Predictive Factors for Hepatocellular Carcinoma Development after Direct-Acting Antiviral Treatment of HCV
Abstract
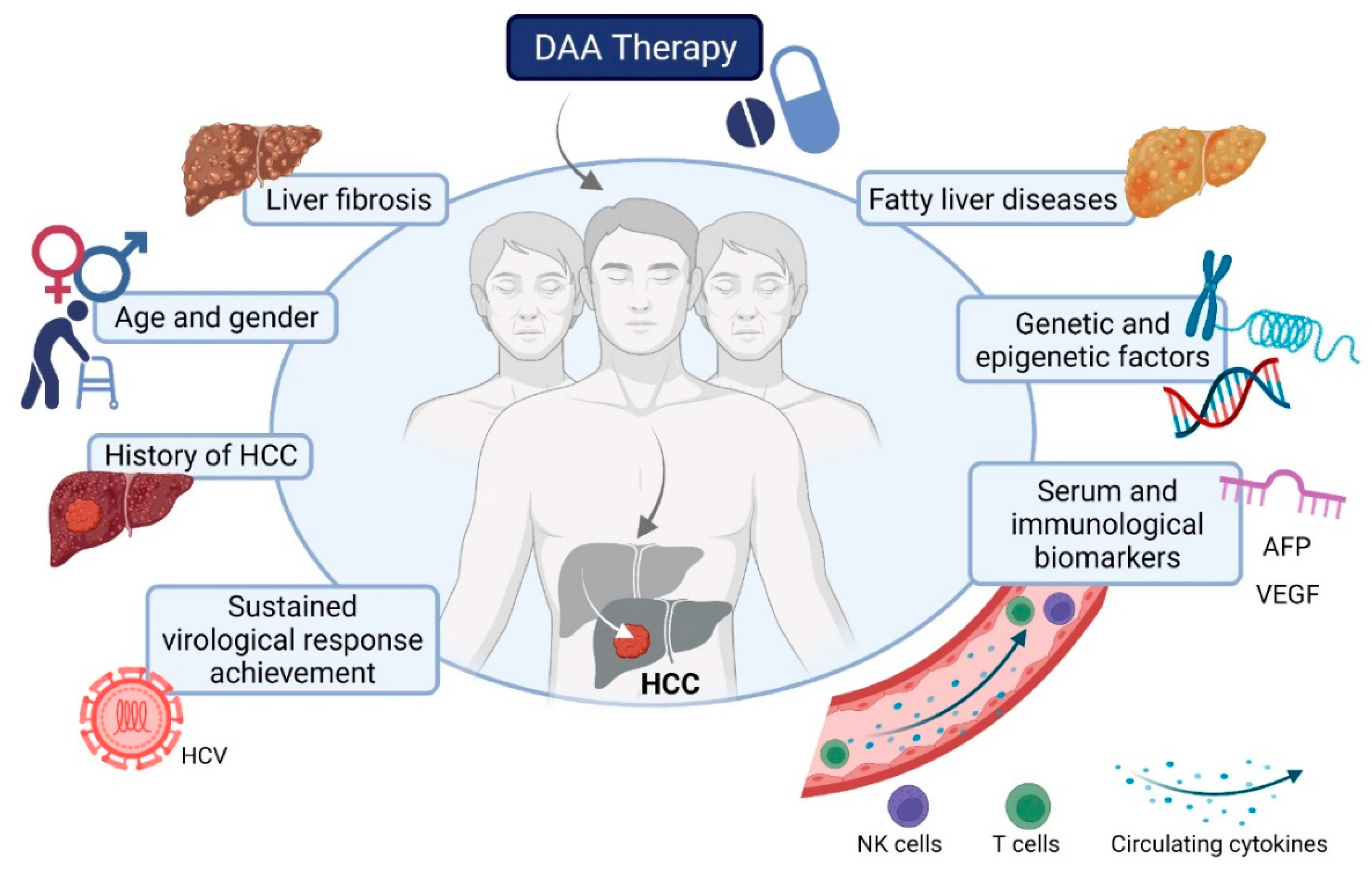
:1. Introduction
2. Predictive Factors and Biomarkers for HCC Development after DAA Treatment of HCV
2.1. Sustained Virological Response Achievement
2.2. History of HCC
2.3. Age and Gender
2.4. Liver Fibrosis
2.5. Serum Biomarkers: AFP and Others
2.6. miRNA
2.7. Underlying Metabolic-Associated Fatty Liver Disease
2.8. Genetic Variants
2.9. Epigenetic Changes
2.10. Immunological Biomarkers
2.11. Predictive Models and Scores
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hirano, J.; Yoshio, S.; Sakai, Y.; Songling, L.; Suzuki, T.; Itoh, Y.; Zhang, H.; Chen, D.V.; Haga, S.; Oomori, H.; et al. Hepatitis C virus modulates signal peptide peptidase to alter host protein processing. Proc. Natl. Acad. Sci. USA 2021, 118, e2026184118. [Google Scholar] [CrossRef] [PubMed]
- Hamdane, N.; Jühling, F.; Crouchet, E.; El Saghire, H.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Roca Suarez, A.A.; et al. HCV-Induced Epigenetic Changes Associated with Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology 2019, 156, 2313–2329.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, D.A.; Casciato, P.C.; Caldirola, M.S.; Gaillard, M.I.; Giadans, C.; Ameigeiras, B.; De Matteo, E.N.; Preciado, M.V.; Valva, P. Chronic Hepatitis C Pathogenesis: Immune Response in the Liver Microenvironment and Peripheral Compartment. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Macek Jilkova, Z.; Afzal, S.; Marche, H.; Decaens, T.; Sturm, N.; Jouvin-Marche, E.; Huard, B.; Marche, P.N. Progression of fibrosis in patients with chronic viral hepatitis is associated with IL-17(+) neutrophils. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36, 1116–1124. [Google Scholar] [CrossRef]
- Fugier, E.; Marche, H.; Thelu, M.A.; Macek Jilkova, Z.; Van Campenhout, N.; Dufeu-Duchesne, T.; Leroy, V.; Zarski, J.P.; Sturm, N.; Marche, P.N.; et al. Functions of liver natural killer cells are dependent on the severity of liver inflammation and fibrosis in chronic hepatitis C. PLoS ONE 2014, 9, e95614. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824–7840. [Google Scholar] [CrossRef]
- Choo, Q.-L.; Kuo, G.; Weiner, A.J.; Overby, L.R.; Bradley, D.W.; Houghton, M. Isolation of a cDNA cLone Derived from a Blood-Borne Non-A, Non-B Viral Hepatitis Genome. Science 1989, 244, 359–362. [Google Scholar] [CrossRef] [Green Version]
- Ghany, M.G.; Lok, A.S.F.; Dienstag, J.L.; Feinstone, S.M.; Hoofnagle, J.H.; Jake Liang, T.; Seeff, L.B.; Cohen, D.E.; Bezerra, J.A.; Chung, R.T. The 2020 Nobel Prize for Medicine or Physiology for the Discovery of Hepatitis C Virus: A Triumph of Curiosity and Persistence. Hepatology 2021, 74, 2813–2823. [Google Scholar] [CrossRef]
- Nahon, P.; Layese, R.; Bourcier, V.; Cagnot, C.; Marcellin, P.; Guyader, D.; Pol, S.; Larrey, D.; De Ledinghen, V.; Ouzan, D.; et al. Incidence of Hepatocellular Carcinoma After Direct Antiviral Therapy for HCV in Patients with Cirrhosis Included in Surveillance Programs. Gastroenterology 2018, 155, 1436–1450.e6. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, F.; Kramer, J.R.; Asch, S.M.; Cao, Y.; Li, L.; El-Serag, H.B. Long-Term Risk of Hepatocellular Carcinoma in HCV Patients Treated with Direct Acting Antiviral Agents. Hepatology 2020, 71, 44–55. [Google Scholar] [CrossRef]
- Rinaldi, L.; Nevola, R.; Franci, G.; Perrella, A.; Corvino, G.; Marrone, A.; Berretta, M.; Morone, M.V.; Galdiero, M.; Giordano, M.; et al. Risk of Hepatocellular Carcinoma after HCV Clearance by Direct-Acting Antivirals Treatment Predictive Factors and Role of Epigenetics. Cancers 2020, 12, 1351. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Green, P.K.; Berry, K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2018, 68, 32–53. [Google Scholar] [CrossRef]
- Frazzoni, L.; Sikandar, U.; Metelli, F.; Sadalla, S.; Mazzella, G.; Bazzoli, F.; Fuccio, L.; Azzaroli, F. Hepatocellular Carcinoma Recurrence after Hepatitis C Virus Therapy with Direct-Acting Antivirals. A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1694. [Google Scholar] [CrossRef]
- Compagnoni, S.; Bruno, E.M.; Madonia, G.; Cannizzaro, M.; Madonia, S. Direct antiviral agents in hepatitis C virus related liver disease: Don’t count the chickens before they’re hatched. World J. Gastroenterol. 2021, 27, 2771–2783. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Angeli, P.; Piovesan, S.; Noventa, F.; Anastassopoulos, G.; Chemello, L.; Cavalletto, L.; Gambato, M.; Russo, F.P.; Burra, P.; et al. Newly diagnosed Hepatocellular Carcinoma in patients with advanced hepatitis C treated with DAAs: A prospective population study. J. Hepatol. 2018, 69, 345–352. [Google Scholar] [CrossRef]
- Carrat, F.; Fontaine, H.; Dorival, C.; Simony, M.; Diallo, A.; Hezode, C.; De Ledinghen, V.; Larrey, D.; Haour, G.; Bronowicki, J.P.; et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet 2019, 393, 1453–1464. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated with Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, K.; Takai, K.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Sustained virological response by direct-acting antivirals reduces the recurrence risk of hepatitis C-related hepatocellular carcinoma after curative treatment. Mol. Clin. Oncol. 2020, 12, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; Tine, F.; Distefano, M.; Licata, A.; Giannitrapani, L.; et al. Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated with Direct-Acting Antiviral Agents. Gastroenterology 2018, 155, 411–421.e4. [Google Scholar] [CrossRef] [Green Version]
- Ooka, Y.; Miho, K.; Shuntaro, O.; Nakamura, M.; Ogasawara, S.; Suzuki, E.; Yasui, S.; Chiba, T.; Arai, M.; Kanda, T.; et al. Prediction of the very early occurrence of HCC right after DAA therapy for HCV infection. Hepatol. Int. 2018, 12, 523–530. [Google Scholar] [CrossRef]
- Mariño, Z.; Darnell, A.; Lens, S.; Sapena, V.; Díaz, A.; Belmonte, E.; Perelló, C.; Calleja, J.L.; Varela, M.; Rodriguez, M.; et al. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: Relevance of non-characterized nodules. J. Hepatol. 2019, 70, 874–884. [Google Scholar] [CrossRef]
- Degasperi, E.; D’Ambrosio, R.; Iavarone, M.; Sangiovanni, A.; Aghemo, A.; Soffredini, R.; Borghi, M.; Lunghi, G.; Colombo, M.; Lampertico, P. Factors Associated with Increased Risk of De Novo or Recurrent Hepatocellular Carcinoma in Patients with Cirrhosis Treated with Direct-Acting Antivirals for HCV Infection. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 1183–1191.e1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, E.; Nomura, H.; Nakamuta, M.; Furusyo, N.; Kajiwara, E.; Dohmen, K.; Kawano, A.; Ooho, A.; Azuma, K.; Takahashi, K.; et al. Development of Hepatocellular Carcinoma by Patients Aged 75-84 with Chronic Hepatitis C Treated with Direct-acting Antivirals. J. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Tokumoto, Y.; Joko, K.; Michitaka, K.; Horiike, N.; Tanaka, Y.; Tada, F.; Kisaka, Y.; Nakanishi, S.; Yamauchi, K.; et al. Sex difference in the development of hepatocellular carcinoma after direct-acting antiviral therapy in patients with HCV infection. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.; Naderian, M.; Sohrabpour, A.A. New Concepts on Reversibility and Targeting of Liver Fibrosis; A Review Article. Middle East J. Dig. Dis. 2018, 10, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, F.; Conti, F.; Brillanti, S.; Andreone, P.; Mazzella, G.; Buonfiglioli, F.; Serio, I.; Verrucchi, G.; Bacchi Reggiani, M.L.; Colli, A.; et al. Hepatocellular carcinoma risk assessment by the measurement of liver stiffness variations in HCV cirrhotics treated with direct acting antivirals. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2018, 50, 573–579. [Google Scholar] [CrossRef]
- Rinaldi, L.; Guarino, M.; Perrella, A.; Pafundi, P.C.; Valente, G.; Fontanella, L.; Nevola, R.; Guerrera, B.; Iuliano, N.; Imparato, M.; et al. Role of Liver Stiffness Measurement in Predicting HCC Occurrence in Direct-Acting Antivirals Setting: A Real-Life Experience. Dig. Dis. Sci. 2019, 64, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Perrella, A.; Guarino, M.; De Luca, M.; Piai, G.; Coppola, N.; Pafundi, P.C.; Ciardiello, F.; Fasano, M.; Martinelli, E.; et al. Incidence and risk factors of early HCC occurrence in HCV patients treated with direct acting antivirals: A prospective multicentre study. J. Transl. Med. 2019, 17, 292. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, N.; Saitoh, S.; Akuta, N.; Sezaki, H.; Suzuki, F.; Fujiyama, S.; Kawamura, Y.; Hosaka, T.; Kobayashi, M.; Suzuki, Y.; et al. Advantage of liver stiffness measurement before and after direct-acting antiviral therapy to predict hepatocellular carcinoma and exacerbation of esophageal varices in chronic hepatitis C. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2020, 50, 426–438. [Google Scholar] [CrossRef]
- Alonso Lopez, S.; Manzano, M.L.; Gea, F.; Gutierrez, M.L.; Ahumada, A.M.; Devesa, M.J.; Olveira, A.; Polo, B.A.; Marquez, L.; Fernandez, I.; et al. A Model Based on Noninvasive Markers Predicts Very Low Hepatocellular Carcinoma Risk After Viral Response in Hepatitis C Virus-Advanced Fibrosis. Hepatology 2020, 72, 1924–1934. [Google Scholar] [CrossRef]
- Pons, M.; Rodríguez-Tajes, S.; Esteban, J.I.; Mariño, Z.; Vargas, V.; Lens, S.; Buti, M.; Augustin, S.; Forns, X.; Mínguez, B.; et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J. Hepatol. 2020, 72, 472–480. [Google Scholar] [CrossRef]
- You, M.W.; Kim, K.W.; Shim, J.J.; Pyo, J. Impact of liver-stiffness measurement on hepatocellular carcinoma development in chronic hepatitis C patients treated with direct-acting antivirals: A systematic review and time-to-event meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Beste, L.A.; Green, P.K.; Singal, A.G.; Tapper, E.B.; Waljee, A.K.; Sterling, R.K.; Feld, J.J.; Kaplan, D.E.; Taddei, T.H.; et al. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients with Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology 2019, 157, 1264–1278.e1264. [Google Scholar] [CrossRef] [Green Version]
- Ioannou, G.N. HCC surveillance after SVR in patients with F3/F4 fibrosis. J. Hepatol. 2021, 74, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Ogawa, E.; Huang, C.F.; Toyoda, H.; Jun, D.W.; Tseng, C.H.; Hsu, Y.C.; Enomoto, M.; Takahashi, H.; Furusyo, N.; et al. HCC risk post-SVR with DAAs in East Asians: Findings from the REAL-C cohort. Hepatol. Int. 2020, 14, 1023–1033. [Google Scholar] [CrossRef]
- Watanabe, T.; Tokumoto, Y.; Joko, K.; Michitaka, K.; Horiike, N.; Tanaka, Y.; Tada, F.; Kisaka, Y.; Nakanishi, S.; Yamauchi, K.; et al. AFP and eGFR are related to early and late recurrence of HCC following antiviral therapy. BMC Cancer 2021, 21, 699. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Chen, G.F.; Niu, X.X.; Zhang, M.; Wang, C.; Shao, Q.; Wu, V.; Wang, Y.; Cheng, G.; Hurwitz, S.J.; et al. Non-alcoholic fatty liver disease is a risk factor for occurrence of hepatocellular carcinoma after sustained virologic response in chronic hepatitis C patients: A prospective four-years follow-up study. Metab. Open 2021, 10, 100090. [Google Scholar] [CrossRef]
- Yoshimasu, Y.; Furuichi, Y.; Kasai, Y.; Takeuchi, H.; Sugimoto, K.; Nakamura, I.; Itoi, T. Predictive factors for hepatocellular carcinoma occurrence or recurrence after direct-acting antiviral agents in patients with chronic hepatitis C. J. Gastrointest. Liver Dis. 2019, 28, 63–71. [Google Scholar] [CrossRef]
- Yasui, Y.; Kurosaki, M.; Komiyama, Y.; Takada, H.; Tamaki, N.; Watakabe, K.; Okada, M.; Wang, W.; Shimizu, T.; Kubota, Y.; et al. Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts early occurrence of hepatocellular carcinoma after sustained virologic response by direct-acting antivirals for hepatitis C virus. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2018, 48, 1131–1139. [Google Scholar] [CrossRef]
- Mucke, V.T.; Thomas, D.; Mucke, M.M.; Waidmann, O.; Zeuzem, S.; Sarrazin, C.; Pfeilschifter, J.; Vermehren, J.; Finkelmeier, F.; Grammatikos, G. Serum sphingolipids predict de novo hepatocellular carcinoma in hepatitis C cirrhotic patients with sustained virologic response. Liver Int. Off. J. Int. Assoc. Study Liver 2019, 39, 2174–2183. [Google Scholar] [CrossRef]
- Faillaci, F.; Marzi, L.; Critelli, R.; Milosa, F.; Schepis, F.; Turola, E.; Andreani, S.; Vandelli, G.; Bernabucci, V.; Lei, B.; et al. Liver Angiopoietin-2 Is a Key Predictor of De Novo or Recurrent Hepatocellular Cancer After Hepatitis C Virus Direct-Acting Antivirals. Hepatology 2018, 68, 1010–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadan, H.K.; Meghezel, E.M.; Abdel-Malek, M.O.; Askar, A.A.; Hetta, H.F.; Mahmoud, A.A.; Abdel-Aal, A.M. Correlation Between Vascular Endothelial Growth Factor and Long-Term Occurrence of HCV-Related Hepatocellular Carcinoma After Treatment with Direct-Acting Antivirals. Cancer Investig. 2021, 39, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Pascut, D.; Cavalletto, L.; Pratama, M.Y.; Bresolin, S.; Trentin, L.; Basso, G.; Bedogni, G.; Tiribelli, C.; Chemello, L. Serum miRNA Are Promising Biomarkers for the Detection of Early Hepatocellular Carcinoma after Treatment with Direct-Acting Antivirals. Cancers 2019, 11, 1773. [Google Scholar] [CrossRef] [Green Version]
- Siphepho, P.Y.; Liu, Y.T.; Shabangu, C.S.; Huang, J.F.; Huang, C.F.; Yeh, M.L.; Yu, M.L.; Wang, S.C. The Impact of Steatosis on Chronic Hepatitis C Progression and Response to Antiviral Treatments. Biomedicines 2021, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Peleg, N.; Issachar, A.; Sneh Arbib, O.; Cohen-Naftaly, M.; Harif, Y.; Oxtrud, E.; Braun, M.; Leshno, M.; Barsheshet, A.; Shlomai, A. Liver steatosis is a major predictor of poor outcomes in chronic hepatitis C patients with sustained virological response. J. Viral Hepat. 2019, 26, 1257–1265. [Google Scholar] [CrossRef]
- Shengir, M.; Elgara, M.; Sebastiani, G. Metabolic and cardiovascular complications after virological cure in hepatitis C: What awaits beyond. World J. Gastroenterol. 2021, 27, 1959–1972. [Google Scholar] [CrossRef]
- Degasperi, E.; Galmozzi, E.; Pelusi, S.; D’Ambrosio, R.; Soffredini, R.; Borghi, M.; Perbellini, R.; Facchetti, F.; Iavarone, M.; Sangiovanni, A.; et al. Hepatic Fat-Genetic Risk Score Predicts Hepatocellular Carcinoma in Patients with Cirrhotic HCV Treated with DAAs. Hepatology 2020, 72, 1912–1923. [Google Scholar] [CrossRef]
- Domovitz, T.; Gal-Tanamy, M. Tracking down the Epigenetic Footprint of HCV-Induced Hepatocarcinogenesis. J. Clin. Med. 2021, 10, 551. [Google Scholar] [CrossRef]
- Perez, S.; Kaspi, A.; Domovitz, T.; Davidovich, A.; Lavi-Itzkovitz, A.; Meirson, T.; Alison Holmes, J.; Dai, C.Y.; Huang, C.F.; Chung, R.T.; et al. Hepatitis C virus leaves an epigenetic signature post cure of infection by direct-acting antivirals. PLoS Genet. 2019, 15, e1008181. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Khera, T.; Strunz, B.; Björkström, N.K. Reversal of Immunity after Clearance of Chronic HCV Infection—All Reset? Front. Immunol. 2020, 11, 2659. [Google Scholar] [CrossRef]
- Ghosh, A.; Romani, S.; Kottilil, S.; Poonia, B. Lymphocyte Landscape after Chronic Hepatitis C Virus (HCV) Cure: The New Normal. Int. J. Mol. Sci. 2020, 21, 7473. [Google Scholar] [CrossRef]
- Jiang, H.J.; Wang, X.X.; Luo, B.F.; Cong, X.; Jin, Q.; Qin, H.; Zhang, H.Y.; Kong, X.S.; Wei, L.; Feng, B. Direct antiviral agents upregulate natural killer cell potential activity in chronic hepatitis C patients. Clin. Exp. Med. 2019, 19, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Aregay, A.; Owusu Sekyere, S.; Deterding, K.; Port, K.; Dietz, J.; Berkowski, C.; Sarrazin, C.; Manns, M.P.; Cornberg, M.; Wedemeyer, H. Elimination of hepatitis C virus has limited impact on the functional and mitochondrial impairment of HCV-specific CD8+ T cell responses. J. Hepatol. 2019, 71, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Hartnell, F.; Esposito, I.; Swadling, L.; Brown, A.; Phetsouphanh, C.; de Lara, C.; Gentile, C.; Turner, B.; Dorrell, L.; Capone, S.; et al. Characterizing Hepatitis C Virus-Specific CD4(+) T Cells Following Viral-Vectored Vaccination, Directly Acting Antivirals, and Spontaneous Viral Cure. Hepatology 2020, 72, 1541–1555. [Google Scholar] [CrossRef]
- Debes, J.D.; van Tilborg, M.; Groothuismink, Z.M.A.; Hansen, B.E.; Schulze Zur Wiesch, J.; von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate with Development of Hepatocellular Carcinoma in Patients with HCV Infection Treated with Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517.e3. [Google Scholar] [CrossRef]
- Macek Jilkova, Z.; Seigneurin, A.; Coppard, C.; Ouaguia, L.; Aspord, C.; Marche, P.N.; Leroy, V.; Decaens, T. Circulating IL-13 Is Associated with De Novo Development of HCC in HCV-Infected Patients Responding to Direct-Acting Antivirals. Cancers 2020, 12, 3820. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Green, P.K.; Beste, L.A.; Mun, E.J.; Kerr, K.F.; Berry, K. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J. Hepatol. 2018, 69, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Wakabayashi, H.; Nakayama, H.; Suzuki, T.; Kuroda, M.; Yoshida, N.; Tojo, J.; Kogure, A.; Rai, T.; Saito, H.; et al. Factors associated with hepatocellular carcinoma occurrence after HCV eradication in patients without cirrhosis or with compensated cirrhosis. PLoS ONE 2020, 15, e0243473. [Google Scholar] [CrossRef]
- Innes, H.; Jepsen, P.; McDonald, S.; Dillon, J.; Hamill, V.; Yeung, A.; Benselin, J.; Went, A.; Fraser, A.; Bathgate, A.; et al. Performance of models to predict hepatocellular carcinoma risk among UK patients with cirrhosis and cured HCV infection. JHEP Rep. 2021, 3, 100384. [Google Scholar] [CrossRef] [PubMed]
- Tani, J.; Morishita, A.; Sakamoto, T.; Takuma, K.; Nakahara, M.; Fujita, K.; Oura, K.; Tadokoro, T.; Mimura, S.; Nomura, T.; et al. Simple scoring system for prediction of hepatocellular carcinoma occurrence after hepatitis C virus eradication by direct-acting antiviral treatment: All Kagawa Liver Disease Group Study. Oncol. Lett. 2020, 19, 2205–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahata, Y.; Sakamori, R.; Yamada, R.; Kodama, T.; Hikita, H.; Hagiwara, H.; Imai, Y.; Hiramatsu, N.; Tamura, S.; Yamamoto, K.; et al. Prediction model for hepatocellular carcinoma occurrence in patients with hepatitis C in the era of direct-acting anti-virals. Aliment. Pharmacol. Ther. 2021, 54, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macek Jilkova, Z.; Saleem, K.; Afzal, S.; Decaens, T. Predictive Factors for Hepatocellular Carcinoma Development after Direct-Acting Antiviral Treatment of HCV. Livers 2021, 1, 313-321. https://doi.org/10.3390/livers1040024
Macek Jilkova Z, Saleem K, Afzal S, Decaens T. Predictive Factors for Hepatocellular Carcinoma Development after Direct-Acting Antiviral Treatment of HCV. Livers. 2021; 1(4):313-321. https://doi.org/10.3390/livers1040024
Chicago/Turabian StyleMacek Jilkova, Zuzana, Komal Saleem, Samia Afzal, and Thomas Decaens. 2021. "Predictive Factors for Hepatocellular Carcinoma Development after Direct-Acting Antiviral Treatment of HCV" Livers 1, no. 4: 313-321. https://doi.org/10.3390/livers1040024
APA StyleMacek Jilkova, Z., Saleem, K., Afzal, S., & Decaens, T. (2021). Predictive Factors for Hepatocellular Carcinoma Development after Direct-Acting Antiviral Treatment of HCV. Livers, 1(4), 313-321. https://doi.org/10.3390/livers1040024





