Exploring Endogenous Tryptamines: Overlooked Agents Against Fibrosis in Chronic Disease? A Narrative Review
Abstract
1. Introduction
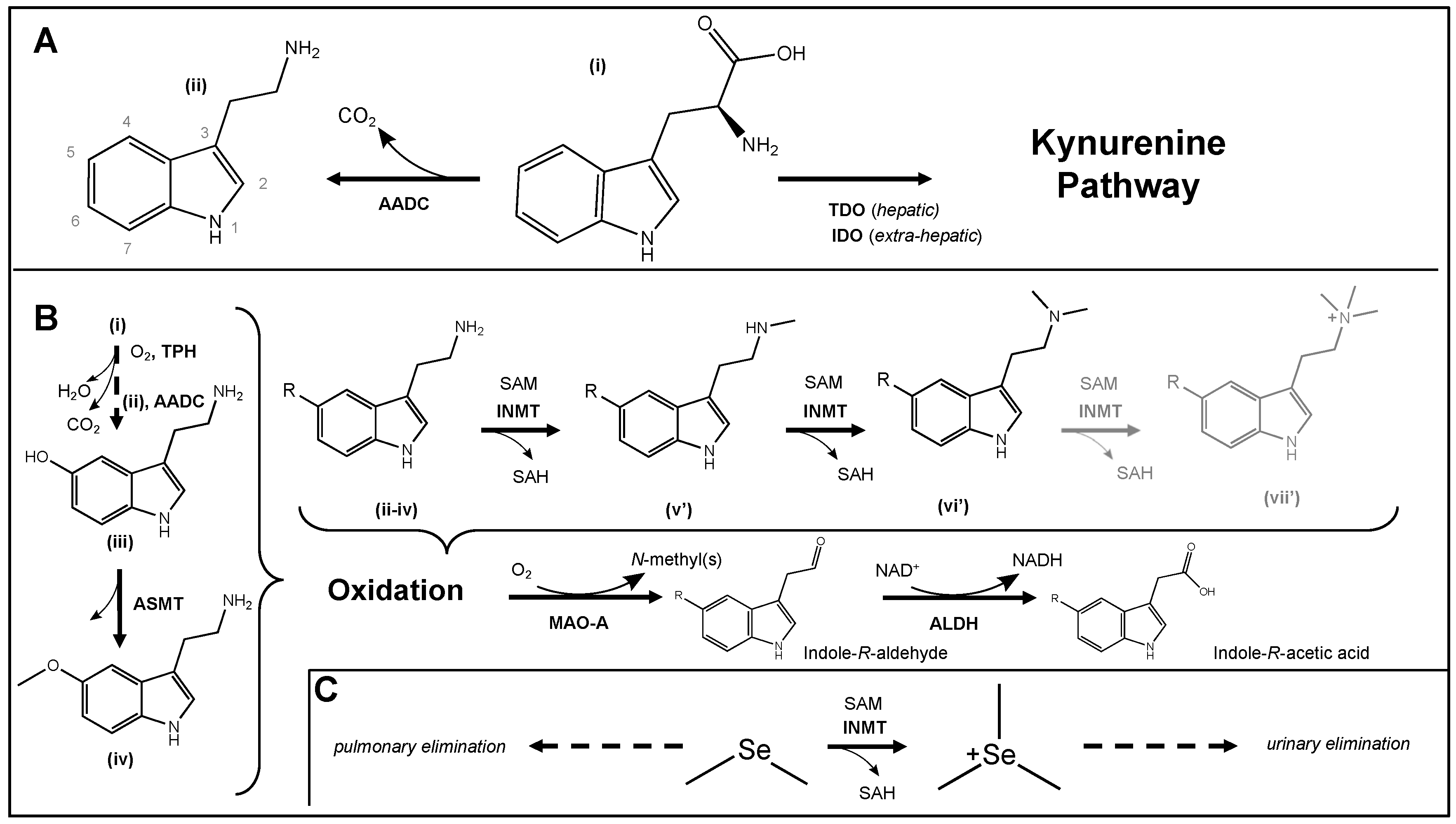
2. Tryptamine Metabolism and Biochemistry
2.1. Redox Biochemistry
2.2. Molecular Biology
2.3. Tryptamine Metabolism and the Gut Microbiome
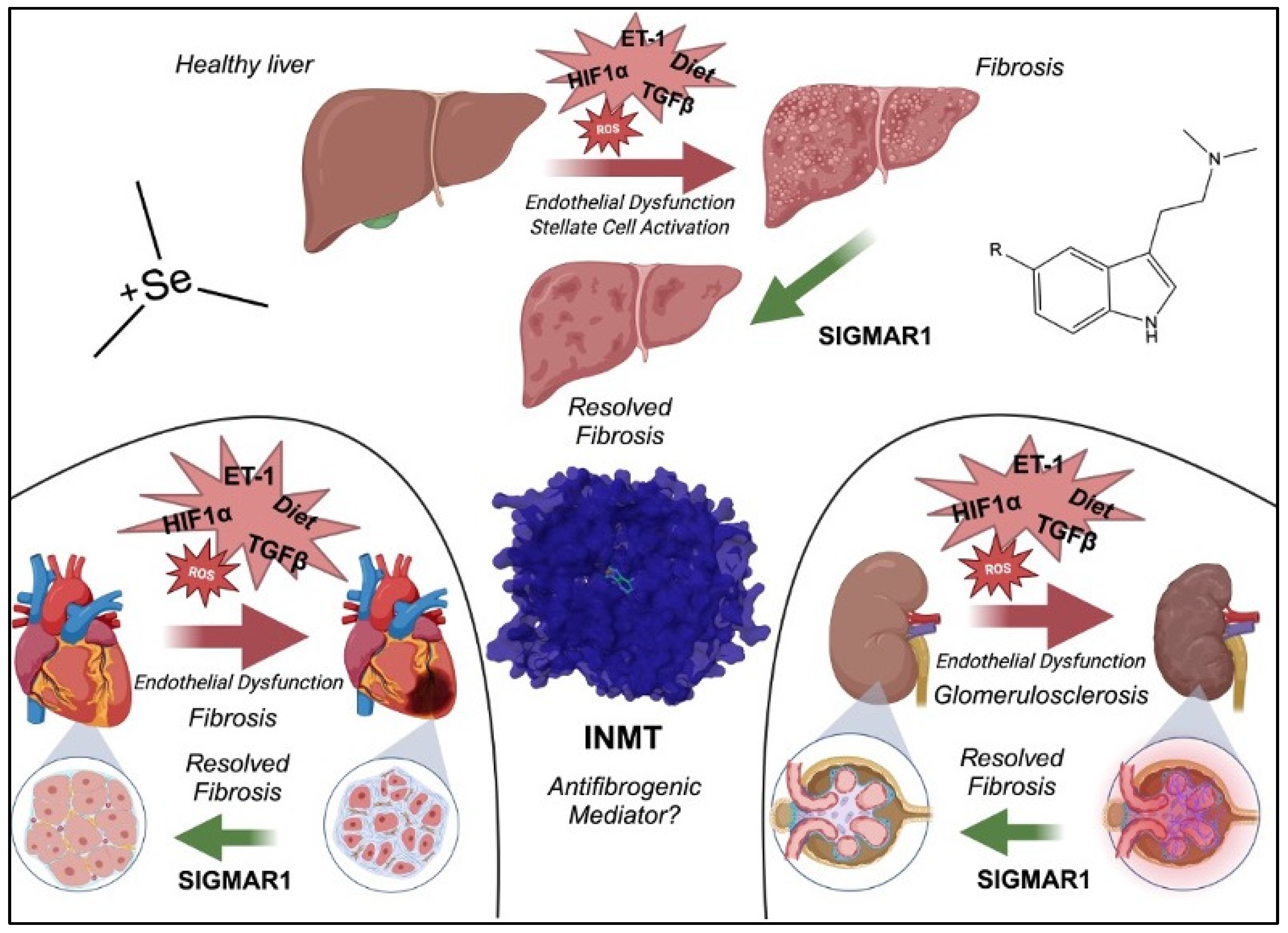
3. INMT in Models of Human Diseases
3.1. INMT in Renal Diseases
3.2. INMT in Hepatic Diseases
3.3. INMT in Cancer
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, N.R.; Preuss, C.V. Controlled Substance Act. In StatPearls; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar] [PubMed]
- Carhart-Harris, R.L.; Goodwin, G.M. The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology 2017, 42, 2105. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Vakharia, S.P.; Netherland, J.; Frederique, K. How the War on Drugs Impacts Social Determinants of Health beyond the Criminal Legal System. Ann. Med. 2022, 54, 2024. [Google Scholar] [CrossRef]
- Strassman, R.J. Human Hallucinogenic Drug Research in the United States: A Present-Day Case History and Review of the Process. J. Psychoact. Drugs 1991, 23, 29–38. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.J. Clinical Investigations of the Therapeutic Potential of Ayahuasca: Rationale and Regulatory Challenges. Pharmacol. Ther. 2004, 102, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Drug Enforcement Administration. Schedules of Controlled Substances: Placement of 5-Methoxy-N,N-Dimethyltryptamine into Schedule I of the Controlled Substances Act. Final Rule Fed. Fed. Regist. 2010, 75, 79296–79300. [Google Scholar]
- McKenna, D.J.; Towers, G.H.N. Biochemistry and Pharmacology of Tryptamines and Beta-Carbolines a Minireview. J. Psychoact. Drugs 1984, 16, 347–358. [Google Scholar] [CrossRef]
- Miller, M.J.; Albarracin-Jordan, J.; Moore, C.; Capriles, J.M. Chemical Evidence for the Use of Multiple Psychotropic Plants in a 1,000-Year-Old Ritual Bundle from South America. Proc. Natl. Acad. Sci. USA 2019, 166, 11207–11212. [Google Scholar] [CrossRef]
- Palamar, J.J.; Le, A. Trends in DMT and Other Tryptamine Use Among Young Adults in the United States. Am. J. Addict. 2018, 27, 578. [Google Scholar] [CrossRef]
- Griffin, M.; Callander, D.; Duncan, D.T.; Palamar, J.J. Differential Risk for Drug Use by Sexual Minority Status among Electronic Dance Music Party Attendees in New York City. Subst. Use Misuse 2020, 55, 230. [Google Scholar] [CrossRef]
- Han, B.H.; Duncan, D.T.; Arcila-Mesa, M.; Palamar, J.J. Co-Occurring Mental Illness, Drug Use, and Medical Multimorbidity among Lesbian, Gay, and Bisexual Middle-Aged and Older Adults in the United States: A Nationally Representative Study. BMC Public. Health 2020, 20, 1123. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, C.; Zeifman, R.J.; Erritzoe, D.; Nutt, D.J.; Carhart-Harris, R.L. Effects of DMT on Mental Health Outcomes in Healthy Volunteers. Sci. Rep. 2024, 14, 3097. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Averill, L.A.; Sepeda, N.D.; Barsuglia, J.P.; Amoroso, T. Psychedelic Treatment for Trauma-Related Psychological and Cognitive Impairment Among US Special Operations Forces Veterans. Chronic Stress 2020, 4, 2470547020939564. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.P.; Venable, K.; Destouni, A.; Billac, G.; Ebenezer, P.; Stadler, K.; Nichols, C.; Barker, S.; Francis, J. Pharmahuasca and DMT Rescue ROS Production and Differentially Expressed Genes Observed after Predator and Psychosocial Stress: Relevance to Human PTSD. ACS Chem. Neurosci. 2022, 13, 257–274. [Google Scholar] [CrossRef]
- Vorobyeva, N.; Kozlova, A.A. Three Naturally-Occurring Psychedelics and Their Significance in the Treatment of Mental Health Disorders. Front. Pharmacol. 2022, 13, 927984. [Google Scholar] [CrossRef]
- Manske, R.H.F. A SYNTHESIS OF THE METHYLTRYPTAMINES AND SOME DERIVATIVES. Can. J. Res. 1931, 5, 592–600. [Google Scholar] [CrossRef]
- Gonçalves de Lima, O. Observações sobre o “vinho da Jurema” utilizado pelos índios Pancarú de Tacaratú (Pernambuco) [Observations on the “vinho de Jurema” used by the Pancaru’ Indians of Tacaratu’ (Pernambuco)]. Ariquivos Inst. Pesqui. Agron. 1946, 4, 45–80. [Google Scholar]
- Szára, S. Dimethyltryptamin: Its Metabolism in Man; the Relation to Its Psychotic Effect to the Serotonin Metabolism. Experientia 1956, 12, 441–442. [Google Scholar] [CrossRef]
- Shinozuka, K.; Tabaac, B.J.; Arenas, A.; Beutler, B.D.; Cherian, K.; Evans, V.D.; Fasano, C.; Muir, O.S. Psychedelic Therapy: A Primer for Primary Care Clinicians—N,N-Dimethyltryptamine and Ayahuasca. Am. J. Ther. 2024, 31, E112–E120. [Google Scholar] [CrossRef]
- Timmermann, C.; Roseman, L.; Schartner, M.; Milliere, R.; Williams, L.T.J.; Erritzoe, D.; Muthukumaraswamy, S.; Ashton, M.; Bendrioua, A.; Kaur, O.; et al. Neural Correlates of the DMT Experience Assessed with Multivariate EEG. Sci. Rep. 2019, 9, 16324. [Google Scholar] [CrossRef]
- Carbonaro, T.M.; Gatch, M.B. Neuropharmacology of N,N-Dimethyltryptamine. Brain Res. Bull. 2016, 126, 74–88. [Google Scholar] [CrossRef]
- Morales-Garcia, J.A.; Calleja-Conde, J.; Lopez-Moreno, J.A.; Alonso-Gil, S.; Sanz-SanCristobal, M.; Riba, J.; Perez-Castillo, A. N,N-Dimethyltryptamine Compound Found in the Hallucinogenic Tea Ayahuasca, Regulates Adult Neurogenesis in Vitro and in Vivo. Transl. Psychiatry 2020, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Bentz, E.N.; Lobayan, R.M.; Martínez, H.; Redondo, P.; Largo, A. Intrinsic Antioxidant Potential of the Aminoindole Structure: A Computational Kinetics Study of Tryptamine. J. Phys. Chem. B 2018, 122, 6386–6395. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Kovacs, A.; Frecska, E.; Rajnavolgyi, E. Psychedelic N,N-Dimethyltryptamine and 5-Methoxy-N,N-Dimethyltryptamine Modulate Innate and Adaptive Inflammatory Responses through the Sigma-1 Receptor of Human Monocyte-Derived Dendritic Cells. PLoS ONE 2014, 9, e106533. [Google Scholar] [CrossRef] [PubMed]
- Dakic, V.; Minardi Nascimento, J.; Costa Sartore, R.; MacIel, R.D.M.; De Araujo, D.B.; Ribeiro, S.; Martins-De-Souza, D.; Rehen, S.K. Short Term Changes in the Proteome of Human Cerebral Organoids Induced by 5-MeO-DMT. Sci. Rep. 2017, 7, 12863. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.H.; Bouso, J.C. Significance of Mammalian N,N-Dimethyltryptamine (DMT): A 60-Year-Old Debate. J. Psychopharmacol. 2022, 36, 905–919. [Google Scholar] [CrossRef]
- Axelrod, J. Enzymatic Formation of Psychotomimetic Metabolites from Normally Occurring Compounds. Science 1961, 134, 343. [Google Scholar] [CrossRef]
- Franzen, F.; Gross, H. Tryptamine, N,N-Dimethyltryptamine, N,N-Dimethyl-5-Hydroxytryptamine and 5-Methoxytryptamine in Human Blood and Urine. Nature 1965, 206, 1052. [Google Scholar] [CrossRef]
- Mandel, L.R.; Ahn, H.S.; VandenHeuvel, W.J.A.; Walker, R.W. Indoleamine-N-Methyl Transferase in Human Lung. Biochem. Pharmacol. 1972, 21, 1197–1200. [Google Scholar] [CrossRef]
- Mozier, N.M.; Mcconnell, K.P.; Hoffman, J.L. S-Adenosyl-L-Methionine:Thioether S-Methyltransferase, a New Enzyme in Sulfur and Selenium Metabolism. J. Biol. Chem. 1988, 263, 4527–4531. [Google Scholar] [CrossRef] [PubMed]
- Palmer, I.S.; Gunsalus, R.P.; Halverson, A.W.; Olson, O.E. Trimethylselenonium Ion as a General Excretory Product from Selenium Metabolism in the Rat. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1970, 208, 260–266. [Google Scholar] [CrossRef]
- Ganther, H.E. Pathways of Selenium Metabolism Including Respiratory Excretory Products. J. Am. Coll. Toxicol. 1986, 5, 1–5. [Google Scholar] [CrossRef]
- Kuehnelt, D.; Engström, K.; Skröder, H.; Kokarnig, S.; Schlebusch, C.; Kippler, M.; Alhamdow, A.; Nermell, B.; Francesconi, K.; Broberg, K.; et al. Selenium Metabolism to the Trimethylselenonium Ion (TMSe) Varies Markedly Because of Polymorphisms in the Indolethylamine N-Methyltransferase Gene. Am. J. Clin. Nutr. 2015, 102, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Torres, B.; Tyler, J.S.; Satyshur, K.A.; Ruoho, A.E. Human Indole(Ethyl)Amine-N-Methyltransferase (HINMT) Catalyzed Methylation of Tryptamine, Dimethylsulfide and Dimethylselenide Is Enhanced under Reducing Conditions—A Comparison between 254C and 254F, Two Common HINMT Variants. PLoS ONE 2019, 14, e0223546. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Kyono, R.; Shibukawa, Y.; Tanaka, Y.K.; Suzuki, N.; Ogra, Y. Differential Molecular Mechanisms of Substrate Recognition by Selenium Methyltransferases, INMT and TPMT, in Selenium Detoxification and Excretion. J. Biol. Chem. 2024, 300, 105599. [Google Scholar] [CrossRef] [PubMed]
- Strassman, R.J. Human Psychopharmacology of N,N-Dimethyltryptamine. Behav. Brain Res. 1995, 73, 121–124. [Google Scholar] [CrossRef]
- Takenaka, M.; Imai, E.; Kaneko, T.; Ito, T.; Moriyama, T.; Yamauchi, A.; Hori, M.; Kawamoto, S.; Okubo, K. Isolation of Genes Identified in Mouse Renal Proximal Tubule by Comparing Different Gene Expression Profiles. Kidney Int. 1998, 53, 562–572. [Google Scholar] [CrossRef]
- Gewin, L.S. Renal Fibrosis: Primacy of the Proximal Tubule. Matrix Biol. 2018, 68–69, 248. [Google Scholar] [CrossRef]
- Thompson, M.A.; Moon, E.; Kim, U.J.; Xu, J.; Siciliano, M.J.; Weinshilboum, R.M. Human Indolethylamine N-Methyltransferase: CDNA Cloning and Expression, Gene Cloning, and Chromosomal Localization. Genomics 1999, 61, 285–297. [Google Scholar] [CrossRef]
- Chu, U.B.; Vorperian, S.K.; Satyshur, K.; Eickstaedt, K.; Cozzi, N.V.; Mavlyutov, T.; Hajipour, A.R.; Ruoho, A.E. Noncompetitive Inhibition of Indolethylamine-N-Methyltransferase by N,N-Dimethyltryptamine and N,N-Dimethylaminopropyltryptamine. Biochemistry 2014, 53, 2956–2965. [Google Scholar] [CrossRef]
- Friedberg, L.M.; Sen, A.K.; Nguyen, Q.; Tonucci, G.P.; Hellwarth, E.B.; Gibbons, W.J.; Jones, J.A. In Vivo Biosynthesis of N,N-Dimethyltryptamine, 5-MeO-N,N-Dimethyltryptamine, and Bufotenine in E. Coli. Metab. Eng. 2023, 78, 61–71. [Google Scholar] [CrossRef]
- Barker, S.A. N,N-Dimethyltryptamine (DMT), an Endogenous Hallucinogen: Past, Present, and Future Research to Determine Its Role and Function. Front. Neurosci. 2018, 12, 536. [Google Scholar] [CrossRef]
- Cameron, L.P.; Olson, D.E. Dark Classics in Chemical Neuroscience: N,N-Dimethyltryptamine (DMT). ACS Chem. Neurosci. 2018, 9, 2344–2357. [Google Scholar] [CrossRef]
- Wallach, J.V. Endogenous Hallucinogens as Ligands of the Trace Amine Receptors: A Possible Role in Sensory Perception. Med. Hypotheses 2009, 72, 91–94. [Google Scholar] [CrossRef]
- Hua, H.; Fu, X.; Wang, W.; Wang, S.; Wang, D.; Wu, Z.; Zhang, Q.; He, T.; Yang, C. A Bibliometric Analysis of Research on Psychedelics for Depression Treatment. Heliyon 2024, 10, e36886. [Google Scholar] [CrossRef]
- Wyatt, R.J.; Mandel, L.R.; Ahn, H.S.; Walker, R.W.; Vanden Heuvel, W.J.A. Gas Chromatographic-Mass Spectrometric Isotope Dilution Determination of N,N-Dimethyltryptamine Concentrations in Normals and Psychiatric Patients. Psychopharmacologia 1973, 31, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Pantano, L.; Agyapong, G.; Shen, Y.; Zhuo, Z.; Fernandez-Albert, F.; Rust, W.; Knebel, D.; Hill, J.; Boustany-Kari, C.M.; Doerner, J.F.; et al. Molecular Characterization and Cell Type Composition Deconvolution of Fibrosis in NAFLD. Sci. Rep. 2021, 11, 18045. [Google Scholar] [CrossRef]
- Steinhauser, S.; Estoppey, D.; Buehler, D.P.; Xiong, Y.; Pizzato, N.; Rietsch, A.; Wu, F.; Leroy, N.; Wunderlin, T.; Claerr, I.; et al. The Transcription Factor ZNF469 Regulates Collagen Production in Liver Fibrosis [PREPRINT]. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Z.; Yang, S.; Fu, J.; Fan, Y.; Wang, N.; Hu, J.; Ma, L.; Peng, C.; Wang, Z.; et al. Kidney Single-Cell Transcriptome Profile Reveals Distinct Response of Proximal Tubule Cells to SGLT2i and ARB Treatment in Diabetic Mice. Mol. Ther. 2022, 30, 1741. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Hacker, A.; Langburt, D.; Dewar, M.; McFadden, M.J.; Zhang, H.; Kuzmanov, U.; Zhou, Y.Q.; Hussain, B.; Ehsan, F.; et al. Myocardial Infarction Induces Cardiac Fibroblast Transformation within Injured and Noninjured Regions of the Mouse Heart. J. Proteome Res. 2021, 20, 2867–2881. [Google Scholar] [CrossRef] [PubMed]
- Degertekin, B.; Ozenirler, S.; Elbeg, S.; Akyol, G. The Serum Endothelin-1 Level in Steatosis and NASH, and Its Relation with Severity of Liver Fibrosis. Dig. Dis. Sci. 2007, 52, 2622–2628. [Google Scholar] [CrossRef] [PubMed]
- van de Lest, N.A.; Bakker, A.E.; Dijkstra, K.L.; Zandbergen, M.; Heemskerk, S.A.C.; Wolterbeek, R.; Bruijn, J.A.; Scharpfenecker, M. Endothelial Endothelin Receptor A Expression Is Associated With Podocyte Injury and Oxidative Stress in Patients With Focal Segmental Glomerulosclerosis. Kidney Int. Rep. 2021, 6, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Smeijer, J.D.; Kohan, D.E.; Rossing, P.; Correa-Rotter, R.; Liew, A.; Tang, S.C.W.; de Zeeuw, D.; Gansevoort, R.T.; Ju, W.; Lambers Heerspink, H.J. Insulin Resistance, Kidney Outcomes and Effects of the Endothelin Receptor Antagonist Atrasentan in Patients with Type 2 Diabetes and Chronic Kidney Disease. Cardiovasc. Diabetol. 2023, 22, 251. [Google Scholar] [CrossRef] [PubMed]
- Lerman, A.; Holmes, D.R.; Bell, M.R.; Garratt, K.N.; Nishimura, R.A.; Burnett, J.C. Endothelin in Coronary Endothelial Dysfunction and Early Atherosclerosis in Humans. Circulation 1995, 92, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Distler, O. How Does Endothelial Cell Injury Start? The Role of Endothelin in Systemic Sclerosis. Arthritis Res. Ther. 2007, 9, S2. [Google Scholar] [CrossRef] [PubMed]
- Villanova, N.; Moscatiello, S.; Ramilli, S.; Bugianesi, E.; Magalotti, D.; Vanni, E.; Zoli, M.; Marchesini, G. Endothelial Dysfunction and Cardiovascular Risk Profile in Nonalcoholic Fatty Liver Disease. Hepatology 2005, 42, 473–480. [Google Scholar] [CrossRef]
- Hu, S.; Hang, X.; Wei, Y.; Wang, H.; Zhang, L.; Zhao, L. Crosstalk among Podocytes, Glomerular Endothelial Cells and Mesangial Cells in Diabetic Kidney Disease: An Updated Review. Cell Commun. Signal. 2024, 22, 136. [Google Scholar] [CrossRef]
- Xie, G.; Wang, X.; Wang, L.; Wang, L.; Atkinson, R.D.; Kanel, G.C.; Gaarde, W.A.; DeLeve, L.D. Role of Differentiation of Liver Sinusoidal Endothelial Cells in Progression and Regression of Hepatic Fibrosis in Rats. Gastroenterology 2012, 142, 918–927.e6. [Google Scholar] [CrossRef]
- Miyao, M.; Kotani, H.; Ishida, T.; Kawai, C.; Manabe, S.; Abiru, H.; Tamaki, K. Pivotal Role of Liver Sinusoidal Endothelial Cells in NAFLD/NASH Progression. Lab. Invest. 2015, 95, 1130–1144. [Google Scholar] [CrossRef]
- Marcuccilli, M.; Chonchol, M. NAFLD and Chronic Kidney Disease. Int. J. Mol. Sci. 2016, 17, 562. [Google Scholar] [CrossRef] [PubMed]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Lafoz, E.; Ruart, M.; Anton, A.; Oncins, A.; Hernández-Gea, V. The Endothelium as a Driver of Liver Fibrosis and Regeneration. Cells 2020, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.S.; Lee, K.H.; Goerdt, S.; Augustin, H.G. Angiodiversity and Organotypic Functions of Sinusoidal Endothelial Cells. Angiogenesis 2021, 24, 289. [Google Scholar] [CrossRef]
- Fujita, T.; Soontrapa, K.; Ito, Y.; Iwaisako, K.; Moniaga, C.S.; Asagiri, M.; Majima, M.; Narumiya, S. Hepatic Stellate Cells Relay Inflammation Signaling from Sinusoids to Parenchyma in Mouse Models of Immune-Mediated Hepatitis. Hepatology 2016, 63, 1325–1339. [Google Scholar] [CrossRef]
- Nagy, N.E.; Holven, K.B.; Roos, N.; Senoo, H.; Kojima, N.; Norum, K.R.; Blomhoff, R. Storage of Vitamin A in Extrahepatic Stellate Cells in Normal Rats. J. Lipid Res. 1997, 38, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.J.; Mandal, C.; Ghee, J.Y.; Yoo, J.A.; Lee, M.J.; Kang, Y.S.; Hyun, Y.Y.; Lee, J.E.; Kim, H.W.; Han, S.Y.; et al. Inhibition of Renal Stellate Cell Activation Reduces Renal Fibrosis. Biomedicines 2020, 8, 431. [Google Scholar] [CrossRef]
- Ezhilarasan, D. Endothelin-1 in Portal Hypertension: The Intricate Role of Hepatic Stellate Cells. Exp. Biol. Med. 2020, 245, 1504. [Google Scholar] [CrossRef]
- Pinzani, M.; Milani, S.; De Franco, R.; Grappone, C.; Caligiuri, A.; Gentilini, A.; Tosti-Guerra, C.; Maggi, M.; Failli, P.; Ruocco, C.; et al. Endothelin 1 Is Overexpressed in Human Cirrhotic Liver and Exerts Multiple Effects on Activated Hepatic Stellate Cells. Gastroenterology 1996, 110, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Giulia Battelli, M.; Bolognesi, A.; Korsmo, H.W.; Ekperikpe, U.S.; Daehn, I.S. Emerging Roles of Xanthine Oxidoreductase in Chronic Kidney Disease. Antioxidants 2024, 13, 712. [Google Scholar] [CrossRef]
- Chao, H.H.; Liu, J.C.; Lin, J.W.; Chen, C.H.; Wu, C.H.; Cheng, T.H. Uric Acid Stimulates Endothelin-1 Gene Expression Associated with NADPH Oxidase in Human Aortic Smooth Muscle Cells. Acta Pharmacol. Sin. 2008, 29, 1301–1312. [Google Scholar] [CrossRef]
- Khosla, U.M.; Zharikov, S.; Finch, J.L.; Nakagawa, T.; Roncal, C.; Mu, W.; Krotova, K.; Block, E.R.; Prabhakar, S.; Johnson, R.J. Hyperuricemia Induces Endothelial Dysfunction. Kidney Int. 2005, 67, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Daehn, I.S.; Duffield, J.S. The Glomerular Filtration Barrier: A Structural Target for Novel Kidney Therapies. Nat. Rev. Drug Discov. 2021, 20, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Meliambro, K.; He, J.C.; Campbell, K.N. Podocyte-Targeted Therapies—Progress and Future Directions. Nat. Rev. Nephrol. 2024, 20, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef] [PubMed]
- Ebefors, K.; Wiener, R.J.; Yu, L.; Azeloglu, E.U.; Yi, Z.; Jia, F.; Zhang, W.; Baron, M.H.; He, J.C.; Haraldsson, B.; et al. Endothelin Receptor-A Mediates Degradation of the Glomerular Endothelial Surface Layer via Pathologic Crosstalk between Activated Podocytes and Glomerular Endothelial Cells. Kidney Int. 2019, 96, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Abbate, M.; Zoja, C.; Morigi, M.; Rottoli, D.; Angioletti, S.; Tomasoni, S.; Zanchi, C.; Longaretti, L.; Donadelli, R.; Remuzzi, G. Transforming Growth Factor-Β1 Is Up-Regulated by Podocytes in Response to Excess Intraglomerular Passage of Proteins: A Central Pathway in Progressive Glomerulosclerosis. Am. J. Pathol. 2002, 161, 2179. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S. Mechanisms and Consequences of TGF-β Overexpression by Podocytes in Progressive Podocyte Disease. Cell Tissue Res. 2012, 347, 129–140. [Google Scholar] [CrossRef]
- Dar, W.A.; Sullivan, E.; Bynon, J.S.; Eltzschig, H.; Ju, C. Ischemia Reperfusion Injury in Liver Transplantation: Cellular and Molecular Mechanisms. Liver Int. 2019, 39, 788. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular Pathophysiology of Ischemic Acute Kidney Injury. J. Clin. Investig. 2011, 121, 4210. [Google Scholar] [CrossRef]
- Mallikarjuna, P.; Zhou, Y.; Landström, M. The Synergistic Cooperation between TGF-β and Hypoxia in Cancer and Fibrosis. Biomolecules 2022, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Takei, Y.; Kawano, S.; Nagano, K.; Tsuji, S.; Masuda, E.; Nishimura, Y.; Okumura, S.; Kashiwagi, T.; Fusamoto, H.; et al. Endothelin-1 Is Involved in the Pathogenesis of Ischemia/Reperfusion Liver Injury by Hepatic Microcirculatory Disturbances. Hepatology 1994, 19, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.; Hayashida, T.; Liang, X.; Schnaper, H.W. Hypoxia-Inducible Factor-1α Promotes Glomerulosclerosis and Regulates COL1A2 Expression through Interactions with Smad3. Kidney Int. 2016, 90, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Clambey, E.T.; McNamee, E.N.; Westrich, J.A.; Glover, L.E.; Campbell, E.L.; Jedlicka, P.; De Zoeten, E.F.; Cambier, J.C.; Stenmark, K.R.; Colgan, S.P.; et al. Hypoxia-Inducible Factor-1 Alpha-Dependent Induction of FoxP3 Drives Regulatory T-Cell Abundance and Function during Inflammatory Hypoxia of the Mucosa. Proc. Natl. Acad. Sci. USA 2012, 109, E2784–E2793. [Google Scholar] [CrossRef] [PubMed]
- Stow, L.R.; Jacobs, M.E.; Wingo, C.S.; Cain, B.D. Endothelin-1 Gene Regulation. FASEB J. 2011, 25, 16. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.R.; Sadik, A.; Sharma, S.; Poschet, G.; Gegner, H.M.; Lanz, T.V.; Lucarelli, P.; Klingmüller, U.; Platten, M.; Heiland, I.; et al. Hypoxia Routes Tryptophan Homeostasis Towards Increased Tryptamine Production. Front. Immunol. 2021, 12, 1. [Google Scholar] [CrossRef]
- Szabo, A.; Kovacs, A.; Riba, J.; Djurovic, S.; Rajnavolgyi, E.; Frecska, E. The Endogenous Hallucinogen and Trace Amine N,N-Dimethyltryptamine (DMT) Displays Potent Protective Effects against Hypoxia via Sigma-1 Receptor Activation in Human Primary IPSC-Derived Cortical Neurons and Microglia-like Immune Cells. Front. Neurosci. 2016, 10, 207848. [Google Scholar] [CrossRef]
- Nasiri-Ansari, N.; Androutsakos, T.; Flessa, C.M.; Kyrou, I.; Siasos, G.; Randeva, H.S.; Kassi, E.; Papavassiliou, A.G. Endothelial Cell Dysfunction and Nonalcoholic Fatty Liver Disease (NAFLD): A Concise Review. Cells 2022, 11, 2511. [Google Scholar] [CrossRef] [PubMed]
- Daehn, I.; Bottinger, E.P. Microvascular Endothelial Cells Poised to Take Center Stage in Experimental Renal Fibrosis. J. Am. Soc. Nephrol. 2015, 26, 767. [Google Scholar] [CrossRef]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The Hallucinogen N,N-Dimethyltryptamine (DMT) Is an Endogenous Sigma-1 Receptor Regulator. Science 2009, 323, 934. [Google Scholar] [CrossRef]
- Kärkkäinen, J.; Forsström, T.; Tornaeus, J.; Wähälä, K.; Kiuru, P.; Honkanen, A.; Stenman, U.H.; Turpeinen, U.; Hesso, A. Potentially Hallucinogenic 5-Hydroxytryptamine Receptor Ligands Bufotenine and Dimethyltryptamine in Blood and Tissues. Scand. J. Clin. Lab. Investig. 2005, 65, 189–199. [Google Scholar] [CrossRef]
- Wang, J.; Xu, D.; Shen, L.; Zhou, J.; Lv, X.; Ma, H.; Li, N.; Wu, Q.; Duan, J. Anti-Inflammatory and Analgesic Actions of Bufotenine through Inhibiting Lipid Metabolism Pathway. Biomed. Pharmacother. 2021, 140, 111749. [Google Scholar] [CrossRef] [PubMed]
- Batalla, M. All-Natural 5-MeO-DMT Sigma Receptor 1 Agonist and Its Therapeutic Impact in Mental and Neurodegener-Ative Diseases through Mitochondrial Activation. Sci. Rev. Biol. 2023, 2, 1–20. [Google Scholar] [CrossRef]
- Sawyer, E.M.; Jensen, L.E.; Meehl, J.B.; Larsen, K.P.; Petito, D.A.; Hurley, J.H.; Voeltz, G.K. SigmaR1 Shapes Rough Endoplasmic Reticulum Membrane Sheets. Dev. Cell 2024, 59, 2566–2577. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Su, X.; Dong, C.; He, Z.; Song, Q.; Song, C.; Zhou, J.; Liao, W.; Wang, C.; Yang, S.; et al. Sigma-1 Receptor Exerts Protective Effects on Ameliorating Nephrolithiasis by Modulating Endoplasmic Reticulum-Mitochondrion Association and Inhibiting Endoplasmic Reticulum Stress-Induced Apoptosis in Renal Tubular Epithelial Cells. Redox Rep. 2024, 29, 2391139. [Google Scholar] [CrossRef]
- Bernard-Marissal, N.; Médard, J.J.; Azzedine, H.; Chrast, R. Dysfunction in Endoplasmic Reticulum-Mitochondria Crosstalk Underlies SIGMAR1 Loss of Function Mediated Motor Neuron Degeneration. Brain 2015, 138, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, C.S.; Aishwarya, R.; Alam, S.; Remex, N.S.; Morshed, M.; Nitu, S.; Miriyala, S.; Panchatcharam, M.; Hartman, B.; King, J.; et al. The Molecular Role of Sigmar1 in Regulating Mitochondrial Function through Mitochondrial Localization in Cardiomyocytes. Mitochondrion 2022, 62, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, R.; Abdullah, C.S.; Morshed, M.; Remex, N.S.; Bhuiyan, M.S. Sigmar1’s Molecular, Cellular, and Biological Functions in Regulating Cellular Pathophysiology. Front. Physiol. 2021, 12, 705575. [Google Scholar] [CrossRef]
- Saleh, S.R.; Younis, F.A.; Abdelrahman, S.S.; Attia, A.A.; El-Demellawy, M.A.; Newairy, A.A.; Ghareeb, D.A. Attenuation of High-Fat High-Sucrose Diet and CCl4-Induced Non-Alcoholic Steatohepatitis in Rats by Activating Autophagy and SIGMAR1/GRP78/ITPR1 Signaling Using Berberine-Loaded Albumin Nanoparticles: In Vivo Prediction and in-Silico Molecular Modeling. J. Pharm. Investig. 2024, 54, 1–24. [Google Scholar] [CrossRef]
- Munguia-Galaviz, F.J.; Miranda-Diaz, A.G.; Cardenas-Sosa, M.A.; Echavarria, R. Sigma-1 Receptor Signaling: In Search of New Therapeutic Alternatives for Cardiovascular and Renal Diseases. Int. J. Mol. Sci. 2023, 24, 1997. [Google Scholar] [CrossRef]
- Zhang, K.K.; Yang, J.Z.; Cheng, C.H.; Wan, J.Y.; Chen, Y.C.; Zhou, H.Q.; Zheng, D.K.; Lan, Z.X.; You, Q.H.; Wang, Q.; et al. Short-Chain Fatty Acids Mitigate Methamphetamine-Induced Hepatic Injuries in a Sigma-1 Receptor-Dependent Manner. Ecotoxicol. Environ. Saf. 2024, 280, 116538. [Google Scholar] [CrossRef] [PubMed]
- Remex, N.S.; Abdullah, C.S.; Aishwarya, R.; Kolluru, G.K.; Traylor, J.; Bhuiyan, M.A.N.; Kevil, C.G.; Orr, A.W.; Rom, O.; Pattillo, C.B.; et al. Deletion of Sigmar1 Leads to Increased Arterial Stiffness and Altered Mitochondrial Respiration Resulting in Vascular Dysfunction. Front. Physiol. 2024, 15, 1386296. [Google Scholar] [CrossRef] [PubMed]
- Mavlyutov, T.A.; Epstein, M.L.; Liu, P.; Verbny, Y.I.; Ziskind-Conhaim, L.; Ruoho, A.E. Development of the Sigma-1 Receptor in C-Terminals of Motoneurons and Colocalization with the N,N′-Dimethyltryptamine Forming Enzyme, Indole-N-Methyl Transferase. Neuroscience 2012, 206, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Frecska, E.; Bokor, P.; Winkelman, M. The Therapeutic Potentials of Ayahuasca: Possible Effects against Various Diseases of Civilization. Front. Pharmacol. 2016, 7, 174229. [Google Scholar] [CrossRef]
- Aguanno, A.; Afar, R.; Albert, V.R. Tissue-Specific Expression of the Nonneuronal Promoter of the Aromatic L-Amino Acid Decarboxylase Gene Is Regulated by Hepatocyte Nuclear Factor. J. Biol. Chem. 1996, 271, 4528–4538. [Google Scholar] [CrossRef]
- Párrizas, M.; Maestro, M.A.; Boj, S.F.; Paniagua, A.; Casamitjana, R.; Gomis, R.; Rivera, F.; Ferrer, J. Hepatic Nuclear Factor 1-α Directs Nucleosomal Hyperacetylation to Its Tissue-Specific Transcriptional Targets. Mol. Cell Biol. 2001, 21, 3234–3243. [Google Scholar] [CrossRef]
- Rebouissou, S.; Imbeaud, S.; Balabaud, C.; Boulanger, V.; Bertrand-Michel, J.; Tercé, F.; Auffray, C.; Bioulac-Sage, P.; Zucman-Rossi, J.; Bertrand, J. HNF1alpha Inactivation Promotes Lipogenesis in Human Hepatocellular Adenoma Independently of SREBP-1 and Carbohydrate-Response Element-Binding Protein (ChREBP) Activation. J. Biol. Chem. 2007, 282, 14437–14446. [Google Scholar] [CrossRef]
- Dean, J.G. Indolethylamine- N-Methyltransferase Polymorphisms: Genetic and Biochemical Approaches for Study of Endogenous N,N,-Dimethyltryptamine. Front. Neurosci. 2018, 12, 232. [Google Scholar] [CrossRef]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics Promote Neuroplasticity Through Activation of Intracellular 5-HT2A Receptors. Science 2023, 379, 700. [Google Scholar] [CrossRef]
- Servillo, L.; Giovane, A.; Balestrieri, M.L.; Cautela, D.; Castaldo, D. N-Methylated Tryptamine Derivatives in Citrus Genus Plants: Identification of N,N,N-Trimethyltryptamine in Bergamot. J. Agric. Food Chem. 2012, 60, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Chadeayne, A.R.; Manke, D.R.; Pham, D.N.K.; Reid, B.G.; Golen, J.A. Active Metabolite of Aeruginascin (4-Hydroxy-N,N,N-Trimethyltryptamine): Synthesis, Structure, and Serotonergic Binding Affinity. ACS Omega 2020, 5, 16940–16943. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.M.; Fitzpatrick, P.F. Mechanisms of Tryptophan and Tyrosine Hydroxylase. IUBMB Life 2013, 65, 350. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the Significance of an Alternate Pathway of Melatonin Synthesis via 5-Methoxytryptamine: Comparisons across Species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef]
- Wiechmann, A.F.; Bok, D.; Horwitzj, J. Localization of Hydroxyindole-O-Methyltransferase in the Mammalian Pineal Gland and Retina. Invest. Ophthalmol. Vis. Sci. 1985, 26, 253–265. [Google Scholar] [PubMed]
- Wyatt, R.J.; Saavedra, J.M.; Axelrod, J. A Dimethyltryptamine-Forming Enzyme in Human Blood. Am. J. Psychiatry 1973, 130, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Zohairi, F.; Khandelia, H.; Hakami Zanjani, A.A. Interaction of Psychedelic Tryptamine Derivatives with a Lipid Bilayer. Chem. Phys. Lipids 2023, 251, 105279. [Google Scholar] [CrossRef]
- Mandel, L.R.; Walker, R.W. The Biosynthesis of 5-Methoxy-N,N-Dimethyltryptamine Invitro. Life Sci. 1974, 15, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Glynos, N.G.; Carter, L.; Lee, S.J.; Kim, Y.; Kennedy, R.T.; Mashour, G.A.; Wang, M.M.; Borjigin, J. Indolethylamine N-Methyltransferase (INMT) Is Not Essential for Endogenous Tryptamine-Dependent Methylation Activity in Rats. Sci. Rep. 2023, 13, 280. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Zhong, S.; Jeong, J.H.; Huang, C.; Chen, X.; Dickinson, S.I.; Dhillon, J.; Yang, L.; Luo, J.L. Targeting INMT and Interrupting Its Methylation Pathway for the Treatment of Castration Resistant Prostate Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 307. [Google Scholar] [CrossRef]
- Cui, K.; Yao, X.; Wei, Z.; Yang, Y.; Liu, X.; Huang, Z.; Huo, H.; Tang, J.; Xie, Y. Poor Prognosis, Hypomethylation, and Immune Infiltrates Are Associated with Downregulation of INMT in Head and Neck Squamous Cell Carcinoma. Front. Genet. 2022, 13, 917344. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, L.P.; Silva, S.R.B.; de Lima-Neto, P.; Monteiro, N.d.K.V. A Mechanistic Insight for the Biosynthesis of N,N-Dimethyltryptamine: An ONIOM Theoretical Approach. Biochem. Biophys. Res. Commun. 2023, 678, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kubicskó, K.; Farkas, Ö. Quantum Chemical (QM:MM) Investigation of the Mechanism of Enzymatic Reaction of Tryptamine and: N,N-Dimethyltryptamine with Monoamine Oxidase A. Org. Biomol. Chem. 2020, 18, 9660–9674. [Google Scholar] [CrossRef] [PubMed]
- Burden, D.A.; Philips, S.R. Kinetic Measurements of the Turnover Rates of Phenylethylamine and Tryptamine in Vivo in the Rat Brain. J. Neurochem. 1980, 34, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422.e21. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Henry, W.S.; Ricq, E.L.; Graham, E.T.; Phadnis, V.V.; Maretich, P.; Paradkar, S.; Boehnke, N.; Deik, A.A.; Reinhardt, F.; et al. Plasticity of Ether Lipids Promotes Ferroptosis Susceptibility and Evasion. Nature 2020, 585, 603. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.A.; Clostio, A.J.; Houston, I.R.; Ruiz, J.; Magtanong, L.; Dixon, S.J.; Watts, J.L. Ether Lipid Deficiency Disrupts Lipid Homeostasis Leading to Ferroptosis Sensitivity. PLoS Genet. 2022, 18, e1010436. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of Plasmalogen Lipids in Health and Disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2012, 1822, 1442–1452. [Google Scholar] [CrossRef]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and Degradation Products of Tryptophan in Solution and Proteins. Free Radic. Biol. Med. 2020, 160, 696–718. [Google Scholar] [CrossRef]
- Banushi, B.; Polito, V. A Comprehensive Review of the Current Status of the Cellular Neurobiology of Psychedelics. Biology 2023, 12, 1380. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.M.; Coimbra, J.B.; Clara, R.O.; Dörr, F.A.; Moreno, A.C.R.; Chagas, J.R.; Tufik, S.; Pinto, E.; Catalani, L.H.; Campa, A. Biosynthesis of N,N-Dimethyltryptamine (DMT) in a Melanoma Cell Line and Its Metabolization by Peroxidases. Biochem. Pharmacol. 2014, 88, 393–401. [Google Scholar] [CrossRef]
- Madrid-Gambin, F.; Fabregat-Safont, D.; Gomez-Gomez, A.; Olesti, E.; Mason, N.L.; Ramaekers, J.G.; Pozo, O.J. Present and Future of Metabolic and Metabolomics Studies Focused on Classical Psychedelics in Humans. Biomed. Pharmacother. 2023, 169, 115775. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S.; Bröer, A. Amino Acid Homeostasis and Signalling in Mammalian Cells and Organisms. Biochem. J. 2017, 474, 1935–1963. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, T.; Camargo, S.M.R.; Herzog, B.; Bordin, M.; Pos, K.M.; Verrey, F. Recycling of Aromatic Amino Acids via TAT1 Allows Efflux of Neutral Amino Acids via LAT2-4F2hc Exchanger. Pflug. Arch. 2007, 454, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Teunis, C.; Nieuwdorp, M.; Hanssen, N. Interactions between Tryptophan Metabolism, the Gut Microbiome and the Immune System as Potential Drivers of Non-Alcoholic Fatty Liver Disease (NAFLD) and Metabolic Diseases. Metabolites 2022, 12, 514. [Google Scholar] [CrossRef]
- Salminen, A. Activation of Aryl Hydrocarbon Receptor (AhR) in Alzheimer’s Disease: Role of Tryptophan Metabolites Generated by Gut Host-Microbiota. J. Mol. Med. 2023, 101, 201–222. [Google Scholar] [CrossRef]
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine Emerges from the Shadows—Current Knowledge on Its Fate and Function. Pharmacol. Ther. 2021, 225, 107845. [Google Scholar] [CrossRef]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef]
- Choi, W.; Moon, J.H.; Kim, H. Serotonergic Regulation of Energy Metabolism in Peripheral Tissues. J. Endocrinol. 2020, 245, R1–R10. [Google Scholar] [CrossRef]
- Shen, L.; Lv, X.; Yang, X.; Deng, S.; Liu, L.; Zhou, J.; Zhu, Y.; Ma, H. Bufotenines-Loaded Liposome Exerts Anti-Inflammatory, Analgesic Effects and Reduce Gastrointestinal Toxicity through Altering Lipid and Bufotenines Metabolism. Biomed. Pharmacother. 2022, 153, 113492. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Félix-Soriano, E.; Wright, K.R.; Shen, H.; Baer, L.A.; Stanford, K.I.; Guo, L.-W. Differential Responses to Sigma-1 or Sigma-2 Receptor Ablation in Adiposity, Fat Oxidation, and Sexual Dimorphism. Int. J. Mol. Sci. 2022, 2022, 10846. [Google Scholar] [CrossRef]
- Guo, M.; Liu, R.; Zhang, F.; Qu, J.; Yang, Y.; Li, X. A New Perspective on Liver Diseases: Focusing on the Mitochondria-Associated Endoplasmic Reticulum Membranes. Pharmacol. Res. 2024, 208, 107409. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.S. Psychedelics and the Human Receptorome. PLoS ONE 2010, 5, 9019. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Multiple Receptors Contribute to the Behavioral Effects of Indoleamine Hallucinogens. Neuropharmacology 2011, 61, 364. [Google Scholar] [CrossRef]
- McKenna, D.; Riba, J. New World Tryptamine Hallucinogens and the Neuroscience of Ayahuasca. Curr. Top. Behav. Neurosci. 2018, 36, 283–311. [Google Scholar]
- Jacob, M.S.; Presti, D.E. Endogenous Psychoactive Tryptamines Reconsidered: An Anxiolytic Role for Dimethyltryptamine. Med. Hypotheses 2005, 64, 930–937. [Google Scholar] [CrossRef]
- Kozell, L.B.; Eshleman, A.J.; Swanson, T.L.; Bloom, S.H.; Wolfrum, K.M.; Schmachtenberg, J.L.; Olson, R.J.; Janowsky, A.; Abbas, A.I. Pharmacologic Activity of Substituted Tryptamines at 5-Hydroxytryptamine (5-HT)2A Receptor (5-HT2AR), 5-HT2CR, 5-HT1AR, and Serotonin Transporter. J. Pharmacol. Exp. Ther. 2023, 385, 62. [Google Scholar] [CrossRef]
- Namkung, J.; Shong, K.E.; Kim, H.; Oh, C.M.; Park, S.; Kim, H. Inhibition of Serotonin Synthesis Induces Negative Hepatic Lipid Balance. Diabetes Metab. J. 2018, 42, 233–243. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092. [Google Scholar] [CrossRef]
- Watanabe, H.; Akasaka, D.; Ogasawara, H.; Sato, K.; Miyake, M.; Saito, K.; Takahashi, Y.; Kanaya, T.; Takakura, I.; Hondo, T.; et al. Peripheral Serotonin Enhances Lipid Metabolism by Accelerating Bile Acid Turnover. Endocrinology 2010, 151, 4776–4786. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Oh, C.M.; Kim, H. Serotonin in the Regulation of Systemic Energy Metabolism. J. Diabetes Investig. 2022, 13, 1639. [Google Scholar] [CrossRef] [PubMed]
- El-Merahbi, R.; Löffler, M.; Mayer, A.; Sumara, G. The Roles of Peripheral Serotonin in Metabolic Homeostasis. FEBS Lett. 2015, 589, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Ruddell, R.G.; Oakley, F.; Hussain, Z.; Yeung, I.; Bryan-Lluka, L.J.; Ramm, G.A.; Mann, D.A. A Role for Serotonin (5-HT) in Hepatic Stellate Cell Function and Liver Fibrosis. Am. J. Pathol. 2006, 169, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Redenšek Trampuž, S.; van Riet, S.; Nordling, Å.; Ingelman-Sundberg, M. Mechanisms of 5-HT Receptor Antagonists in the Regulation of Fibrosis in a 3D Human Liver Spheroid Model. Sci. Rep. 2024, 14, 1396. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeong, W.; Yun, C.; Kim, H.; Oh, C.M. Serotonergic Regulation of Hepatic Energy Metabolism. Endocrinol. Metab. 2021, 36, 1151. [Google Scholar] [CrossRef]
- Shajib, M.S.; Khan, W.I. The Role of Serotonin and Its Receptors in Activation of Immune Responses and Inflammation. Acta Physiol. 2015, 213, 561–574. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Gülçin, I. Measurement of Antioxidant Ability of Melatonin and Serotonin by the DMPD and CUPRAC Methods as Trolox Equivalent. J. Enzym. Inhib. Med. Chem. 2008, 23, 871–876. [Google Scholar] [CrossRef]
- Azouzi, S.; Santuz, H.; Morandat, S.; Pereira, C.; Côté, F.; Hermine, O.; El Kirat, K.; Colin, Y.; Le Van Kim, C.; Etchebest, C.; et al. Antioxidant and Membrane Binding Properties of Serotonin Protect Lipids from Oxidation. Biophys. J. 2017, 112, 1863. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-Methylenedioxymethamphetamine, Lysergic Acid Diethylamide, and Metabolites of the Catecholamine Neurotransmitters Are Agonists of a Rat Trace Amine Receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.D.; Gainetdinov, R.R.; Hoener, M.C.; Shahid, M. Pharmacology of Human Trace Amine-Associated Receptors: Therapeutic Opportunities and Challenges. Pharmacol. Ther. 2017, 180, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Vaganova, A.N.; Katolikova, N.V.; Murtazina, R.Z.; Kuvarzin, S.R.; Gainetdinov, R.R. Public Transcriptomic Data Meta-Analysis Demonstrates TAAR6 Expression in the Mental Disorder-Related Brain Areas in Human and Mouse Brain. Biomolecules 2022, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Sotnikova, T.D.; Caron, M.G.; Gainetdinov, R.R. Trace Amine-Associated Receptors as Emerging Therapeutic Targets. Mol. Pharmacol. 2009, 76, 229. [Google Scholar] [CrossRef] [PubMed]
- Jragh, D.; Chandrasekhar, B.; Yousif, M.H.M.; Oriowo, M.A. Vasodilator Effect of Trace Amines, 3-Iodothyronamine, and RO5263397 in the Rat Perfused Kidney: Comparison with Tryptamine. Pharmacology 2023, 108, 368–378. [Google Scholar] [CrossRef]
- Zhu, C.P.; Liu, S.Q.; Wang, K.Q.; Xiong, H.L.; Aristu-Zabalza, P.; Boyer-Díaz, Z.; Feng, J.F.; Song, S.H.; Luo, C.; Chen, W.S.; et al. Targeting 5-Hydroxytryptamine Receptor 1A in the Portal Vein to Decrease Portal Hypertension. Gastroenterology 2024, 167, 993–1007. [Google Scholar] [CrossRef]
- Housset, C.; Rockey, D.C.; Bissell, D.M. Endothelin Receptors in Rat Liver: Lipocytes as a Contractile Target for Endothelin 1. Proc. Natl. Acad. Sci. USA 1993, 90, 9266–9270. [Google Scholar] [CrossRef]
- Rockey, D.C.; Weisiger, R.A. Endothelin Induced Contractility of Stellate Cells from Normal and Cirrhotic Rat Liver: Implications for Regulation of Portal Pressure and Resistance. Hepatology 1996, 24, 233–240. [Google Scholar] [CrossRef]
- Gabriel, A.; Kuddus, R.H.; Abdul, S.R.; Watkins, W.D.; Gandhi, C.R. Superoxide-Induced Changes in Endothelin (ET) Receptors in Hepatic Stellate Cells. J. Hepatol. 1998, 29, 614–627. [Google Scholar] [CrossRef]
- Rockey, D.C. Stellate Cells and Portal Hypertension. In Stellate Cells in Health and Disease; Academic Press: Cambridge, MA, USA, 2015; pp. 125–144. ISBN 9780128005446. [Google Scholar]
- Albadawy, R.; Agwa, S.H.A.; Khairy, E.; Saad, M.; El Touchy, N.; Othman, M.; El Kassas, M.; Matboli, M. Circulatory Endothelin 1-Regulating Rnas Panel: Promising Biomarkers for Non-Invasive Nafld/Nash Diagnosis and Stratification: Clinical and Molecular Pilot Study. Genes 2021, 12, 1813. [Google Scholar] [CrossRef]
- Okamoto, T.; Koda, M.; Miyoshi, K.; Onoyama, T.; Kishina, M.; Matono, T.; Sugihara, T.; Hosho, K.; Okano, J.; Isomoto, H.; et al. Antifibrotic Effects of Ambrisentan, an Endothelin-A Receptor Antagonist, in a Non-Alcoholic Steatohepatitis Mouse Model. World J. Hepatol. 2016, 8, 933. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Singh, S.; Tanaka, N.; Umemura, T. Role of G Protein-Coupled Receptors in Hepatic Stellate Cells and Approaches to Anti-Fibrotic Treatment of Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 773432. [Google Scholar] [CrossRef] [PubMed]
- Böhm, F.; Pernow, J. The Importance of Endothelin-1 for Vascular Dysfunction in Cardiovascular Disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Yanagisawa, M. Endothelin: 30 Years from Discovery to Therapy. Hypertension 2019, 74, 1232–1265. [Google Scholar] [CrossRef]
- Shi-Wen, X.; Chen, Y.; Denton, C.P.; Eastwood, M.; Renzoni, E.A.; Bou-Gharios, G.; Pearson, J.D.; Dashwood, M.; Du Bois, R.M.; Black, C.M.; et al. Endothelin-1 Promotes Myofibroblast Induction through the ETA Receptor via a Rac/Phosphoinositide 3-Kinase/Akt-Dependent Pathway and Is Essential for the Enhanced Contractile Phenotype of Fibrotic Fibroblasts. Mol. Biol. Cell 2004, 15, 2707–2719. [Google Scholar] [CrossRef]
- Dupuis, J.; Stewart, D.J.; Cernacek, P.; Gosselin, G. Human Pulmonary Circulation Is an Important Site for Both Clearance and Production of Endothelin-1. Circulation 1996, 94, 1578–1584. [Google Scholar] [CrossRef]
- Lassén, E.; Daehn, I.S. Clues to Glomerular Cell Chatter in Focal Segmental Glomerulosclerosis: Via Endothelin-1/ET A R. Kidney Int. Rep. 2021, 6, 1758–1760. [Google Scholar] [CrossRef]
- Lassén, E.; Daehn, I.S. Molecular Mechanisms in Early Diabetic Kidney Disease: Glomerular Endothelial Cell Dysfunction. Int. J. Mol. Sci. 2020, 21, 9456. [Google Scholar] [CrossRef]
- Qi, H.; Casalena, G.; Shi, S.; Yu, L.; Ebefors, K.; Sun, Y.; Zhang, W.; D’Agati, V.; Schlondorff, D.; Haraldsson, B.; et al. Glomerular Endothelial Mitochondrial Dysfunction Is Essential and Characteristic of Diabetic Kidney Disease Susceptibility. Diabetes 2017, 66, 763–778. [Google Scholar] [CrossRef]
- Daehn, I.; Casalena, G.; Zhang, T.; Shi, S.; Fenninger, F.; Barasch, N.; Yu, L.; D’Agati, V.; Schlondorff, D.; Kriz, W.; et al. Endothelial Mitochondrial Oxidative Stress Determines Podocyte Depletion in Segmental Glomerulosclerosis. J. Clin. Investig. 2014, 124, 1608. [Google Scholar] [CrossRef]
- Cho, J.-J.; Hocher, B.; Herbst, H.; Jia, J.-D.; Ruehl, M.; Hahn, E.G.; Otto Riecken, E.; Schuppan, D. An Oral Endothelin-A Receptor Antagonist Blocks Collagen Synthesis and Deposition in Advanced Rat Liver Fibrosis. Gastroenerology 2000, 118, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Örmeci, N. Endothelins and Liver Cirrhosis. Portal Hypertens. Cirrhosis 2022, 1, 66–72. [Google Scholar] [CrossRef]
- Lambers, C.; Roth, M.; Zhong, J.; Campregher, C.; Binder, P.; Burian, B.; Petkov, V.; Block, L.H. The Interaction of Endothelin-1 and TGF-Β1 Mediates Vascular Cell Remodeling. PLoS ONE 2013, 8, e73399. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Shaul, P.W.; Borok, Z.; Willis, B.C. Endothelin-1 Induces Alveolar Epithelial–Mesenchymal Transition through Endothelin Type A Receptor–Mediated Production of TGF-Β1. Am. J. Respir. Cell Mol. Biol. 2007, 37, 38. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhu, J.; Wang, R.; Chen, X.; Mi, L.; Walz, T.; Springer, T.A. Latent TGF-β Structure and Activation. Nature 2011, 474, 343. [Google Scholar] [CrossRef] [PubMed]
- Hiepen, C.; Mendez, P.L.; Knaus, P. It Takes Two to Tango: Endothelial TGFβ/BMP Signaling Crosstalk with Mechanobiology. Cells 2020, 9, 1965. [Google Scholar] [CrossRef]
- Goumans, M.J.; Liu, Z.; Ten Dijke, P. TGF-β Signaling in Vascular Biology and Dysfunction. Cell Res. 2008, 19, 116–127. [Google Scholar] [CrossRef]
- Chen, P.Y.; Qin, L.; Li, G.; Wang, Z.; Dahlman, J.E.; Malagon-Lopez, J.; Gujja, S.; Cilfone, N.A.; Kauffman, K.J.; Sun, L.; et al. Endothelial TGF-β Signalling Drives Vascular Inflammation and Atherosclerosis. Nat. Metab. 2019, 1, 912. [Google Scholar] [CrossRef]
- Blann, A.D.; Wang, J.M.; Wilson, P.B.; Kumar, S. Serum Levels of the TGF-Beta Receptor Are Increased in Atherosclerosis. Atherosclerosis 1996, 120, 221–226. [Google Scholar] [CrossRef]
- Ashcroft, G.S. Bidirectional Regulation of Macrophage Function by TGF-β. Microbes Infect. 1999, 1, 1275–1282. [Google Scholar] [CrossRef]
- Ueshima, E.; Fujimori, M.; Kodama, H.; Felsen, D.; Chen, J.; Durack, J.C.; Solomon, S.B.; Coleman, J.A.; Srimathveeravalli, G. Macrophage-Secreted TGF-Β1 Contributes to Fibroblast Activation and Ureteral Stricture after Ablation Injury. Am. J. Physiol. Ren. Physiol. 2019, 317, F52. [Google Scholar] [CrossRef]
- Ten Hove, M.; Smyris, A.; Booijink, R.; Wachsmuth, L.; Hansen, U.; Alic, L.; Faber, C.; Höltke, C.; Bansal, R. Engineered SPIONs Functionalized with Endothelin a Receptor Antagonist Ameliorate Liver Fibrosis by Inhibiting Hepatic Stellate Cell Activation. Bioact. Mater. 2024, 39, 406. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Í.; Varga, V.; Dvorácskó, S.; Farkas, A.E.; Körmöczi, T.; Berkecz, R.; Kecskés, S.; Menyhárt, Á.; Frank, R.; Hantosi, D.; et al. N,N-Dimethyltryptamine Attenuates Spreading Depolarization and Restrains Neurodegeneration by Sigma-1 Receptor Activation in the Ischemic Rat Brain. Neuropharmacology 2021, 192, 108612. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Fontanilla, D.; Gopalakrishnan, A.; Chae, Y.K.; Markley, J.L.; Ruoho, A.E. The Sigma-1 Receptor Protects against Cellular Oxidative Stress and Activates Antioxidant Response Elements. Eur. J. Pharmacol. 2012, 682, 12. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.A.; Seki, S.M.; Fernández-Castañeda, A.; Beiter, R.M.; Eccles, J.D.; Woodfolk, J.A.; Gaultier, A. Modulation of the Sigma-1 Receptor-IRE1 Pathway Is Beneficial in Preclinical Models of Inflammation and Sepsis. Sci. Transl. Med. 2019, 11, eaau5266. [Google Scholar] [CrossRef]
- Das, E.; Sahu, K.K.; Roy, I. The Functional Role of Ire1 in Regulating Autophagy and Proteasomal Degradation under Prolonged Proteotoxic Stress. FEBS J. 2023, 290, 3270–3289. [Google Scholar] [CrossRef]
- An, Y.; Qi, Y.; Li, Y.; Li, Z.; Yang, C.; Jia, D. Activation of the Sigma-1 Receptor Attenuates Blood-Brain Barrier Disruption by Inhibiting Amyloid Deposition in Alzheimer’s Disease Mice. Neurosci. Lett. 2022, 774, 136528. [Google Scholar] [CrossRef]
- Gaultier, A.; Hollister, M.; Reynolds, I.; Hsieh, E.H.; Gonias, S.L. LRP1 Regulates Remodeling of the Extracellular Matrix by Fibroblasts. Matrix Biol. 2010, 29, 22–30. [Google Scholar] [CrossRef]
- Brailoiu, E.; Chakraborty, S.; Brailoiu, G.C.; Zhao, P.; Barr, J.L.; Ilies, M.A.; Unterwald, E.M.; Abood, M.E.; Taylor, C.W. Choline Is an Intracellular Messenger Linking Extracellular Stimuli to IP3-Evoked Ca2+ Signals through Sigma-1 Receptors. Cell Rep. 2019, 26, 330. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Jiang, X. One Carbon Metabolism and Early Development: A Diet-Dependent Destiny. Trends Endocrinol. Metab. 2021, 32, 579–593. [Google Scholar] [CrossRef]
- Shioda, N.; Ishikawa, K.; Tagashira, H.; Ishizuka, T.; Yawo, H.; Fukunaga, K. Expression of a Truncated Form of the Endoplasmic Reticulum Chaperone Protein, Σ1 Receptor, Promotes Mitochondrial Energy Depletion and Apoptosis. J. Biol. Chem. 2012, 287, 23318–23331. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation by-Products and Their Impact on Host Health. Microbiome 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Korsmo, H.W.; Jiang, X.; Caudill, M.A. Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients 2019, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 5214. [Google Scholar] [CrossRef]
- Andrikopoulos, P.; Aron-Wisnewsky, J.; Chakaroun, R.; Myridakis, A.; Forslund, S.K.; Nielsen, T.; Adriouch, S.; Holmes, B.; Chilloux, J.; Vieira-Silva, S.; et al. Evidence of a Causal and Modifiable Relationship between Kidney Function and Circulating Trimethylamine N-Oxide. Nat. Commun. 2023, 14, 5843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How Gut Microbiota Contributes to Heart Failure. Transl. Res. 2021, 228, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The Severity of Nonalcoholic Fatty Liver Disease Is Associated with Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Kearns, R. The Kynurenine Pathway in Gut Permeability and Inflammation. Inflammation 2024, 47, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct Signatures of Gut Microbiome and Metabolites Associated with Significant Fibrosis in Non-Obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells 2022, 11, 2296. [Google Scholar] [CrossRef]
- Long, Q.; Luo, F.; Li, B.; Li, Z.; Guo, Z.; Chen, Z.; Wu, W.; Hu, M. Gut Microbiota and Metabolic Biomarkers in Metabolic Dysfunction–Associated Steatotic Liver Disease. Hepatol. Commun. 2024, 8, e0310. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.G.; Kim, S.M.; Caussy, C.; Fu, T.; Guo, J.; Bassirian, S.; Singh, S.; Madamba, E.V.; Bettencourt, R.; Richards, L.; et al. A Universal Gut-Microbiome-Derived Signature Predicts Cirrhosis. Cell Metab. 2020, 32, 878–888.e6. [Google Scholar] [CrossRef] [PubMed]
- Crost, E.H.; Coletto, E.; Bell, A.; Juge, N. Ruminococcus Gnavus: Friend or Foe for Human Health. FEMS Microbiol. Rev. 2023, 47, fuad014. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, S.; Kumar, R.M.S.; Priya, P.S.; Haridevamuthu, B.; Nayak, S.P.R.R.; Chulenbayeva, L.; Almagul, K.; Arockiaraj, J. Gut Enterobacteriaceae and Uraemic Toxins—Perpetrators for Ageing. Exp. Gerontol. 2023, 173, 112088. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Xiao, H.; Lin, C.; Wong, H.L.X.; Lam, Y.Y.; Gong, M.; Wu, G.; Ning, Z.; Huang, C.; Zhang, Y.; et al. Gut Microbiota-Derived Tryptamine and Phenethylamine Impair Insulin Sensitivity in Metabolic Syndrome and Irritable Bowel Syndrome. Nat. Commun. 2023, 14, 4986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Wang, X.; Ren, X.; Liu, Y. Changes of Intestinal Bacterial Microbiota in Coronary Heart Disease Complicated with Nonalcoholic Fatty Liver Disease. BMC Genom. 2019, 20, 862. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; Desantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic Kidney Disease Alters Intestinal Microbial Flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Shah, N.B.; Allegretti, A.S.; Nigwekar, S.U.; Kalim, S.; Zhao, S.; Lelouvier, B.; Servant, F.; Serena, G.; Thadhani, R.I.; Raj, D.S.; et al. Blood Microbiome Profile in CKD: A Pilot Study. Clin. J. Am. Soc. Nephrol. 2019, 14, 692–701. [Google Scholar] [CrossRef]
- Seki, N.; Kimizuka, T.; Gondo, M.; Yamaguchi, G.; Sugiura, Y.; Akiyama, M.; Yakabe, K.; Uchiyama, J.; Higashi, S.; Haneda, T.; et al. D-Tryptophan Suppresses Enteric Pathogen and Pathobionts and Prevents Colitis by Modulating Microbial Tryptophan Metabolism. iScience 2022, 25, 104838. [Google Scholar] [CrossRef]
- Tang, Z.; Yu, S.; Pan, Y. The Gut Microbiome Tango in the Progression of Chronic Kidney Disease and Potential Therapeutic Strategies. J. Transl. Med. 2023, 21, 689. [Google Scholar] [CrossRef]
- Yang, J.Z.; Zhang, K.K.; Shen, H.W.; Liu, Y.; Li, X.W.; Chen, L.J.; Liu, J.L.; Li, J.H.; Zhao, D.; Wang, Q.; et al. Sigma-1 Receptor Knockout Disturbs Gut Microbiota, Remodels Serum Metabolome, and Exacerbates Isoprenaline-Induced Heart Failure. Front. Microbiol. 2023, 14, 1255971. [Google Scholar] [CrossRef]
- López-Torres, C.D.; Torres-Mena, J.E.; Castro-Gil, M.P.; Villa-Treviño, S.; Arellanes-Robledo, J.; del Pozo-Yauner, L.; Pérez-Carreón, J.I. Downregulation of Indolethylamine N-Methyltransferase Is an Early Event in the Rat Hepatocarcinogenesis and Is Associated with Poor Prognosis in Hepatocellular Carcinoma Patients. J. Gene Med. 2022, 24, e3439. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dixon, E.E.; Xuanyuan, Q.; Guo, J.; Yoshimura, Y.; Debashish, C.; Niesnerova, A.; Xu, H.; Rouault, M.; Humphreys, B.D. High Resolution Spatial Profiling of Kidney Injury and Repair Using RNA Hybridization-Based in Situ Sequencing. Nat. Commun. 2024, 15, 1396. [Google Scholar] [CrossRef] [PubMed]
- Nuño-Ayala, M.; Guillén, N.; Arnal, C.; Lou-Bonafonte, J.M.; de Martino, A.; García-de-Jalón, J.A.; Gascón, S.; Osaba, L.; Osada, J.; Navarro, M.A. Cystathionine β-Synthase Deficiency Causes Infertility by Impairing Decidualization and Gene Expression Networks in Uterus Implantation Sites. Physiol. Genom. 2012, 44, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Groza, T.; Gomez Lopez, F.L.; Mashhadi, H.H.; Muñoz-Fuentes, V.; Gunes, O.; Wilson, R.; Cacheiro, P.; Frost, A.; Keskivali-Bond, P.; Vardal, B.; et al. The International Mouse Phenotyping Consortium: Comprehensive Knockout Phenotyping Underpinning the Study of Human Disease. Nucleic Acids Res. 2023, 51, D1038–D1045. [Google Scholar] [CrossRef] [PubMed]
- Urabe, Y.; Tanikawa, C.; Takahashi, A.; Okada, Y.; Morizono, T.; Tsunoda, T.; Kamatani, N.; Kohri, K.; Chayama, K.; Kubo, M.; et al. A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1. PLoS Genet. 2012, 8, e1002541. [Google Scholar] [CrossRef]
- Peto, K.; Nemeth, N.; Mester, A.; Magyar, Z.; Ghanem, S.; Somogyi, V.; Tanczos, B.; Deak, A.; Bidiga, L.; Frecska, E.; et al. Hemorheological and Metabolic Consequences of Renal Ischemia-Reperfusion and Their Modulation by N,N-Dimethyl-Tryptamine on a Rat Model. Clin. Hemorheol. Microcirc. 2018, 70, 107–117. [Google Scholar] [CrossRef]
- Nemes, B.; Pető, K.; Németh, N.; Mester, A.; Magyar, Z.; Ghanem, S.; Sógor, V.; Tánczos, B.; Deák, Á.; Kállay, M.; et al. N,N-Dimethyltryptamine Prevents Renal Ischemia-Reperfusion Injury in a Rat Model. Transplant. Proc. 2019, 51, 1268–1275. [Google Scholar] [CrossRef]
- Xu, Y.; Li, L.; Tang, P.; Zhang, J.; Zhong, R.; Luo, J.; Lin, J.; Zhang, L. Identifying Key Genes for Diabetic Kidney Disease by Bioinformatics Analysis. BMC Nephrol. 2023, 24, 305. [Google Scholar] [CrossRef] [PubMed]
- Balzer, M.S.; Doke, T.; Yang, Y.W.; Aldridge, D.L.; Hu, H.; Mai, H.; Mukhi, D.; Ma, Z.; Shrestha, R.; Palmer, M.B.; et al. Single-Cell Analysis Highlights Differences in Druggable Pathways Underlying Adaptive or Fibrotic Kidney Regeneration. Nat. Commun. 2022, 13, 4018. [Google Scholar] [CrossRef]
- Liu, J.; Kumar, S.; Dolzhenko, E.; Alvarado, G.F.; Guo, J.; Lu, C.; Chen, Y.; Li, M.; Dessing, M.C.; Parvez, R.K.; et al. Molecular Characterization of the Transition from Acute to Chronic Kidney Injury Following Ischemia/Reperfusion. JCI Insight 2017, 2, e94716. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.G.; Liu, T.; Huff, S.; Sheler, B.; Barker, S.A.; Strassman, R.J.; Wang, M.M.; Borjigin, J. Biosynthesis and Extracellular Concentrations of N,N-Dimethyltryptamine (DMT) in Mammalian Brain. Sci. Rep. 2019, 9, 9333. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A Single–Cell Type Transcriptomics Map of Human Tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef] [PubMed]
- Lake, B.B.; Menon, R.; Winfree, S.; Hu, Q.; Ferreira, R.M.; Kalhor, K.; Barwinska, D.; Otto, E.A.; Ferkowicz, M.; Diep, D.; et al. An Atlas of Healthy and Injured Cell States and Niches in the Human Kidney. Nature 2023, 619, 585–594. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Yamada, H.; Matsuhashi, K.; Okada, W.; Tanaka, Y.K.; Suzuki, N.; Ogra, Y. Production of a Urinary Selenium Metabolite, Trimethylselenonium, by Thiopurine S-Methyltransferase and Indolethylamine N-Methyltransferase. Chem. Res. Toxicol. 2020, 33, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- MacParland, S.A.; Liu, J.C.; Ma, X.Z.; Innes, B.T.; Bartczak, A.M.; Gage, B.K.; Manuel, J.; Khuu, N.; Echeverri, J.; Linares, I.; et al. Single Cell RNA Sequencing of Human Liver Reveals Distinct Intrahepatic Macrophage Populations. Nat. Commun. 2018, 9, 4383. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, O.; Königshofer, P.; Brusilovskaya, K.; Hofer, B.S.; Bareiner, K.; Simbrunner, B.; Jühling, F.; Baumert, T.F.; Lupberger, J.; Trauner, M.; et al. Transcriptomic Signatures of Progressive and Regressive Liver Fibrosis and Portal Hypertension. iScience 2024, 27, 109301. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-H.; Oh, J.-H.; Selvaraj, S.; Park, S.-M.; Choi, M.-S.; Spanel, R.; Yoon, S.; Borlak, J.; Lee, E.-H.; Oh, J.-H.; et al. Immunogenomics Reveal Molecular Circuits of Diclofenac Induced Liver Injury in Mice. Oncotarget 2016, 7, 14983–15017. [Google Scholar] [CrossRef] [PubMed]
- Kyritsi, K.; Chen, L.; O’Brien, A.; Francis, H.; Hein, T.W.; Venter, J.; Wu, N.; Ceci, L.; Zhou, T.; Zawieja, D.; et al. Modulation of the Tryptophan Hydroxylase 1/Monoamine Oxidase-A/5-Hydroxytryptamine/5-Hydroxytryptamine Receptor 2A/2B/2C Axis Regulates Biliary Proliferation and Liver Fibrosis During Cholestasis. Hepatology 2020, 71, 990–1008. [Google Scholar] [CrossRef]
- Arroyo, N.; Villamayor, L.; Díaz, I.; Carmona, R.; Ramos-Rodríguez, M.; Muñoz-Chápuli, R.; Pasquali, L.; Toscano, M.G.; Martín, F.; Cano, D.A.; et al. GATA4 Induces Liver Fibrosis Regression by Deactivating Hepatic Stellate Cells. JCI Insight 2021, 6, e150059. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Rebolledo-Jaramillo, B.; Zong, Y.; Wang, L.; Russo, P.; Hancock, W.; Stanger, B.Z.; Hardison, R.C.; Blobel, G.A. Function of GATA Factors in the Adult Mouse Liver. PLoS ONE 2013, 8, e83723. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Cortes, V.; Barbarigo, V.; Badimon, L. Low Density Lipoprotein Receptor-Related Protein 1 Modulates the Proliferation and Migration of Human Hepatic Stellate Cells. J. Cell Physiol. 2012, 227, 3528–3533. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y. Interdisciplinary Advances Reshape the Delivery Tools for Effective NASH Treatment. Mol. Metab. 2023, 73, 101730. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, A.N.; Chinnarasu, S.; Ding, Y.; Xian, X.; Herz, J.; Jaeschke, A.; Hui, D.Y. Low-Density Lipoprotein Receptor–Related Protein-1 Dysfunction Synergizes with Dietary Cholesterol to Accelerate Steatohepatitis Progression. J. Biol. Chem. 2018, 293, 9674. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, L.; Li, X.; Song, J.; Wu, X.; Zhou, S. Inositol-Requiring Enzyme 1-Mediated Endoplasmic Reticulum Stress Triggers Apoptosis and Fibrosis Formation in Liver Cirrhosis Rat Models. Mol. Med. Rep. 2015, 11, 2941–2946. [Google Scholar] [CrossRef]
- Watson, J.D.; Prokopec, S.D.; Smith, A.B.; Okey, A.B.; Pohjanvirta, R.; Boutros, P.C. TCDD Dysregulation of 13 AHR-Target Genes in Rat Liver. Toxicol. Appl. Pharmacol. 2014, 274, 445–454. [Google Scholar] [CrossRef]
- Kozaczek, M.; Bottje, W.; Greene, E.; Lassiter, K.; Kong, B.; Dridi, S.; Korourian, S.; Hakkak, R. Comparison of Liver Gene Expression by RNAseq and PCR Analysis after 8 Weeks of Feeding Soy Protein Isolate- or Casein-Based Diets in an Obese Liver Steatosis Rat Model. Food Funct. 2019, 10, 8218–8229. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Yan, C.; Li, C.; Zhang, L.; Zhang, L.; Liang, E.; Liu, T.; Mao, J. Effect of Metformin on Nonalcoholic Fatty Liver Based on Meta-Analysis and Network Pharmacology. Medicine 2022, 101, E31437. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Butticè, G.; Sengupta, P.K.; Smith, B.D. Major Histocompatibility Class II Transactivator (CIITA) Mediates Repression of Collagen (COL1A2) Transcription by Interferon γ (IFN-γ). J. Biol. Chem. 2004, 279, 41319–41332. [Google Scholar] [CrossRef]
- Winau, F.; Hegasy, G.; Weiskirchen, R.; Weber, S.; Cassan, C.; Sieling, P.A.; Modlin, R.L.; Liblau, R.S.; Gressner, A.M.; Kaufmann, S.H.E. Ito Cells Are Liver-Resident Antigen-Presenting Cells for Activating T Cell Responses. Immunity 2007, 26, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gong, N.; Xue, L.; Billingsley, M.M.; El-Mayta, R.; Shepherd, S.J.; Alameh, M.G.; Weissman, D.; Mitchell, M.J. Ligand-Tethered Lipid Nanoparticles for Targeted RNA Delivery to Treat Liver Fibrosis. Nat. Commun. 2023, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Tsurusaki, S.; Tsuchiya, Y.; Koumura, T.; Nakasone, M.; Sakamoto, T.; Matsuoka, M.; Imai, H.; Yuet-Yin Kok, C.; Okochi, H.; Nakano, H.; et al. Hepatic Ferroptosis Plays an Important Role as the Trigger for Initiating Inflammation in Nonalcoholic Steatohepatitis. Cell Death Dis. 2019, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Wang, B.; Huang, R.; Zhang, C.; Fu, P.; Ma, L. Ferroptosis in Organ Fibrosis: From Mechanisms to Therapeutic Medicines. J. Transl. Int. Med. 2024, 12, 22. [Google Scholar] [CrossRef]
- Cho, S.S.; Yang, J.H.; Lee, J.H.; Baek, J.S.; Ku, S.K.; Cho, I.J.; Kim, K.M.; Ki, S.H. Ferroptosis Contribute to Hepatic Stellate Cell Activation and Liver Fibrogenesis. Free Radic. Biol. Med. 2022, 193, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Lei, Z.G.; Chu, K.; Lam, O.J.H.; Chiang, C.Y.; Zhang, Z.J. N,N-Dimethyltryptamine, a Natural Hallucinogen, Ameliorates Alzheimer’s Disease by Restoring Neuronal Sigma-1 Receptor-Mediated Endoplasmic Reticulum-Mitochondria Crosstalk. Alzheim. Res. Ther. 2024, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosi, N.; Apolloni, S. Fibrotic Scar in Neurodegenerative Diseases. Front. Immunol. 2020, 11, 1394. [Google Scholar] [CrossRef] [PubMed]
- Judd, J.M.; Jasbi, P.; Winslow, W.; Serrano, G.E.; Beach, T.G.; Klein-Seetharaman, J.; Velazquez, R. Inflammation and the Pathological Progression of Alzheimer’s Disease Are Associated with Low Circulating Choline Levels. Acta Neuropathol. 2023, 146, 565. [Google Scholar] [CrossRef]
- Szegeczki, V.; Perényi, H.; Horváth, G.; Hinnah, B.; Tamás, A.; Radák, Z.; Ábrahám, D.; Zákány, R.; Reglodi, D.; Juhász, T. Physical Training Inhibits the Fibrosis Formation in Alzheimer’s Disease Kidney Influencing the TGFβ Signaling Pathways. J. Alzheimers Dis. 2021, 81, 1195. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Psallida, S.; Panagopoulos, F.; Stratigou, T.; Geladari, E.; Karampela, I.; Tsilingiris, D.; Dalamaga, M. The Interplay Between Dietary Choline and Cardiometabolic Disorders: A Review of Current Evidence. Curr. Nutr. Rep. 2024, 13, 152–165. [Google Scholar] [CrossRef]
- Strupp, B.J.; Powers, B.E.; Velazquez, R.; Ash, J.A.; Kelley, C.M.; Alldred, M.J.; Strawderman, M.; Caudill, M.A.; Mufson, E.J.; Ginsberg, S.D. Maternal Choline Supplementation: A Potential Prenatal Treatment for Down Syndrome and Alzheimer’s Disease. Curr. Alzheimer Res. 2015, 13, 97–106. [Google Scholar] [CrossRef]
- Velazquez, R.; Ferreira, E.; Knowles, S.; Fux, C.; Rodin, A.; Winslow, W.; Oddo, S. Lifelong Choline Supplementation Ameliorates Alzheimer’s Disease Pathology and Associated Cognitive Deficits by Attenuating Microglia Activation. Aging Cell 2019, 18, e13037. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Lei, P.; Zhou, H.; Liang, R.; Zhu, R.; Wang, W.; Zhou, L.; Sun, Y. Sigma-1 Receptor Protects against Ferroptosis in Hepatocellular Carcinoma Cells. J. Cell Mol. Med. 2019, 23, 7349–7359. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Long, J.; Zuo, B.; Li, Y.; Song, Y.; Yu, M.; Xun, Z.; Wang, Y.; Wang, X.; Sang, X.; et al. Development and Validation of a Selenium Metabolism Regulators Associated Prognostic Model for Hepatocellular Carcinoma. BMC Cancer 2023, 23, 451. [Google Scholar] [CrossRef] [PubMed]
- Sonet, J.; Bulteau, A.L.; Touat-hamici, Z.; Mosca, M.; Bierla, K.; Mounicou, S.; Lobinski, R.; Chavatte, L. Selenoproteome Expression Studied by Non-Radioactive Isotopic Selenium-Labeling in Human Cell Lines. Int. J. Mol. Sci. 2021, 22, 7308. [Google Scholar] [CrossRef]
- Jianfeng, W.; Yutao, W.; Jianbin, B. Indolethylamine-N-Methyltransferase Inhibits Proliferation and Promotes Apoptosis of Human Prostate Cancer Cells: A Mechanistic Exploration. Front. Cell Dev. Biol. 2022, 10, 805402. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, B.; Wang, J.; Wu, L.; Tan, Q.; Ji, C. Low Expression of INMT Is Associated with Poor Prognosis but Favorable Immunotherapy Response in Lung Adenocarcinoma. Front. Genet. 2022, 13, 946848. [Google Scholar] [CrossRef]
| Receptor | DMT | 5-HO-DMT | 5-MeO-DMT |
|---|---|---|---|
| 5-HTR1A | Agonist | Agonist | Agonist |
| 5-HTR1B | Partial Agonist | Partial Agonist | Partial Agonist |
| 5-HTR1D | Weak Agonist | Unknown | Weak Agonist |
| 5-HTR2A | Agonist | Agonist | Agonist |
| 5-HTR2B | Agonist | Agonist | Agonist |
| 5-HTR2C | Agonist | Agonist | Agonist |
| 5-HTR7 | Agonist | Unknown | Agonist |
| SIGMAR1 | Agonist | Agonist | Agonist |
| TAAR1 | Agonist | Unknown | Agonist |
| TAAR6 | Predicted Agonist | Unknown | Predicted Agonist |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korsmo, H.W. Exploring Endogenous Tryptamines: Overlooked Agents Against Fibrosis in Chronic Disease? A Narrative Review. Livers 2024, 4, 615-637. https://doi.org/10.3390/livers4040043
Korsmo HW. Exploring Endogenous Tryptamines: Overlooked Agents Against Fibrosis in Chronic Disease? A Narrative Review. Livers. 2024; 4(4):615-637. https://doi.org/10.3390/livers4040043
Chicago/Turabian StyleKorsmo, Hunter W. 2024. "Exploring Endogenous Tryptamines: Overlooked Agents Against Fibrosis in Chronic Disease? A Narrative Review" Livers 4, no. 4: 615-637. https://doi.org/10.3390/livers4040043
APA StyleKorsmo, H. W. (2024). Exploring Endogenous Tryptamines: Overlooked Agents Against Fibrosis in Chronic Disease? A Narrative Review. Livers, 4(4), 615-637. https://doi.org/10.3390/livers4040043






