Validation and Comparison of Non-Invasive Tests for the Exclusion of High-Risk Varices in Compensated Advanced Chronic Liver Disease
Abstract
1. Introduction
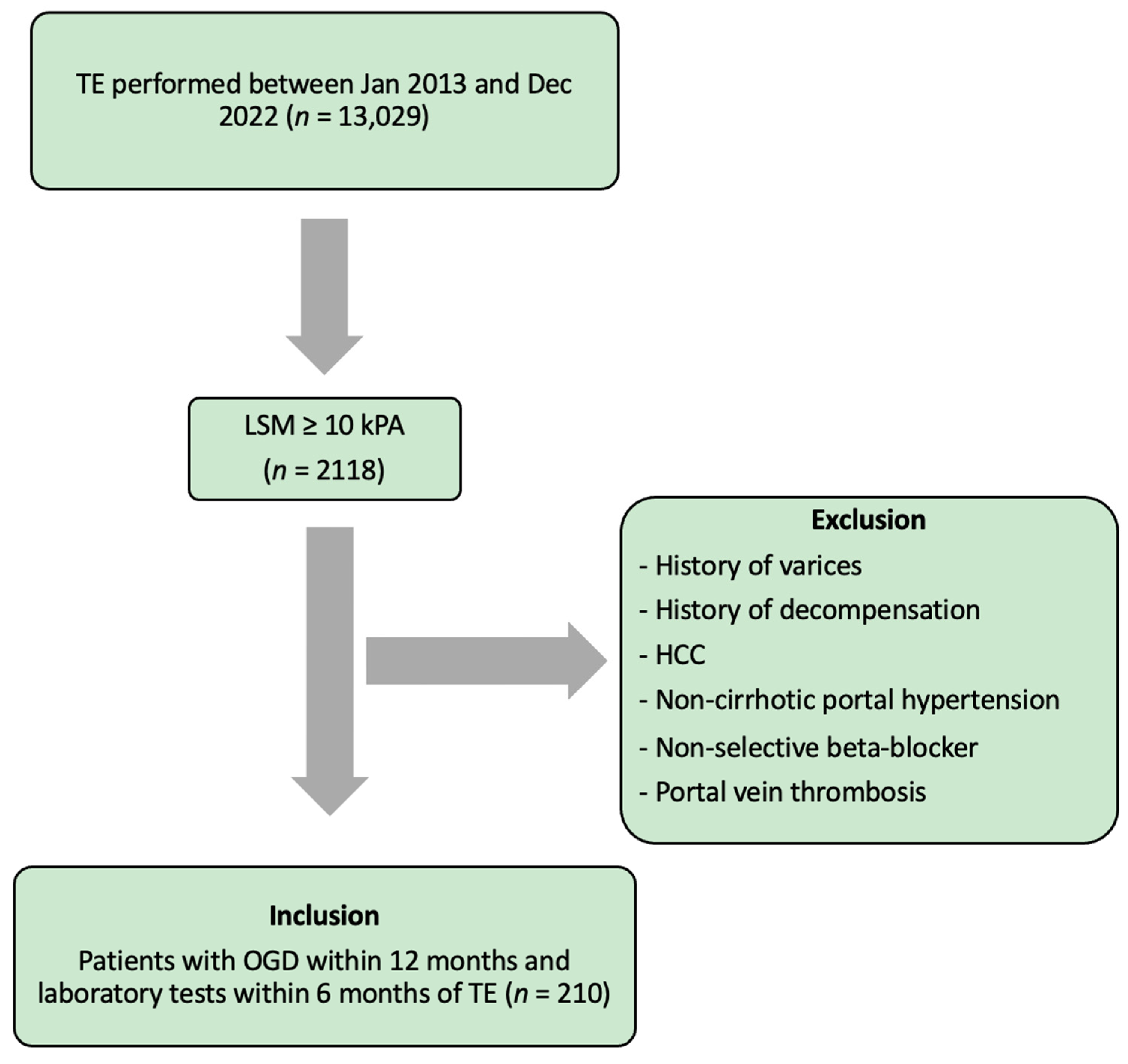
2. Methods
2.1. Data Collection
2.1.1. Transient Elastography
2.1.2. Calculation of NIT Indices
- -
- FIB-4 [20] = (age [years] × AST [U/L])/(PLT [109/L] × ALT [U/L]1/2)
- -
- APRI [21] = ((AST [U/L]/ULN)/PLT [×109/L])) × 100
- -
- MELD [18] = 0.957 × Logₑ(creatinine [mg/dL]) + 0.378 × Logₑ(bilirubin [mg/dL]) + 1.120 × Logₑ(INR) + 0.643
- -
- MELD-Na [19] = MELD − Na − 0.025 × MELD × (140 − Na) + 140
- -
- FIPS [16] = 1.43 × log10 (bilirubin [mg/dL]) − 1.71/(creatinine [mg/dL]) + 0.02 × (age [years]) − 0.02 × (albumin [g/L])
- -
- EVendo [15] = ((8.5 × INR + AST [U/L]/35)/(PLT [×103/μL]/150) + (blood urea nitrogen [mg/dL]/20) + (Hb [g/dL]/15)) + 1 if ascites present
- -
- B6C for excluding HRVs was used as described in the Baveno VI consensus (PLT > 150 × 109/L and LSM < 20 kPa) [11].
- -
- E6BC was used as proposed by Augustin et al. (PLT > 110 × 109/L and LSM < 25 kPa) [12].
2.1.3. Assessment of Varices
2.2. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Performance and Safety of the NITs in Patients with cACLD
3.3. Performance and Safety of B6C and EVendo in cACLD Subgroups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Franchis, R.; Primignani, M. Natural history of portal hypertension in patients with cirrhosis. Clin. Liver Dis. 2001, 5, 645–663. [Google Scholar] [CrossRef] [PubMed]
- LaBrecque, D.; Khan, A.G.; Sarin, S.K.; Le Mair, A.W. Esophageal Varices. World Gastroenterology Organisation Global Guidelines; WGO: Milwaukee, WI, USA, 2014. [Google Scholar]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. J. Hepatol. 2017, 65, 310–335. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Beste, L.; Curry, M.; Bonder, A.; Waljee, A.; Saini, S. Suboptimal Implementation of Evidence-based Therapy for Acute Variceal Hemorrhage: A Systematic Review of Observational Studies. Clin. Gastroenterol. Hepatol. 2017, 15, 1373–1381.e7. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; García-Pagán, J.C. Prevention of variceal rebleeding. Lancet 2003, 361, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Simonetto, D.A.; Liu, M.; Kamath, P.S. Portal Hypertension and Related Complications: Diagnosis and Management. Mayo Clin. Proc. 2019, 94, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Nicolini, G.; Angeloni, S.; Rinaldi, V.; De Santis, A.; Merkel, C.; Attili, A.F.; Riggio, O. Incidence and natural history of small esophageal varices in cirrhotic patients. J. Hepatol. 2003, 38, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Kheraj, R.; Garud, S.; Neeman, N.; Nathanson, L.A.; Kelly, C.P.; Sawhney, M.; Landon, B.; Doyle, R.; Rosenberg, S.; et al. The incidence and cost of unexpected hospital use after scheduled outpatient endoscopy. JAMA Intern. Med. 2010, 170, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Stanley, A.J.; Hayes, P.C.; Patch, D.; Millson, C.; Mehrzad, H.; Austin, A.; Ferguson, J.W.; Olliff, S.P.; Hudson, M.; et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015, 64, 1680–1704. [Google Scholar] [CrossRef] [PubMed]
- Issaka, R.B.; Feld, L.D.; Kao, J.; Hegarty, E.; Snailer, B.; Kalra, G.; Tomizawa, Y.; Strate, L. Real-World Data on the Impact of COVID-19 on Endoscopic Procedural Delays. Clin. Transl. Gastroenterol. 2021, 12, e00365. [Google Scholar] [CrossRef]
- de Franchis, R.; Baveno, V.I.F. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef]
- Augustin, S.; Pons, M.; Maurice, J.B.; Bureau, C.; Stefanescu, H.; Ney, M.; Blasco, H.; Procopet, B.; Tsochatzis, E.; Westbrook, R.H.; et al. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. J. Hepatol. 2017, 66, 1980–1988. [Google Scholar] [CrossRef]
- Schacher, F.C.; Neto, G.J.; Mattos, A.A. Screening for esophageal varices in cirrhotic patients—Non-invasive methods. Ann. Hepatol. 2019, 18, 673–678. [Google Scholar] [CrossRef]
- Sebastiani, G.; Tempesta, D.; Fattovich, G.; Castera, L.; Halfon, P.; Bourliere, M.; Noventa, F.; Angeli, P.; Saggioro, A.; Alberti, A. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: Results of a multicenter, large-scale study. J. Hepatol. 2010, 53, 630–638. [Google Scholar] [CrossRef]
- Dong, T.S.; Kalani, A.; Aby, E.S.; Le, L.; Luu, K.; Hauer, M.; Kamath, R.; Lindor, K.D.; Tabibian, J.H. Machine Learning-based Development and Validation of a Scoring System for Screening High-Risk Esophageal Varices. Clin. Gastroenterol. Hepatol. 2019, 17, 1894–1901.e1. [Google Scholar] [CrossRef]
- Bettinger, D.; Sturm, L.; Pfaff, L.; Hahn, F.; Kloeckner, R.; Volkwein, L.; Praktiknjo, M.; Lv, Y.; Han, G.; Huber, J.P.; et al. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival. J. Hepatol. 2021, 74, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Stafylidou, M.; Paschos, P.; Katsoula, A.; Malandris, K.; Ioakim, K.; Bekiari, E.; Haidich, A.-B.; Akriviadis, E.; Tsapas, A. Performance of Baveno VI and Expanded Baveno VI Criteria for Excluding High-Risk Varices in Patients with Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1744–1755.e11. [Google Scholar] [CrossRef]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. J. Hepatol. 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Biggins, S.W.; Kim, W.R.; Terrault, N.A.; Saab, S.; Balan, V.; Schiano, T.; Benson, J.; Therneau, T.; Kremers, W.; Wiesner, R.; et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006, 130, 1652–1660. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Wai, C.-T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.-F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Srinivasan, S.; Sundaram, S.; Emura, F.; Reddy, N.; Faigel, D.O.; Repici, A.; Parasa, S.; Sharma, P. Ongoing Global Impact of the COVID-19 Pandemic on Endoscopy: A Subsequent International Survey of 121 Centers From 35 Countries. Gastroenterology 2022, 162, 328–330.e3. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Hsieh, W.-Y.; Su, C.-W.; Hou, M.-C.; Wang, Y.-P.; Hsin, I.-F.; Yang, T.-C.; Liao, W.-C.; Lin, H.-C.; Lee, F.-Y.; et al. Combination of albumin-bilirubin grade and platelets to predict a compensated patient with hepatocellular carcinoma who does not require endoscopic screening for esophageal varices. Gastrointest. Endosc. 2018, 88, 230–239.e2. [Google Scholar] [CrossRef]
- Maurice, J.B.; Brodkin, E.; Arnold, F.; Navaratnam, A.; Paine, H.; Khawar, S.; Dhar, A.; Patch, D.; O’Beirne, J.; Mookerjee, R.; et al. Validation of the Baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J. Hepatol. 2016, 65, 899–905. [Google Scholar] [CrossRef]
- Bae, J.; Sinn, D.H.; Kang, W.; Gwak, G.-Y.; Choi, M.S.; Paik, Y.-H.; Lee, J.H.; Koh, K.C.; Paik, S.W. Validation of the Baveno VI and the expanded Baveno VI criteria to identify patients who could avoid screening endoscopy. Liver Int. 2018, 38, 1442–1448. [Google Scholar] [CrossRef]
- Gaete, M.I.; Díaz, L.A.; Arenas, A.; González, K.; Cattaneo, M.; Fuster, F.; Henríquez, R.; Soza, A.; Arrese, M.; Barrera, F.; et al. Baveno VI and Expanded Baveno VI criteria successfully predicts the absence of high-risk gastro-oesophageal varices in a Chilean cohort. Liver Int. 2020, 40, 1427–1434. [Google Scholar] [CrossRef]
- Hu, Y.; Wen, Z. Validation and comparison of non-invasive prediction models based on liver stiffness measurement to identify patients who could avoid gastroscopy. Sci. Rep. 2021, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. J. Hepatol. 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Alswat, K.; Alanazi, M.; Bashmail, A.; Alkhamash, M.; Alqahtani, S.; Al-Hamoudi, W.; Abdo, A. Validation of the EVendo score for the prediction of varices in cirrhotic patients. Saudi J. Gastroenterol. 2022, 28, 378–384. [Google Scholar] [CrossRef] [PubMed]
- De Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R.; et al. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef]
- Augustin, S.; Pons, M.; Genesca, J. Validating the Baveno VI recommendations for screening varices. J. Hepatol. 2017, 66, 459–460. [Google Scholar] [CrossRef]
- de Franchis, R. Updating consensus in portal hypertension: Report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J. Hepatol. 2000, 33, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Jakab, S.S.; Garcia-Tsao, G. Screening and Surveillance of Varices in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2019, 17, 26–29. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 210) | No HRVs (n = 184) | HRVs (n = 26) | p Value | |
|---|---|---|---|---|
| Age (years) | 57 (49–64) | 57 (49.25–64) | 58.5 (48.5–62.25) | 0.809 |
| Male, n (%) | 138 (65.7) | 118 (64.1) | 20 (76.9) | 0.198 |
| Aetiology, n (%) ALD NAFLD HBV HCV Other | 33 (15.7) 66 (31.4) 12 (5.7) 88 (41.9) 11 (5.3) | 29 (15.8) 55 (29.9) 12 (6.5) 78 (42.4) 10 (5.4) | 4 (15.4) 11 (42.3) 0 (0) 10 (38.5) 1 (3.8) | 0.578 |
| LSM (kPA) | 20.25 (12.8–32.45) | 19.9 (12.83–31.1) | 25.2 (12.65–35.3) | 0.551 |
| Hb (g/L) | 140.5 (124.75–150.25) | 145.5 (126.75–151) | 140 (119–154.5) | 0.023 |
| WCC (×109/L) | 5.9 (4.4–7.6) | 5.95 (4.375–7.675) | 4.3 (3.8–5.9) | 0.017 |
| PLT (×109/L) | 149.5 (91.25–202.25) | 135 (79.75–181.75) | 89 (61–124.5) | <0.001 |
| Bilirubin (µmol/L) | 12 (8–20) | 10 (8.25–15.75) | 16 (12–28) | 0.004 |
| Albumin (g/L) | 37 (34–40) | 38 (36–39.75) | 35 (29–38) | 0.128 |
| ALT (U/L) | 46 (29–77) | 54.5 (35.25–86.75) | 43 (34.5–84) | 0.988 |
| AST (U/L) | 44 (30–79) | 44 (34.75–78.75) | 75 (42.5–106.5) | 0.406 |
| Na (mmol/L) | 139 (138–141) | 139 (138–140) | 140 (136.5–141) | 0.415 |
| Creatinine (µmol/L) | 64 (55–76) | 64 (55–76.5) | 63 (50–68) | 0.207 |
| Urea (mmol/L) | 4.5 (3.4–5.8) | 4.8 (3.7–6.25) | 3.8 (2.6–4.85) | 0.743 |
| Fe (µg/L) | 115 (46.5–311) | 112.5 (50.25–319) | 206 (35–260) | 0.897 |
| INR | 1.1 (1–1.2) | 1.1 (1–1.2) | 1.2 (1.05–1.25) | 0.009 |
| AFP (IU/mL) | 3 (2–7) | 3 (2–6.75) | 6 (3–7.5) | 0.150 |
| Child–Pugh Class, n (%) | 0.492 | |||
| A | 193 (91.9) | 170 (92.4) | 23 (88.5) | |
| B | 17 (8.1) | 14 (7.6) | 3 (11.5) |
| Variable | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| β | OR | 95% CI | p Value | β | OR | 95% CI | p Value | |
| WCC | −0.476 | 0.621 | 0.477–0.808 | <0.001 * | ||||
| PLT | −0.019 | 0.981 | 0.972–0.990 | <0.001 * | −0.019 | 0.981 | 0.972–0.990 | <0.001 * |
| Bilirubin | 0.036 | 1.037 | 1.003–1.072 | 0.032 | ||||
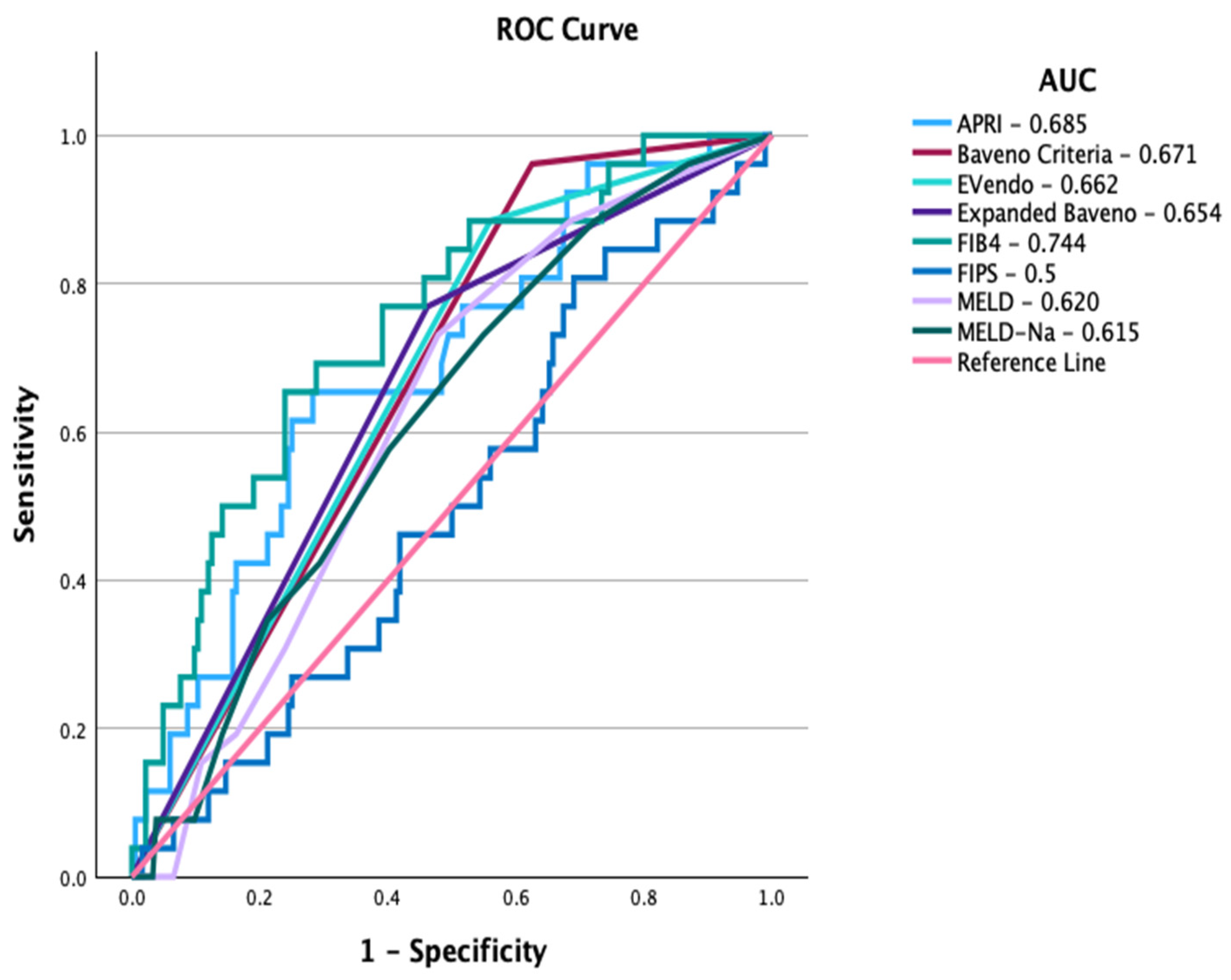
| Cut-Off | AUC | Sensitivity | Specificity | PPV | NPV | +LR | −LR | |
|---|---|---|---|---|---|---|---|---|
| Baveno VI | PLT > 150 and LSM < 20 | 0.671 | 96.2 | 38 | 18 | 98.6 | 1.55 | 0.1 |
| Expanded Baveno | PLT > 110 and LSM < 25 | 0.654 | 76.9 | 53.8 | 19 | 94.3 | 1.66 | 0.43 |
| FIB4 | 4.409 | 0.744 | 65.4 | 76.1 | 27.9 | 94 | 2.74 | 0.45 |
| APRI | 1.372 | 0.685 | 65.4 | 71.7 | 24.6 | 93.6 | 2.31 | 0.48 |
| FIPS | −2.762 | 0.5 | 80.8 | 31 | 14.2 | 91.9 | 1.17 | 0.62 |
| MELD | 7.5 | 0.620 | 73.1 | 52.2 | 17.8 | 93.2 | 1.53 | 0.52 |
| MELD-Na | 8.5 | 0.615 | 73.1 | 45.1 | 15.8 | 92.2 | 1.33 | 0.6 |
| EVendo | 3.9 | 0.662 | 88.5 | 44 | 18.3 | 95.4 | 1.58 | 0.26 |
| Cut-Off | Spared OGD (%) | Missed HRVs (%) | |
|---|---|---|---|
| Baveno VI | PLT > 150 and LSM < 20 | 33.8 | 1.41 |
| Expanded Baveno | PLT > 110 and LSM < 25 | 50 | 5.71 |
| FIB4 | 4.409 | 71 | 6.04 |
| APRI | 1.372 | 67.1 | 6.38 |
| FIPS | −2.762 | 29.5 | 8.06 |
| MELD | 7.5 | 49 | 6.8 |
| MELD-Na | 8.5 | 42.8 | 7.77 |
| EVendo | 3.9 | 40 | 3.57 |
| Spared OGD (%) | Missed HRVs (%) | |||
|---|---|---|---|---|
| B6C | EVendo | B6C | EVendo | |
| HBV | 50% (6/12) | 50% (6/12) | 0% (0/6) | 0% (0/6) |
| HCV | 31.8% (28/88) | 30.7% (27/88) | 0% (0/28) | 0% (0/27) |
| ALD | 21.2% (7/33) | 30.3% (10/33) | 0% (0/7) | 0% (0/10) |
| NAFLD | 43.9% (29/66) | 56.1% (37/66) | 3.4% (1/29) | 8.1% (3/37) |
| Other | 9.1% (1/11) | 36.4% (4/11) | 0% (0/1) | 0% (0/4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurup, R.; Kalo, E.; Read, S.; Ma, W.S.; George, J.; Ahlenstiel, G. Validation and Comparison of Non-Invasive Tests for the Exclusion of High-Risk Varices in Compensated Advanced Chronic Liver Disease. Livers 2024, 4, 182-192. https://doi.org/10.3390/livers4020014
Kurup R, Kalo E, Read S, Ma WS, George J, Ahlenstiel G. Validation and Comparison of Non-Invasive Tests for the Exclusion of High-Risk Varices in Compensated Advanced Chronic Liver Disease. Livers. 2024; 4(2):182-192. https://doi.org/10.3390/livers4020014
Chicago/Turabian StyleKurup, Rajiv, Eric Kalo, Scott Read, Wai See Ma, Jacob George, and Golo Ahlenstiel. 2024. "Validation and Comparison of Non-Invasive Tests for the Exclusion of High-Risk Varices in Compensated Advanced Chronic Liver Disease" Livers 4, no. 2: 182-192. https://doi.org/10.3390/livers4020014
APA StyleKurup, R., Kalo, E., Read, S., Ma, W. S., George, J., & Ahlenstiel, G. (2024). Validation and Comparison of Non-Invasive Tests for the Exclusion of High-Risk Varices in Compensated Advanced Chronic Liver Disease. Livers, 4(2), 182-192. https://doi.org/10.3390/livers4020014






