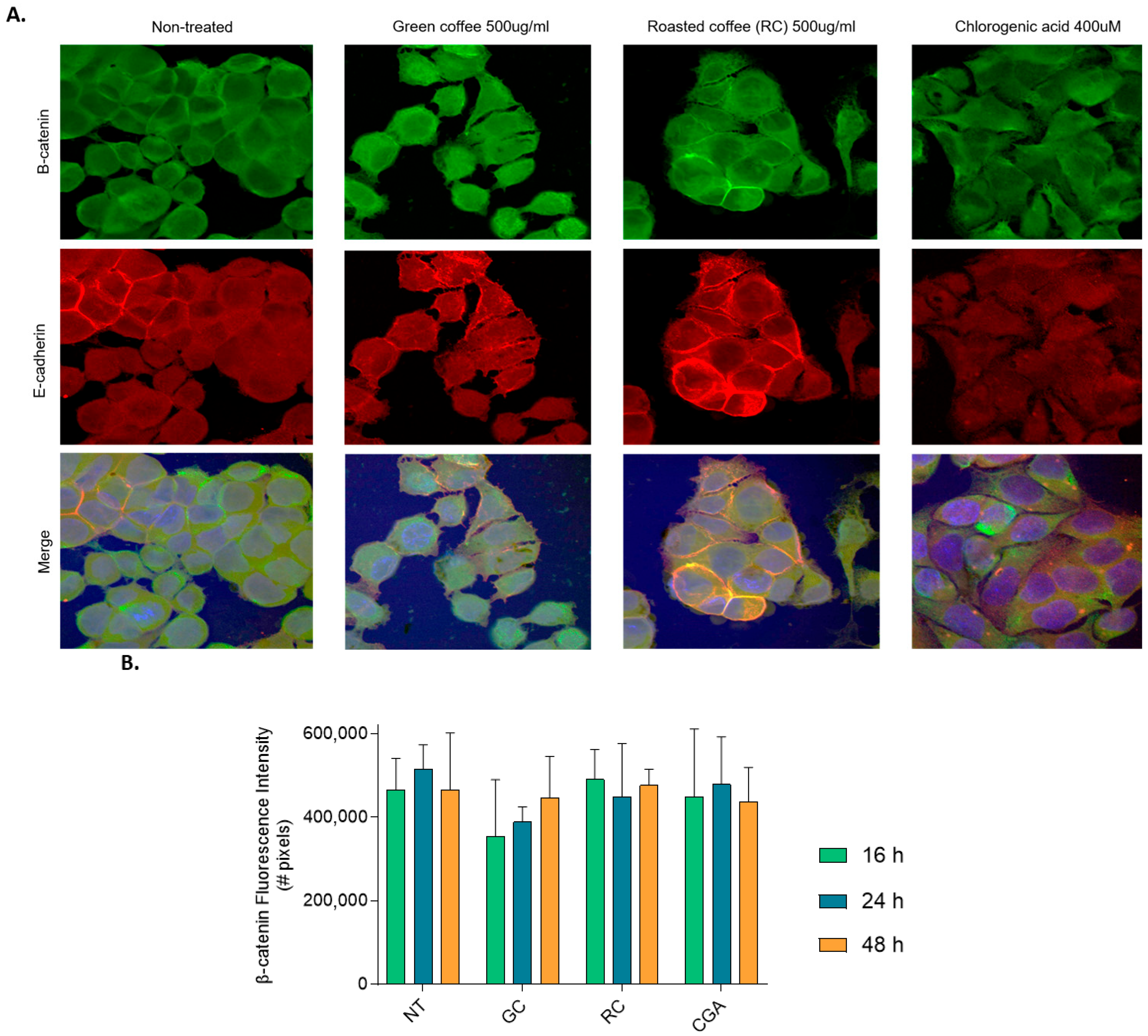
To assess the subcellular localization of β-catenin and E-cadherin, cells were seeded on glass coverslips and treated for 16, 24, and 48 h with coffee extracts or CGA. After treatment, cells were fixed in 4% paraformaldehyde for 10 min, permeabilized with 0.1% Triton X-100, and stored in PBS/Glycerol (50:50) solution at −20 °C until further use. Cells were subsequently washed with 1X PBS and blocked with fetal bovine serum for 45 min. Cells were then incubated with a rabbit anti-E-cadherin polyclonal antibody (CD324, Thermo Fisher info antibody) diluted 1:500 in TBS-T at 4 °C overnight. Cells were then washed three times with PBS for 10 min, followed by incubation with an anti-rabbit IgG-IgG-conjugated secondary antibody (Alexa Fluor 546, Thermo Fisher) diluted 1:200 was added at 4 °C for 60 min. Cells were washed again three times with PBS for 10 min, followed by incubation with a mouse anti-β-catenin polyclonal antibody (PA5-77934, Ivitrogen) diluted 1:500 at 4 °C for 60 min, washed three times with PBS for 10 min and an anti-mouse IgG-conjugated secondary antibody (Alexa Fluor 488, Thermo Fisher) diluted 1:500 was added at 4 °C for 60 min. Finally, the slides were washed three times with PBS, incubated with 2 µg/mL Hoechst 33258 (H1398, Thermo Fisher) at 4 °C for 10 min, and washed three times with PBS. Slides were mounted using Fluorsave reagent (Thermo Fisher). Images were visualized using an FV1000 Olympus laser scanner confocal microscope from Evident corporation, Tokyo, Japan confocal microscope coupled to a spinning disk (DSU) and epifluorescence (Thermo Fisher) with three reading channels (Hoechst: blue, β-catenin: green, E-cadherin: red). For image analysis, the images were converted to 8-bit TIFF format, image processing was standardized for the β-catenin and E-cadherin channels, the “Despeckles” function was applied, and they were segmented using the “Li” algorithm. Finally, colocalization images between both channels were obtained using the “DNA” function of the “Image Calculator” tool. The number of positive pixels for the signals of the markers used, their respective area value (µm2), and the percentage of colocalization between β-catenin and E-cadherin were quantified.
2.8. Statistical Analysis
All the experiments were performed using cells from at least three independent cultures (n = 3) and two replicates for each experimental condition. Normality tests were performed using the Kolmogorov–Smirnov or the Shapiro–Wilk test. The statistical significance of differences between the means of the experimental groups was evaluated using one-way and two-way analysis of variance (ANOVA) and the t-Student test as a post-test. The results are presented as the mean ± standard deviation (mean ± SD). Significant differences were considered when p ≤ 0.05. Analyses were performed using GraphPad Prism software 9 (Version 9.4.1, GraphPad Software Inc., San Diego, CA, USA).
4. Discussion
Discovering novel, effective chemopreventive strategies remains a challenge for HCC since conventional therapies based on radiotherapy, chemotherapy, and surgical resection are still ineffective, and are in some cases even associated with a high recurrence rate [
23,
24]. Although high coffee intake is associated with reduced risk of HCC development, there are still discrepancies between studies, and further analysis, including specific groups such as patients with viral hepatitis B or C infection, is needed [
25].
The evaluation of coffee components as treatment for HCC is challenging, due to the presence of many bioactive compounds in coffee, which vary depending on the species of coffee plant, level of roasting, and the brewing method [
17,
26]. CGA, the main phenolic acid present in green coffee beans, has a high antioxidant potential and is hepatoprotective by reducing inflammation and fibrosis [
20,
26]. In addition, it has antiproliferative effects on HCC cells in in vivo models [
16]. In a previous study from our group, coffee extracts were characterized. We identified CGA as the most abundant phenolic compound, and demonstrated that CGA reduced cell viability and migration in colorectal adenocarcinoma-derived cells [
22].
Understanding the role of signaling pathways involved in cancer has become a strategy to find new molecular therapeutic targets [
27]. Current evidence shows that the Wnt/β-catenin signaling pathway plays a pivotal role in the tumorigenesis, progression, and therapeutic resistance of hepatocellular carcinoma (HCC) cells [
28]. Its abnormal activation—through mutations in CTNNB1, inactivation of regulatory proteins like APC or AXIN, or external factors like HBV/HCV infection and alcohol—drives proliferation, metastasis, immune evasion, and cancer stem cell maintenance in HCC [
29]. Given its central role, the Wnt/β-catenin axis has become an attractive therapeutic target, with a variety of inhibitors, monoclonal antibodies, and decoy receptors in development [
29]. However, clinical translation remains limited due to the involvement of the Wnt/β-catenin pathway in normal tissue homeostasis and concerns about toxicity, thus prompting interest in less toxic, natural inhibitors.
Several bioactive compounds such as curcumin, berberine, emodin, and resveratrol have been reported to downregulate β-catenin activity, suppress Wnt ligand interactions, or promote β-catenin degradation in preclinical HCC models [
30,
31]. Recent findings suggest that natural products such as dietary polyphenols and compounds found in coffee have potential as modulators of the Wnt/β-catenin pathway, and at the same time show reduced toxicity and adverse effects [
32]. Coffee is rich in bioactive compounds, including a variety of polyphenols, and several studies have shown that coffee consumption is associated with reduced risk of liver cancer [
33]. This protection can be partially explained by the suppression of Wnt/β-catenin signaling by coffee components via inhibition of oxidative stress and kinase activity [
34,
35]. These dietary compounds may therefore act as effective chemopreventive, chemotherapeutic, or adjuvant agents, offering a complementary strategy to conventional treatments for Wnt-driven liver cancer.
The present study evaluated cytotoxic and antiproliferative effects of green and roasted coffee extracts (GC and RC) and chlorogenic acid (CGA) on HepG2 cells, a hepatocellular carcinoma (HCC) derived cell line. Our findings support that both coffee extracts and CGA show significant antiproliferative effects, demonstrated by inhibition of cell viability and cell proliferation, induction of cell death, and modulation of the Wnt/β-catenin signaling pathway. Both GC and RC decreased HepG2 cell viability, with RC being more potent than GC. These findings are similar to what was previously reported for colon cancer cells treated with the same coffee extracts [
22]. Likewise, other studies using HepG2 cells treated with different coffee bean extracts (CBEs) and black instant coffee (BICs) have shown similar results, with BICs being more potent than CBEs, regarding antioxidant and cell killing capacity [
36]. Taken together, these results support the idea that bioactive compounds generated during the roasting process are critical in the biological effects of coffee [
37].
Analysis of the coffee extracts revealed distinct compositions between the green and roasted varieties. Green coffee extract (GC) exhibited a chlorogenic acid concentration 4.4 times greater than that of roasted coffee extract (RC) [
22]. In contrast, levels of xanthines, such as theobromine and caffeine, were found to be slightly higher in RC compared to GC. Biologically, these differences could lead to varying effects: high chlorogenic acid in GC has been linked to chemopreventive, antioxidant, anti-inflammatory, and anti-cancer properties, while the increased xanthines in RC might influence cellular metabolism and pathways related to neuroprotection and potentially modulating cancer progression, with a similar proportion or lesser that the caffeic derivatives due to decomposition during the roasting process [
19,
38].
CGA showed moderate effects in reducing cell viability at the higher concentrations used in this study (800 and 1600 µM), while CGA treatment was able to induce significant activation of caspase-3 (800 µM) and showed significant reduction in cell proliferation (200–800 µM) (
Figure 1 and
Figure 3). These results are comparable to those reported by Yan et al., who demonstrated that CGA reduces cell viability in HepG2 cells, although at lower doses. Interestingly, cell cycle analysis showed that CGA induced S-phase arrest in vitro and decreased tumor progression and tumor size in a murine model with HepG2 xenografts. Furthermore, in this study, the biological effects of CGA were associated with the inhibition of ERK1/2 in HepG2 cells and reduction in matrix metalloproteinase 2 (MMP-2) expression [
16,
39].
In our study, we observed that coffee extracts and CGA significantly inhibit DNA synthesis and suppress cell proliferation. These results suggest that, in addition to cytotoxicity, these compounds interfere with the cell cycle and cell proliferation. Previous studies showed that polyphenols downregulate cyclin expression and cell cycle progression pathways such as the PI3K/AKT signaling pathway in experimental liver cancer models [
40]. Likewise, caffeine, one of the most significant bioactive compounds present in coffee, inhibits the proliferation, migration, and invasion of HepG2 and Huh-7 cells via modulation of the Akt and extracellular signal-regulated kinase (ERK)1/2 signaling pathways [
18].
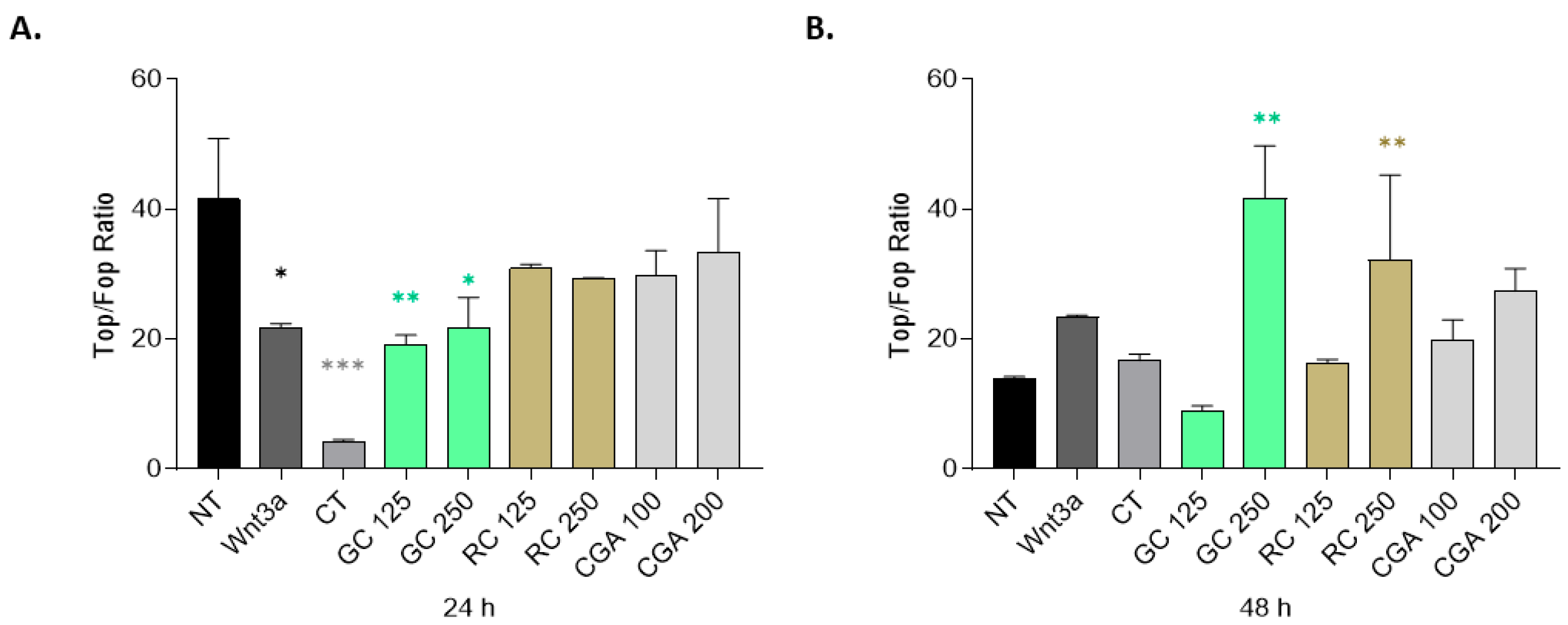
Our findings also demonstrated that the effects of green coffee extract (GC), roasted coffee extract (RC), and chlorogenic acid (CGA) are partially mediated via modulation of the Wnt/β-catenin signaling pathway. In the TOP/FOP (
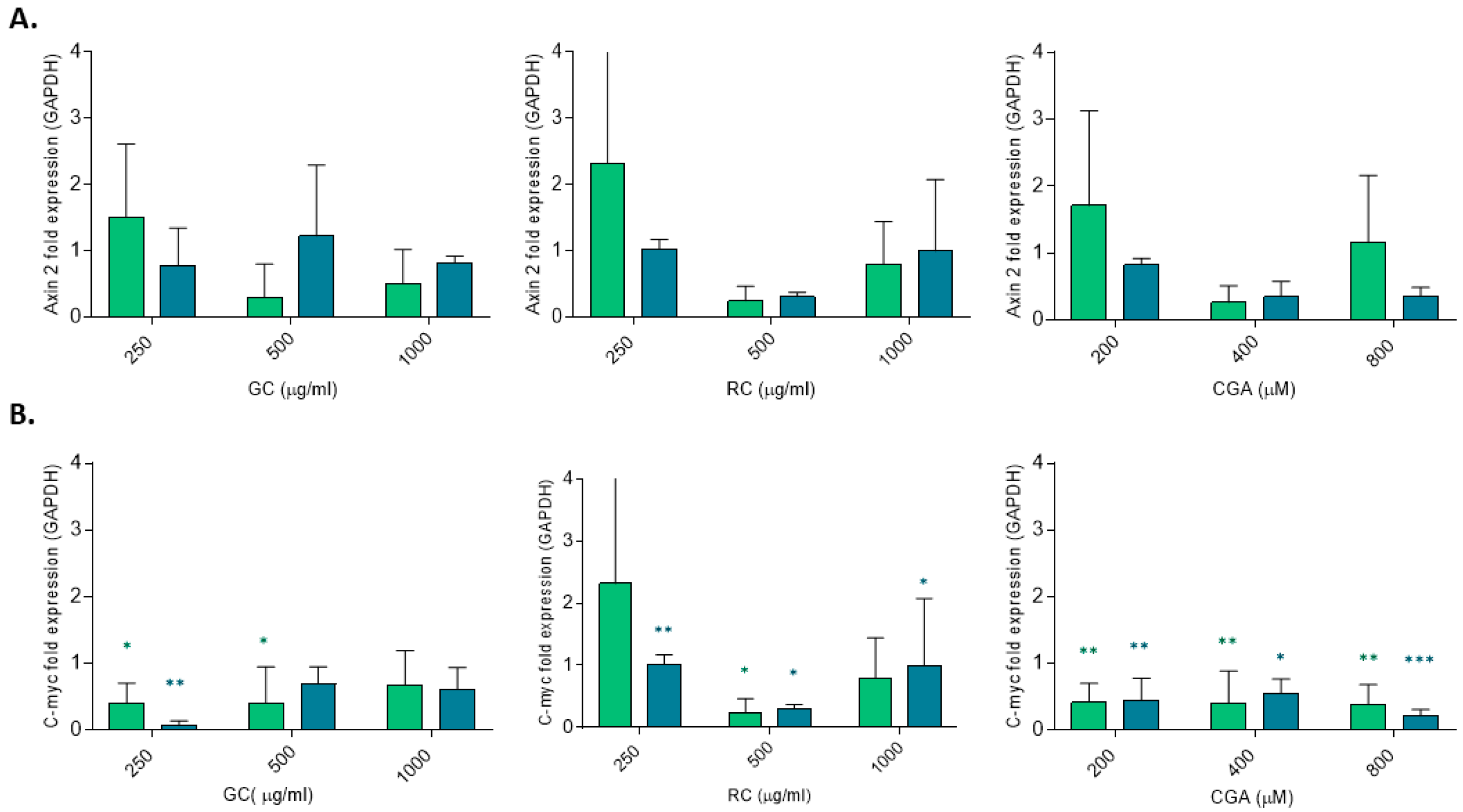
Figure 4) reporter assay, only green coffee-treated HepG2 cells showed a significant reduction in transcriptional activity and a reduced expression of β-catenin RNA, measured by qPCR (
Figure 5), whereas CGA significantly reduced c-Myc mRNA levels. This observation highlights the role of c-Myc as Wnt/β-catenin target gene in cancer cell proliferation and in hepatocarcinogenesis. Similar results were reported by Zhao et al. (2023) [
41], observing that hydrogen sulfide (H
2S) suppresses H
2O
2-induced proliferation of HepG2 cells by inhibiting downstream effectors of the Wnt/β-catenin pathway, including Cyclin D1 and TCF4 [
41]. These shared effects suggest that both CGA and GC may disrupt mitogenic signals driven by β-catenin-mediated gene transcription.
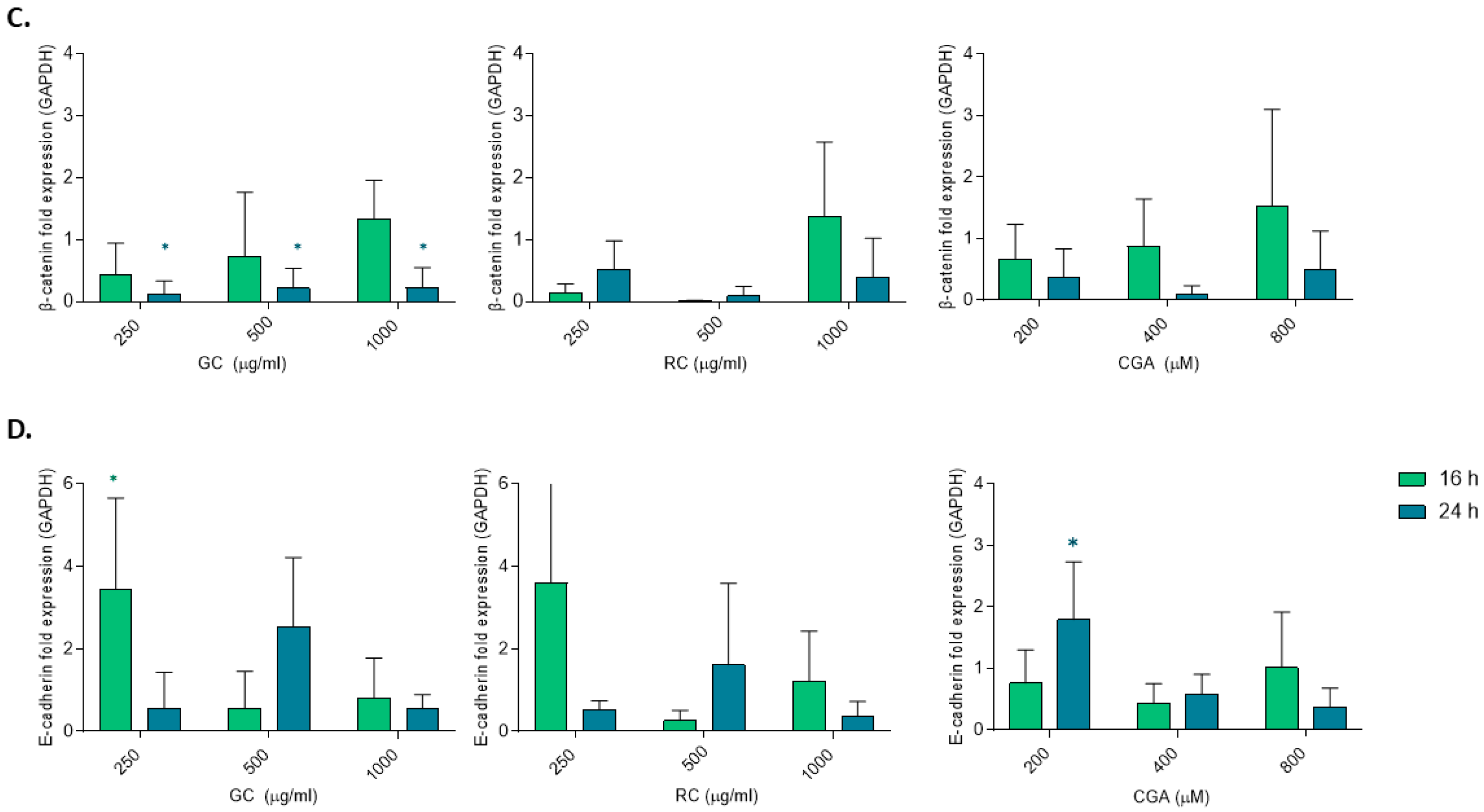
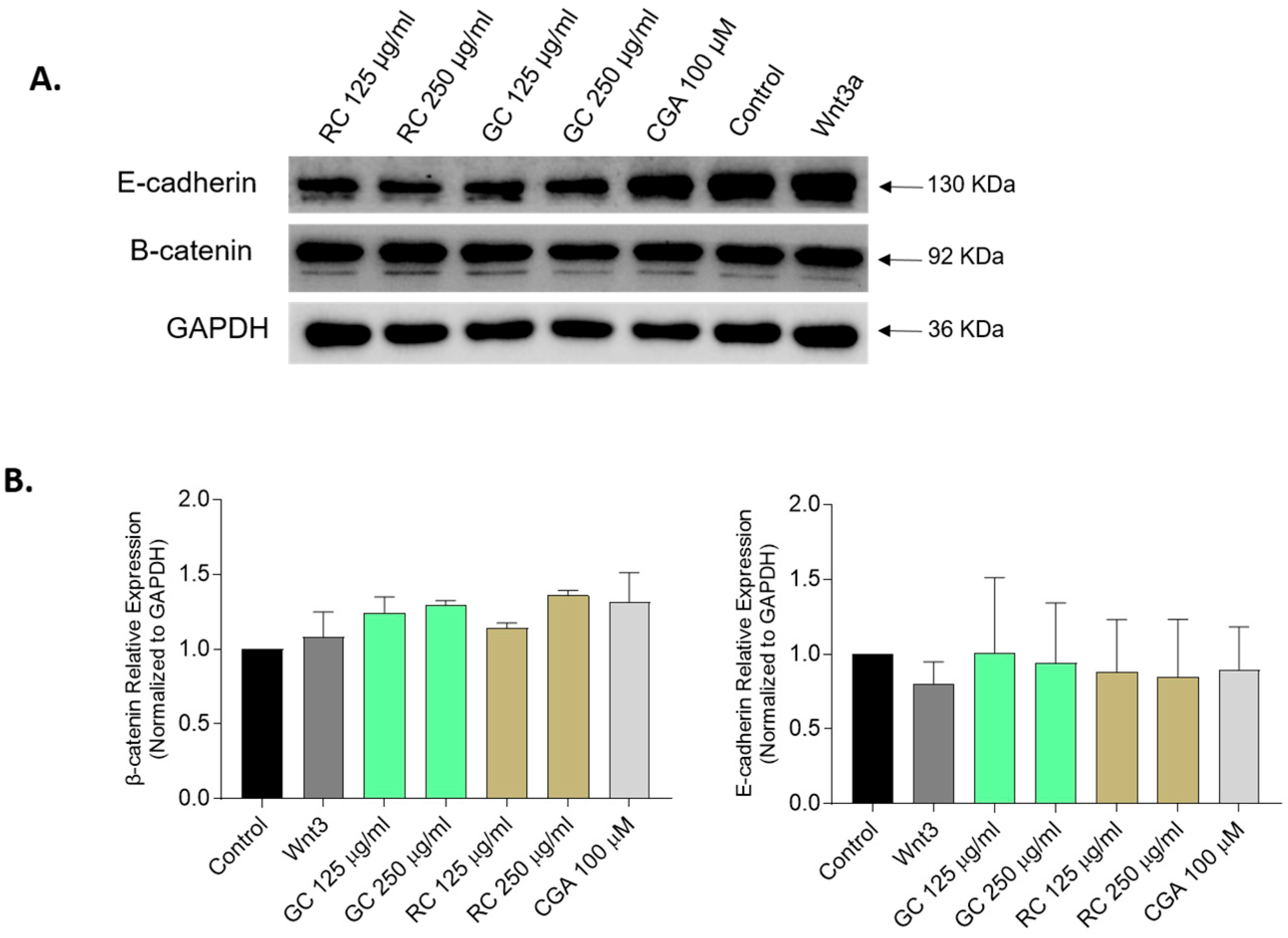
Our data also revealed an upregulation of E-cadherin mRNA levels following treatment with low doses of GC and CGA, indicating restoration of the epithelial phenotype and reduced metastatic potential. We did observe partial reduction in β-catenin mRNA levels, but we did not observe any changes in AXIN2 mRNA levels. In addition, although GC at 24 h significantly downregulated β-catenin mRNA level, this was not accompanied by changes in β-catenin or E-cadherin protein levels (
Figure 6). Promising findings have been reported before: Fako et al. (2016) demonstrated that pimozide treatment reduces β-catenin and EpCAM mRNA and protein levels in HepG2 cells [
42], but also highlighted the complexity of β-catenin regulation due to mutations in CTNNB1, such as the exon 3–4 truncation present in HepG2 cells. Such mutations confer β-catenin stabilization, which could explain why mRNA modulation does not translate to reduced protein levels in our system.
The effect of CGA as an anti-cancer compound in HCC has been reported before by others. For instance, Yang et al. (2021) reported that CGA contributes to anti-HCC activity by modulating the AKT/Cyclin D1/p21/p27 axis [
43]. Given that Cyclin D1 is also a β-catenin target, this finding supports the crosstalk between the Wnt/β-catenin and AKT signaling pathways as a target of CGA. We observed that CGA treatment consistently reduces c-Myc mRNA, with limited effects on β-catenin protein, suggesting that CGA is targeting downstream β-catenin target genes rather than core protein pathway regulators themselves.
c-Myc expression is known to be regulated by the Wnt/β-catenin dependent and independent signaling pathways in hepatocellular carcinoma (HCC) [
4]. It has been shown that receptor tyrosine kinase (RTK)-mediated pathways such as MAPK/ERK, PI3K/AKT, and JAK/STAT can upregulate c-Myc transcription and enhance its protein stability [
8]. Likewise, EGFR activation can stimulate Ras/MAPK signaling, promoting c-Myc expression independently of Wnt/β-catenin signaling. Similarly, c-Myc signaling through its ligand HGF activates downstream PI3K/AKT and STAT3 cascades, both of which contribute to increased c-Myc transcription and translation. Additionally, FGF19-FGFR4 signaling, often co-amplified with the CCND1 oncogene, has been implicated in liver tumorigenesis and may upregulate c-Myc through transcriptional crosstalk [
29]. These alternative regulatory pathways highlight c-Myc as a key driver of cell proliferation and tumor progression in HCC, even when Wnt/β-catenin is not the dominant oncogenic pathway. Altogether, these findings suggest that dietary polyphenols such as CGA, like other bioactive compounds from coffee, are effective in targeting Wnt-driven oncogenic transcription in HCC. However, further investigation to understand in more detail the mechanisms involved in their anti-cancer effects is needed.
The main question in the present study was exploring the effect of coffee extracts on the Wnt/β-catenin pathway regulation, and we have found that in HepG2 cells with the used treatments, there was a lack of significant regulation of this pathway, but we observed a clear downregulation of c-Myc mRNA expression. c-Myc itself has also been proposed as a therapeutic target, playing a key role in HCC therapy [
44].
Both the present study as well as our previous study using colorectal carcinoma (CRC) cell lines [
45], demonstrate that chlorogenic acid (CGA) and coffee extracts exhibit antiproliferative and cytotoxic effects, which correlate with the ability to modulate the Wnt/β-catenin signaling pathway. In both cancer types, CGA consistently downregulated key oncogenic targets (such as c-Myc in HCC and Cyclin D1 in CRC), although the effects at the protein level vary between these models, likely due to intrinsic differences in the molecular background of these cell lines. In the HCC study, we observed partial inhibition of β-catenin-mediated transcription (notably with green coffee), whereas in the CRC study, we showed stronger and more consistent effects in SW480 cells, which display a higher baseline level of Wnt/β-catenin.
In addition, both studies highlighted the influence of coffee bean processing on bioactive compound content, showing differential biological features that can translate in differential molecular mechanisms, suggesting that the composition and biological properties of green coffee vs. roasted coffee extracts should be further explored. While CGA is a prominent component of both GC and RC extracts used in this study, attributing the observed cellular effects solely to CGA is challenging. The extracts contain a complex mixture of compounds, including other phenolic acids, xanthines (caffeine and theobromine), and melanoidins (particularly in TC). Therefore, the effects could stem from CGA alone, synergistic/additive interactions between multiple compounds, specific compound ratios, or even the activity of non-CGA components. Disentangling the individual contributions of each compound would require further experiments that isolate and test each component, and various combinations thereof, to fully elucidate the mechanisms at play [
22]. These findings collectively support the potential of CGA and coffee polyphenols as chemotherapeutic and adjunctive agents to target Wnt/β-catenin driven tumors.