Effects of Chronic Elevation in Plasma Membrane Cholesterol on the Function of Human Na+/Taurocholate Cotransporting Polypeptide (NTCP) and Organic Cation Transporter 1 (OCT1)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Cell Culture, Cholesterol Treatment, and Uptake Experiments
2.3. Cholesterol Quantification
2.4. Surface Biotinylation and Western Blotting
2.5. Statistical Analysis
3. Results
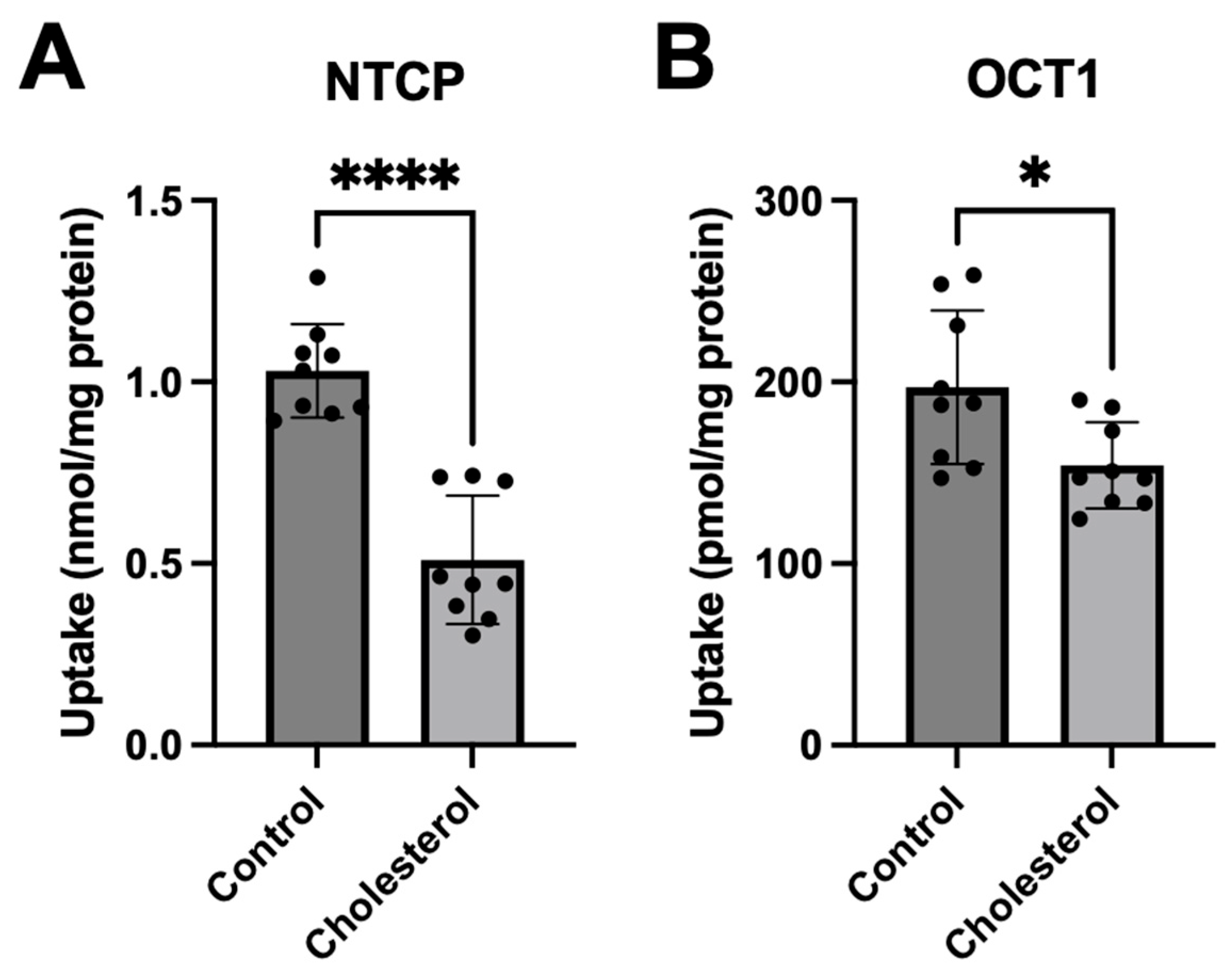
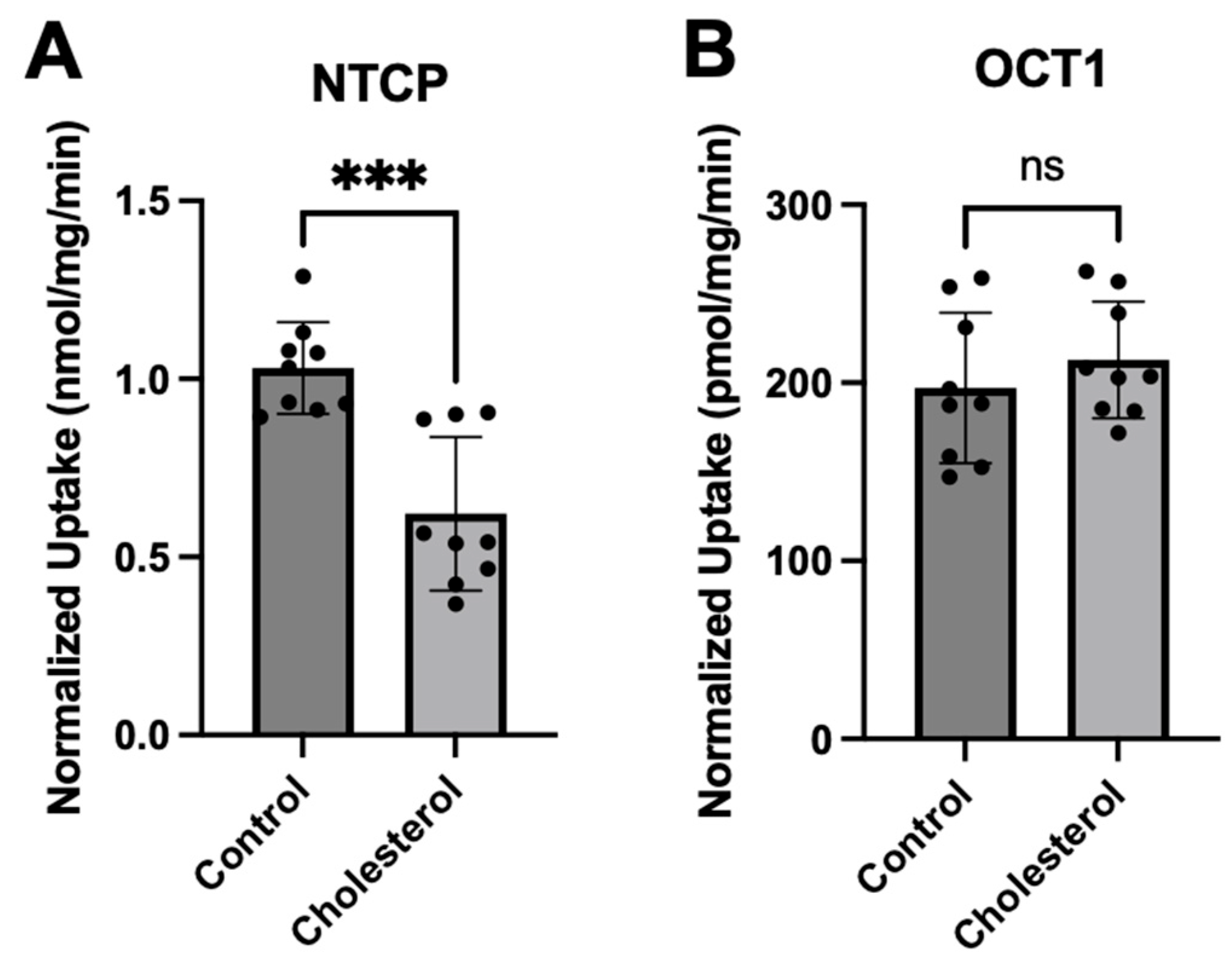
3.1. Functional Consequences of a Chronic Increase in Plasma Membrane Free Cholesterol Levels
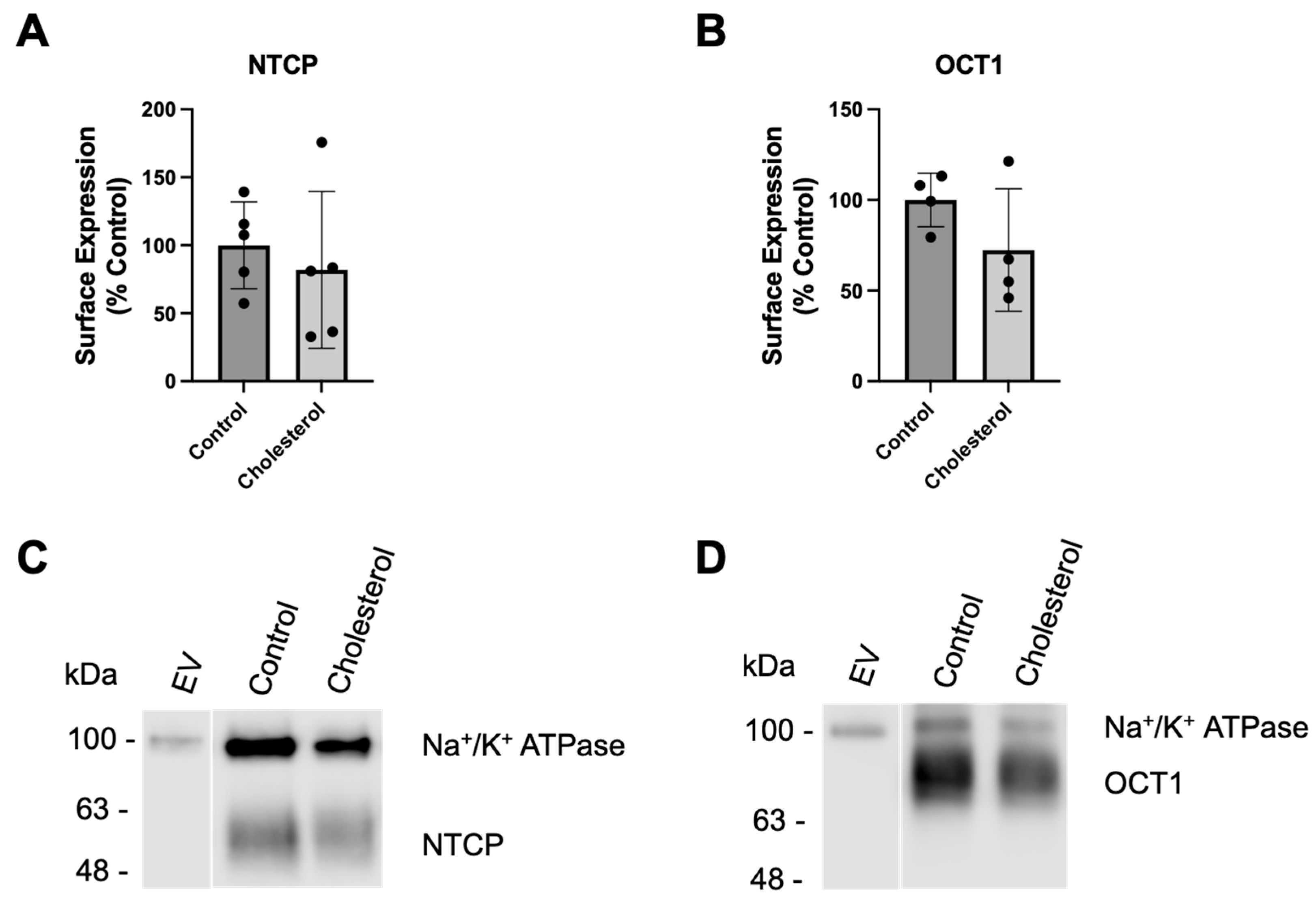
3.2. Characterizing the Effect of a Chronic Increase in Free Cholesterol on Transporter Expression and Function
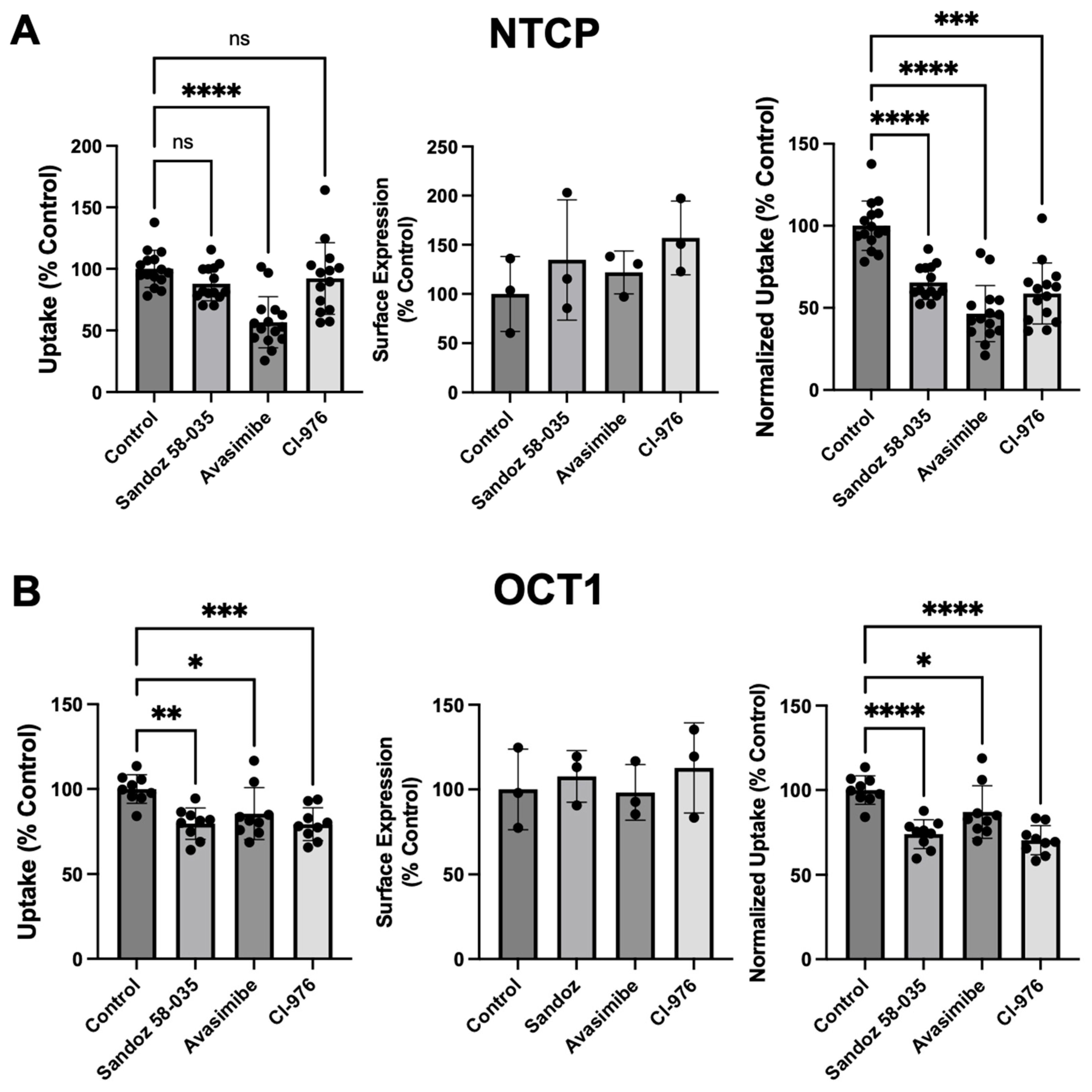
3.3. Effect of ACAT Inhibitors on Function and Expression of NTCP and OCT1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarke, J.D.; Novak, P.; Lake, A.D.; Hardwick, R.N.; Cherrington, N.J. Impaired N-linked glycosylation of uptake and efflux transporters in human non-alcoholic fatty liver disease. Liver Int. 2017, 37, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007, 46, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Ioannou, G.N. The Role of Cholesterol in the Pathogenesis of NASH. Trends Endocrinol. Metab. 2016, 27, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, X.H.; Ou, X.; Ouyang, X.P.; Tang, C.K. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog. Lipid Res. 2021, 83, 101109. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- van der Wulp, M.Y.; Verkade, H.J.; Groen, A.K. Regulation of cholesterol homeostasis. Mol. Cell Endocrinol. 2013, 368, 1–16. [Google Scholar] [CrossRef]
- Kullak-Ublick, G.A.; Glasa, J.; Boker, C.; Oswald, M.; Grutzner, U.; Hagenbuch, B.; Stieger, B.; Meier, P.J.; Beuers, U.; Kramer, W.; et al. Chlorambucil-taurocholate is transported by bile acid carriers expressed in human hepatocellular carcinomas. Gastroenterology 1997, 113, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Nies, A.T.; Koepsell, H.; Winter, S.; Burk, O.; Klein, K.; Kerb, R.; Zanger, U.M.; Keppler, D.; Schwab, M.; Schaeffeler, E. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 2009, 50, 1227–1240. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Meier, P.J. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J. Clin. Investig. 1994, 93, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Claro da Silva, T.; Polli, J.E.; Swaan, P.W. The solute carrier family 10 (SLC10): Beyond bile acid transport. Mol. Asp. Med. 2013, 34, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Slijepcevic, D.; Kaufman, C.; Wichers, C.G.; Gilglioni, E.H.; Lempp, F.A.; Duijst, S.; de Waart, D.R.; Elferink, R.P.; Mier, W.; Stieger, B.; et al. Impaired Uptake of Conjugated Bile Acids and Hepatitis B Virus Pres1-Binding in Na+ -Taurocholate Cotransporting Polypeptide Knockout Mice. Hepatology 2015, 62, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Craddock, A.L.; Love, M.W.; Daniel, R.W.; Kirby, L.C.; Walters, H.C.; Wong, M.H.; Dawson, P.A. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am. J. Physiol. 1998, 274, G157–G169. [Google Scholar] [CrossRef] [PubMed]
- Kullak-Ublick, G.A.; Ismair, M.G.; Kubitz, R.; Schmitt, M.; Haussinger, D.; Stieger, B.; Hagenbuch, B.; Meier, P.J.; Beuers, U.; Paumgartner, G. Stable expression and functional characterization of a Na+-taurocholate cotransporting green fluorescent protein in human hepatoblastoma HepG2 cells. Cytotechnology 2000, 34, 1–9. [Google Scholar] [CrossRef]
- Fujino, H.; Saito, T.; Ogawa, S.; Kojima, J. Transporter-mediated influx and efflux mechanisms of pitavastatin, a new inhibitor of HMG-CoA reductase. J. Pharm. Pharmacol. 2005, 57, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.H.; Tirona, R.G.; Leake, B.F.; Glaeser, H.; Lee, W.; Lemke, C.J.; Wang, Y.; Kim, R.B. Drug and bile acid transporters in rosuvastatin hepatic uptake: Function, expression, and pharmacogenetics. Gastroenterology 2006, 130, 1793–1806. [Google Scholar] [CrossRef]
- Greupink, R.; Dillen, L.; Monshouwer, M.; Huisman, M.T.; Russel, F.G. Interaction of fluvastatin with the liver-specific Na+-dependent taurocholate cotransporting polypeptide (NTCP). Eur. J. Pharm. Sci. 2011, 44, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.A.; Qiu, X.; Rotter, C.J.; Kimoto, E.; Piotrowski, M.; Varma, M.V.; Ei-Kattan, A.F.; Lai, Y. Quantitative assessment of the contribution of sodium-dependent taurocholate co-transporting polypeptide (NTCP) to the hepatic uptake of rosuvastatin, pitavastatin and fluvastatin. Biopharm. Drug Dispos. 2013, 34, 452–461. [Google Scholar] [CrossRef]
- Chen, H.; Huang, M.; Zhang, D.; Wang, H.; Wang, D.; Li, M.; Wang, X.; Zhu, R.; Liu, J.; Ma, L. Metformin’s effect on metabolic dysfunction-associated steatotic liver disease through the miR-200a-5p and AMPK/SERCA2b pathway. Front. Pharmacol. 2024, 15, 1477212. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, K.; Gautam, N.; Sivakumar, K.; Paydak, H.; Mehta, J.L. Metformin: Its salutary effects beyond diabetes mellitus. J. Investig. Med. 2025, 73, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Idowu, J.Y.; Hagenbuch, B. Free Cholesterol Affects the Function and Localization of Human Na+/Taurocholate Cotransporting Polypeptide (NTCP) and Organic Cation Transporter 1 (OCT1). Int. J. Mol. Sci. 2022, 23, 8457. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, M.J.; Miller, H.; Idowu, J.Y.; Zitzow, J.D.; Chang, S.C.; Hagenbuch, B. Perfluoroalkyl Carboxylic Acids Interact with the Human Bile Acid Transporter NTCP. Livers 2021, 1, 221–229. [Google Scholar] [CrossRef]
- Boxberger, K.H.; Hagenbuch, B.; Lampe, J.N. Ligand-dependent modulation of hOCT1 transport reveals discrete ligand binding sites within the substrate translocation channel. Biochem. Pharmacol. 2018, 156, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Faussner, A.; Deininger, M.M.; Weber, C.; Steffens, S. Direct addition of poly-lysine or poly-ethylenimine to the medium: A simple alternative to plate pre-coating. PLoS ONE 2022, 17, e0260173. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, M.J.; Malhotra, S.; Fenton, A.W.; Swint-Kruse, L.; Karanicolas, J.; Hagenbuch, B. A clinically relevant polymorphism in the Na(+)/taurocholate cotransporting polypeptide (NTCP) occurs at a rheostat position. J. Biol. Chem. 2021, 296, 100047. [Google Scholar] [CrossRef] [PubMed]
- Iaea, D.B.; Mao, S.; Lund, F.W.; Maxfield, F.R. Role of STARD4 in sterol transport between the endocytic recycling compartment and the plasma membrane. Mol. Biol. Cell. 2017, 28, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Llaverias, G.; Laguna, J.C.; Alegret, M. Pharmacology of the ACAT inhibitor avasimibe (CI-1011). Cardiovasc. Drug Rev. 2003, 21, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Field, F.J.; Albright, E.; Mathur, S. Inhibition of acylcoenzyme A: Cholesterol acyltransferase activity by PD128O42: Effect on cholesterol metabolism and secretion in CaCo-2 cells. Lipids 1991, 26, 1–8. [Google Scholar] [CrossRef]
- Bastiaanse, E.M.; Hold, K.M.; Van der Laarse, A. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovasc. Res. 1997, 33, 272–283. [Google Scholar] [CrossRef]
- Scanga, R.; Scalise, M.; Xiu, F.; Galluccio, M.; Console, L.; Visentin, M.; Indiveri, C. Impact of 7-ketocholesterol on the function and stability of the LAT1 transporter. Biochem. Pharmacol. 2025, 239, 117075. [Google Scholar] [CrossRef] [PubMed]
- Demel, R.A.; De Kruyff, B. The function of sterols in membranes. Biochim. Biophys. Acta. 1976, 457, 109–132. [Google Scholar] [CrossRef]
- Li, H.; Papadopoulos, V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 1998, 139, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M. Cholesterol and the interaction of proteins with membrane domains. Prog. Lipid Res. 2006, 45, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Sutter, M.L.; Console, L.; Fahner, A.F.; Samodelov, S.L.; Gai, Z.; Ciarimboli, G.; Indiveri, C.; Kullak-Ublick, G.A.; Visentin, M. The role of cholesterol recognition (CARC/CRAC) mirror codes in the allosterism of the human organic cation transporter 2 (OCT2, SLC22A2). Biochem. Pharmacol. 2021, 194, 114840. [Google Scholar] [CrossRef]
- Kumari, R.M.; Khatri, A.; Chaudhary, R.; Choudhary, V. Concept of lipid droplet biogenesis. Eur. J. Cell Biol. 2023, 102, 151362. [Google Scholar] [CrossRef] [PubMed]
- Meuwese, M.C.; Franssen, R.; Stroes, E.S.; Kastelein, J.J. And then there were acyl coenzyme A: Cholesterol acyl transferase inhibitors. Curr. Opin. Lipidol. 2006, 17, 426–430. [Google Scholar] [CrossRef]
- Post, S.M.; Zoeteweij, J.P.; Bos, M.H.; de Wit, E.C.; Havinga, R.; Kuipers, F.; Princen, H.M. Acyl-coenzyme A: Cholesterol acyltransferase inhibitor, avasimibe, stimulates bile acid synthesis and cholesterol 7alpha-hydroxylase in cultured rat hepatocytes and in vivo in the rat. Hepatology 1999, 30, 491–500. [Google Scholar] [CrossRef] [PubMed]

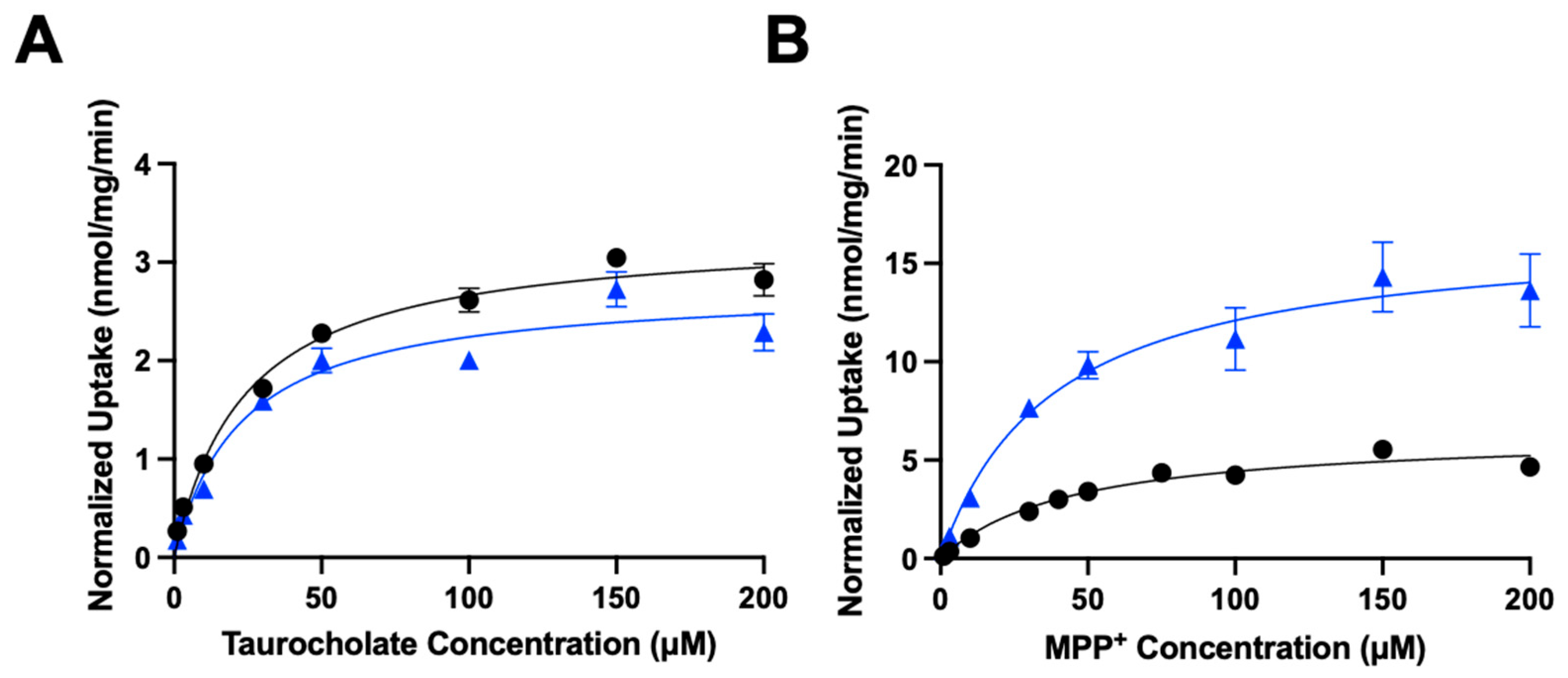
| Transporter | Parameters | Control | Cholesterol |
|---|---|---|---|
| NTCP | Km (µM) | 24.0 ± 4.0 | 22.7 ± 6.7 |
| Vmax (nmol/mg/min) | 3.3 ± 0.1 | 2.7 ± 0.2 | |
| Vmax/Km (µL/mg/min) | 138 ± 23 | 119 ± 36 | |
| OCT1 | Km (µM) | 44.4 ± 9.7 | 38.0 ± 6.3 |
| Vmax (nmol/mg/min) | 6.4 ± 0.5 | 16.7 ± 0.9 | |
| Vmax/Km (µL/mg/min) | 144 ± 33 | 439 ± 77 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idowu, J.Y.; McKimens, C.; Hagenbuch, B. Effects of Chronic Elevation in Plasma Membrane Cholesterol on the Function of Human Na+/Taurocholate Cotransporting Polypeptide (NTCP) and Organic Cation Transporter 1 (OCT1). Livers 2025, 5, 45. https://doi.org/10.3390/livers5030045
Idowu JY, McKimens C, Hagenbuch B. Effects of Chronic Elevation in Plasma Membrane Cholesterol on the Function of Human Na+/Taurocholate Cotransporting Polypeptide (NTCP) and Organic Cation Transporter 1 (OCT1). Livers. 2025; 5(3):45. https://doi.org/10.3390/livers5030045
Chicago/Turabian StyleIdowu, Jessica Y., Caylie McKimens, and Bruno Hagenbuch. 2025. "Effects of Chronic Elevation in Plasma Membrane Cholesterol on the Function of Human Na+/Taurocholate Cotransporting Polypeptide (NTCP) and Organic Cation Transporter 1 (OCT1)" Livers 5, no. 3: 45. https://doi.org/10.3390/livers5030045
APA StyleIdowu, J. Y., McKimens, C., & Hagenbuch, B. (2025). Effects of Chronic Elevation in Plasma Membrane Cholesterol on the Function of Human Na+/Taurocholate Cotransporting Polypeptide (NTCP) and Organic Cation Transporter 1 (OCT1). Livers, 5(3), 45. https://doi.org/10.3390/livers5030045






