Abstract
After the first release of synthalin B (dodecamethylenbiguanide) in 1928 and its later retraction in the 1940s in Germany, the retraction of phenformin (N-Phenethylbiguanide) and of Buformin in the USA (but not outside) because of the lethal complication of acidosis seemed to have put an end to the era of the biguanides as oral antidiabetics. The strongly hygroscopic metformin (1-1-dimethylbiguanide), first synthesized 1922 and resuscitated as an oral antidiabetic (type 2 of the elderly) compound first released in 1959 in France and in other European countries, was used in the first large multicenter prospective long-term trial in England in the UKPDS (1977–1997). It was then released in the USA after a short-term prospective trial in healthy overweight “young” type 2 diabetics (mean age 53 years) in 1995 for oral treatment of type 2 diabetes. It was, however, prescribed to mostly multimorbid older patients (above 60–65 years of age). Metformin is now the most used oral drug for type 2 diabetes worldwide. While intravenous administration of biguanides does not have any glucose-lowering effect, their oral administration leads to enormous increase in their intestinal concentration (up to 300-fold compared to that measured in the blood), to reduced absorption of glucose from the diet, to increased excretion of glucose through the stool, and to decrease in insulin serum level through increased hepatic uptake and decreased production. Intravenously injected F18-labeled glucose in metformin-treated type 2 diabetics accumulates in the small and even more in the large intestine. The densitometry picture observed in metformin-treated overweight diabetics is like that observed in patients after bowel-cleansing or chronically taking different types of laxatives, where the accumulated radioactivity can even reach values observed in colon cancer. The glucose-lowering mechanism of action of metformin is therefore not only due to inhibition of glucose uptake in the small intestine but also to “attraction” of glucose from the hepatocyte into the intestine, possibly through the insulin-mediated uptake in the hepatocyte and its secretion into the bile. Furthermore, these compounds have also a diuretic effect (loss of sodium and water in the urine) Acute gastrointestinal side effects accompanied by fluid loss often lead to the drugs’ dose reduction and strongly limit adherence to therapy. Main long-term consequences are “chronic” dehydration, deficiency of vitamin B12 and of iron, and, as observed for all the biguanides, to “chronic” increase in fasting and postprandial lactate plasma level as a laboratory marker of a clinical condition characterized by hypotension, oliguria, adynamia, and evident lactic acidosis. Metformin is not different from the other biguanides: synthalin B, buformin, and phenformin. The mechanism of action of the biguanides as antihyperglycemic substances and their side effects are comparable if not even stronger (abdominal pain, nausea, vomiting, diarrhea, fluid loss) to those of laxatives.
1. Introduction: Development of Industrial Food Production, Hypercaloric Nutrition, Overweight/Obesity, and Type 2 Diabetes and Increasing Popularity of Biguanides
Beginning in the middle of the 20th century, the so-called developed countries, namely Germany, Italy, Japan, the United States of America, and also the United Kingdom, France, Finland, Sweden, and Norway, experienced a steady growth (3–5-fold) in the population size [1]. Those countries have mainly contributed to the worldwide pandemic increase in obesity and overweight. Obesity is also increasing in the formerly called developing world (emerging countries, low-income countries, etc.), inducing a change in the definition of malnutrition, as it now comprises not only undernourishment but also overfeeding, especially in children [2,3,4,5]. Japan represents an exception among the Western-world countries, as the overweight and obesity numbers are relatively lower [6] since agriculture and livestock production is limited by the scarcity of land that can be used for agriculture and by the state control of the import of food products from abroad [7,8].
Nevertheless, there are still several hundred million people worldwide suffering from hunger [9]. The global overfeeding can be explained by the epochal change and enormous increase in food production, which began in the middle of the 19th century, when soil fertilization, the industrialization of agriculture [10,11,12], and the livestock sector started to grow [13,14] first in the Western world but then expanded into the rest of the world along with globalization, especially after the Second World War [15,16]. In the middle of the 20th century, it became obvious that cigarette smoke was the first cause of coronary heart disease and of lung cancer in the United Kingdom and in the United States of America [17,18,19,20,21,22,23]. It became apparent that consumption of alcoholic beverages would increase mortality [24].
Immediately after these publications, an alternative explanation for the development of arteriosclerosis also inducible by starvation [25] was offered by the biologist Ancel Kyes [26], namely hypercholesterolemia attributed to the different composition of animal fat in the food of men in north Karelia, south-east of Finland on the Russian border, although the study found a much lower BMI than that found in American men [27,28]. Finnish women from the same area with the same hypercholesterolemia were not as much affected as men [29,30].
It has to be mentioned that the true risk factors, namely cigarette smoke and consumption of alcoholic beverages, were not discussed, as it was not emphasized that these men had a normal body mass index [31,32,33,34,35,36].
The meat industry more greatly favored the hypothesis of exaggerated consumption of carbohydrates and sugar being the cause of overweight and of the co-morbidities induced by hypercaloric nutrition, including alcoholic beverages [37,38,39,40]. This hypothesis was denied in scientific contributions published in the world literature; in the meantime, however, the explosion of the overweight pandemic [41,42,43,44] can be attributed to the development of a “food addiction” [45]. It has to be pointed out that being overweight changes the “normal” physiology. In fact, fat accumulates in the liver in the abdominal cavity but also around the intrathoracic organs, heart, and lungs [46,47,48]. Overweight also is accompanied by increase in blood volume, which is however not proportional to the increase in body weight [49,50,51]. This may have serious consequences for the kidneys [49] if one considers that blood volume physiologically decreases with increasing age [52,53,54,55], mostly due to dehydration [53] and hypoalbuminemia [56], accompanied by increased lactate production [57,58], leading to subclinical “chronic” acidosis, especially in obese type 2 diabetics [59,60,61,62].
Increase in blood volume without a corresponding increase in the vascular bed may explain the development of hypertension in obese patients [63]. In addition, increased body weight is accompanied by changes in the largest organ of the body, the skin. Increased dryness of the skin is a physiological change in obese persons (inadequate hydration) that is accompanied by increased blood flow in the capillaries, increased transdermal fluid loss, and increased sweat gland activity. The latent peripheral tissue hypoxia [64] could explain the increased plasma D-dimer found in patients with hypertension and diabete type 2 [65].
As increase in endocrine pancreas function is time-limited, many obese patients develop hyperglycemia [66] mostly in the presence of increased insulin level (compared to lean persons), which, however, becomes insufficient to keep the glucose serum level under the prediabetic limit of 125 mg/dL [67]. While the overweight pandemic expands, the use of different drugs to contrast the “comorbidities” has also expanded, including the number of persons suffering from different side effects from the single drugs. As those drugs considered adequate for appetite reduction are considered life-style drugs [68], and diffusion of statin use [69,70] only distracts the attention from the causes of the obesity pandemic, a surgical subspecialty, namely bariatric surgery [71], appears to be the only measure to reduce food uptake as the therapeutic escape solution for type 2 diabetes. Preventive nutritional suggestions (reduction in calorie uptake, especially by reducing uptake of sweets, fat [72,73], and alcoholic beverages) remain mainly unsuccessful [73]. The treatment of “comorbidities” caused by the diffusion of this lifestyle continues to be dealt with by increasing the number of prescriptions of statins (“statinization”, oral antidiabetics, and diuretics [74]), aggravating maybe the most serious obesity-associated “comorbidity”, namely chronic kidney disease [75,76].
Metformin (1,1-dimethylbiguanide), a water-soluble, strong basic compound [77] belonging to the biguanides group, is mostly used as antidiabetic drug for obese patients. It has a molecular weight of 129.16, while metformin hydrochloride (Glucophage) has a m.w. of 165.62. In 1957, the first successful use of this biguanide in overweight diabetics was published in France [77]; nowadays, about 200 million doses/day worldwide are utilized for the treatment of multimorbid, older, overweight persons with type 2 diabetes alone or in combination with other orally administered antidiabetic compounds or with insulin [78,79]. The review of the literature of the last 100 years strongly suggests that biguanides can hardly be considered classical drugs, as they do not bind to proteins and do not undergo any biotransformation. They not only induce loss of water and of nutrients, especially through glucose excretion into the stool, but they also increase water loss through the urine, creating a constant danger of kidney failure because of dehydration, often accentuated by regular consumption of alcoholic beverages in elderly overweight/obese diabetics [80]. This danger may be further increased by administration of diuretics as antihypertensive drugs.
2. History of Biguanides: From the First Animal Experiments to the Production of the First Synthetic Compounds to Their First Oral Use as Substitute of Insulin or in Addition to Insulin in Overweight Type 2 Diabetics
On the basis of the assumption [81] that parathyroids are involved in glucose metabolism, Watanabe K administered guanidine hydrochloride subcutaneously into rabbits, which were kept without food and water. A decrease in glucose serum level was observed, which was paralleled by a deterioration of general conditions until death ensued. He also observed the development of marked acidosis in those animals that refused food uptake [82].
Frank, Northemann, and Wagner in 1926 studied the effect of orally administered synthalin (aminophenthylguanidine) compared to that of subcutaneously administered insulin, namely on the effect of peripheral glucose consumption and on glucose storage as glycogen [83].
The authors calculated that 1 mg synthalin administered orally generated the same effect as one unit insulin [83,84] in the reduction in the amount of glucose eliminated in the urine. Furthermore, authors found that synthalin was able to reduce the serum glucose level to below the glycosuria concentration, to abolish acidotic episodes, and to reduce the symptoms of the disease, namely polyuria and polydipsia. Comparable results were obtained by oral and subcutaneous application of the substance.
After the first supporting experiences were published [85,86,87,88,89,90], Frank, Northman, and Wagner published their next experience with the “new” biguanide synthalin B (dodecamethylendiguanidin) [88]. This was a further molecular development of the drug in the attempt to reduce the gastrointestinal side effects observed with synthalin [89]. Initially, the substance induced diarrhea, which was more frequent than under synthalin but less strong and more temporary. Indication for this therapy was diabetes in older patients who were normally reluctant to take the prescribed diet, while the treatment of choice for the young diabetic patient remained insulin administration. Synthalin B was approved in Germany and made available for further studies in England and in the United States of America [91]. These experiences, especially those performed with synthalin A (diguanidinodecamethylene), delivered some support but also confirmed the high frequency of gastrointestinal side effects [89,90].
Bischoff and coworkers [91] and Batherwick and coworkers [92] reported about a special observation concerning the “poison” synthalin administered parenterally to rabbits. The fasting animals developed hypoglycemia accompanied by an increase in serum urea concentration just before dying. Authors called the damage of the tubular epithelium (while the glomeruli were intact) “nephritis”.
Short-term results obtained with oral administration were unsuccessful.
The data were summarized and also critically commented upon in an article in the scientific journal Nature in January 1928 [93]. The author repeated the judgment that synthalin was not “a substitute for insulin,” but the first results obtained with that substance would encourage the continuation of the search for better compounds with insulin-like effects [93]. Werner and Bell synthesized [94,95] a strong hygroscopic 1-1-dimethylguanidine hydrochloride, which was tested for comparison with synthalin by the pharmacologists Hesse and Taubmann [96]. They confirmed that biguanide derivatives like synthalin were able to reduce the glucose serum level up to cramp level and that glucose administration was able to reduce the cramps but not to ameliorate the general conditions of the animals (rabbit). Compared to the insulin effect, biguanides were not able to induce glycogen synthesis in the liver. On the contrary, compound-induced hepatic glycogen depletion was observable. All the authors underlined the finding of Watanabe [82] that the substances synthalin and the biguanides caused lactic acidosis. Minot and coworkers thoroughly studied the lactic acidosis production induced by guanidine intoxication [97].
The authors found that vomiting and diarrhea in addition to the tendency toward hypoglycemia were even aggravated by administration of bicarbonate. They also mentioned that in some cases, retention of nitrogen could be due to “nephritis” (see Blatherwick et al.). The authors also stated that “fluid administration to improve kidney function is an important feature whenever the specific means employed to combat the acidosis are unsuccessful. The removal of guanidine through increased kidney function may be an important feature in the adjustment of acid-base equilibrium by continuous venoclysis with glucose or Ringer solution” [97]. Because of the unsatisfactory safety, the search for new guanidine-compounds continued [98,99], and synthalin remained an oral antidiabetic drug on the German market until 1940.
Then, retraction from the market was effectuated because of seldom-occurring but fatal acidosis attributed to anaerobic glycolysis [98] and also because of possible fatal hepatotoxicity [99]. At the beginning of the 1950s, however, synthalin received interest again because of reports about its specific toxic effect on pancreatic alpha-cells [100,101]. At the same time, several other (“new”) biguanides were synthesized and tested in comparison also with synthalin, but the side effects, especially the tendency toward lactate production due to anaerobic glycolysis, were quite similar to those of the other biguanides [101].
Buformin and phenformin [102,103,104,105,106,107,108,109] were developed and have been used for treatment of obese type 2 diabetics even up to today.
It was shown that phenformin administered orally for three days to hyperinsulin responders, namely obese non-diabetics, before oral but not intravenous administration of glucose was able to reduce both glucose and insulin serum levels to the level of normal subjects. A similar effect was also observed in obese diabetics [108]. These results strongly suggested a reduction in glucose absorption and also a lack of induction of intestinal hormones by phenformin. Stimulation of the beta cells to synthesize more insulin was hypothesized.
Walker and Linton, during the two years of the use of phenformin, observed in at least 100 patients “a worrying and indeed dangerous complication”, namely ketonuria and acidosis in the presence of normoglycemia or mild hyperglycemia [107].
Ketonuria with or without acidosis was observed in about one third of all cases, always without a significant rise of blood sugar levels. The suggested measures to combat ketosis (“metabolic starvation”) were (a) increased dietary carbohydrate, (b) reduction in the dose of phenformin, and (c) supplementary use of insulin. These measures with additional alkali administration were not successful in a patient who died a few hours after admission, and autopsy failed to find the lesions possibly responsible for his death [107]. Walker RS, however, mentioned efforts to clarify the mechanisms of acidosis development under phenformin therapy and reported that the patients controlled under phenformin had increased basal blood lactate levels exaggerated by the administered compound and by exercise, accompanied by a marked fall in the alkali reserve of a magnitude many times greater than that observed in normal people.
Dehydration, as the cause of hypotension and kidney injury, was not discussed either at the clinical or at autopsy level.
At that time, Beaser in his report [109] about the short-term treatment of 10 overweight diabetic patients with a combination of phenformin, sulfonylurea, and insulin did not find toxic side effects but mentioned that “the potential usefulness of phenethylguanidine is circumscribed by gastrointestinal intolerance, which supervenes in many patients before the most effective dosage levels are reached”. In the following decades, the focus was turned to the glucose serum level [110] and less toward the systemic consequences of the gastrointestinal fluid loss due, on one hand, to the reduced fluid uptake as a consequence of nausea to loss of appetite and the increased loss through the intestine and, on the other hand, to an increased loss of water through the kidneys [111,112].
Bratusch-Marrain and coworkers [110] studied the effect of buformin on carbohydrate metabolism in healthy men. The authors found that buformin altered the lactate-balance. In fact, splanchnic lactate output was increased after glucose ingestion, while hepatic glucose output was not increased under buformin treatment. The arterial blood glucose concentration after ingestion of glucose was significantly reduced by buformin pretreatment. Buformin treatment induced a marked rise of splanchnic lactate production [110].
In a study performed in Japan [113] on 13 overweight diabetics randomly divided into two groups, buformin was given in a daily dose of 150 mg (50 mg after breakfast, lunch, and dinner) for two weeks, while the other group was administered a hypocaloric diet (20 kcal/kg b.w.) along with physiotherapy for two weeks. Buformin treatment not only significantly lowered fasting plasma glucose and plasma insulin-levels, but it also increased hepatic but not peripheral glucose uptake when compared to the diet-alone group [113].
While phenformin was definitively withdrawn from the market in the USA because of lactic acidosis development [114], buformin was only partially taken from the market. Both are still used in many countries outside of the United States of America [109,110,115].
The use of metformin (dimethylbiguanide of Wagner and Bell) as a “new” biguanide was first described by Mehnert H and Seitz W [116] and by Sterne 1957 [117] and first approved in France 1959. It was used in the UK, where the lowering effect on body weight was described when compared to the opposite result obtained with chlorpropamide after twelve months (but not after a further six months) of treatment in obese diabetics [118]. By that time, “some non-tissue-forming catabolic process” should be hypothesized if reduction in food intake was not the explanation for the tendency of weight loss in obese diabetics [118], as also observed with phenformin.
Despite some critical case reports about the occurrence of fatal acidosis [119] and the long-term low efficacy (mostly justified by improvement of microvascular changes) on a subgroup of overweight type 2 diabetics [120] and mainly based on the short-term (29 weeks) effect on glucose serum level as surrogate marker [121], the published trial results (Table 1) were considered to favor the introduction of metformin as an oral antihyperglycemic drug.

Table 1.
Metformin therapy in type 2 diabetes prospective trials.
The findings of the short-term trial [121] were sufficient for approval of metformin as one more oral antidiabetic drug in the United States in August 1995, while the first cases of lactic acidosis were reported as mostly attributed to “comorbidities” [122], which were then listed as contraindications [123] for metformin therapy. A confirmatory long-term prospective trial under similar short-term conditions was not requested by the FDA [123,124].
Metformin was defined as the “cousin” of phenformin by Campbell and colleagues [124] and not much different from phenformin and buformin [108,125] and from the other biguanides described in the previous decades (see above).
3. Pharmacokinetic and Pharmacodynamic of Biguanides
“To produce Its Characteristic Effects a Drug Must Be Present in Appropriate Concentration at Its Site of Action”([126])
After oral administration of metformin [127,128], like the other biguanide hydrochloride [129,130], about 30–60% of the compound [129] is absorbed, while a large part accumulates in the small intestine to reach a 300-fold larger concentration than that measured in the blood [131], from the first part of the small intestine into the venous blood of the portal vein, first released through the “fenestrated” hepatic sinusoidal endothelium of the liver to quickly reach the hepatocytes [132]. It was first supposed to be transported through the OCT1-hepatocyte membrane transporter [133,134]. Genetic variations of this transporter were supposed to be the basis for the different efficacy of the drug. However, this could not be confirmed in fasting healthy individuals with reduced-function OCT1-diplotypes [135]. Treatment of those individuals with 2000 mg metformin daily for a week did not modify plasma glucose-, insu], and treatment peptide concentration. While plasma glucose appeared to be lower during the first 2–3 h, the mean plasma lactate value increased significantly during metformin treatment, remaining within the normal range.
The absorbed part of the orally administered metformin is released from the liver into the systemic circulation and eliminated unchanged (up to five times faster than creatinine) through the kidney by glomerular filtration (but also by tubular secretion) and can be found up to almost 100% in the urine. After intravenous administration, Pentikäinen found some C14-labeled metformin in the saliva but not in the feces [131].
After oral administration of C14-metformin, the bioavailability in three healthy volunteers was 61% and 51% of the administered dose 48 h later. The recovery in the feces of one volunteer from whom the stool was collected for one week was 29.4% and 90.5%, together with the activity recovered in the urine. Metformin was not bound to proteins in vivo or in vitro [131].
C11-metformin (9.5 microSv/MBq = ca.1.1 micrograms), first injected intravenously, allowed the study of its biodistribution in humans by the PET scan technique [133]. Most of the activity was found in the liver, the kidneys, and the urinary bladder. Most of the absorbed compound was cleared from the blood 20 min after injection. Dosimetry calculations were performed for the stomach-, small intestine-, liver-, kidney-, and bladder contents. It was demonstrated that hepatic metformin uptake is very rapid and fully reversible, but the accumulation of the activity was higher than after intravenous administration since, although slower, the tracer delivery came from the portal blood through the liver first. Two hours after the oral ingestion of the tracer, the bulk of the radioactivity was still found in the intestine (Figure 1 lower panel), and no further observation of the fate of the radioactive metformin was possible.
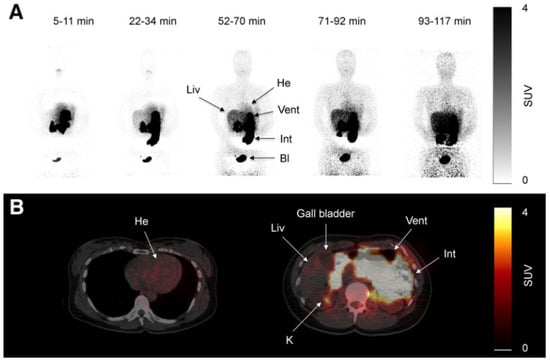
Figure 1.
Scans of C11-metformin administered to humans intravenously and orally (upper (A) and lower (B) panel, respectively) taken at different times after administration. Gormensen LC et al. ([133], with permission).
It is understandable that the study performed by Pentikäinen and colleagues [131] with 14C-labeled metformin could only be repeated by the addition of “cold” metformin to the 14C-labeled compound. This would most probably accelerate the intestinal passage as most of the conventional drug-containing tablets also containing polyethylenglycol (3–6000) as an additive, which is a laxative.
Although an active transport of metformin through the membrane of the hepatocyte was extensively discussed by Gromensen et al. [133], it has to be mentioned that hepatic densitometry pictures similar to those observed after intravenous and oral administration of C11-metformin can also be observed after the injection of C11-nicotine [136] or even of C11-donepezil, a high-affinity antagonist of acetylcholinesterase normally used as drug in the treatment of patients with Parkinson’s disease [137,138]. It cannot be ruled out that there is a diffusion into the space of Disse through the fenestrae of the sinusoidal endothelial cells [132] and that the compound is then washed out from the interstitium back into the hepatic vein. It may be therefore more appropriate to speak about hepatic metformin “extraction” [139] than about uptake.
The kidney-to-blood activity ratio was identical independently of the administration route of the radioactive metformin. Some discrete uptakes of the tracer was found in the salivary glands, and discrete uptake was found also in the intestine. No activity was found in the gallbladder. Significant amounts of the tracer passed to the small intestine 10 min after ingestion.
Despite the increasing popularity, the mechanism of action of metformin (gluco-phage of Sterne) has been elusive until the present time.
Especially after the report of Bonora et al. [140] and of Sum et al. [141] on the lack of metabolic changes after intravenous administration of metformin hydrochloride, attention concentrated mostly on pharmacokinetic studies performed after oral administration of immediate- or retarded-release metformin formulations and of different dosages at different times of day.
Bonora and colleagues [140] found no significant change in the fasting plasma level of glucose, insulin, C-peptide, glucagon, and growth hormone after intravenous injection of 1 g of metformin as a bolus in 15 non-diabetic subjects (4 males and 11 females). Sum et al. [141] performed a double-blind randomized crossover study in nine type 2 diabetic patients (six female and three male; HbA1c 8.8 vs. 8.6 on oral metformin therapy (Glucophage, Lypha).
After a 10-hour fasting period and having omitted the morning metformin dose, D-glucose, [3-H3]-glucose, and metformin/NaCl were injected intravenously. Metformin was continuously infused to achieve a serum concentration at a lower therapeutic level and then (after the first 120 min) increased to reach a high therapeutic range of 5–7 mg/L. Glucose was infused to reach and then maintain a concentration of 5 mmol. Blood samples were collected at 10-minute intervals. Urine samples were also collected. After the first increase in metformin serum level from 1.64 to 6.57 at the end of the second step, a continuous decrease in metformin level was measured under NaCl infusion.
The study was repeated between 10 and 20 days after the first study, and no difference was found between the two study days in fasting glucose, insulin plasma levels, and metformin concentration. No difference was found between control and metformin studies in hepatic glucose disposal, peripheral glucose disposal, and glucose oxidation.
Although the authors did not underline this important finding, they observed no change in blood lactate concentrations. These results demonstrated that there was no acute metabolic effect of increasing metformin serum concentration in type 2 diabetics on hepatic glucose production or peripheral glucose disposal, and contrary to the previous observation, after oral administration, no influence of intravenously administered metformin on lactate production was found [141].
McCright and coworkers [142] performed a pharmacokinetics study in obese patients with type 2 diabetes who were intolerant to metformin and compared the numbers obtained in 19 obese diabetics who tolerated oral metformin. Under fasting conditions, 500 mg metformin was orally administered. Peak serum concentration of metformin was reached at 2.5 h (2.1/2.0 mg/L, respectively) in both groups, and the continuous decrease for 24 h follow-up was also comparable [142]. Lactate serum concentration increased to 2.4 mmol/L one hour after the peak of metformin serum concentration in both groups. The curve of the decrease in the serum lactate concentration during the following hours was even more flat than that of metformin. The peak of lactate measured early after metformin ingestion was of course higher than that measured 24 h after administration of higher doses of metformin to their patients with a lower BMI, as reported by De Fronzo 1995 [121].
4. Mechanisms of Action (Lowering Effect of Serum Glucose) of Metformin and the Other Biguanides Therapy
An attempt to explain the mechanism of action of biguanides in lowering glucose serum level was again discussed by Sterne [143]. After eight years of personal research, the following was clear for him:
(1) In the diabetic patient, biguanides do not act by metabolic effect. (2) There is an absence of hypoglycemic effect in non-diabetic patients under biguanides treatment (dimethyl in France n-butyl in Germany and phenethyl in USA [78]), as biguanides do not stimulate insulin production. (3) The development of lactic acidosis so far observed may be due to many other reasons, especially coexisting comorbidities. (4) There was not enough support for the hypothesis of biguanide-induced deviation of glycolysis toward the anaerobic pathway.
Despite many additional publications, however, the mechanism of action of metformin (and of the biguanides) has been a matter of discussion until now [144,145,146,147,148,149,150,151].
It is, however, important to underline the following established certainties:
(1) About 40–60% of the orally administered compounds are excreted with the stool; (2) the absorbed compounds are completely excreted unchanged through the kidney; (3) no damage is observed in tissues like liver, kidney, and muscle after administration of the compounds, and only a negligible amount of the biguanide is detectable in those tissues; (4) an increase in serum lactate can be detected, especially when the compounds are administered orally after a meal and in obese persons but not after intravenous administration; (5) although an increase in lactate level can be measured in the portal blood after oral administration, lactate concentration in arterial blood is higher than that in the hepatic vein, and this can be explained, at least in part, by the use of lactate in the hepatocellular gluconeogenesis but also by the lactate production in peripheral tissues; (6) in most of the cases of hospitalization reported under biguanide therapy, dehydration and hypotension combined with acute kidney failure (or acute on chronic) dominate the clinical picture, with lactate acidosis being the marker of anaerobic glycolysis in the hypoxic tissues and reduced renal clearance, as it may be an increased serum biguanide concentration; and (7) injection of lactate in healthy animals does not have any pathological consequence.
In experiments performed in metformin-treated fa/fa rats, Penicaud et al. [144] demonstrated that there was no glucose utilization in insulin-dependent organs, and it was even decreased in skeletal muscle. The authors found increased glucose utilization in the stomach and small intestine of the metformin-treated rats, as confirmed by Ikeda et al. [152], by Abdul-Ghani [153], and also recently by experiments in mice [154]. The experiments demonstrated that metformin does not exert its hypoglycemic action through an increased insulin secretion, but it rather causes reduced intestinal absorption of glucose and therefore increased glucose excretion in the stool [144,147] and most probably also of serum-glucose reaching the intestine through the bile [154].
Lalau et al. observed a clinical case of lactic acidosis in a 65 year-old patient who presented with a massive elevation of metformin serum concentration but with only a mild increase in serum creatinine in the presence of an acute intestinal occlusion. The clinical picture, however, resolved soon after the abdominal operation [155]. This experience stimulated Lalau and coworkers to reproduce this clinical situation in an animal experiment, where authors could show that obstruction of the terminal ileum may induce a short-term increase in metformin serum concentration without a concomitant increase in serum creatinine and serum lactate. Among other possibilities, the authors proposed hypovolemia as possible cause of metformin serum retention [156]. They called the increase in serum metformin “retention”, intended as temporarily reduced renal elimination.
Elevated metformin serum concentration without an increase in serum creatinine concentration could be the consequence of reduced urine production persisting not long enough (duration of the experiment 4 days), confirming the 5-fold faster metformin renal clearance compared to that of creatinine.
Bailey also underlined glucose utilization in the intestine even in the fasting state, in which glucose is used from the vascular compartment [157].
Besides the inhibitory effect of glucose absorption in the small intestine, a reduction in the glucose production in the liver is still under discussion. This is, however, difficult to understand, as intravenously injected metformin does not have any effect on glycemia [140,141]. Furthermore, there is no established therapeutic or “toxic” concentration of metformin reported in the literature, which could be used for therapy monitoring or diagnostic purposes in diabetics under biguanide therapy, as recently assessed by Lalau et al. [158], and also important given the enormous variability of the serum level, especially in obese persons [131], possibly due to a different compliance and/or hydration status, which can change suddenly through symptoms like vomiting, diarrhea, and fever, leading to emergency clinical conditions. As stated by Lalau and coworkers, caution should be used when attributing a causative role for lactate acidosis to metformin therapy [158].
A further indirect mechanism of the glucose-lowering effect of orally administered metformin has been postulated, namely through an increase in incretin GLP-1-production in the small intestine [153], eventually mediated by bile salts [159]. On the other hand, it was demonstrated ex vivo in the perfused rat ileum that only carbohydrate luminal perfusion increases the release of glucagon-like peptide-1 into the mesenteric vein [160] through the uptake of glucose into the intestinal epithelial cells. In fact, an increase in GLP-1 production could be detected in the portal blood of diabetic and non-diabetic cirrhotic patients after the introduction of a study meal (69 g of carbohydrates) into the small intestine, but no significant difference in the peripheral insulin serum level was found [161] between diabetic and non-diabetic patients. As GLP-1 should reduce glycemia by stimulating insulin production in the pancreas only in the presence of hyperglycemia, an increased insulin serum level under metformin therapy should be the precondition for the indirect hypoglycemic effect. The insulin serum concentration under metformin (as is the case for buformin and for phenformin) therapy, however, does not increase either. Instead, it remains unchanged, or it is decreased, as has been repeatedly demonstrated.
The administration of GLP-1 RA exenatide in type 2 diabetics seems, on the contrary, to function by a mechanism like that of metformin by inducing a significant reduction in body weight in a short-term treatment [162] due to nausea, loss of appetite, and abdominal discomfort.
Recently, a further attempt was made to explain the early loss of body weight after starting oral metformin therapy. It was found that the blood of diabetic overweight patients treated with metformin contained increased amounts of GDF15 [163,164], a hormone considered to act as an appetite suppressor by directly interacting with specific receptors in the brain. On the other hand, however, a decrease in the release of ghrelin (the classical appetite stimulus) into the blood but also an increase in an appetite-suppressing metabolite [165] was reported.
It was hypothesized that production of the hormone could, however, possibly be due to a metformin-induced change in the intestinal microbiota. On the other hand, this hypothesis has been critically reviewed [166,167]. Still, it cannot be excluded that metformin acts as a stressor for intestinal cells and that local GDF15-production together with increased lactate and serotonin [168] production may represent a sign of local hypoxia.
In a retrospective analysis, Kim and coworkers [169] described an accumulation of intravenously injected F18-FDG in the intestinal lumen of patients suffering from non-inflammatory diarrhea or constipation. Gontier and colleagues [170] retrospectively studied the localization of F18-FDG injected in type 2 diabetics and found a significant increase in the radioactively-labeled glucose in the wall but also in the lumen of the small bowel and the colon in patients treated with metformin compared to that of non-metformin-treated diabetics, which was not different from that of the non-diabetic controls [170]. The authors stated that “the digestive tract is the only tissue responsible for a large glucose utilization enhancement”.
Morita and coworkers [171], by using positron emission tomography (PET)-MRI, recently found that the maximum standardized uptake value (SUVmax) of F18-FDG in the intestine (jejunum, ileum, and right or left hemicolon) of metformin-treated diabetics was higher than that of the control group. More importantly, the study permitted to differentiate the SUVmax of the intestinal wall from that of the intestinal lumen. The SUVmax of the intraluminal space in metformin-treated diabetics was greater than that of the controls (Figure 2). On the contrary, the SUVmax of the intestinal wall was similar in both groups [171]. A temporarily increased accumulation of the injected tracer seems to be observed (Figure 3) also in the liver of metformin-treated diabetics up to 48 h after interruption of the oral uptake of the drug [171,172,173], suggesting a persisting uptake of the radioactive glucose mediated by circulating insulin as consequence of the “metabolic starvation”(?) induced by the biguanide.
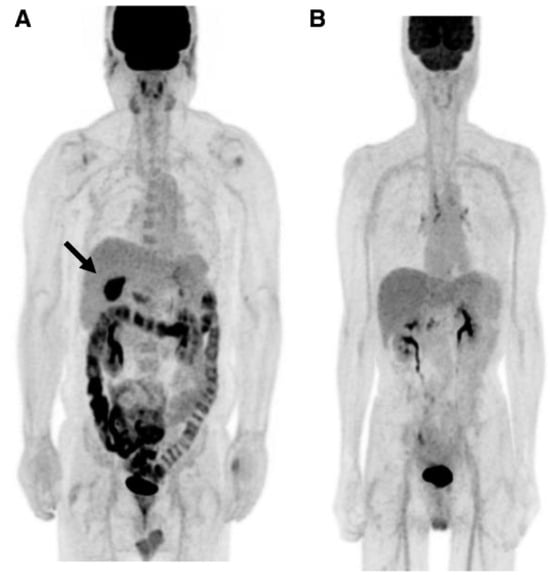
Figure 2.
PET-images taken 60 min after intravenous administration of F18-FDG in a diabetic patient treated with metformin (A) and in a control patient (B). In (A), radioactivity has accumulated in the last portion of the ileum and in the colon (right hemicolon, stronger than left hemicolon). The indication for the study was that gall bladder cancer was confirmed by the accumulation of the tracer in the gallbladder (arrow). From Morita Y et al. [171].
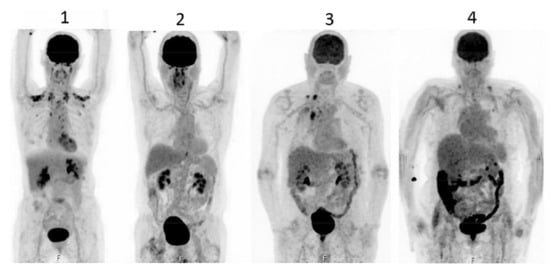
Figure 3.
PET scan was performed in diabetics at different times after interruption of metformin therapy. In patient 4, interruption time was shorter than 48 h and showed strong accumulation of the tracer in the colon. From Schreuder N et al. [173].
In a pivotal animal study, Massollo and coworkers found a significantly increased intestinal uptake of intravenously injected radioactive glucose in mice that were fed metformin for 4 weeks. At the same time, the serum glucose concentration decreased, as did the whole-body radioactivity. The latter finding does not support the assumption of an insulin-mediated glucose uptake in the muscle but could indicate a reduction in peripheral blood volume. Authors also stated that “the data indicates that the metabolic action of metformin cannot be directly attributed to the presence of therapeutic drug concentrations; they reflect rather phenotypic modification in the gut cells that occur after a relatively long time and persist at least 2 days after drug disappearance” ([174], page 263).
Accumulation of F18-FDG in the large intestine (Figure 4) has been found also in persons who regularly use laxatives [175,176]. SUVmax can even reach levels that simulate those of colorectal neoplasms (Figure 5) in patients with chronic constipation [177].
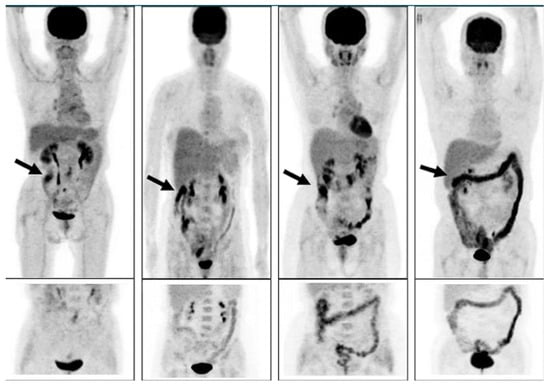
Figure 4.
PET pictures of early scans (upper row) and of late FDG scans (lower row) after oral administration of laxatives. The arrows in the upper row of PET scans show the different patterns of accumulation of the tracers in the large intestine. From Chen Y-K et al. ([176], with permission).
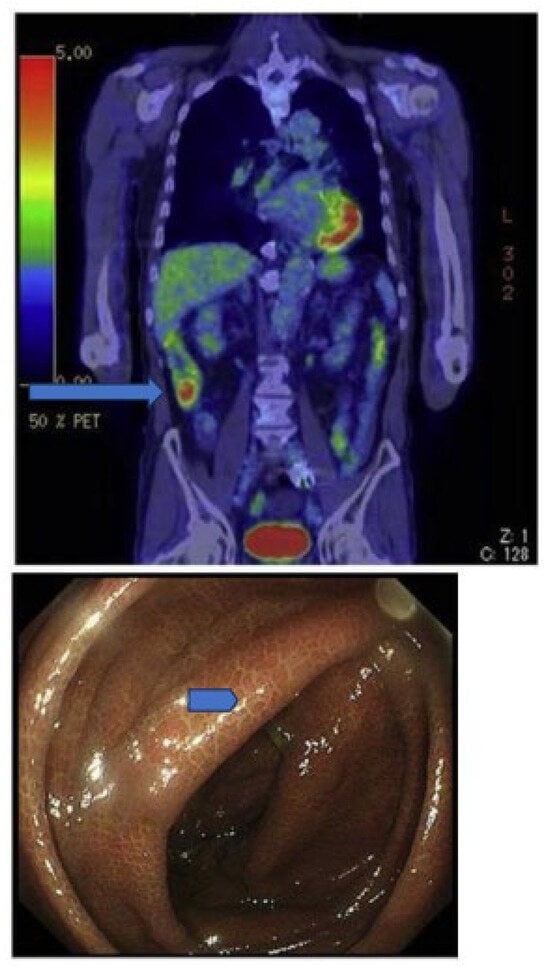
Figure 5.
Accumulation of radioactivity after intravenous administration in the coecum and ascending colon (upper panel, long arrow) of a patient using anthraquinone laxatives, as demonstrated by the presence of melanosis coli at colonoscopy performed to exclude colon cancer (lower panel, short arrow). From Katsumata R [177].
It seems justified to conclude that biguanides such as phenformin [178] and metformin [179] not only induce glucose “malabsorption” after oral administration, but they may also “attract” glucose from the systemic circulation into the lumen of the small bowel, which is then transported and further concentrated and excreted from the large intestine as stool, together with the remaining compound [180].
Under the influence of intestinal metformin, the reduced glucose uptake from the intestine into the portal blood could also be compensated by an increase in an insulin-mediated uptake [181,182] of glucose from the systemic circulation into the liver and from there excreted through the bile [144,183] into the small bowel and then excreted with the stool. Through the effect of metformin, radioactive glucose then reaches the large intestine, there achieving the concentration necessary to be captured by the dosimetry scan (whole-body PET scan).
The reduction in intestinal absorption of glucose [170], of other nutrients [184], and of some life-saving drugs [185] by metformin resembles that of “irritant laxatives” [186,187] that produce peristalsis and not only reduce absorption of glucose and other food components [188,189] and increase the serum level of homocysteine [190] but also induce a reduction in protein digestion [191] and also, even more importantly, cause the loss of water [192]. The latter, together with increased loss of sodium and water with the biguanide through the kidney may explain the dehydration and the increase in the creatinine serum level described in metformin-treated overweight type 2 diabetics. It may even worsen the latent splanchnic and peripheral tissue hypoperfusion, leading to an increase in the production and decrease in the elimination of lactate, to hyperferritinemia, to an increase in hepcidin serum level, and to hyposideremic anemia as an acute-phase response to tissue damage [193,194].
5. Short- and Long-Term Side Effects of Metformin and Other Biguanides
5.1. Short-Term Side Effects of Therapy with Biguanides
As described by Frank and colleagues [82], after the first administration, the biguanide synthalin causes nausea, loss of appetite, increased intestinal movements, gastric pain, vomiting, and also diarrhea”. For this reason, they developed a therapeutic procedure consisting of increasing the dose for the first 3–4 days, followed by an interruption of drug administration for 1–2 days. It is therefore understandable that many diabetics, especially the older ones, cannot tolerate the medication even when starting with the lower dose of 500 mg, as those side effects may be accompanied by dizziness, tiredness, abdominal cramps, asthenia, myalgia, and an altered metallic taste [193,194], as confirmed to be more frequent in patients treated with metformin than in the placebo-treated control diabetics.
Although the side effects are like those caused by cytotoxic anticancer drugs [195], in the case of the biguanides, they have been attributed in part to the intestinal disturbance of the bile salt metabolism [196,197,198,199]. The increase in bile salt elimination in the stool has been observed also in patients with chronic constipation treated with elobixibat, a laxative approved in Japan for treatment of chronic constipation [200], and is probably similar to what has been discussed recently for other antihyperglycemic laxatives [201,202].
Biguanides can cause, besides the above-mentioned side effects, flatulence, abdominal bloating, heartburn, headache, agitation, and chills. It is therefore not surprising that aggravation of dehydration may be the consequence, especially in elderly, overweight patients. Reduced blood volume could even be the mechanism behind the observed effect of metformin on blood pressure when administered to hypertensive non-obese men [203,204], as the strong renal clearance of the compound and sodium with water [205] resembles the action of diuretics [206,207].
As increase in lactate serum level under orally administered biguanides is a continuum in the reporting from emergency and ICU-hospitalized overweight/obese diabetics [90,111,155,156,158,208], and as biguanides are also used alone or in combination with other drugs for the purpose of weight loss in non-diabetics, it is worth mentioning here the study published in 1978 by Czyzyk and coworkers [209], who studied the effect of the three main biguanides, namely buformin, phenformin, and metformin, on blood lactate levels in 56 healthy subjects (30 men and 26 women). Of special interest is the effect of alcohol and fructose, a very popular sweetener, and of exercise during administration of the biguanides. Authors found only minimal differences in the effect of the individual biguanides. The highest increase in blood lactate was observed after intravenous fructose loading, leading to an increase in glucose serum level. The lactate increase under biguanide was also further increased after oral ethanol, with metformin showing the highest lactate increase 4 to 6 h after ethanol administration compared to phenformin and buformin. The effect of exercise (15 min duration) was studied twice in 18 volunteers (7 men and 11 women; mean age 29 years). Before the second experiments, biguanides were administered orally. While biguanides did not cause a significant reduction in glucose serum level, all the drugs caused a significant rise in blood lactate and a significant fall in bicarbonate levels but no change in blood pH. This has to be taken into consideration when talking about metformin-associated lactate acidosis (MALA, [158]).
In 1978, Luft and coworkers published [210] a review on cases of lactate acidosis after treatment with biguanides. (Most of the patients reported were treated with buformin or phenformin but also with metformin.)
More than 50% of the patients died soon after hospitalization, showing advanced renal insufficiency and higher lactate acidosis [208]. The most commonly reported clinical signs were dehydration and pallor. Patients were frequently treated with vasopressors and volume expanders, but the cornerstone of the treatments reported was alkalinization with bicarbonate administration, hemodialysis, insulin, and glucose.
The suggestions based on the clinical examination of the published data failed to establish the proper sequence of events after treatment with biguanides, considering that the hypoglycemic effect of the drugs is due to their intestinal accumulation, local fluid loss, and elimination of water, with the nutrients and the absorbed part of the compound cleared through the urine, leading to prerenal insufficiency.
The eventual accumulation of different amounts of the biguanides in the blood and the increase in serum lactate concentration can both be seen as signs of reduced kidney function due to dehydration.
Both symptoms could be improved by mere fluid administration.
It can therefore be concluded that measurement of the metformin serum level dispensable for the determination of the therapeutic procedure and risk of death [208].
Development of increased serum lactate under biguanide treatment has been known since the first administration of the compounds as oral therapeutics in elderly obese type 2 diabetics. It was well known at the beginning of the UKPDS trial, as it was stated that “no clinically evident case of lactate acidosis was reported to the authors” from the patients treated with metformin in that first long-term report [120]. No statistically significant increase in lactate serum level was reported in the short-term study of De Fronzo and colleagues [121], where determination of fasting lactate was part of the monitoring during the 29 weeks of the trial. It has, however, pointed out that only overweight, non-obese (mean BMI 29), and relatively young (not yet elderly; mean age 53 years), healthy type 2 diabetics were originally admitted to the trials (Table 1).
After the publication of the American study testifying to the safety of metformin use [121] and the approval of the compound for oral therapy in elderly overweight/obese type 2 diabetics, the first 10 cases of lactate acidosis with three cases of death were reported to the FDA [122], and the presence of comorbidities, which were an exclusion criterion for participation to the trials (see Table 1), was found as a possible contributory factor for the development of acidosis (congestive heart failure, hypoxia, chronic lung disease, sepsis, and impaired renal function).
Seven further deaths under metformin therapy were reported six months later by Innerfield [211]. The report was sharply criticized by the authors of the American study, and a further study was announced by the FDA [212]. Additional cases reported to the FDA in the period from May 1995 to June 1996 were described by the FDA in 1998 [123]. Twenty of the forty-seven hospitalized patients died; the mean age of these patients was 77 years (compared to 53 years of the patients in the study of de Fronzo), and sixteen had a serum creatinine above 1.5 mg/dL, while eight were also treated with furosemide, the most effective, aggressive diuretic drug. The body mass index was not reported. In that report, it was stated that the upper limit of lactate was considered to be “normal” (2.7 mmol/L) in those patients treated with metformin in the first short-term study [121], while seven had a lactate serum level between 2.7 and 4.9 mmol/L, therefore not meeting the criteria for lactate acidosis previously published by one of the reporting authors. Blood pressure values and hydration status were not mentioned.
In that report, it was suggested to restrict metformin therapy to healthy diabetics who are under 80 years old, although, again, the age of the patients included in the trial [121] was 53 years.
In spite of the fact that lactate acidosis cases were reported to the FDA immediately after the release of metformin [211,212], suggesting that the compound was similar to the other biguanides [101,213], such a life-threatening complication is considered to be significantly more seldom. It has been described especially in diabetics with reduced kidney function [214,215,216,217] until recently [218] and in emergency conditions such as severe COVID-19-infection [157,219,220,221,222,223] in hospitalized diabetics.
The above-mentioned intestinal effects and adverse events can not only be the cause of the temporary dose reduction or withdrawal of metformin intake but also the long-term complete lack of adherence to the therapy [224,225,226,227].
On the contrary, the loss of body weight observed especially at the beginning (but not at later time points) of metformin therapy [228,229] has been seen (not always, [121]) as an achievement, as it is known that overweight, obese, elderly diabetics do not comply with diet restrictions.
Loss of body weight, however, can be seen as a possibly dangerous acute consequence of the gastrointestinal side effects of the compound. In fact, there could not only be a negative effect of the metformin on food- but also on fluid intake and increased diuresis with consequent dehydration, tissue hypoperfusion, hypoxia-induced tissue damage, and consequent increased lactate production, especially in type 2 diabetics performing exercise [230].
The consequent kidney “injury” could account for lactate accumulation and lead to acute lactic acidosis (just as marker of dehydration), the symptoms of which may be similar to the side effects of metformin, with the main distinctive characteristic of dehydration, namely hypotension, tissue hypoxia with increase in anaerobic glycolysis, diffuse peripheral inflammation, peripheral tissue injury fibrinolysis, and provisional clot formation [231].
It has to be kept in mind that oral metformin administration alone can also induce a decrease in plasma insulin, serum cholesterol, and triglycerides and a significant decrease in blood pressure, as was found when metformin (850 mg twice a day) was given for 6 weeks to nine non-obese, non-diabetic, non-smoking men suffering from mild, non-treated hypertension [205]. A significant marked decrease in both systolic and diastolic blood pressure was observed within the first three weeks of therapy. The blood pressure increased again within two months after interruption of the metformin administration and mildly decreased again during the next three years of follow-up under dietary restrictions.
This phenomenon must be taken into consideration when interpreting the complication observed in five patients with type 2 diabetes and hypertension treated with metformin and an angiotensin receptor blocker. The patients were admitted to the emergency room because of severe metabolic acidosis (pH 6.60–6.94); high lactate 14–23 mmol/L) and elevated serum creatinine as signs of acute kidney failure in patients previously having normal kidney function. The clinical conditions were precipitated by severe dehydration with circulatory and respiratory collapse. Also, in these cases, however, the importance of replenishment of the blood volume (sufficient fluid and eventually albumin infusions) was not as emphasized [96] as it should have been.
5.2. Long-Term Side Effects of Metformin Therapy
In their 10-year follow-up report [232] after the end of the UKPDS [233,234], Holman and coworkers described that the metformin-group patients were followed-up for a period of 17.7 years, with 8.8 years of post-trial follow-up. Overall mortality was 44% (51.5% cardiovascular and 24.2% cancer as the cause of death). Baseline differences in surrogate marker glycated hemoglobin disappeared after the first year of intensive therapy. Although there was no difference in blood pressure levels, and plasma creatinine was not significantly different within the two groups, the plasma creatinine levels in the metformin group were 15% higher than those in the conventional therapy group. As about 10% of the patients were treated with diuretics because of hypertension, both metformin and diuretics can eventually increase dehydration, which also naturally develops with increasing age in elderly persons.
Metformin treatment in type 2 diabetics is associated with early development of anemia, which is most probably due, although not solely, to the inhibition of vitamin B12 absorption [235,236,237,238]. Vitamin B12 absorption inhibition could also explain the development of peripheral neuropathy in elderly diabetics treated with metformin [239].
Anemia and iron deficiency under long-term metformin treatment (>5 years) was described by Ahmed and colleagues [240].
6. Adherence to the Prescribed Dose of Metformin and Other Biguanides
After several reports claiming the positive effects of orally administered drugs including metformin alone or in combination with other oral substances and also with insulin were published, stating “new medications for attempting to nearly normalize blood glucose levels could not have arrived at a better time” [241], there was great enthusiasm about metformin, the first drug that did not induce an increase in body weight and did not induce increase in insulin serum level but did build up continuously and was poorly absorbed (>30% eliminated with the stool) and quickly eliminated, unchanged, via urine; the enthusiasm was only speculative. In addition, dose-dependent gastrointestinal side effects caused the drug to be withdrawn in up to 50% of the study patients [122,242].
The results of the large UKPDS trial, which started 1977 in England, contributed to the enormous “popularity” of the substance [228,229]. The UKPDS reported positive results in the reduction in microvascular consequences of type 2 diabetes in the subgroup of overweight diabetics treated with metformin [229,232]. They represented, despite the several critical comments [233,234], especially about the statistical significance of the obtained results, the definitive establishment of long-term oral therapy alone and/or in combination with insulin and other oral antidiabetic drugs. In one of the first reports of the UKPDS trial, the first data about adherence to protocol after 3-year follow-up were given [243] for the 2741 (of the first 3000) who were still attending the clinics (91%). While 83% of those patients allocated to insulin and 90% of those allocated to sulphonylurea were on the allocated therapy, 30% of those allocated to diet policy had to be reallocated as having secondary diet failure. The adherence to protocol of the metformin (sub)group was not mentioned. Adherence to protocol of this group of patients was not mentioned in the 10-year follow-up report [232]. In this report, however, it was found that the change in body weight was similar in the metformin and in the conventional control (diet only) groups. Of the metformin group, 82% of person-years (3035 of 3682) were still treated with metformin alone (mean dose 2550 mg/day) or in combination. The number of deaths among those patients allocated to the combination therapy metformin and sulphonylurea (median follow-up 6.6 years) with an adherence of 62% person-years was increased with a diabetes-related mortality risk increase of 96% (p = 0.039). It was stated that after 3 years of monotherapy, more than 50 of the patients needed an additional drug, and after 9 years, most of the patients under combination therapy needed insulin administration [243,244].
In the short-term prospective trial (29 weeks) of DeFronzo and coworkers [121], 78% of the patients allocated to metformin were taking 2550 mg/day of the drug after the 5-week titration phase. Before end of the 29 weeks of the study duration, 22% of the patients in the metformin group withdrew from the study as well as 28% in the placebo group (12% because of treatment failure vs. 1% in the metformin group, protocol 1). In protocol 2, 25% of the patients in the metformin group withdrew before the end of the 29 weeks, mainly because of gastrointestinal side effects.
This was the first randomized, prospective trial showing that metformin can induce a change in the lactate serum level even if the level remained within a “normal” range. Interestingly the study showed no significant change in body weight in the metformin group compared with the placebo group.
This means that adherence to the first and the only curative therapeutic intervention for type 2 diabetes of the “elderly” (perhaps young old) with overweight/obesity, namely diet (restriction of the caloric intake), was negligible.
Adherence to dietetic prescription and to anti-diabetic, antihypertensive drug uptake was judged by means of the information given by the patients and contained in the questionnaire filled out by the patients at the yearly visit when fasting glucose-, creatinine-, and glycated hemoglobin level together with blood pressure were measured. In year 6 to 10 of follow-up after the UKPDS trial was closed in 1997, visits were continued every 3 years. Patients unable to attend the visits were sent the questionnaires, as was done for their general doctors at the end of the study in September 2007 [229]. Of the 2729 patients assigned to receive intensive therapy, 489 (17.5%) had died at the end of the trial in 1997, and of the 2118 available for the post-trial follow-up, 674 (31.8%) died within 10 years, and 1010 (37%) completed the 10 year-monitoring. Of the 1118 patients allocated to the conventional therapy (diet only), 213 (19%) at the end of the trial in 1997 and a further 324 (36.8%) of the 880 available died during the post-trial follow-up study, while 379 completed the post-trial monitoring (33.3%). While 48% of the patients allocated to the conventional therapy had died at the end of the post-trial monitoring, the data available from the group of those patients allocated to the diet and intensive therapy allow the conclusion that 49.3% died within the 30 years of the two trials [230]. Of the 342 patients allocated to the intensive therapy with metformin, 50 died during the UKPDS trial, and 279 were available for the 10 year-follow-up trial. Of those, 102 died (29.8%), and 136 (39.8%) completed the post-trial monitoring, while 44.4% of the originally allocated patients had died.
While the mean age did not differ between the two groups (conventional vs. intensive therapy), body weight, fasting glucose value, and glycated hemoglobin value were similar; more patients allocated to the intensive therapy were receiving a combination of oral and insulin therapy (64%) than those who were originally allocated to the conventional therapy (46%). In addition, the creatinine serum level in those allocated to intensive therapy with metformin was 15% higher than that in the conventional therapy group. Unfortunately, failure to adhere to the main curative intervention in overweight patients, namely dietetic restriction, was not discussed, as the attention was concentrated on drug administration.
The question of adherence to therapy in type 2 overweight/obese, elderly patients has concentrated more on the drug prescription and less on the curative dietetic therapy.
The aggressive approach for improvement of adherence has been justified by a calculation of a possible reduction in expenses for additional drug costs and for management of comorbidities attributed mainly to the glycemic control, considered a chronic disease in itself [245,246,247].
Although the introduction of extended-release (ER) metformin may have improved tolerability and adherence to the compound uptake, its superiority in glycemic control is not demonstrated [247].
The issue still is, however, the costs of the long-term consequences of administration of a compound that can be considered to act as a laxative (and a diuretic) by withdrawing water from the intestine and from the systemic circulation in elderly, obese, dehydrated patients with an increased basal lactate serum level (although still within the normal range).
Therefore, the question is justified as to whether it is rational to try to increase adherence to metformin therapy in the attempt to eliminate the “barriers”, especially when the cause of non-adherence is the large number of side effects, especially when prescribed without paying attention to the numerous contraindications [245].
7. Contraindications
Every clinical decision nowadays is based on justification from the literature [248], called evidence-based decision. This attempt to reduce healthcare costs was thought to be objective and led to the development of guidelines that could be followed worldwide. The basis for the development of evidence has been (multiple) multicenter (possibly international), prospective, randomized, double-blind trials, mostly financed by the pharmaceutical industry, whose results have been published in international journals. The results should have been acceptable for national and international release authorities, such as the American Food and Drug Administration (FDA), which regularly discusses the structure of the trials in advance with the pharmaceutical company planning and financing the trial.
In the last decades, it has become clear that clinical trial conditions are often different from those of the real-world application of the results obtained under study conditions [249], as immediately happened after the release of metformin in 1995/1996 [211,212], when only healthy, overweight, relatively young diabetics were included in the two prospective trials, but the compound was then administered to older patients with comorbidities. Those results should be a guide for the clinician who is asked to judge the advantages compared to the possible disadvantages of the drug before prescribing it to a patient.
To achieve this goal, the clinician, however, must have knowledge about the literature and about the criteria that led to the choice to recruit the specific study patients at the beginning of the randomization process. It is, on the other hand, clear that it is almost impossible to have available results of at least one prospective trial for each clinical situation in each single patient.
In fact, often, the results from retrospective analyses (especially those published after the release of a drug) are even used for guideline purposes. Such studies are used to perform and publish meta-analyses in the attempt to answer so-far unaddressed questions, often extrapolating the answer to the “special question” from subgroup analysis with doubtful statistical significance.
Despite the long history of the use of synthetic biguanides as oral antidiabetic drugs in overweight/obese diabetics and of the common side effects, the basis for the interpretation of these side effects and for what is considered the real danger arising from administration, namely lactate acidosis (as sign of anaerobic glycolysis and hypoxia caused by dehydration), is still considered to be unclear [153].
Although metformin is the most prescribed oral drug for type 2 diabetes in obese patients worldwide, the other two biguanides, namely buformin and phenformin, are still available in many countries outside the USA, such as Europe, South America, and Asia [250]; the controversy about the fundamental question and the real reasons for avoiding metformin (and the other biguanides) therapy is still ongoing.
It is still not yet clear how orally administered metformin (and other biguanides) cause the main side effects, namely abdominal discomfort, nausea, vomiting, loss of appetite, and bad taste (paralleled by intestinal lactate production).
L-lactate is a product of anaerobic glycolysis taking place mainly in the muscle even under normal conditions [251], and its clearance takes place mainly in the liver, where it is converted into glucose (under fasting conditions), with a potential increase in the capacity of the normal liver up to sixfold [158,251]. Lactate, however, also increases after a meal [252], and the increase seems to be due to increased release from the liver [253].
Serum lactate concentration is increased after exercise, especially after a meal, in overweight type 2 diabetics [230].
A recent report underlined the importance of hydration status when judging the effect of exercise on the lactate serum concentration even in young, non-obese people [254,255].
The clearance capacity of the liver can be temporarily impaired only by a strong reduction in the blood flow into the liver (over 75%) by an acute-phase situation induced by endotoxemia or massive liver failure. On the other hand, about 30% of lactate is cleared by the kidney, and every acute condition reducing this capacity will induce an increase in serum concentration of lactate, which, however, is not the cause of the clinical picture characterized by the massive reduction in the blood volume and resulting in hypotonia and diffuse peripheral tissue hypoxia.
Chronically reduced kidney function will contribute to an increase in lactate blood concentration only in the contemporary presence of a reduced clearance capacity of the liver. Normal liver function could lead to an underestimation of the danger of incipient kidney failure.
Elderly (above 60–65 years of age), overweight/obese type 2 diabetics represent the largest group of type 2 diabetics [254]. They suffer from potential dehydration [255] and subclinical hypoxia.
Doar and coworkers [256], after having shown that the fasting blood lactate level is higher in obese than in non-obese subjects independently of their glucose tolerance test-results, demonstrated that the level of lactate remains elevated in the diabetics (maturity onset with a mean age of 50 years) after glucose administration up to the end of the test. Removal of infused lactate was impaired in obese type 2 diabetics but not significantly impaired in obese non-diabetics (mean age 25.4 years), although the fasting lactate levels were similar. Increasing dehydration has been found with increasing age, weight, and metabolic syndrome conditions, which may serve as explanation for fasting and postprandial increased lactate levels [256]. It is therefore understandable that conditions leading to a worsening of peripheral oxygen delivery due to further reduction in blood volume may precipitate the clinical picture of hypovolemia and shock outside of septicemia and sepsis, such as therapy with diuretics like furosemide and/or with the laxatives frequently used in depressed, constipated elderly diabetics [257,258,259]. These conditions may all have in common the last consequence of fluid withdrawal from the systemic circulation through the skin (e.g., heat) [260,261], kidney (diuretics), or intestine (chronic diarrhea induced by laxatives and other drugs, e.g., metformin) [262,263,264] or even be responsible for clinical pictures like mesenteric ischemia [265,266,267,268].
Metabolic acidosis induced by increased production of lactate in the hypoxemic peripheral tissues (subclinical dehydration of the obese elderly diabetic type 2) and/or reduced clearance mainly by decreased kidney function (mainly prerenal due to dehydration) may become a marker of acute clinical worsening due to sudden fluid loss in case of an acute illness like viral infections of the respiratory tract, as may be the case for influenza or coronavirus infections [269], or gastrointestinal viruses like noroviruses. Hypovolemic shock may be the common clinical emergency complicated by so-called multiorgan failure, such as acute kidney failure (AKI) and pulmonary insufficiency (ARDS) [269].
It is important to clearly identify the cause of the emergency in order to start with the proper therapeutic approach intended first to gradually reestablish the normal hydration and blood volume by administration of fluids and albumin. It is not necessary to administer bicarbonate in the assumption that acidosis is the cause of the clinical emergency condition [270]. Under these preconditions, the use of substances like biguanides represents an unjustified danger for diabetics when considering the long list of contraindications beginning with an age and body mass index higher than that of the two main prospective studies [120,122], which should be added to the long list published by Sulkin et al. (1997) [271] alongside dehydration, obstipation, depression, and polypharmacy.
8. Conclusions
Metformin, the latest and most often prescribed biguanide to overweight/obese, mostly elderly type 2 diabetics, does not seem to differ from the previous biguanides used as oral antidiabetics. The mechanisms of the serum glucose-lowering effect and the acute and long-term consequences are like those of conventional laxatives apart from abdominal symptoms and the taste and appetite disturbance frequently caused by biguanides. Dehydration, prerenal kidney injury, and worsening of lactate acidosis (as marker of hypoxia) are the most serious and, for older, multimorbid patients, life-threatening consequences of protracted metformin therapy (Figure 6). Rehydration is the first mandatory therapeutic intervention in patients developing symptoms like adynamia, hypotension, weakness, and oliguria.
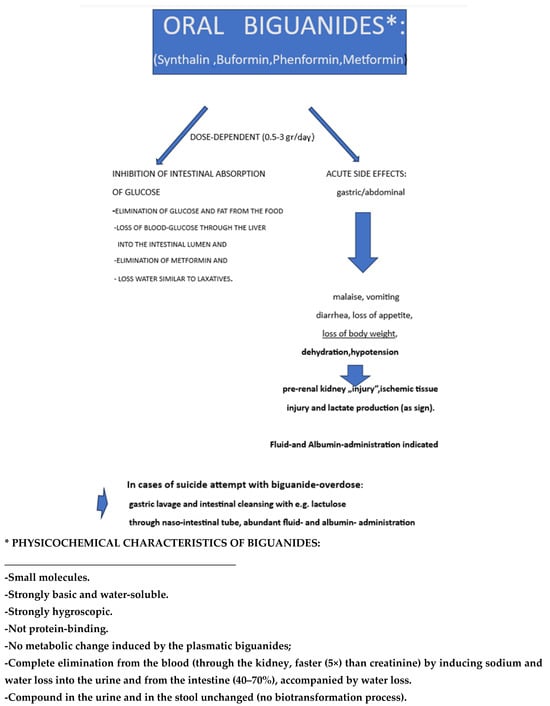
Figure 6.
Graphical Abstract of the content of the review.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Estimated Global Population from 10,000BCE to 2100 (in Millions) Statista 2024. Available online: https://www.statista.com/statistics/1006502/global-population-ten-thousand-bc-to-2050/ (accessed on 23 July 2025).
- The GBD 2015 Obesity Collaborators. Health effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 372, 13–27. [Google Scholar]
- New WHO Report: Europe Can Reverse Its Obesity “Epidemic”. Available online: https://www.who.int/europe/news/item/03-05-2022-new-who-report--europe-can-reverse-its-obesity--epidemic#:~:text=The%20new%20WHO%20report%20outlines,achieving%20environmentally%20sustainable%20food%20systems (accessed on 23 July 2025).
- WHO Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 May 2025).
- Kerac, M.; McGrath, M.; Connell, N.; Kompala, C.H.; Moore, W.H.; Bailey, J.; Bansma, R.; Berkley, J.A.; Briend, A.; Collins, S.; et al. “Severe Malnutrition”: Thinking deeply, communicating simply. BMJ Glob. Health 2020, 5, e003023. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Ohmori, K.; Miura, C.; Suzuki, Y.; Nakaya, N.; Fujita, K.; Sato, Y.; Tsubono, Y.; Tsuji, I.; Fukao, A.; et al. Body Mass Index and Mortality in Japan: The Miyagi Cohort Study. J. Epidemiol. 2004, 14, S33–S38. [Google Scholar] [CrossRef]
- Nippon.com. Japan Sets New Record Low for Food Self-Sufficiency on a Production Value Basis. Available online: https://www.nippon.com/en/japan-data/h01758/ (accessed on 23 July 2025).
- Klonowska-Siwak, E. The Japanese Food Market and the Japanese Business Culture. Available online: https://www.eu-japan.eu/sites/default/files/imce/workshops/EU-Japan%20Centre_EKS_30%20June%202022.pdf (accessed on 23 July 2025).
- WHO Hunger Numbers Stubbornly High for Three Consecutive Years as Global Crises Deepening Report. 1 in 11 People World Wide Faced Hunger in 2023, 1 in 5 in Africa. Available online: https://www.who.int/news/item/24-07-2024-hunger-numbers-stubbornly-high-for-three-consecutive-years-as-global-crises-deepen--un-report (accessed on 23 July 2025).
- Rosenfeld, L. Justus Liebig and Animal Chemistry. Clin. Chem. 2003, 49, 1696–1707. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Half of the World’s Habitable Land is Used for Agriculture. Our World Data 2019. 16 February 2024. Available online: https://ourworldindata.org/global-land-for-agriculture (accessed on 22 July 2025).
- World Food And Agriculture-Statistical Yearbook 2023. FAO: Rome, Italy, 2023. [CrossRef]
- Cordain, L.; Brand, J.; Eaton, S.B.; Man, N.; Holt, S.M.A.; Steta, J.D. Plant-Animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gather diets. Am. J. Clin. Nutr. 2000, 71, 682–692. [Google Scholar] [CrossRef]
- Stafford, N. History: The Changing notion of food. Nature 2010, 468, 16–17. [Google Scholar] [CrossRef]
- Wright, I. Livestock, Engine for Economic Growth and Sustainability CFSHLPE. Available online: https://www.fao.org/cfs/cfs-hlpe/insights/news-insights/news-detail/livestock-engine-for-economic-growth-and-sustainability/en (accessed on 23 July 2025).
- The World Bank. Moving Towards Sustainability: The Livestock Sector and the World Bank. Available online: https://www.worldbank.org/en/topic/agriculture/brief/moving-towards-sustainability-the-livestock-sector-and-the-world-bank (accessed on 23 July 2025).
- Doll, R.; Hill, A.B. Lung cancer and other causes of death in relation to smoking. A second report on mortality of british doctors. BMJ 1956, 2, 1070–1081. [Google Scholar] [CrossRef]
- Hopkinson, N.S. Smoking and Lung Cancer-70 long years on. BMJ 2024, 384, q443. [Google Scholar] [CrossRef]
- Dawber, T.R.; Moore, E.; Mann, G.V. II. coronary heart disease in the Framingham study. Am. J. Public Health 1957, 47, 4–24. [Google Scholar] [CrossRef]
- Hammond, C.; Horn, D. Smoking and death rates-report on forty-four months of follow-up of 187,783 men. JAMA 1958, 166, 1294–1308. [Google Scholar] [CrossRef]
- Sheps, M.C. Shall we Count the Living or the Dead? N. Engl. J. Med. 1958, 259, 1210–1214. [Google Scholar] [CrossRef]
- Doyle, J.T.; Dawber, T.R.; Kannel, W.B.; Heslin, S.A.; Kahn, H.A. Cigarette smoking and coronary heart disease. Combined experience of the Albany and Framingham Studies. N. Engl. J. Med. 1962, 266, 796–801. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R. Mortality in relation to smoking20 years observations on male british doctors. BMJ 1976, 2, 1525–1536. [Google Scholar] [CrossRef]
- Doll, R.D.; Peto, R.; Hall, E.; Wheatley, K.; Gray, R. Mortality in relation to consumption of alcohol:13 years observation of male british doctors. BMJ 1994, 309, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Freund, K.; Belanger, A.J.; DÀgostino, R.B.; Kanner, W.B. The Health Risk of Smoking the framingham Study: 34 years of follow-up. Ann. Epidemiol. 1992, 3, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Magowska, A.M. Historical Perspectives. The changing face of hunger: From fasting to the concept of atherogenesis. Adv. Physiol. Edu. 2020, 44, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Anderson, J.T.; Grande, F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957, 2, 959–966. [Google Scholar] [CrossRef]
- Roine, P.; Pekkarinen, M.; Karvonen, M.J.; Kihlberg, J. Diet and cardiovascular disease in Finland. Lancet 1958, 2, 173–175. [Google Scholar] [CrossRef]
- Huijbregets, P.; Feskens, E.; Raesanen, L.; Fidanza, F.; Nissinen, A.; Menotti, A.; Kromhout, D. Dietary pattern and 20 year Mortality in elderly men in Finland, Italy, and the Nederlands: Longitudinal cohort study. BMJ 1997, 315, 13–17. [Google Scholar] [CrossRef]
- Karvonen, M.; Orma, E.; Keys, A.; Fidanza, F.; Brozek, J. Cigarette Smoking, Serum-Cholesterol, Blood-Pressure and Body Fatness. Observations In Finland. Lancet 1959, 492–494. [Google Scholar] [CrossRef]
- Keys, A.; Karvonen, M.J.; Fidanza, F. Serum-Cholesterol Studies in Finland. Lancet 1958, 2, 175–178. [Google Scholar] [CrossRef]
- Menotti, A.; Puddu, P.E.; Kafatos, A.G.; Tolonen, H.; Adachi, H.; Jacobs, D.R., Jr. Cardiovascular Mortality in 10 Cohorts of Middle-Aged Men Followed up 60 Years until Extinction: The seven Countries Study. J. Cardiovasc. Dev. Dis. 2023, 10, 201. [Google Scholar] [CrossRef]
- Puddu, P.E.; Piras, P.; Kafatos, A.; Adachi, H.; Tolonen, H.; Menotti, A. Competing Risks of Coronary Heart Disease Mortality versus Other Causes of Death in 10 Cohorts of Middle-Aged Men of the Seven Countries Study Followed for 60 Years to Extinction. J. Cardiovasc. Dev. Dis. 2023, 10, 482. [Google Scholar] [CrossRef] [PubMed]
- Bucheley, R.W.; Drake, R.M.; Breslow, L. Relationship of Amount of Cigarette Smoking to Coronary Heart Disease Mortality Rates in Men. Circulation 1958, 18, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, T.K.M.; Puska, P.; Berg, M.A.K.; Pokusajeva, S.; Moisejeva, N.; Uhanov, M.; Artemjev, A. Health- related Behaviours in the Republic of Karelia, Russia, and North Karelia, Finland. Int. J. Behav. Med. 1994, 1, 285–304. [Google Scholar] [CrossRef] [PubMed]
- YLE. Health Report: Abuse a “Big Problem” in Finland, Main Cause of Preventable Deaths. 30 December 2022. Available online: https://yle.fi (accessed on 23 July 2025).
- Manthey, J.; Probst, C.; Kilian, C.; Moskalewicz, J.; Sierosławski, J.; Karlsson, T.; Rehm, J. Unrecorded Alcohl Consumption in Seven European Union Countries. Eur. Addict. Res. 2020, 26, 316–325. [Google Scholar] [CrossRef]
- EUROSTAT. Alcohol Consumption Statistics. Statistics Explained. July 2021. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Alcohol_consumption_statistics (accessed on 23 July 2025).
- Garcia, M.J.; McNamara, P.M.; Gordon, T.; Kannell, W.B. Morbidity and mortality in diabetics in the Framingham population. Sixteen-year follow-up study. Diabetes 1974, 23, 105–111. [Google Scholar] [CrossRef]
- Mayes, C.R.; Thompson, D.B. What Should We Eat? Biopolitics, Ethics, and Nutritional Scientism. J. Bioethical Inq. 2015, 12, 587–599. [Google Scholar] [CrossRef]
- Mayes, C. Healthy Eating Policy: Racial Liberalism, Global Connections and Contested Science. Food Ethics 2023, 8, 1. [Google Scholar] [CrossRef]
- Shintani, H.; Shintani, T. Effects of antidiabetic drugs that cause glucose excretion directly from the body on mortality. Med. Drug Discov. 2020, 8, 100062. [Google Scholar] [CrossRef]
- Antoniotti, E. Comè´Cambiata La Salute Degli Italiani 1861–2011: Centocinquant‘Anni.Quotidianosanita’.it. 16 March 2011. Available online: https://www.quotidianosanita.it/cronache/articolo.php?articolo_id=3269 (accessed on 23 July 2025).
- Liu, B.; Hu, Y.; Rai, S.K.; Wang, M.; Hu, F.B.; Sun, Q. Low-Carbohydrate Diet Macronutrient Quality and Weight Change. JAMA Netw. Open 2023, 6, e2349552. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.C.; Sedgmond, J.; Mizey, L.; Chambers, C.D.; Lawrence, N. Food addiction: Implications for the diagnosisi and treatment of overeating. Nutrients 2019, 11, 2086. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Pedley, A.; Hoffman, U.; Massaro, J.M.; Levy, D.; Long, M.T. Visceral and Intrahepatic fat are associated with cardiometabolic risk factors above other ectopic fat depots: The Framingham Heart Study. Am. J. Med. 2018, 131, 684–692. [Google Scholar] [CrossRef]
- Manka, P. Liver, Heart, Death Can This Sequence Be Broken? Dig. Dis. Sci. 2023, 68, 3490–3491. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Bacopoulou, F.; Markousis-Mavrogenis, G.; Chrousos, G.; Charmandari, E. Cardiovascular Imaging in Obesity. Nutrients 2021, 13, 744. [Google Scholar] [CrossRef]
- Lemmens, H.J.; Bernstein, D.P.; Brodsky, J.B. Estimating Blood Volume in Obese and Morbidity Obese Patients. Obes. Surg. 2006, 16, 773–776. [Google Scholar] [CrossRef]
- Csige, I.; Ujvarosy, D.; Szabo, Z.; Lörincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The impact of Obesity on the cardiovascular System. J. Diabetes Res. 2018, 2018, 3407306. [Google Scholar] [CrossRef]
- Stapleton, P.A.; James, M.E.; Goodwill, A.G.; Fresbee, J.C. Obesity and Vascular Dysfunction. Pathophysiology 2008, 15, 79–89. [Google Scholar] [CrossRef]
- Faizan, U.; Rouster, A.S. Nutrition and Hydration Requirements in Children and Adults. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutritionand hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Flatharta, T.O.; Flynn, A.; Mulkerrin, E.C. Heat-related chronic kidney disease mortality in the young and old: Differing mechanisms, potentially similar solutions? BMJ Evid. Based Med. 2019, 24, 45–47. [Google Scholar] [CrossRef]
- Gunanithi, K.; Sakthidasan, S. Serum Albumin levels in uncontrolled type II diabetes mellitus-An observational study. MedPulse. Int. J. Biochem. 2021, 20, 13–17. [Google Scholar]
- DiNicolantonio, J.J.; O´Keefe, J. Low-grade metabolic acidosis as a driver of chronic disease: A 21st century public health crisis. Open Heart 2021, 8, e001730. [Google Scholar] [CrossRef]
- Alpern, R.J.; Sakhaee, K. The clinical Spectrum of Chronic Metabolic Acidosis: Homeostatic Mechanisms Produce Significant Morbidity. Am. J. Kid. Dis. 1997, 29, 291–302. [Google Scholar] [CrossRef]
- Doar, J.W.H.; Cramp, D.G. The effects of Obesity and Maturity-Onset Diabetes Mellitus on L(+) Lactic Acid Metabolism. Clin. Sci. 1970, 39, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.O.; Hoogeveen, R.C.; Brancati, F.L.; Astor, B.C.; Ballantyne, C.M.; Schmidt, M.I.; Young, J.H. Association of Blood Lactate with type 2 diabetes: The Atherosclerosis Risk in Communities Carotid MRI Study. Int. J. Epidemiol. 2010, 39, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Bertossi, D. Insights on the role of L-lactate as a signaling molecule in skin aging. Biogerontology 2023, 24, 709–726. [Google Scholar] [CrossRef] [PubMed]
- De-Cleva, R.; Cardia, L.; Veira-Carducci, A.; Greve, J.M.; Santo, M.A. Lactate can be a Marker of Metabolic Syndrome in Severe Obesity? ABCD. Arq. Bras. Cir. Dig. 2021, 34, e1579. [Google Scholar] [CrossRef]
- Re, R.N. Obesity-Related Hypertension. Ochsener J. 2009, 9, 133–136. [Google Scholar]
- Trayurn, P. Hypoxia and Adipose Tissue Function and Dysfunction in Obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef]
- Rodriguez, A.J.; Boonya-Ananta, M.T.; Gonzalez, M.; Du Le, V.N.; Fine, J.; Palacios, C.; McShane, M.J.; Cote´, G.L.; Ramella-Roman, J.C. Skin optical properties in the obese and their relation to body mass index: A review. J. Biomed. Opt. 2022, 27, 030902. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Shin, J.H. Association between D-dimer and long-term mortality in patients with acute severe hypertension visiting the emergency department. Cinical Hypertens. 2023, 29, 16. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.J.; Faerch, K. Estimating insulin sensitivity and beta cell function: Perspectives from the modern pandemics of obesity and type 2 diabetes. Diabetologia 2012, 55, 2863–2867. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.H.; Shukla, A.P.; Igel, L.I.; Kumar, R.B.; Aronne, L.J. Pharmacotherapy for Obesity. Endocrinol. Metab. Clin. N. Am. 2016, 45, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Guadamuz, J.S.; Shooshtari, A.; Qato, D.M. Global, regional and national trends in statin utilasation in high-income and low/middle-income countries, 2015–2020. BMJ Open 2022, 12, e061350. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, insulin Resistanc, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Coucouls, A.P.; Daigle, C.R.; Arterburn, D.E. Long-term outcomes of metabolic/bariatric surgery in adults. BMJ 2023, 383, e071027. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, C.K. Time for new Approach to Reducing Cardiovascular Disease: Is Limitation on Saturated Fat Meat Consumption Still Justified. Am. J. Med. 2020, 133, 1009–1010. [Google Scholar] [CrossRef]
- Lee, C.D.; Hardin, C.; Longo, D.L.; Ingelfinger, J.R. Nutrition in Medicine—A New Review Article Series. N. Engl. J. Med. 2024, 390, 1324–1325. [Google Scholar] [CrossRef]
- Blais, J.E.; Wie, Y.; Yap, K.K.W.; Alwafi, H.; Ma, T.T.; Brauer, R.; Lau, W.C.Y.; Man, K.K.C.; Siu, C.W.; Tan, K.C.B.; et al. Trends in Lipi-modifying agent use in 83 countries. Atherosclerosis 2021, 328, 44–51. [Google Scholar] [CrossRef]
- Kang, H.; Hong, S.H. Risk of Kidney Dysfunction from Polypharmacy among Older Patients: A Nested Case-Control Study of the South Korean Senior Cohort. Sci. Rep. 2019, 9, 10440. [Google Scholar] [CrossRef]
- Oosting, I.J.; Colombijn, J.M.T.; Kaasenbrood, L.; Liabeuf, S.; Laville, S.M.; Hooft, L.; Bots, M.; Verhaar, M.C.; Vernoij, R.W.M. Polypharmacy in Patients with CKD A Systematic Review and Meta-Analysis. Kidney360 2024, 5, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Viollet, B. Le nouvelles promesses de la metformine. Vers une meilleure comprehension de ses mechanismes d’action. Medicine/Sciences 2014, 30, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Staub, H. Übersichten.40 Jahre Insulin und intermediärer Stoffwechsel. Kli. Wschr. 1965, 42, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. The origin of type 2 diabetes medications. Br. J. Diabetes 2022, 22, 112–120. [Google Scholar] [CrossRef]
- Yamagushi, H.; Sekiguchi, N.; Hirano, A.; Oshima, A.; Imai, T. Metformin-associated Lactic Acidosis Induced by Excessive Acohol Consumption. Intern. Med. 2024, 63, 1745–1749. [Google Scholar] [CrossRef]
- Watanabe, C.K. Studies in the metabolic changes induced by administration of guanidine bases. II. The influence of guanidine upon urinary ammonia and acid excretion. J. Biol. Chem. 1918, 34, 51–63. [Google Scholar] [CrossRef]
- Watanabe, C.K. Studies in the metabolic changes induced by the administration of guanidine bases: VI. The influence of Guanidine Acidosis on the Fat Content of the Blood. J. Biol. Chem. 1922, 1, 195–200. [Google Scholar]
- Frank, E.; Northmann, M.; Wagner, A. Über Synthetisch Dargestellte Körper mit Insulin artiger Wirkung auf den Normalen und diabetischen Organismus. Kli. Wochenschr. 1926, 45, 2100–2107. [Google Scholar] [CrossRef]
- Banting, F.C.; Best, C.H. The internal secretion of the pancreas. J. Lab. Clin. Med. 1922, 7, 251–266. [Google Scholar]
- Calvert, E.G.B. Observations on the treatment of Diabetes By Synthalin. Lancet 1927, 5430, 649–651. [Google Scholar] [CrossRef]
- Anonymus. Synthalin in the treatment of Diabetes. Preliminary reports to the medical research council. Lancet 1927, 5427, 517–521. [Google Scholar] [CrossRef]
- Bodo, R.; Marks, A. The relation of synthalin to carbohydrate metabolism. J. Physiol. 1928, 65, 83–99. [Google Scholar] [CrossRef]
- Ringer, A.I.; Bilon, S.; Harris, M.M.; Landy, A. Synthalin. its use in the treatment of Diabetes. Arch. Int. Med. 1928, 41, 453–471. [Google Scholar] [CrossRef]
- Staub, H.; Küng, O. Zum.Synthalin Mechanismus. Klin. Woch. 1928, 7, 1365–1366. [Google Scholar] [CrossRef]
- Frank, E.; Nothmann, M.; Wagner, A. Über die experimentelle und klinische Wirkung des Dodekamethylendiguanids (Synthalin B). Klin. Woch. 1928, 42, 1996–2000. [Google Scholar] [CrossRef]
- Bischoff, F.; Sahyun, M.; Long, L. Guanidine Structure and Hypoglycemia. J. Biol. Chem. 1929, 81, 325–349. [Google Scholar] [CrossRef]
- Blatherwick, N.R.; Sahyun, M.; Hill, E. Some effects of Synthalin on Metabolism. J. Biol. Chem. 1927, 75, 671–683. [Google Scholar] [CrossRef]
- Anonymus. Insulin and Synthalin. Nature 1928, 3039, 151–153. [Google Scholar]
- Werner, E.A.; Bell, J. CXXVII—The preparation of Guanidine by the interaction of Dicyanodiamide and Ammonium Thiocyanate. J. Chem. Soc. Trans. 1920, 117, 1133–1136. [Google Scholar] [CrossRef]
- Werner, E.A.; Bell, J. The Preparation of methylguanidine, and of BB-dimethylguanidine by the interaction of dicyanodiamid, and methylammonium and dimethylammonium chlorides respectively. J. Chem. Soc. Trans. 1922, 121, 1790–1794. [Google Scholar] [CrossRef]
- Hesse, E.; Taubmann, G. Die Wirkung des Biguanids und seiner Derivate auf den Zuckerstoffwechsel. NNaunyn-Schmiedebergs Arch. Für Exp. Pathol. Und Pharmakol. 1929, 142, 290–308. [Google Scholar] [CrossRef]
- Minot, A.S.; Dodd, K.; Saunders, J.M. The acidosis of guanidine intoxication. J. Clin. Investig. 1934, 13, 917–932. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J. Effect of guanidine and synthalin on the citric Acid Metabolism. Acta Medica Scand. 1946, 125, 82–94. [Google Scholar] [CrossRef]
- Goldner, M.G. Oral hypoglycemic agents Past and Present. AMA Arch. Intern. Med. 1958, 102, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C. Hydropic Degeneration of the aCells of The Pancreatic Islets Produced by Synthalin A. J. Path. Bact. 1952, 56, 575584. [Google Scholar]
- Ferner, H.; Runge, W. Synthalin A as elective Mitotic Poison Acting on aCells of the Islet of Langerhans. Science 1955, 122, 420. [Google Scholar] [CrossRef]
- Bloom, A.; Richards, J.G. Phenformin as Adjuvant Oral Therapy in Diabetes. Br. Med. J. 1961, 1, 1796–1799. [Google Scholar] [CrossRef]
- Krall, L.P.; Camerini-Davalos, R. Clinical Trials with DBI, a New Nonsulfonylurea Oral Hypoglycemic Agent. AMA Archiv. Int. Med. 1958, 102, 25–31. [Google Scholar] [CrossRef]
- Williams, R.H.; Tanner, D.C.; Odell, W.D. Hypoglycemic Actions of Phenethyl-Amyl-, and Isoamyl-Diguanide. Diabetes 1958, 7, 87–92. [Google Scholar] [CrossRef]
- Unger, G.; Freedman, L.; Shapiro, S.L. Pharmacological Studies of a New Oral Hypoglycemic Drug. Proc. Soc. Exp. Biol. Med. 1957, 95, 190–192. [Google Scholar] [CrossRef]
- Tranquada, R.E.; Kleeman, C.; Brown, J. Some effects of Phenylmethylbiguanide on Human Hepatic Metabolism as Measured by Hepatic Vein Catheterization. Diabetes 1960, 9, 207–214. [Google Scholar] [CrossRef]
- Walker, R.S.; Linton, A.; Thomson, W.S.T. Mode of action and Side effects of Phenformin Hydrochloride. BMJ 1960, 2, 1567–1569. [Google Scholar] [CrossRef]
- Grodsky, G.M.; Karam, J.H.; Pavlatos, F.C.; Forsham, P.H. Reduction by Phenformin of excessive Insulin Levels after glucose loading in Obese Diabetic Subjectes. Metabolism 1963, 12, 278–286. [Google Scholar][Green Version]
- Beaser, S. Therapy of Diabetes Mellitus with Combinations of Drugs Given Orally. N. Engl. J. Med. 1958, 259, 1207–1210. [Google Scholar] [CrossRef]
- Walker, R.S. Preliminary Observations on Phenethyldiguanide. BMJ 1959, 2, 405–406. [Google Scholar] [CrossRef]
- Bratusch-Marrain, P.R.; Korn, A.; Waldhäusl, W.K.; Gasic, S.; Nowotny, P. Effect of Buformin on Splanchnic Carbohydrate and Substrate Metabolism in Healthy Man. Metabolism 1981, 30, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Phillps, P.J.; Scicchitano, R.; Clarkson, A.R.; Gilmore, A.R. Metformin-associated lactic acidosis. Aust. N. Z. J. Med. 1978, 8, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, A.; Stockley, C.; Keal, J.; Rolan, P.; Bochner, F. Reduction of Metformin renal tubular secretion by cimetidine in man. J. Clin. Pharmacol. 1987, 231, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Komatsu, R.; Matsuyama, T. Short-term effect of buformin, a biguanide, on insulin sensitivity, soluble fraction of tumor necrosis factor receptor and serum lipids in overweight patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2004, 66, 133–138. [Google Scholar] [CrossRef]
- Misbin, R.I. Phenformin-Associated Lactic Acidosis: Pathogenesis and Treatment. Ann. Int. Med. 1977, 87, 591–595. [Google Scholar] [CrossRef]
- Knatterud, G.; Meinert, C.L.; Klimt, C.R.; Osborne, R.K.; Martin, D.B. Effects of Hypoglycemic Agents on Vascular Complications in Patients With Adult-Onset Diabetes. JAMA 1971, 217, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, H.; Seitz, W. Weitere Ergebnisse der Diabetesbehandlung mit bluzuckersenkenden Biguaniden. Münch. Med. Wschr. 1958, 100, 1849–1851. [Google Scholar] [PubMed]
- Sterne, J. Du nouveau dans les antidiabetiques. La NNdimethylamino guanyl guanidine (NNDG). Maroc. Med. 1957, 36, 1295–1296. [Google Scholar]
- Clarke, B.F.; Duncan, U.P. Comparison of chlorpropamide and metformin treatment on weight and blood glucose response and uncontrolled obese diabetics. Lancet 1968, 1, 123–126. [Google Scholar] [CrossRef]
- Lebacq, E.G.; Tirzmalis, A. Metformin and Lactic Acidosis. Lancet 1972, 12, 314–315. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study Group. Prospective diabetes study group 16. Overview of 6 years therapy of type 2 diabetes: A progressive disease. Diabetes 1995, 44, 1249–1258. [Google Scholar] [CrossRef]
- De Fronzo, R.; Goodmann, A. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995, 333, 541–549. [Google Scholar] [CrossRef]
- Guriguian, J.; Green, L.; Misbin, R.J.; Stadel, B.; Fleming, G.A. Efficacy of Metformn in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1996, 334, 269. [Google Scholar]
- Misbin, R.I.; Green, L.; Stadel, B.V.; Gueriguian, J.L.; Gubbi, A.; Fleming, G.A.N. Lactic acidosis in patients with diabetes treated with metformin. N. Engl. J. Med. 1998, 338, 265–266. [Google Scholar] [CrossRef]
- Campbell, R.K.; White, J.R.; Saulie, B.A. Metformin: A new Oral Biguanide. Clin. Ther. 1996, 18, 360–370. [Google Scholar] [CrossRef]
- Krishnamurthy, M.; Sahouria, J.J.; Desai, R.; Caguiat, J. Buformin induced lactic acidosis-A symptom of Modern Health Care Malady. J. Am. Geriatr Soc. 2004, 52, 1785. [Google Scholar] [CrossRef] [PubMed]
- Fingl, E.; Woodbury, D.M. General Principles. 1. Pharmacokinetics 2–26 pp In The Pharmacological Basis of Therapeutics, 5th ed.; Goodman, L.S., Gilman, A., Eds.; Macmillan Publishing Inc: New York, NY, USA.
- Sambol, N.C.; Chiang, J.; O´Conner, M.; Liu, C.Y.; Lin, E.T.; Goodman, A.M.; Benet, L.Z.; Karam, J.H. Pharmacokinetics and Pharmacodynamics of Metformin in Healthy Subjects and Patients with Non-Insulin-Dependent Diabetes Mellitus. J. Clin. Pharmacol. 1996, 36, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Sombol, N.C.; Chiang, J.; Lin, E.T.; Goodman, A.M.; Liu, C.h.L.; Benet, L.Z.; Cogan, M.G. Kidney Function and Age Are Both Predictors of Pharmacokinetics of Metformin. J. Clin. Pharmacol. 1995, 35, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Lintz, W.; Berger, W.; Aenishaenslin, W.; Kutova, V.; Baerlocher, C.h.; Kapp, J.P.; Beckmann, R. Buthylbiguanide Concentration in Plasma, Liver, and intestine after intravenous and Oral Administration to man. Eur. J. Clin. Pharmacol. 1974, 7, 433–448. [Google Scholar] [CrossRef]
- Alkalay, D.; Khermani, L.; Wagner, W.H.; Bartlett, M.F. Pharmacokinetics of Phenformin in Man. J. Clin. Pharmacol. 1975, 15, 446–448. [Google Scholar] [CrossRef]
- Pentikäinen, P.J.; Neuvonen, P.J.; Penttilä, A. Pharmacokinetics of Metformin After intravenous and Oral Administration to Man. Eur. J. Clin. Pharmacol. 1979, 16, 185–202. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Fraser, R.; Hilmer, S.; Rivory, L.P.; McLean, A.J. The Hepatic Sinusoid in Aging and Cirrhosis. Effects on Hepatic Substrate Disposition and Drug Clearance. Clin. Pharmacokinet. 2005, 44, 187–200. [Google Scholar] [CrossRef]
- Gormensen, L.C.; Sundelin, E.I.; Jensen, J.B.; Vendelbo, M.H.; Jakobsen, S.; Munk, O.L.; Christensen, M.M.H.; Broesen, K.; Froekiaer, J.; Jessen, N. In vivo Imaging of Human C11-Metformin in Peripheral Organs: Dosimetry, Biodistribution, and Kinetic Analyses. J. Nucl. Med. 2016, 57, 1920–1926. [Google Scholar] [CrossRef]
- Shu, Y.; Sheardown, S.A.; Brown, C.; Owen, R.P.; Zhang, S.; Castro, R.A.; Ianculescu, A.G.; Yue, L.; Lo, J.C.; Burchard, E.G.; et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Investig. 2007, 117, 1422–1431. [Google Scholar] [CrossRef]
- Christensen, M.M.H.; Hoejnd, K.; Hother-Nielsen, O.; Stage, T.B.; Damkler, P.; Beck-Nielsen, H.; Brosen, K. Steady-state pharmacokinetics of metformin is independent of OCT1 genotype in healthy volunteers. Eur. J. Clin. Pharmacol. 2015, 71, 691–697. [Google Scholar] [CrossRef]
- Garg, P.K.; Lokitz, S.J.; Nazih, R.; Garg, S. Biodistribtution and Radiation Dosimetry of C11-Nicotine from Whole-Body PET Imaging in Humans. J. Nucl. Med. 2017, 58, 473–478. [Google Scholar] [CrossRef]
- Gierloeff, T.; Jakobsen, S.; Nahimi, A.; Munk, O.L.; Bender, D.; Alstrup, A.K.O.; Vase, K.H.; Hansen, S.B.; Brooks, D.J.; Berghammer, P. In Vivo Imaging of Human Acetylcholinesterase Density in Peripheral Organs using C11-Donezepezil: Dosimetry, Biodistribution, and Kinetic Analyses. J. Nucl. Med. 2014, 55, 1818–1824. [Google Scholar]
- Gierloeff, T.; Fedorova, T.; Knudsen, K.; Munk, O.L.; Nahimi, A.; Jacobsen, S.; Danielsen, E.H.; Terkelsen, A.J.; Hansen, J.; Pavese, N.; et al. Imaging acetylcholinesterase density in peripheral organs in Parkinson’s disease with C11-donepezil PET. Brain 2015, 138, 653–663. [Google Scholar]
- Chou, C.-H. Uptake and Dispersion of Metformin in the Isolated Perfused Rat Liver. J. Pharm. Pharmacol. 2000, 52, 1011–1016. [Google Scholar] [CrossRef]
- Bonora, E.; Cigolini, M.; Bosello, O.; Zancanaro, C.; Capretti, L.; Zavaroni, I.; Coscelli, C.; Butturini, U. Lack of effect of intravenous metformin on plasma concentrations of glucose, insulin, C-peptide, glucagon and growth hormone in non-diabetic subjects. Curr. Med. Res. Opin. 1984, 9, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Sum, C.F.; Webster, J.M.; Johnson, A.B.; Catalano, C.; Cooper, B.G.; Taylor, R. The effect of intravenous Metformin on Glucose Metabolism During Hyperglicemia in Type 2 Diabetes. Diabetes Med. 1992, 9, 61–65. [Google Scholar] [CrossRef] [PubMed]
- McCreight, L.J.; Stage, T.B.; Connelly, P.; Lonergaum, M.; Nielsen, F.; Prelin, C.; Adamsky, J.; Broesen, K.; Pearson, E.R. Pharmacokinetics of metformin in patients with gastrointestinal intolerance. Diabetes Obes. Metab. 2018, 20, 1593–1601. [Google Scholar] [CrossRef]
- Sterne, J. The Present State of Knowledge on the Mode of Action of the Antidiabetic Diguanides. Metabolism 1964, 13, 781–798. [Google Scholar] [CrossRef]
- Penicaud, L.; Hitier, Y.; Ferre, P.; Girard, J. Hypoglycemic effect of metformin in genetically obese(fa/fa) rats results from an increased utilization of blood glucose by intestine. Biochem. J. 1989, 262, 881–885. [Google Scholar] [CrossRef]
- Sahi, J.; Grepper, S.; Smith, C. Hepatocytes as a Tool in drug metabolism, Transport and Safety Elimination in Drug Discvery. Curr. Drug Iscovery Technol. 2010, 7, 188–198. [Google Scholar]
- Bailey, J.C. Metformin and intestinal glucose handling. Diabetes Metab. Rev. 1995, 11 (Suppl. 1), S23–S32. [Google Scholar] [CrossRef]
- Wilcock, C.; Bailey, C.J. Reconsideration of inhibitory effect of metformin on intestinal glucose absorption. J. Pharm. Pharmacol. 1991, 43, 120–121. [Google Scholar] [CrossRef]
- Christensen, M.M.H.; Hojhund, K.; Hother-Nielsen, O.; Stage, T.B.; Damkier, P.; Beck-Nielsen, H.; Brosen, K. Endogenous glucose production increase in response to metformin treatment in the glycogen-depleted state in humans:a randomized trial. Diabetologia 2015, 58, 2494–2502. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xie, C.; Wu, H.; Jones, K.I.; Horowitz, M.; Rayner, C.K. Metformin reduces the rate of small intestinal glucose absorption in type 2 diabetes. Diabetes Obes. Metab. 2017, 119, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Horakova, O.; Kroupova, P.; Bardova, K.; Buresova, J.; Janowska, P.; Kopecky, J.; Rossmeisl, M. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci. Rep. 2019, 9, 6156. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.W. Metformin:60-year odyssey with the journey still continuing-a personal commentary from Professor Ian Campbell. Curr. Med. Res. Opin. 2022, 38, 55–58. [Google Scholar] [CrossRef]
- Ikeda, T.; Iwata, K.; Murakami, H. Inhibition Effect of Metformin on Intestinal Glucose Absorption in the perfused Rat Intestine. Biochem. Pharmacol. 2000, 59, 887–890. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.; DeFronzo, R.A. Is The time To change the Type 2 Diabetes Treatment Paradigm? Yes! GLP-1 Ras Should Replace Metformin in the Type 2 Diabetes Algorithm. Diabetes Care 2017, 40, 1121–1127. [Google Scholar] [CrossRef]
- Tobar, N.; Rocha, G.Z.; Santos, A.; Guadagnini, D.; Assalin, H.B.; Camargo, J.A.; Goncalves, A.E.S.S.; Pallis, F.R.; Oliveira, A.G.; Rocco, S.A.; et al. Metformin acts in the gut and induces gut-liver cross-talk. PNAS 2023, 120, e2211933120. [Google Scholar] [CrossRef]
- Lalau, J.D.; Race, J.-M.; Brinquin, L. Lactic Acidosisin MetforminTherapy. Diabetes Care 1998, 21, 1360–1362. [Google Scholar]
- Lalau, J.D.; RaceJ-MAndrelli, P.; Lacroix, C.; Canarelli, J.P. Metformin retention Independent of Renal Failure in Intestinal Occlusion. Diabetes Metab. 2000, 27, 24–28. [Google Scholar]
- Bailey, C.J.; Gwilt, M. Diabetes, Metformin and Clinical Course of COVID-19: Outcomes, Mechanisms and Suggestions on the Therapeutic Use of Metformin. Front. Pharmacol. 2022, 13, 78459. [Google Scholar] [CrossRef] [PubMed]
- Lalau, J.D.; Kajbal, F.; Protti, A.; Christensen, M.M.; De Bore, M.-E.; Wirnsperger, N. Metformin-associated lactic-acidosis (MALA): Moving towards a new paradigm. Diabetes Obes. Metab. 2017, 19, 1502–1512. [Google Scholar] [CrossRef]
- Bronden, A.; Alber, A.; Rohde, U.; Rehfeld, J.F.; Holst, J.J.; Vilsboell, T.; Knop, F.K. Single-dose Metformin enhances Bile-Acid-induced Glucagon-like Peptide-1 Secretion in Patients with type-2 Diabetes. J. Clin. Endocrinol. Metab. 2017, 102, 4153–4162. [Google Scholar] [CrossRef]
- Ritzel, U.; Fromme, A.; Otteleben, M.; Leonhardt, U.; Ramadori, G. Release of glucagon-like peptide-1(GLP-1) by carbohydrates in the perfused rat ileum. Acta Diabetol. 1997, 34, 18–21. [Google Scholar] [CrossRef]
- Raddatz, D.; Nolte, W.; Roßbach, C.; Leonhardt, U.; Buchwald, A.; Scholz, K.H.; Ramadori, G. Measuring the effect of a Study Meal on Portal Concentrations of Glucagon-Like Peptide-1 (GLP-1) in Non Diabetic and Diabetic Patients with Liver Cirrhosis: Transjugular Intrahepatic Portosystemic Stent Shunt(TIPSS) as a New Method for Metabolic Measurement. Exp. Clin. Endocrinol. Diabetes 2008, 116, 461–467. [Google Scholar]
- Li, B.; Hu, Y.; Wang, G.; Liu, L. The effect of exenatide on fasting bile acids in newly diagnosed type 2 diabetes mellitus patients, a pilot study. BMC Pharmacol. Toxicol. 2020, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Coll, A.P.; Chen, M.; Taskar, P.; Rimmington, D.; Patel, S.; Tadross, J.A.; Cimino, I.; Yang, M.; Welsh, P.; Virtue, S.; et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2019, 578, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Kincald, J.W.R.; Rimmington, D.; Tadross, J.A.; Cimino, I.; Zwetkova, I.; Kaser, A.; Richards, P.; Patel, S.; O´Rahilly, S.; Coll, A.P. The gastrointestinal tract is a major source of the acute metformin-stimulated rise in GD15. Sci. Rep. 2024, 14, 1899. [Google Scholar] [CrossRef]
- Scott, B.; Day, E.A.; Lynch, L.; O´Brien, K.L.; Scanlan, J.; Cromwell, G.; Scannal, A.L.; Mc Donnell, E.; Finlay, D.K. Metformin and Feeding increase levels of appetite-suppressing metabolite Lac-Phe in humans. Nat. Metab. 2024, 6, 651658. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Kamishnyl, A. Effects of metformin on the gut microbiota: A systematic review. Mol. Metab. 2023, 77, 101805. [Google Scholar] [CrossRef]
- Larsson, A.; Ericson, U.; Jonsson, D.; Miari, M.; Athanassiadis, P.; Baldanzi, G.; Brunkwall, L.; Hellstrand, S.; Klinge, B.; Melander, O.; et al. New Connections of Medication use and polypharmacy with the gut microbiota composition and functional potential in a large population. Sci. Rep. 2024, 14, 23723. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, L.X.; Boenisch, H.; Goethert, M.; Moldering, S.G.; Racke, K.; Ramadori, G.; Miller, K.J.; Schwoerer, H. Effects of Metformin on Intestinal 5-Hydroxytriptamine (5-HT) Release on 5-HT3-receptors. Naunyn Schmiedbergers Arch. Pharmacol. 2000, 361, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, J.K.; Kim, B.T. Relationship between gastrointestinal F-18-fluorodoxyglucose accumulation and gastrointestinal symptoms in whole-body PET. Clin. Positron. Imaging 1999, 2, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gontier, E.; Fourme, E.; Wartski, M.; Blondet, C.; Bonardel, G.; Le Stanc, E.; Mantzarides, M.; Foehrenbach, H.; Pecking, A.-P.; Alberini, J.-L. High and typical F18-FDG bowel uptake in patients treated with metformin. Eur. J. Med. Mol. Imaging 2008, 35, 95–99. [Google Scholar] [CrossRef]
- Morita, Y.; Nogami, M.; Sakaguchi, K.; Okada, Y.; Hirota, Y.; Sugawara, K.; Tamori, Y.; Zeng, F.; Murokami, T.; Ogawa, W. Enhanced Release of Glucose into Intraluminal Space of the Intestine Associated With Metformin Treatment as Revealed by [18F] Fluorodeoxyglucose PET-MRI. Diabetes Care 2020, 43, 1796–1802. [Google Scholar] [CrossRef]
- Özülkur, T.; Özülkur, F.; Mert, M.; Özpacaci, T. Clearance of high intestinal F18-FDG uptake associated with metformin after stopping the drug. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1011–1017. [Google Scholar] [CrossRef]
- Schreuder, N.; Klarenbeek, H.; Vendel, B.N.; Jager, P.L.; Kosterink, J.G.W.; van Puijenbroek, E.P. Discontinuation of metformin to prevent metformin-induced high colonic FDG uptake: Is 48 h sufficient? Ann. Neclear Med. 2020, 34, 833–839. [Google Scholar] [CrossRef]
- Massollo, M.; Marini, C.; Brignone, M.; Emionite, B.; Salani Briondato, M.; Capitano, S.; Fitz, F.; Democrito, A.; Amaro, A.; Morbelli, S.; et al. Metformin Temporal and Localized Effects on Gut Glucose Metabolism Assessed Using F18-DG PET in Mice. J. Nucl. Med. 2013, 54, 259–266. [Google Scholar] [CrossRef]
- Tu, D.G.; Chen, C.-R.; Wang, Y.-W.; Tu, C.-W.; Huang, Y.C. Bowel-cleansing methods affecting PET-CT Image interpretation. Nucl. Med. Commun. 2011, 32, 570–574. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Chen, J.-H.; Tsui, C.-C.; Chou, H.-H.; Cheng, R.-H.; Chiu, J.-S. Use of Laxative-augmented Contrast Medium in the Evaluation of Colorectal Foci at FDG PET. Radiology 2011, 259, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, R.; Manabe, N.; Ayaki, M.; Tanikawa, T.; Fujita, M.; Ono, Y.; Fujiwara, H.; Suehiro, M.; Monobe, Y.; Kato, K.; et al. Increased Colonic Fluorodeoxyglucose Uptake in Melanosis Coli-A Case Series of Three Patients. Gastro. Hep. Adv. 2022, 1, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Czyzyk, A.; Tawcki, J.; Sadowsky, J.; Ponnikowska, I.; Szezepanik, Z. Effect of biguanides on intestinal absorption of glucose. Diabetes 1968, 17, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, P.; Bolli, P.; Arbenz, U.; Keiser, G. Intestinale Absorptionsstörung infolge Metformin behandlung (Zur Frage der Wirkungsweise der Biguanide). Diabetologia 1969, 5, 405–412. [Google Scholar] [CrossRef]
- Paleari, L.; Burhenne, J.; Weiss, J.; Foersch, S.; Roth, W.; Parodi, A.; Gnant, M.B.; Bachleitner-Hofmann, T.; Scherer, D.; Ulrich, C.M.; et al. High Accumulation of Metformin in Colonic Tissue of Subjects With Diabetes or the Metabolic Syndrome. Gastroenterology 2018, 154, 1543–1545. [Google Scholar] [CrossRef]
- Madison, L.L. Role of insulin in the hepatic Handling of glucose. Arch. Int. Med. 1969, 123, 284–292. [Google Scholar] [CrossRef]
- Balks, H.-J.; Jungermann, K. Regulation of peripheral insulin/glucagon levels by rat liver. Eur. J. Biochem. 1984, 141, 645–650. [Google Scholar] [CrossRef]
- Guzelian, P.H.; Boyer, J.L. Glucose reabsorption from bile. J. Clin. Investig. 1974, 53, 526–535. [Google Scholar] [CrossRef]
- Infante, M.; Leoni, M.; Caprio, M.; Fabbri, A. Long-term Metformin therapy and Vitamin B12 deficiency: An association to bear in mind. World J. Diabetes 2021, 12, 916–931. [Google Scholar] [CrossRef]
- Wijnen, J.C.F.; Van De Riet, I.R.; Lijfering, W.M.; Van Der Meer, F.J.M. Metformin use decreases the anticoagulant effect of phenprocoumon. J. Thromb. Haemost. 2014, 12, 887–890. [Google Scholar] [CrossRef]
- Baker, E.H.; Sandle, G.I. Complications of Laxative Abuse. Ann. Rev. Med. 1996, 47, 127–134. [Google Scholar] [CrossRef]
- Hart, S.L.; McColl, I. The effect of the laxative oxyphenisatin on the intestinal absorption of glucose in rat and man. Br. J. Pharmac. Chemother. 1968, 32, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Sturtzel, B.; Dietrich, A.; Wagner, K.H.; Gisinger, C.; Elmafda, I. The status of vitamins B6, B12, Folate, and of homocysteine in Geriatric home residents receiving laxatives or dietary fiber. J. Nutr. Health 2010, 14, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Yamada, U. Iron deficiency anemia induced by magnesium overuse: A case report. Biopsychosoc. Med. 2019, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.E.; Takkinen, S.; Johansson, B.; Dotevall, G.; Melander, A.; Berg, S. McClearn Laxative treatment elevates plasma homocysteine: A study on a population-based Swedish sample of old people. Eur. J. Clin. Pharmacol. 2004, 60, 45–49. [Google Scholar]
- Kelley, C.J.; Verdegaal, A.A.; Anderson, B.W.; Shaw, W.L.; Bencivenga-Barry, N.A.; Fogga-Stognew, E.; Goodman, A.L. Metformin inhibits Digestive Proteases and Impairs Protein Digestion in Mice. J. Bil. Chem. 2023, 299, 105363. [Google Scholar] [CrossRef]
- Han, Y.; Yun, C. Metformin Inhibits Na+/H+Exchanger NHE3 Resulting in Intestinal Water Loss. Front. Physiol. 2022, 13, 867244. [Google Scholar] [CrossRef]
- Naz, N.; Malik, I.A.; Sheikh, N.; Ahmad, S.; Kahn, S.; Blaschke, M.; Schultze, F.; Ramadori, G. Ferroportin-1 is a nuclear negative acute-phase protein in rat liver: A comparison with other iro-transport proteins. Lab. Investig. 2012, 92, 842–856. [Google Scholar] [CrossRef]
- Ahmad, S.; Moriconi, F.; Naz, N.; Sultan, S.; Sheikh, N.; Ramadori, G.; Malik, I.A. Ferritin L and Ferritin H are differentially located within hepatic and extra hepatic organs under physiological and acute-phase conditions. Int. J. Clin. Exp. Pathol. 2013, 6, 622–629. [Google Scholar]
- Vidon, N.; Chaussade, S.; Noel, M.; Franchisseur, C.; Huchet, B.; Bernier, J.J. Metformin in the digestive tract. Diabetes Res. Clin. Pract. 1988, 4, 223–229. [Google Scholar] [CrossRef]
- Ghosal, S.; Ghosal, S. The Side Effects of Metformin-A Review. J. Diabetes Metab. Disord. 2019, 6, 030. [Google Scholar] [CrossRef] [PubMed]
- Wickham, R.J. Revisiting the physiology of Nausea and vomiting-challenging the paradigma. Support. Care Cancer 2019, 28, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Caspary, W.F.; Creutzfeldt, W. Inhibition of Bile salt absorption by blood sugar lowering biguanides. Diabetologia 1975, 11, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Scarpello, J.H.B.; Hodgson, E.; Howlett, H.C.S. Effect of Metformin on Bile salt Circulation and Intestinal Motility in Type 2 Diabetes Mellitus. Diabet. Med. 1998, 15, 651–656. [Google Scholar] [CrossRef]
- Sansome, D.J.; Xie, C.; Veedfald, S.; Horowitz, M.; Rayner, C.K.; Wu, T. Mechanisms of glucose-lowering by metformin in type 2 diabetes: Role of bile acids. Diabetes Obes. Metab. 2019, 22, 141–148. [Google Scholar] [CrossRef]
- Metry, M.; Krug, S.A.; Karra, V.K.; Kane, M.A.; Fink, J.C.; Shu, Y.; Wang, H.; Polii, J.E. Differential effects of metformin-mediated BSEP repression on pravastatin and bile acid pharmacokinetics in humans: A randomized controlled trial. Clin. Transl. Sci. 2022, 15, 2468–2478. [Google Scholar] [CrossRef]
- Nakajima, A.; Ishizaki, S.; Matsuda, K.; Kurosu, S.; Taniguchi, S.; Gilberg, P.G.; Mattsson, J.P.; Hasumuma, T.; Camillen, M. Impact of elobixibat on serum and fecal bile acid levels and constipation symptoms in patients with chronic constipation. J. Gastroenterol. Hepatol. 2022, 37, 883–890. [Google Scholar] [CrossRef]
- Spiller, R. Inhibiting glucose absorption to treat constipation. Lancet Gastroenterol. Hepatol. 2018, 3, 588–589. [Google Scholar] [CrossRef]
- Chu, N.; Ling, J.; Jie, H.; Leung, K.; Poon, E. The potential role of lactulose pharmacotherapy in the Treatment and prevention of diabetes. Front. Endocrinol. 2022, 13, 956203. [Google Scholar] [CrossRef]
- Landin-Wilhelmsen, K. Metformin and blood pressure. J. Clin. Pharm. Ther. 1992, 17, 75–79. [Google Scholar] [CrossRef]
- Gudmundsdottir, H.; Aksnes, H.; Heldal, K.; Krogh, A.; Froyshov, S.; Rudberg, N.; Os, I. Metformin and antihypertensive therapy with drugs blocking the renin angiotensin system, a cause of concern? Clin. Nephrol. 2006, 66, 380–385. [Google Scholar] [CrossRef]
- Hashimoto, H.; Nomura, N.; Shoda, W.; Isobe, K.; Kikuchi, H.; Yamamoto, K.; Fujimaru, T.; Ando, F.; Mori, T.; Okado, T.; et al. Metformin Increases urinary sodium excretion by reducing phosphorylation of the sodium-chloride cotransporter. Metabolism 2018, 85, 23–31. [Google Scholar] [CrossRef]
- Mueller, L.; Moser, M.; Prazak, J.; Fuster, D.G.; Schefold, J.C.; Zuercher, P. Metformin’s Role in Hyperlactatemia and Lactic Acidosis in ICU Patients: A Systematic Review. Pharmacology 2023, 108, 213–223. [Google Scholar] [CrossRef]
- Czyzyk, K.A.; Lao, B.; Bartosiewicz, W.; Szczepanik, Z.; Orlowska, K. The Effect of Short-Term Administration of Antidiabetic Biguanide Derivatives On The Blood Lactate Levels in Healthy Subjects. Diabetologia 1978, 2, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Luft, D.; Schmülling, R.M.; Eggstein, M. Lactic acidosis in biguanide-treated diabetics. A review of 330 cases. Diabetologia 1978, 14, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Innerfield, J.R. Metformin-associated mortality in US Studies. N. Engl. J. Med. 1996, 334, 1611–1612. [Google Scholar] [PubMed]
- Stadei, B.V.; Guerigulian, J.; Fleming, G.A. Metformin-associated lactic acidosis. N. Engl. J. Med. 1996, 334, 1613. [Google Scholar]
- Unger, R.H.; Madison, L.L.; Carter, N.W. Tolbutamide-Phenformin in Ketoacidosis-Resistant Patients. JAMA 1960, 174, 2132–2136. [Google Scholar] [CrossRef]
- Cornfield, J. The university Group Diabetes Program. A Further Statistical Analysis of the Mortality Findings. JAMA 1971, 217, 1676–1687. [Google Scholar] [CrossRef]
- Palmer, J.P. Farewell to Phenformin for treating diabetes Mellitus. Ann. Int. Med. 1975, 83, 567–568. [Google Scholar]
- William, D.A.; Marples, J. Biguanides and Lacticacidosis. Lancet 1977, 309, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, E.; Mathieu, S.; Ball, A. Metformin associated lactic acidosis. BMJ 2009, 339, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Blough, B.; Moreland, A.; Mora, A. Metformin-induced lactic acidosis with emphasis on the anion gap. Proc. (Bayl. Univ. Med. Cent.) 2015, 28, 31–33. [Google Scholar] [CrossRef]
- Pena Porta, C.M.; Villafuerte Ledesma, H.M.; DEVera Floristan, C.V.; Ferrer Dufol, A.; Salvador Gomez, T.; Alvarez Lipe, R. Incidence, factors related to presentation, course and mortality of metformin-associated lactic acidosis in the healthcare area of a tertiary hospital. Nefrologia 2019, 39, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Faggian, G.; Cesaro, A.; Faggian, R.; Del Piano, C.; Vitagliano, A.; Del Piano, D.; Salzano, M.; Diglio, A.; Faggian, A. Insufficienza renale acuta, acidosi lattica e metformina: Due casi clinici e review della letteratura. G. Ital. Nefrol. 2023, 3, 1–8. [Google Scholar]
- Cheng, X.; Liu, M.Y.-M.; Li, H.; Zhang, X.; Lei, F.; Quin, J.J.; Chen, Z.; Deng, K.Q.; Lin, L.; Chen, M.-M.; et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020, 32, 537–547. [Google Scholar] [CrossRef]
- Vordoni, A.; Theofilis, P.; Vlachopanos, G.; Koukoulaki, M.; Kalaitzidis, R.G. Metfromin-associated lactic acidosis and acute kidney injury in the era of COVID-19. Front. Biosci. Sch. 2021, 13, 202–207. [Google Scholar] [CrossRef]
- Takayama, K.; Obata, Y.; Maruo, Y.; Yamaguchi, H.; Kosugi, M.; Irie, Y.; Hazama, Y.; Yasuda, T. Metformin-associated Lactic Acidosis with Hypoglycemia during Covid-19 Pandemic. Intern. Med. 2022, 61, 2333–23337. [Google Scholar] [CrossRef]
- Donelly, L.A.; Morris, A.D.; Pearson, E.R. Adherence in patients transferred from immediate release metformin to a sustained release formulation: A population-based study. Diabetes Obes. Metab. 2009, 11, 338–342. [Google Scholar] [CrossRef]
- Rashid, M.; Warraich, N.Y.; Laique, T.; Shujaat, K.; Zawar, S.; Munir, A. Reasons of NON-Compliance to metformin among type 2 diabetics attending diabetic clinic in Lahore. J. Akhtar Saeed Med. Dent. Coll. 2019, 1, 111–116. [Google Scholar] [CrossRef]
- Christofides, E.A. Practical Insight Into Improving Adherence to Metformin Therapy in Patients With Type 2 Diabetes. Clin. Diabetes J. Org. 2019, 37, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.A.; Gonzalez, J.S.; Tripputi, M.T.; Dagogo-Jack, S.; Matulik, M.J.; Montez, M.G.; Tadros, S.; Edelstein, S.L. Long-term metformin adherence in the Diabetes Prevention Program Outcomes Study. BMJ Open Diab. Res. Care 2020, 8, e001537. [Google Scholar] [CrossRef] [PubMed]
- UKPDS (33). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- UKPDS (34). Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Heden, T.A.; Liu, Y.; Kanaley, J.A. Exercise Timing and Blood Lactate Concentration in Individuals With Type 2 Diabetes. Appl. Physiol. Nutr. Metab. 2017, 42, 732–737. [Google Scholar] [CrossRef]
- Tapia, P.; Soto, D.; Bruhn, A.; Alegria, L.; Jarufe, N.; Luengo, C.; Kattan, E.; Regueria, T.; Meissner, A.; Menchaca, R. Impairment of exogenous lactate clearance in experimental hyperdynamic septic shock is not related to total liver hypoperfusion. Crit. Care 2015, 19, 88. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, A.W. 10-Year Follow-up of intensive Glucose Control in Type 2 Diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Nathan, D.M. Some answers, more controversy, from UKPDS. Commentary. Lancet 1998, 352, 832–833. [Google Scholar] [CrossRef]
- Ewart, R.M. The UKPDS: What was the question? Lancet 1999, 353, 1882. [Google Scholar] [CrossRef]
- Tomkin, G.H.; Hadden, D.R.; Weaver, J.A.; Montgomery, D.A.D. Vitamin-B12 Status of Patients on long-term Metformin Therapy. BMJ 1971, 2, 685–687. [Google Scholar] [CrossRef]
- Jager, J.; Kooy, A.; Lehret, P.H.; Wulfele, M.G.; Kolk, J.; Bets, D.; Verbung, J.; Donker, A.J.M.; Stehouver, C.D.A. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: Randomized placebo-controlled trial. BMJ 2010, 340, c2181. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, C.W.; Fang, S.; Lee, H.S.; Park, J.S. Association between metformin dose and vitamin B12 deficiency in patients with type 2 diabetes. Medicine 2019, 98, e17918. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.; Dennis, J.M.; Coleman, R.I.; Sattar, N.; Hattersley, A.T.; Holman, R.R.; Pearson, E.R. Risk of anemia with metformin Use in Type 2 diabetes: A Mastermind Study. Diabetes Care 2020, 43, 2493–2499. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.C.; Kancherla, V.; Khakharia, A.; Allen, L.L.; Phillips, L.S.; Rhee, M.K.; Wilson, P.W.F.; Vaughan, C.P. Long-term metformin treatment and risk of peripheral neuropathy in older Veterans. Diabetes Res. Clin. Pract. 2020, 170, 108486. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.H.; Fadl, N.N.; Kotob, S.E. Impact of long-term Metformin therapy on hepcidin and Iron status in type II Diabetic Patients. Int. J. Pharm. Clin. Res. 2015, 7, 185–193. [Google Scholar]
- Hermann, L.S.; Schersten, B.; Bitzen, P.-O.; Kjellström, T.; Lindgärde, F.; Melander, A. Therapeutic Comparison of Metformin and Sulfonylurea, Alone and in Various Combinations. A double-blind controlled study. Diabetes Care 1994, 17, 1100–1109. [Google Scholar] [CrossRef]
- Dandona, P.; Fonseca, V.; Mier, A.; Beckett, A. Diarrhea and Metformin in Diabetic Clinic. Diabetes Care 1983, 6, 472–474. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Group Study (UKPDS). VIII. Study Design, Prog RRress and Performance. Diabetologia 1991, 34, 877–890. [Google Scholar]
- Turner, R.C.; Cull, C.A.; Fright, V.; Holman, R.R. Glycemic Control with Diet, Sulphonylurea, Metformin, or Insulin in Patients with Type 2 Diabetes Mellitus. Progressive Requirement of multiple Therapies (UKPDS 49). JAMA 1998, 231, 2005–2012. [Google Scholar]
- Flory, J.H.; Mushlin, A.I. Effect of Cost and Formulation in Persistence and Adherence to Initial Metformin in Therapy For Type 2 Diabetes. Diabetes Care 2020, 43, e62–e67. [Google Scholar] [CrossRef]
- Piragine, E.; Petri, D.; Martelli, A.; Calderone, V.; Lucentoforte, E. Adherence to Oral Antidiabetic Drugs in Patients with Type 2 Diabetes: Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1981. [Google Scholar] [CrossRef]
- Mammutovic, A.; Lekic, L.; Zamovic, I.; Mamovic, N.; Kikanovic, A.; Pavlovic, A. Investigations Affecting Adherence to Metformin Therapy In Type 2 Diabetes. Medis. Med. Sci. Res. 2024, 3, 43–51. [Google Scholar]
- Greenes, R.A. A Brief History of Clinical Decision Support. Technical, Social, Cultural, Economic, and Governmental Perspectives. Clin. Decis. Support 2014, 2, 49–109. [Google Scholar] [CrossRef]
- Wang, S.; Hoyte, C. Review of biguanide (Metformin) Toxicity. J. Intensive Care Med. 2018, 34, 863–876. [Google Scholar] [CrossRef]
- Agyekum, A.K.; Klarie, E.; Walsh, M.C.; Nyachoti, C.M. Postprandial portal glucose and lactate fluxes, insulin production, and portal vein-drained viscera oxygen consumption in growing pigs fed a high-fiber diet supplemented with a multi-enzyme cocktail. Anim. Sci. 2016, 94, 3760–3770. [Google Scholar] [CrossRef] [PubMed]
- Dietze, O.; Wicklmayr, M.; Hepp, K.D.; Bogener, W.; Mehnert, H.; Czempiel, H.; Henftling, H.G. On Gluconeogenesis of Human Liver. Accelerated Hepatic Glucose Formation Induced by Increased Precursor Supply. Diabetologia 1976, 12, 555–561. [Google Scholar] [CrossRef]
- Davis, M.A.; Williams, P.E.; Cherrington, A.D. Effect of a mixed meal on hepatic lactate and gluconeogenic precursor metabolism in dogs. Am. J. Physiol. 1984, 247 Pt 1, E362–E369. [Google Scholar] [CrossRef]
- Green, J.M.; Hornsby, J.H.; Pritchett, R.C.; Pritchett, K. Lactate Treshold Comparison in Anaerobic vs Aerobic Athletes and Untrained Partecipants. Int. J. Exerc. Sci. 2014, 7, 329–358. [Google Scholar] [CrossRef]
- Chang, T.; Ravi, N.; Plegue, M.A.; Sonneville, K.R.; Davis, M.M. Inadequate Hydration, BMI, and Obesity Among US Adults: NHANES 2009–2012. Ann. Fam. Med. 2016, 14, 320–324. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynod-Simon, A.; Sobotka, L.; Vanasselt, D.; et al. ESPEN Practical Guidelines Clinical Nutrition And Hydration In Griatrics. Clin. Nutr. 2022, 41, 958–989. [Google Scholar] [CrossRef]
- Doar, J.W.; Cramp, D.G.; Maw, D.S.; Seed, M.; Wynn, V. Blood Pyruvate and Lactate Levels During Oral and Intravenous Glucose Tolerance Tests in Diabetes Mellitus. Clin. Sci. 1970, 39, 253–269. [Google Scholar] [CrossRef]
- Badescu, S.V.; Tataru, C.; Kobilinsea, L.; Georgescu, E.L.; Zahiv, D.M.; Zagrean, L. The association between Diabetes mellitus and Depression. J. Med. Life 2016, 9, 120–125. [Google Scholar]
- Wei, L.; Ji, L.; Miao, Y.; Han, X.; Li, Y.; Wang, Z.; Fu, J.; Guo, L.; Su, Y.; Zhang, Y. Constipation in DM are associated with both poor glycemic control and Diabetic Complications: Current Status and Future Directions. Biomed. Pharmacother. 2023, 165, 115202. [Google Scholar] [CrossRef] [PubMed]
- Haines, S.T. Treating Constipation in the Patient With Diabetes. Diabetes Educ. 1995, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Darienski, R.; Mihaylova, V.; Handjieva-Darienska, T. The link between Obesity and the skin. Front. Nutr. 2022, 9, 855573. [Google Scholar] [CrossRef] [PubMed]
- Svensson, R.; Hahn, R.G.; Zdolsek, J.H.; Bahlmann, H. Plasma volume expansion reveals hidden metabolic acidosis in patients with diabetic ketoacidosis. Intensive Care Mediicne Exp. 2022, 10, 36. [Google Scholar] [CrossRef]
- Délia, J.A.; Segal, A.R.; Pharm, D.; Weinrauch, L.A. Metformin-SGL2, Dehydrationn, and Acidosis Potential. J. Am. Geriatr. Soc. 2017, 65, e101–e102. [Google Scholar] [CrossRef]
- Dobrica, E.C.; Gaman, M.A.; Cozma, M.A.; Bratu, O.G.; Stoian, A.P.; Diaconu, C.C. Polypharmacy in Type 2 Diabetes Mellitus: Insights from an Internal Medicine Department. Medicina 2019, 55, 436. [Google Scholar] [CrossRef]
- Ghiraldi, E.M.; Nourian, A.; Chen, M.; Friedlander, J.I. Investigating Fluid Intake in an Underserved Community: What Factors Are Associated ewith Low Urine Volume on 24-Hour Urine Collection? J. Endocrinol. 2021, 35, 17231728. [Google Scholar] [CrossRef]
- Andraska, E.A.; Tran, L.A.; Haga, L.M.; Mark, A.K.; Madigan, M.C.; Makaroun, M.S.; Eslami, M.H.; Chaer, R.A. Contemporary management of acute and chronic mesenteric ischemia:10-year experience from a multihospital healthcare system. J. Vasc. Surg. 2022, 75, 1624–1635. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Hastings, C.; Johnson, K.; Slaven, E. Metformin-Associated Lactic Acidosis Presenting like Acute Mesenteric Ischemia. J. Emerg. Med. 2019, 57, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.S.; Bronander, K.A. Metformin-associated lactic acidosis Masquerading as Ischemic Bowel. Am. J. Med. 2012, 125, e9. [Google Scholar] [CrossRef] [PubMed]
- Canfora, A.; Ferronetti, A.; Marte, G.; Di Maio, V.; Mauriello, C.; Maida, P.; Bottino, V.; Aprea, G.; Amato, B. Predictive Factors of Intestinal necrosis in acute mesenteric Ischemia. Open. Med. 2019, 14, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G. Albumin Infusion and Critically Ill COVID-19 Patients: Hemodilution and Anticoagulation. Int. J. Mol. Med. 2021, 22, 7126. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Anday, M.M.; Fernandez-Fernandez, C.; MoorignaRamadori-Bayad, D.; Castro-Quintela, E.; Dominguez-Montero, A. Sodium Bicarbonate Therapy in Patients with Metabolic Acidosis. Sci. World J. 2014, 21, 627673. [Google Scholar] [CrossRef]
- Sulkin, T.V.; Bosman, D.; Krentz, A.J. Contraindications to Metformin Therapy in Patients with NIDDM. Diabetes Care 1997, 20, 925–928. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).