Preventive Effect of Gamma-Oryzanol on Physiopathological Process Related to Nonalcoholic Fatty Liver Disease in Animals Submitted to High Sugar/Fat Diet
Abstract
:1. Introduction
2. Methods
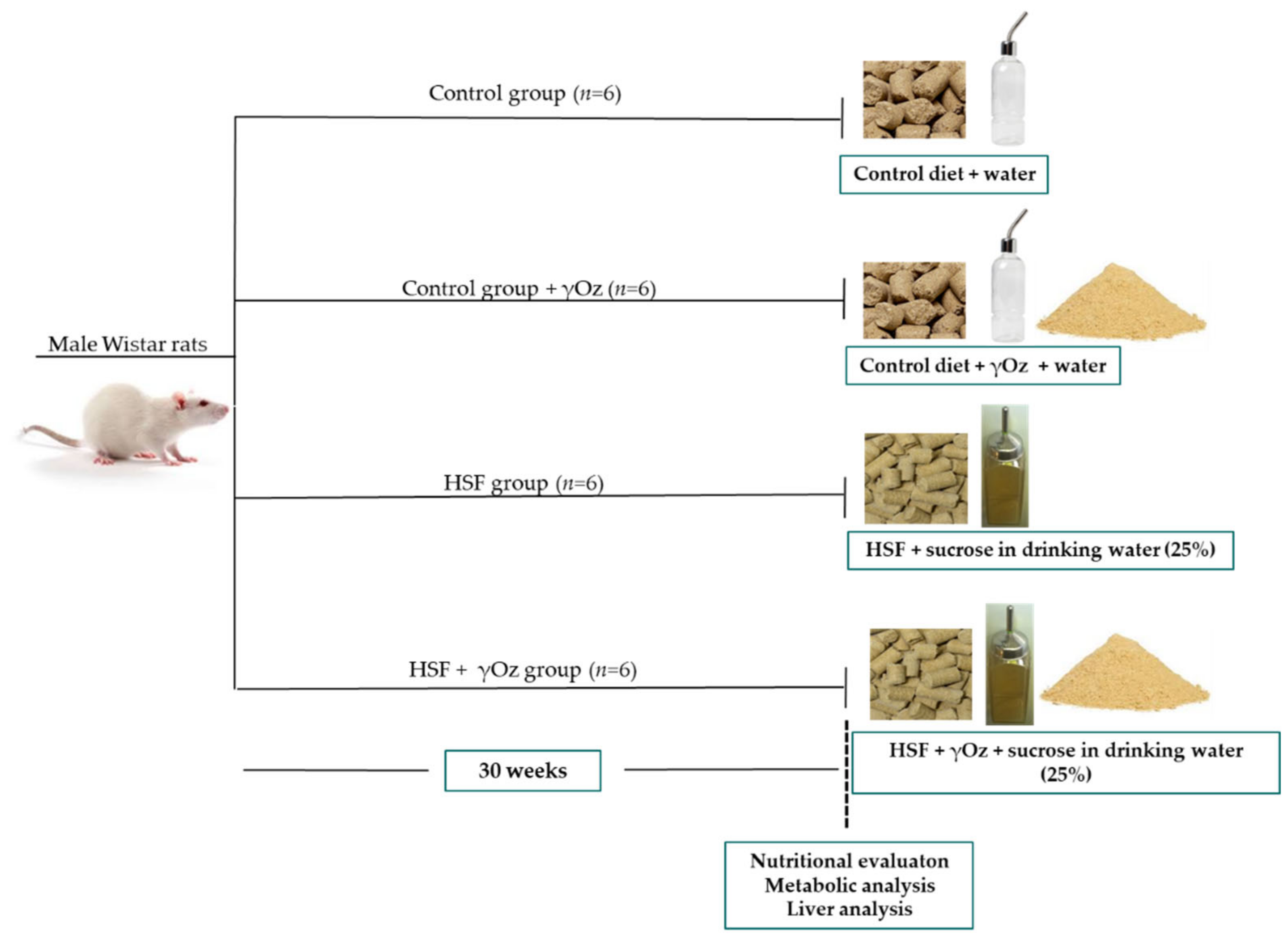
2.1. Experimental Protocol
2.2. Gamma-Oryzanol
2.3. Nutritional Parameters
2.4. Metabolic and Hormonal Analysis
2.5. Liver Evaluation
2.5.1. Preparation of Liver for Analysis
2.5.2. Inflammatory Markers
2.6. Oxidative Stress Markers
Malondialdehyde Levels (MDA)
2.7. Protein Carbonylation
2.8. Histological Analysis
2.9. Hepatic Triglycerides Levels
2.10. Statistical Analysis
3. Results
3.1. Nutritional Intake
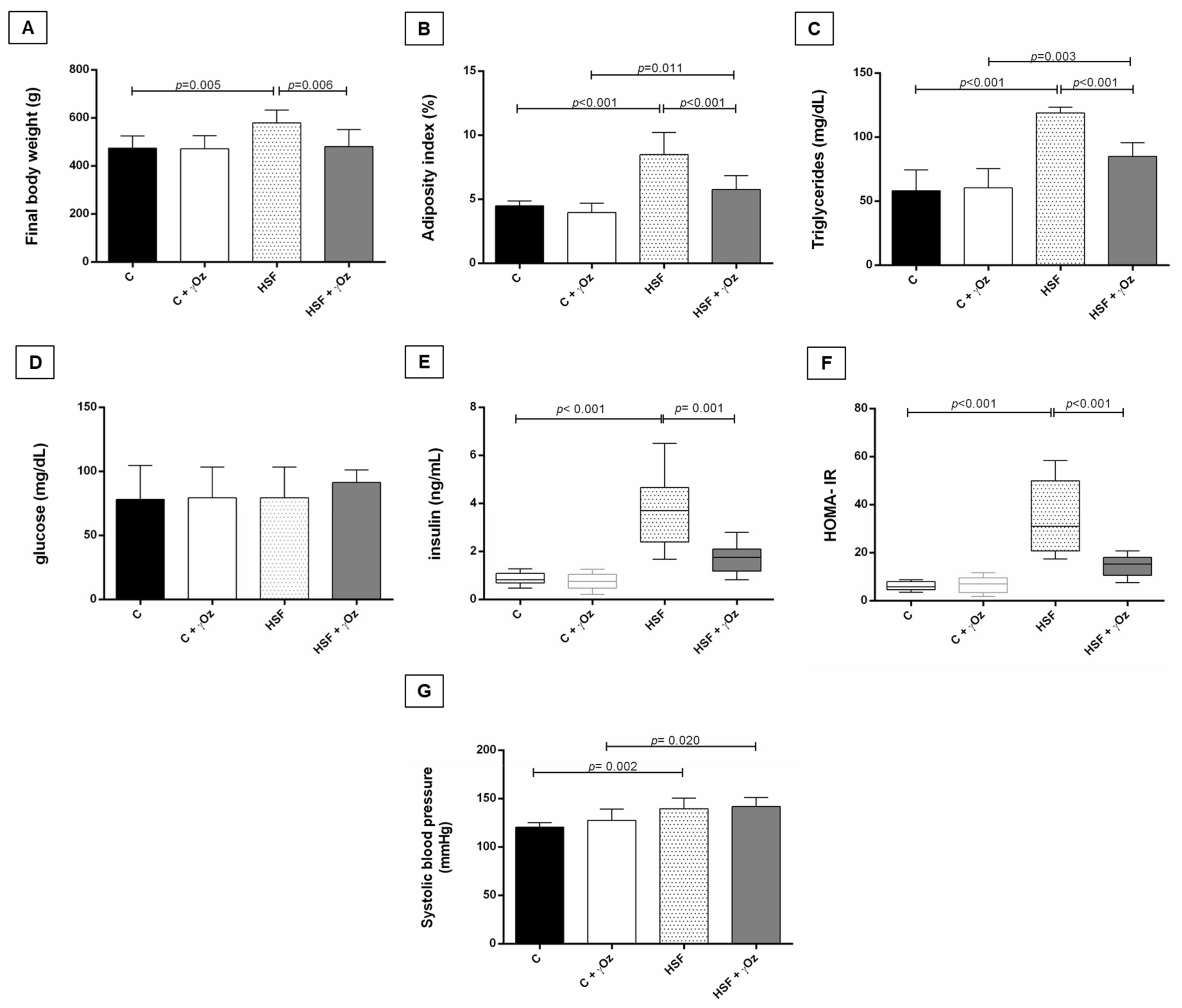
3.2. Metabolic Syndrome Parameters
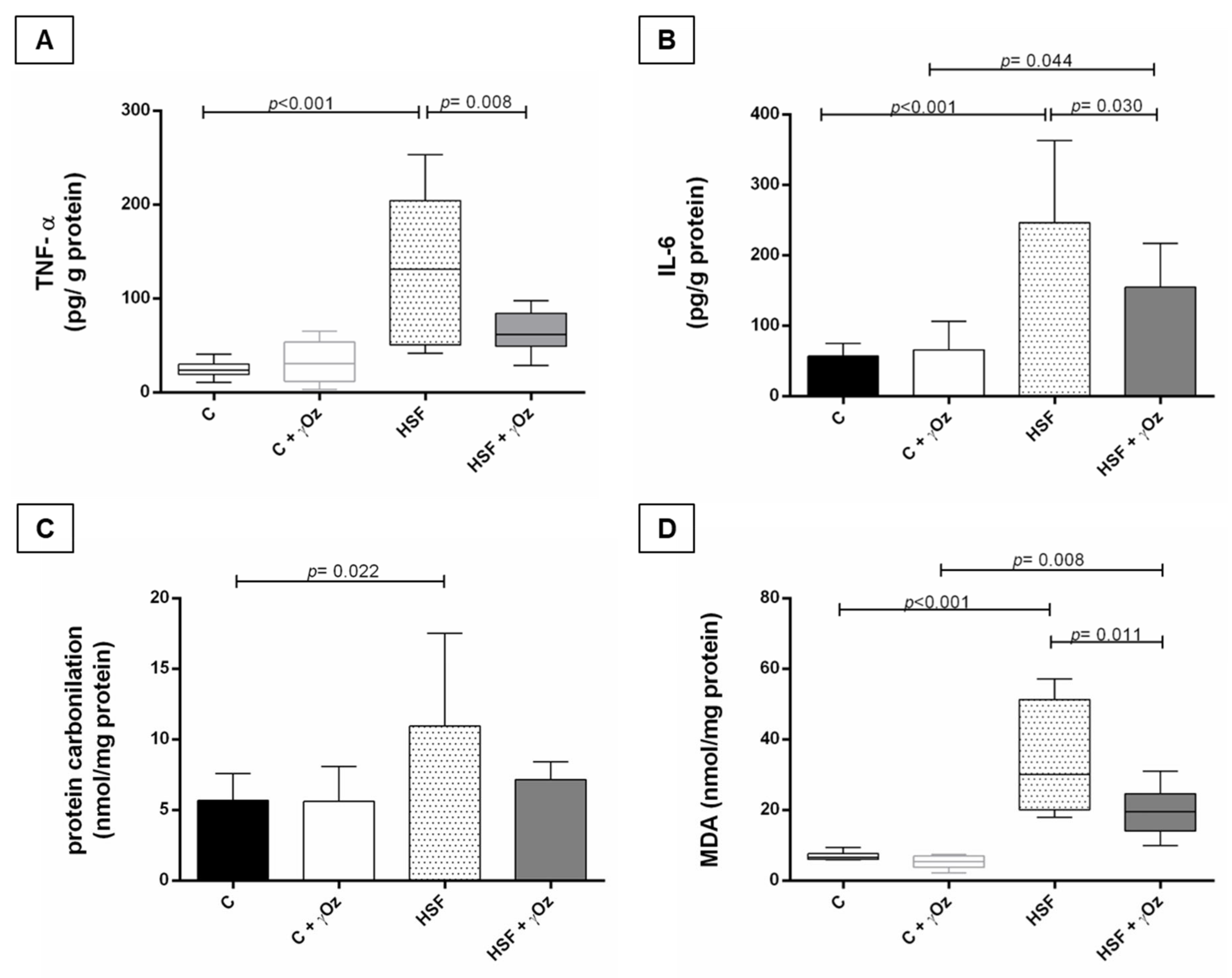
3.3. Oxidative Stress and Inflammatory Parameters
3.4. Hepatic Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muhammad, A. Non-alcoholic fatty liver disease, an overview. Integr. Med. 2019, 18, 42–49. [Google Scholar]
- Tomic, D.; Kemp, W.W.; Roberts, S.K. Nonalcoholic fatty liver disease: Current concepts, epidemiology and management strategies. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1103–1115. [Google Scholar] [CrossRef]
- Costa, M.R.; Garcia, J.L.; Silva, C.C.V.D.A.; Ferron, A.J.T.; Francisqueti-Ferron, F.V.; Hasimoto, F.K.; Gregolin, C.S.; de Campos, D.H.S.; de Andrade, C.R.; Ferreira, A.L.D.A.; et al. Lycopene Modulates Pathophysiological Processes of Non-Alcoholic Fatty Liver Disease in Obese Rats. Antioxidants 2019, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Tsukamoto, H. Inflammation in alcoholic and nonalcoholic fatty liver disease: Friend or foe? Gastroenterology 2016, 150, 1704–1709. [Google Scholar] [CrossRef] [Green Version]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Valenzuela, R.; Videla, L.A. Crosstalk mechanisms in hepatoprotection: Thyroid hormone-docosahexaenoic acid (DHA) and DHA-extra virgin olive oil combined protocols. Pharmacol. Res. 2018, 132, 168–175. [Google Scholar] [CrossRef]
- Minatel, I.O.; Francisqueti, F.V.; Corrêa, C.R.; Pace, G.; Lima, P. Antioxidant Activity of γ -Oryzanol: A Complex Network of Inter-actions. Int. J. Mol. Sci. 2016, 17, 1107. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, A.; Sulaiman, A.; Sert, M.; Khan, M.S.A.; Khan, M.A. Functional and Therapeutic Potential of γ-Oryzanol. In Funct Foods—Phytochem Heal Promot Potential; IntechOpen: London, UK, 2021; p. 97666. [Google Scholar] [CrossRef]
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of Ferulic Acid and γ-Oryzanol on High-Fat and High-Fructose Diet-Induced Metabolic Syndrome in Rats. PLoS ONE 2015, 10, e0118135. [Google Scholar] [CrossRef] [Green Version]
- Francisqueti, F.V.; Ferron, A.J.T.; Hasimoto, F.K.; Alves, P.H.R.; Garcia, J.L.; Santos, K.C. Gamma Oryzanol Treats Obesity-Induced Kidney Injuries by Modulating the Adiponectin Receptor 2/PPAR- α Axis. Oxid. Med. Cell. Longev. 2018, 2018, 1278392. [Google Scholar] [CrossRef] [Green Version]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Effect of Oryzanol and Ferulic Acid on the Glucose Metabolism of Mice Fed with a High-Fat Diet. J. Food Sci. 2010, 76, H7–H10. [Google Scholar] [CrossRef]
- Francisqueti, F.V.; Minatel, I.O.; Ferron, A.J.T.; Bazan, S.G.Z.; Silva, V.D.S.; Garcia, J.L.; De Campos, D.H.S.; Ferreira, A.L.; Moreto, F.; Cicogna, A.C.; et al. Effect of Gamma-Oryzanol as Therapeutic Agent to Prevent Cardiorenal Metabolic Syndrome in Animals Submitted to High Sugar-Fat Diet. Nutrients 2017, 9, 1299. [Google Scholar] [CrossRef] [Green Version]
- IBGE. Instituto Brasileiro de Geografia e Estatística, Coordenação de Trabalho e Rendimento. Pesquisa de Orçamentos Familiares: 2008-2009. Análise Do Consumo Aliment. Pessoal No Brasil. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv50063.pdf (accessed on 16 May 2022).
- Samarghandian, S.; Farkhondeh, T.; Samini, F.; Borji, A. Protective Effects of Carvacrol against Oxidative Stress Induced by Chronic Stress in Rat’s Brain, Liver, and Kidney. Biochem. Res. Int. 2016, 2016, 2645237. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectropho-tometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a General NAFLD Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [Green Version]
- Parra-Vargas, M.; Sandoval-Rodriguez, A.; Rodriguez-Echevarria, R.; Dominguez-Rosales, J.A.; Santos-Garcia, A.; Armendariz-Borunda, J. Delphinidin Ameliorates Hepatic Triglyceride Accumulation in Human HepG2 Cells, but Not in Diet-Induced Obese Mice. Nutrients 2018, 10, 1060. [Google Scholar] [CrossRef] [Green Version]
- Caturano, A.; Acierno, C.; Nevola, R.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Salvatore, T.; Adinolfi, L.E.; Sasso, F.C. Non-Alcoholic Fatty Liver Disease: From Pathogenesis to Clinical Impact. Processes 2021, 9, 135. [Google Scholar] [CrossRef]
- WHO. World Health Organisation Obesity and Overweight Fact Sheet; WHO: Geneva, Switzerland, 2016; Volume 1. [Google Scholar]
- Francisqueti-Ferron, F.V.; Garcia, J.L.; Ferron, A.J.T.; Maia, E.T.N.; Gregolin, C.S.; Silva, J.P.D.C.; dos Santos, K.C.; Lo, T.C.; Siqueira, J.S.; de Mattei, L.; et al. Gamma-oryzanol as a potential modulator of oxidative stress and inflammation via PPAR-y in adipose tissue: A hypothetical therapeutic for cytokine storm in COVID-19? Mol. Cell. Endocrinol. 2020, 520, 111095. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, D.-H.; Ahn, J.; Lee, H.; Choi, W.H.; Jang, Y.J.; Ha, T.-Y. γ-Oryzanol Enhances Adipocyte Differentiation and Glucose Uptake. Nutrients 2015, 7, 4851–4861. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Di Zhao, H.L. Adipose tissue dysfunction and the pathogenesis of metabolic syndrome. World J. Hypertens. 2013, 3, 18–26. [Google Scholar] [CrossRef]
- Lafontan, M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2014, 40, 16–28. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Olza, J.; Gil, Á.; Aguilera, C.M. Oxidative stress and inflammation in obesity and metabolic syndrome. In Obesity: Oxidative Stress and Dietary Antioxidants; Academic Press: Cambridge, MA, USA, 2018; pp. 1–15. [Google Scholar] [CrossRef]
- Justo, M.L.; Claro, C.; Zeyda, M.; Stulnig, T.M.; Herrera, M.D.; Rodríguez-Rodríguez, R. Rice bran prevents high-fat diet-induced inflammation and macrophage content in adipose tissue. Eur. J. Nutr. 2015, 55, 2011–2019. [Google Scholar] [CrossRef]
- Kalra, A.; Yetiskul, E.; Wehrle, C.; Al, E. Physiology, Liver; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Mattei, L.; Francisqueti-Ferron, F.V.; Garcia, J.L.; Ferron, A.J.T.; Silva, C.C.V.D.A.; Gregolin, C.S.; Nakandakare-Maia, E.T.; Silva, J.d.C.P.; Moreto, F.; Minatel, I.O.; et al. Antioxidant and anti-inflammatory properties of gamma- oryzanol attenuates insulin resistance by increasing GLUT- 4 expression in skeletal muscle of obese animals. Mol. Cell. Endocrinol. 2021, 537, 111423. [Google Scholar] [CrossRef]
- Ma, Z.; Chu, L.; Liu, H.; Wang, W.; Li, J.; Yao, W.; Yi, J.; Gao, Y. Beneficial effects of paeoniflorin on non-alcoholic fatty liver disease induced by high-fat diet in rats. Sci. Rep. 2017, 7, 44819. [Google Scholar] [CrossRef] [Green Version]
- Freitas, I.; Boncompagni, E.; Tarantola, E.; Gruppi, C.; Bertone, V.; Ferrigno, A.; Milanesi, G.; Vaccarone, R.; Tira, M.E.; Vairetti, M. In Situ Evaluation of Oxidative Stress in Rat Fatty Liver Induced by a Methionine- and Choline-Deficient Diet. Oxidative Med. Cell. Longev. 2016, 2016, 9307064. [Google Scholar] [CrossRef] [Green Version]


| Nutritional Values | ||
|---|---|---|
| Protein (% of ingredients) | 20.0 | 18.0 |
| Carbohydrate (% of ingredients) | 60.0 | 53.5 |
| Fat (% of ingredients) | 4.00 | 16.5 |
| % of unsaturated | 69.0 | 47.0 |
| % of saturated | 31.0 | 53.0 |
| % Energy from protein | 22.9 | 16.6 |
| % Energy from carbohydrate | 66.8 | 49.2 |
| % Energy from fat | 10.4 | 34.2 |
| Energy (kcal/g) | 3.59 | 4.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francisqueti-Ferron, F.V.; Silva, J.P.d.C.; Garcia, J.L.; Ferron, A.J.T.; Kano, H.T.; Silva, C.C.V.d.A.; Costa, M.R.; Nai, G.A.; Moreto, F.; Corrêa, C.R. Preventive Effect of Gamma-Oryzanol on Physiopathological Process Related to Nonalcoholic Fatty Liver Disease in Animals Submitted to High Sugar/Fat Diet. Livers 2022, 2, 146-157. https://doi.org/10.3390/livers2030013
Francisqueti-Ferron FV, Silva JPdC, Garcia JL, Ferron AJT, Kano HT, Silva CCVdA, Costa MR, Nai GA, Moreto F, Corrêa CR. Preventive Effect of Gamma-Oryzanol on Physiopathological Process Related to Nonalcoholic Fatty Liver Disease in Animals Submitted to High Sugar/Fat Diet. Livers. 2022; 2(3):146-157. https://doi.org/10.3390/livers2030013
Chicago/Turabian StyleFrancisqueti-Ferron, Fabiane Valentini, Janaina Paixão das Chagas Silva, Jéssica Leite Garcia, Artur Junio Togneri Ferron, Hugo Tadashi Kano, Carol Cristina Vágula de Almeida Silva, Mariane Róvero Costa, Gisele Alborghetti Nai, Fernando Moreto, and Camila Renata Corrêa. 2022. "Preventive Effect of Gamma-Oryzanol on Physiopathological Process Related to Nonalcoholic Fatty Liver Disease in Animals Submitted to High Sugar/Fat Diet" Livers 2, no. 3: 146-157. https://doi.org/10.3390/livers2030013
APA StyleFrancisqueti-Ferron, F. V., Silva, J. P. d. C., Garcia, J. L., Ferron, A. J. T., Kano, H. T., Silva, C. C. V. d. A., Costa, M. R., Nai, G. A., Moreto, F., & Corrêa, C. R. (2022). Preventive Effect of Gamma-Oryzanol on Physiopathological Process Related to Nonalcoholic Fatty Liver Disease in Animals Submitted to High Sugar/Fat Diet. Livers, 2(3), 146-157. https://doi.org/10.3390/livers2030013






