Reaction Capsule Design for Interaction of Heavy Liquid Metal Coolant, Fuel Cladding, and Simulated JOG Phase at Accident Conditions
Abstract
1. Introduction
2. Materials and Methods
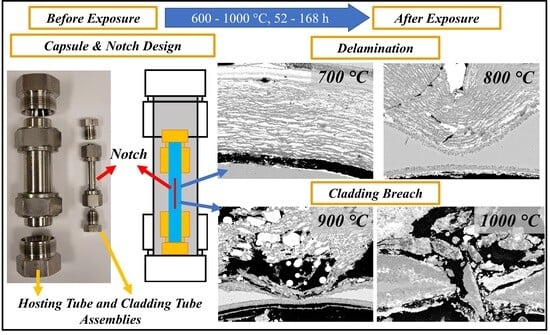
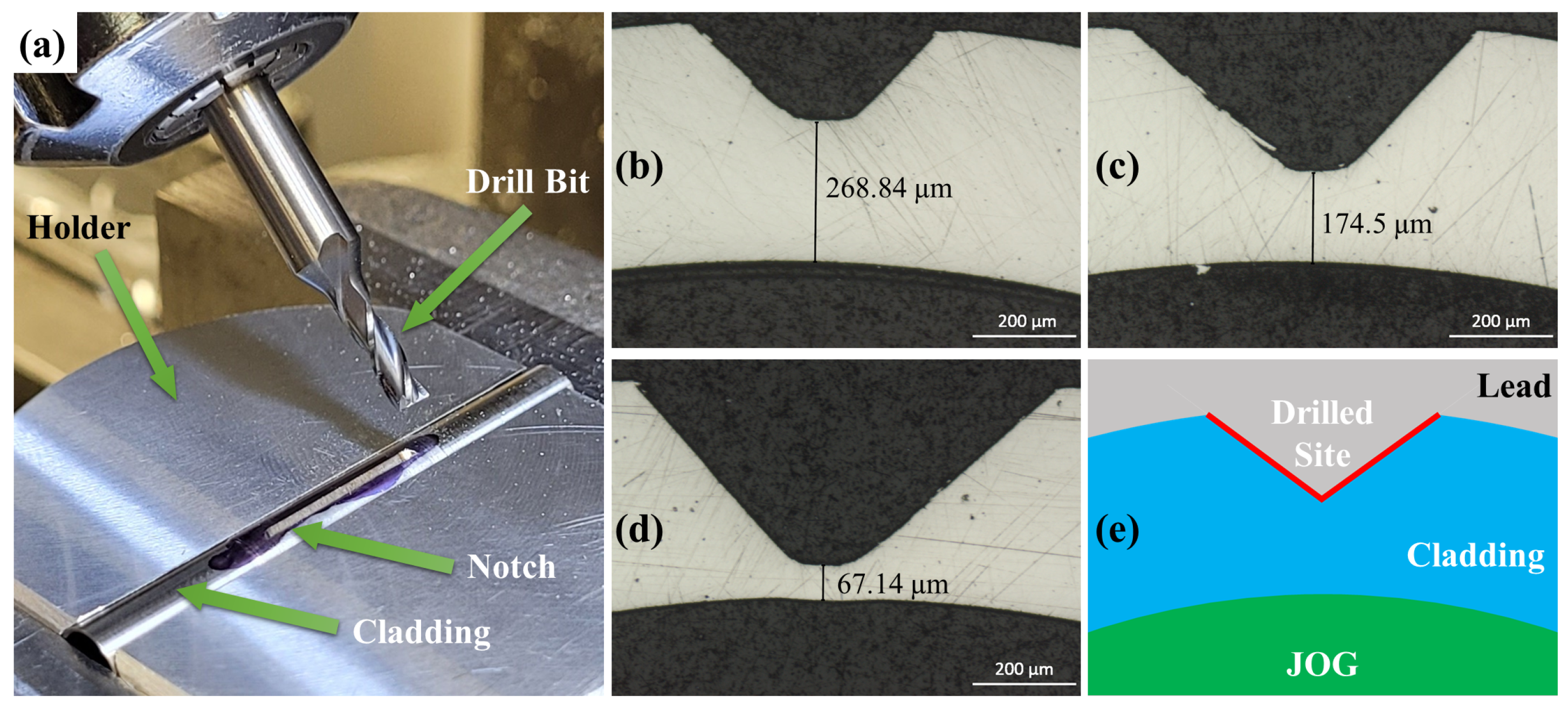
2.1. Design of Reaction Capsule
2.2. Metallic Materials
2.3. Coolant and JOG Chemical Composition
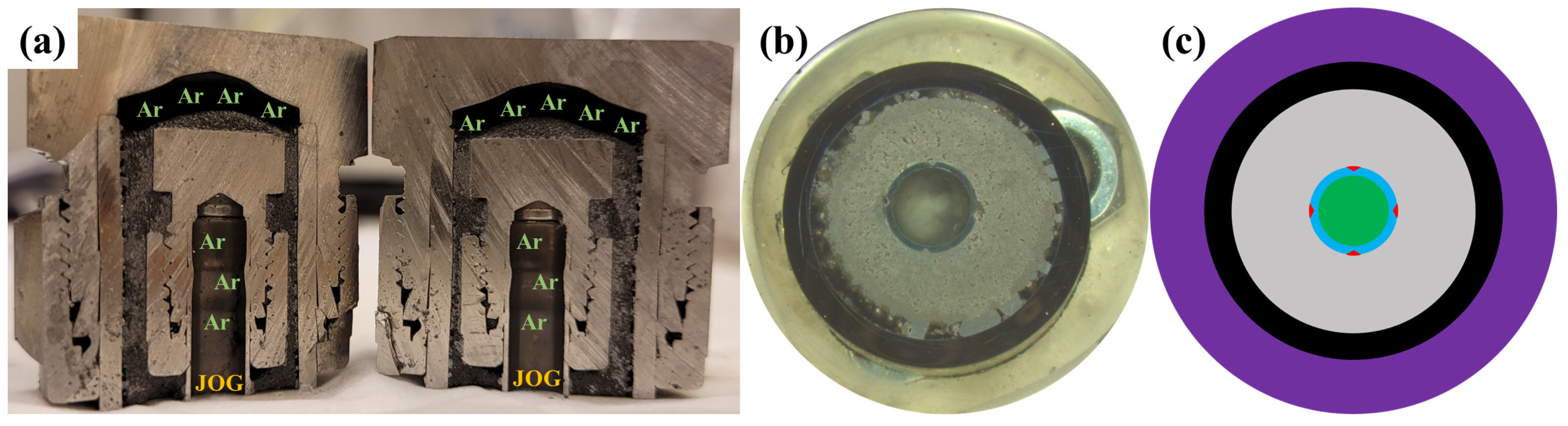
2.4. Assembling and Exposure Experiments
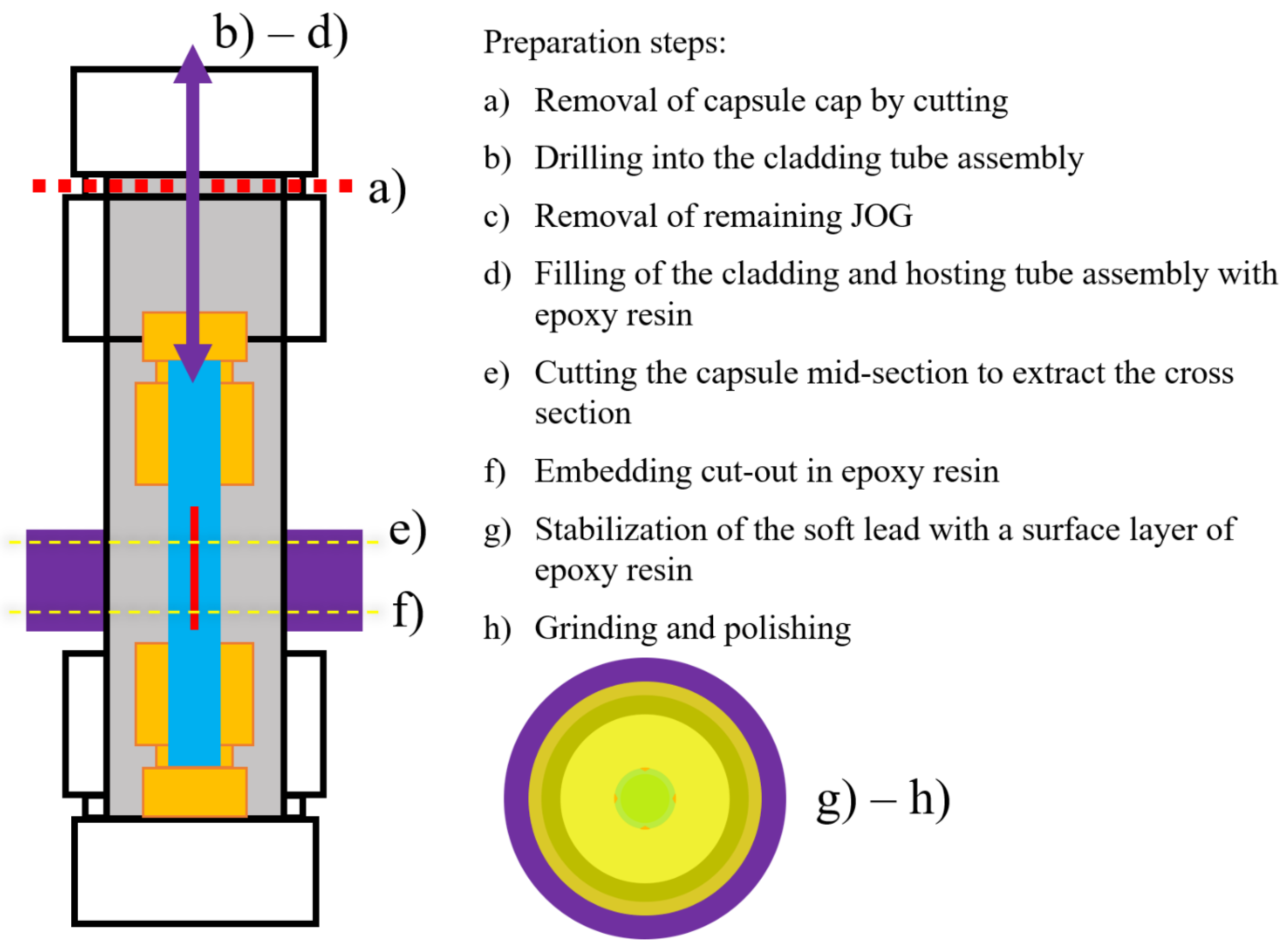
2.5. JOG Extraction and Cross-Section Preparation
2.6. Characterization
3. Results
3.1. Capsule Performance
3.2. Cladding Tube Thinning Investigation
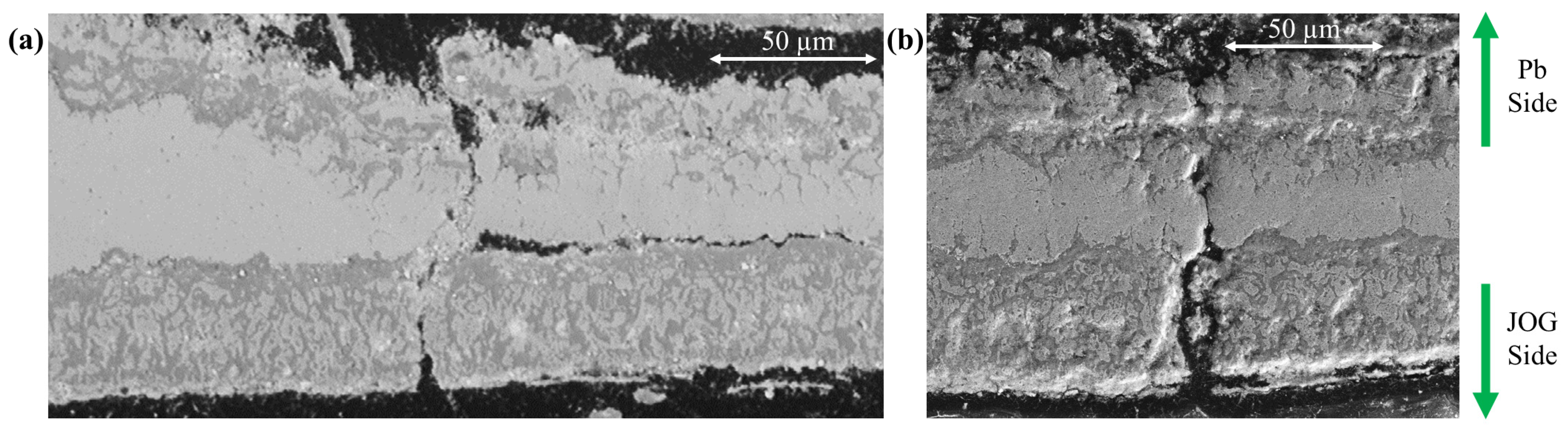
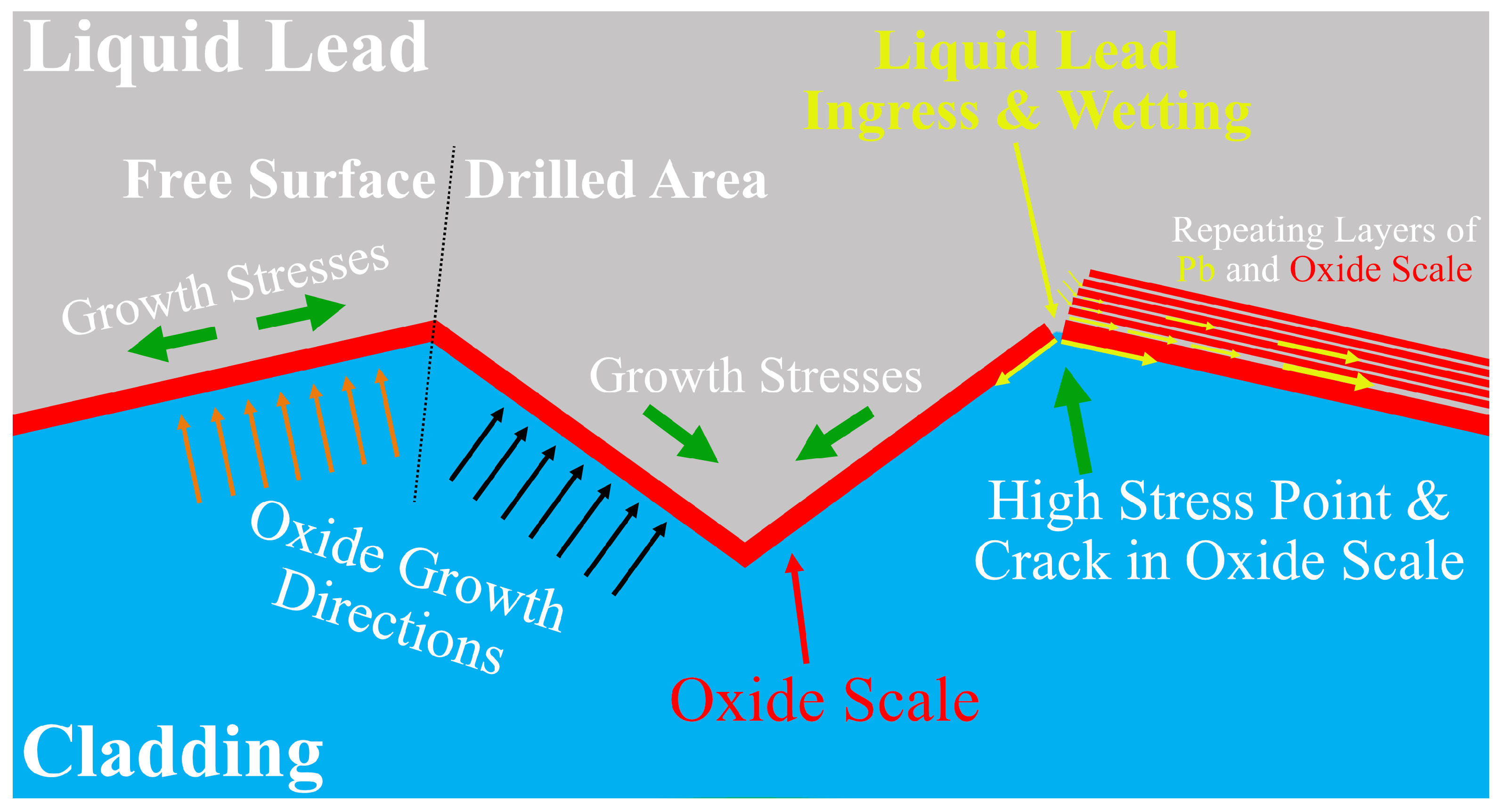
3.3. Corrosion and Breach Characteristics of the Cladding Tube
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, J.E. Generation IV International Forum: A decade of progress through international cooperation. Prog. Nucl. Energy 2014, 77, 240–246. [Google Scholar] [CrossRef]
- Murty, K.L.; Charit, I. Structural materials for Gen-IV nuclear reactors: Challenges and opportunities. J. Nucl. Mater. 2008, 383, 189–195. [Google Scholar] [CrossRef]
- Fazio, C.; Sobolev, V.; Aerts, A.; Gavrilov, S.; Lambrinou, K.; Schuurmans, P.; Gessi, A.; Agostini, P.; Ciampichetti, A.; Martinelli, L. Handbook on Lead-Bismuth Eutectic Alloy and Lead Properties, Materials Compatibility, Thermal-Hydraulics and Technologies; Organisation for Economic Co-Operation and Development: Paris, France, 2015. [Google Scholar]
- Roy, M.; Martinelli, L.; Ginestar, K.; Favergeon, J.; Moulin, G. Dissolution and oxidation behaviour of various austenitic steels and Ni rich alloys in lead-bismuth eutectic at 520 °C. J. Nucl. Mater. 2016, 468, 153–163. [Google Scholar] [CrossRef]
- Buckthorpe, D. Introduction to Generation IV nuclear reactors. In Structural Materials for Generation IV Nuclear Reactors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–22. [Google Scholar]
- Alemberti, A.; Caramello, M.; Frignani, M.; Grasso, G.; Merli, F.; Morresi, G.; Tarantino, M. ALFRED reactor coolant system design. Nucl. Eng. Des. 2020, 370, 110884. [Google Scholar] [CrossRef]
- Abderrahim, H.A.; Baeten, P.; De Bruyn, D.; Heyse, J.; Schuurmans, P.; Wagemans, J. MYRRHA, a Multipurpose hYbrid Research Reactor for High-end Applications. Nucl. Phys. News 2010, 20, 24–28. [Google Scholar] [CrossRef]
- Delville, R.; Stergar, E.; Verwerft, M. Results of a New Production of Nuclear-Grade 1.4970 ‘15-15Ti’Stainless Steel Fuel Cladding Tubes for GEN IV Reactors. In Proceedings of the International Conference on Nuclear Engineering, Prague, Czech Republic, 7–11 July 2014. [Google Scholar]
- Kautz, E.; Gwalani, B.; Yu, Z.; Varga, T.; Geelhood, K.; Devaraj, A.; Senor, D. Investigating zirconium alloy corrosion with advanced experimental techniques: A review. J. Nucl. Mater. 2023, 585, 154586. [Google Scholar] [CrossRef]
- Henry, J.; Maloy, S.A. Irradiation-resistant ferritic and martensitic steels as core materials for Generation IV nuclear reactors. In Structural Materials for Generation IV Nuclear Reactors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 329–355. [Google Scholar]
- Séran, J.L.; Le Flem, M. Irradiation-resistant austenitic steels as core materials for Generation IV nuclear reactors. In Structural Materials for Generation IV Nuclear Reactors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–328. [Google Scholar]
- Frazer, D.; Qvist, S.; Parker, S.; Krumwiede, D.L.; Caro, M.; Tesmer, J.; Maloy, S.A.; Wang, Y.Q.; Hosemann, P. Degradation of HT9 under simultaneous ion beam irradiation and liquid metal corrosion. J. Nucl. Mater. 2016, 479, 382–389. [Google Scholar] [CrossRef]
- Uwaba, T.; Ito, M.; Maeda, K. Diametral strain of fast reactor MOX fuel pins with austenitic stainless steel cladding irradiated to high burnup. J. Nucl. Mater. 2011, 416, 350–357. [Google Scholar] [CrossRef]
- Maeda, K.; Asaga, T. Change of fuel-to-cladding gap width with the burn-up in FBR MOX fuel irradiated to high burn-up. J. Nucl. Mater. 2004, 327, 1–10. [Google Scholar] [CrossRef]
- Barani, T.; Pizzocri, D.; Cappia, F.; Luzzi, L.; Pastore, G.; Van Uffelen, P. Modeling high burnup structure in oxide fuels for application to fuel performance codes. part I: High burnup structure formation. J. Nucl. Mater. 2020, 539, 152296. [Google Scholar] [CrossRef]
- Yurechko, M.; Schroer, C.; Skrypnik, A.; Wedemeyer, O.; Tsisar, V.; Konys, J. Steel T91 subjected to static stress in lead-bismuth eutectic at 450–550 °C and low oxygen concentration. J. Nucl. Mater. 2018, 512, 423–439. [Google Scholar] [CrossRef]
- Ejenstam, J.; Halvarsson, M.; Weidow, J.; Jönsson, B.; Szakalos, P. Oxidation studies of Fe10CrAl–RE alloys exposed to Pb at 550 °C for 10,000 h. J. Nucl. Mater. 2013, 443, 161–170. [Google Scholar] [CrossRef]
- Hojna, A.; Di Gabriele, F.; Klecka, J. Characteristics and Liquid Metal Embrittlement of the steel T91 in contact with Lead-Bismuth Eutectic. J. Nucl. Mater. 2016, 472, 163–170. [Google Scholar] [CrossRef]
- Schroer, C.; Voss, Z.; Wedemeyer, O.; Novotny, J.; Konys, J. Oxidation of steel T91 in flowing lead-bismuth eutectic (LBE) at 550 °C. J. Nucl. Mater. 2006, 356, 189–197. [Google Scholar] [CrossRef]
- Polekhina, N.A.; Litovchenko, I.Y.; Almaeva, K.V.; Pinzhin, Y.P.; Akkuzin, S.A.; Tyumentsev, A.N.; Chernov, V.M.; Leontyeva-Smirnova, M.V. Behavior of 12% Cr low-activation ferritic-martensitic steel EK-181 after holding in a static lead melt at 600 °C for 3000 hours. J. Nucl. Mater. 2021, 545, 152754. [Google Scholar] [CrossRef]
- Lambrinou, K.; Charalampopoulou, E.; Van der Donck, T.; Delville, R.; Schryvers, N. Corrigendum to “Dissolution corrosion of 316L austenitic stainless steels in contact with static liquid lead-bismuth eutectic (LBE) at 500 °C”. Nucl. Mater. 2017, 490, 9–27. J. Nucl. Mater. 2017, 490, 345. [Google Scholar] [CrossRef]
- Schroer, C.; Wedemeyer, O.; Novotny, J.; Skrypnik, A.; Konys, J. Selective leaching of nickel and chromium from Type 316 austenitic steel in oxygen-containing lead–bismuth eutectic (LBE). Corros. Sci. 2014, 84, 113–124. [Google Scholar] [CrossRef]
- Glasbrenner, H.; Konys, J.; Mueller, G.; Rusanov, A. Corrosion investigations of steels in flowing lead at 400 °C and 550 °C. J. Nucl. Mater. 2001, 296, 237–242. [Google Scholar] [CrossRef]
- Košek, L.; Rozumová, L.; Hojná, A.; Aparicio, C.; Vronka, M.; Vít, J. Mechanism of localized corrosion issues of austenitic steels exposed to flowing lead with 10−7 wt.% oxygen at 480 °C up to 16,000 h. J. Nucl. Mater. 2022, 572, 154045. [Google Scholar] [CrossRef]
- Vogt, J.-B.; Proriol Serre, I. A Review of the Surface Modifications for Corrosion Mitigation of Steels in Lead and LBE. Coatings 2021, 11, 53. [Google Scholar] [CrossRef]
- Cautaerts, N.; Delville, R.; Dietz, W.; Verwerft, M. Thermal creep properties of Ti-stabilized DIN 1.4970 (15-15Ti) austenitic stainless steel pressurized cladding tubes. J. Nucl. Mater. 2017, 493, 154–167. [Google Scholar] [CrossRef]
- Zhang, J.S.; Li, N. Review of the studies on fundamental issues in LBE corrosion. J. Nucl. Mater. 2008, 373, 351–377. [Google Scholar] [CrossRef]
- Bassini, S.; Antonelli, A.; Di Piazza, I.; Tarantino, M. Oxygen sensors for Heavy Liquid Metal coolants: Calibration and assessment of the minimum reading temperature. J. Nucl. Mater. 2017, 486, 197–205. [Google Scholar] [CrossRef]
- Rosseel, K.; Lim, J.; Marino, A.; Gladinez, K.; Gonzalez-Prieto, B.; Aerts, A. HELIOS3: A stirred bubble column for oxygen addition or reduction in lead-bismuth eutectic. Nucl. Eng. Des. 2020, 365, 110716. [Google Scholar] [CrossRef]
- Ni-Pb Binary Phase Diagram 0–100 at.% Pb: Datasheet from “PAULING FILE Multinaries Edition—2022”. SpringerMaterials. Available online: https://materials.springer.com/isp/phase-diagram/docs/c_0905795 (accessed on 24 April 2023).
- Klecka, J.; Di Gabriele, F.; Hojna, A. Mechanical properties of the steel T91 in contact with lead. Nucl. Eng. Des. 2015, 283, 131–138. [Google Scholar] [CrossRef]
- Cr-Pb Binary Phase Diagram 0–100 at.% Pb: Datasheet from “PAULING FILE Multinaries Edition—2022”. SpringerMaterials. Available online: https://materials.springer.com/isp/phase-diagram/docs/c_0900789 (accessed on 24 April 2023).
- Müller, G.; Schumacher, G.; Zimmermann, F. Investigation on oxygen controlled liquid lead corrosion of surface treated steels. J. Nucl. Mater. 2000, 278, 85–95. [Google Scholar] [CrossRef]
- Weisenburger, A.; Schroer, C.; Jianu, A.; Heinzel, A.; Konys, J.; Steiner, H.; Muller, G.; Fazio, C.; Gessi, A.; Babayan, S.; et al. Long term corrosion on T91 and AISI1 316L steel in flowing lead alloy and corrosion protection barrier development: Experiments and models. J. Nucl. Mater. 2011, 415, 260–269. [Google Scholar] [CrossRef]
- Fazio, C.; Balbaud, F. Corrosion phenomena induced by liquid metals in Generation IV reactors. In Structural Materials for Generation IV Nuclear Reactors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–74. [Google Scholar]
- Maeda, K.; Tanaka, K.; Asaga, T.; Furuya, H. Distributions of volatile fission products in or near the fuel-cladding gap of the FBR MOX fuel pins irradiated to high burn-up. J. Nucl. Mater. 2005, 344, 274–280. [Google Scholar] [CrossRef]
- Haste, T.; Payot, F.; Bottomley, P.D.W. Transport and deposition in the Phebus FP circuit. Ann. Nucl. Energy 2013, 61, 102–121. [Google Scholar] [CrossRef]
- Tourasse, M.; Boidron, M.; Pasquet, B. Fission product behaviour in phenix fuel pins at high burnup. J. Nucl. Mater. 1992, 188, 49–57. [Google Scholar] [CrossRef]
- Imoto, S. Chemical-State of Fission-Products in Irradiated UO2. J. Nucl. Mater. 1986, 140, 19–27. [Google Scholar] [CrossRef]
- Wallez, G.; Raison, P.E.; Smith, A.L.; Clavier, N.; Dacheux, N. High-temperature behavior of dicesium molybdate Cs2MoO4: Implications for fast neutron reactors. J. Solid State Chem. 2014, 215, 225–230. [Google Scholar] [CrossRef]
- Smith, A.L.; Vlieland, J.; Pignié, M.C.; Abbink, M.; Mikaelian, G.; Benigni, P. New insights into the Cs-Mo-O system: Experimental studies of the Cs2MoO4-MoO3 pseudo-binary system. Thermochim. Acta 2021, 696, 178825. [Google Scholar] [CrossRef]
- Konings, R.J.; Wiss, T.; Benes, O. Predicting material release during a nuclear reactor accident. Nat. Mater. 2015, 14, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Van Hattem, A.; Vlieland, J.; Dankelman, R.; Thijs, M.A.; Wallez, G.; Dardenne, K.; Rothe, J.; Konings, R.J.M.; Smith, A.L. Structural Studies and Thermal Analysis in the Cs2MoO4-PbMoO4 System with Elucidation of beta-Cs2Pb(MoO4)2. Inorg. Chem. 2023, 62, 6981–6992. [Google Scholar] [CrossRef] [PubMed]
- Vigier, J.-F.; Popa, K.; Tyrpekl, V.; Gardeur, S.; Freis, D.; Somers, J. Interaction study between MOX fuel and eutectic lead–bismuth coolant. J. Nucl. Mater. 2015, 467, 840–847. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, K.; Fang, X.D.; Xiong, Z.C.; Wei, L.H.; Zhu, P.C.; Li, X.Y. Oxidation behavior of 316L austenitic stainless steel in high temperature air with long-term exposure. Mater. Res. Express 2020, 7, 066517. [Google Scholar] [CrossRef]
- Nowak, W.J. Effect of Surface Roughness on Oxidation Resistance of Stainless Steel AISI 316Ti During Exposure at High Temperature. J. Mater. Eng. Perform. 2020, 29, 8060–8069. [Google Scholar] [CrossRef]
- Predel, B. (Ed.) Ac-Ag … Au-Zr · Introduction. In Landolt-Börnstein—Group IV Physical Chemistry; Springer: Berlin/Heidelberg, Germany, 2006; Volume 12A: “Ac-Ag…Au-Zr”, pp. 1–23. [Google Scholar]
- Smith, A.L.; Thi, T.N.P.; Gueneau, C.; Dumas, J.C.; Epifano, E.; van Burik, W.; Dupin, N. Thermodynamic modelling assessment of the ternary system Cs-Mo-O. Calphad-Comput. Coupling Phase Diagr. Thermochem. 2021, 75, 102350. [Google Scholar] [CrossRef]
- Stainless Steel Seamless Tubing and Tube Support Systems. Available online: https://www.swagelok.com/downloads/webcatalogs/en/ms-01-181.pdf (accessed on 19 June 2023).
- Zinkle, S.J.; Busby, J.T. Structural materials for fission & fusion energy. Mater. Today 2009, 12, 12–19. [Google Scholar] [CrossRef]
- Wakai, E.; Takaya, S.; Matsui, Y.; Nagae, Y.; Kato, S.; Suzudo, T.; Yamaguchi, M.; Aoto, K.; Nogami, S.; Hasegawa, A.; et al. Irradiation damages of structural materials under different irradiation environments. J. Nucl. Mater. 2021, 543, 152503. [Google Scholar] [CrossRef]

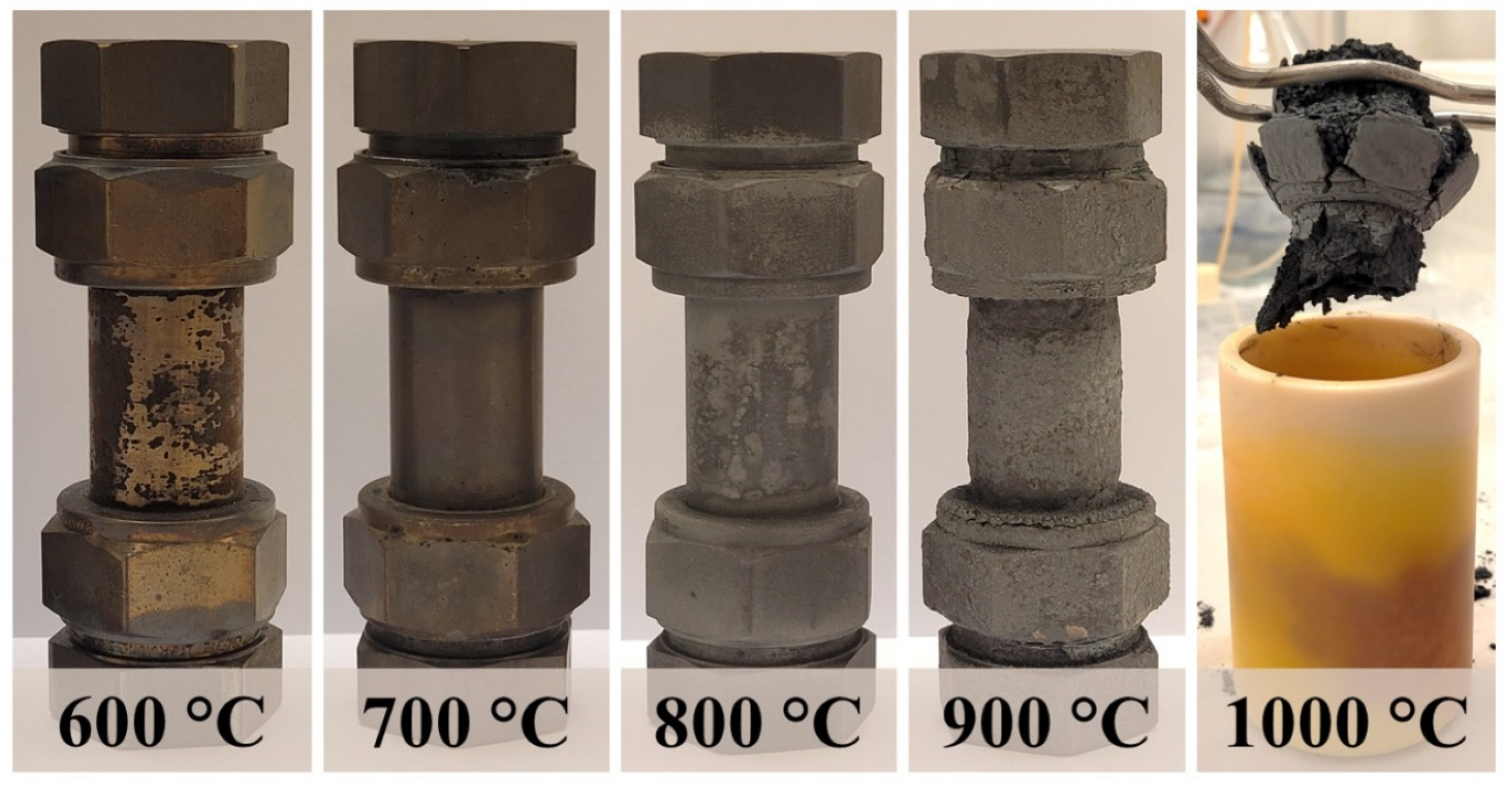
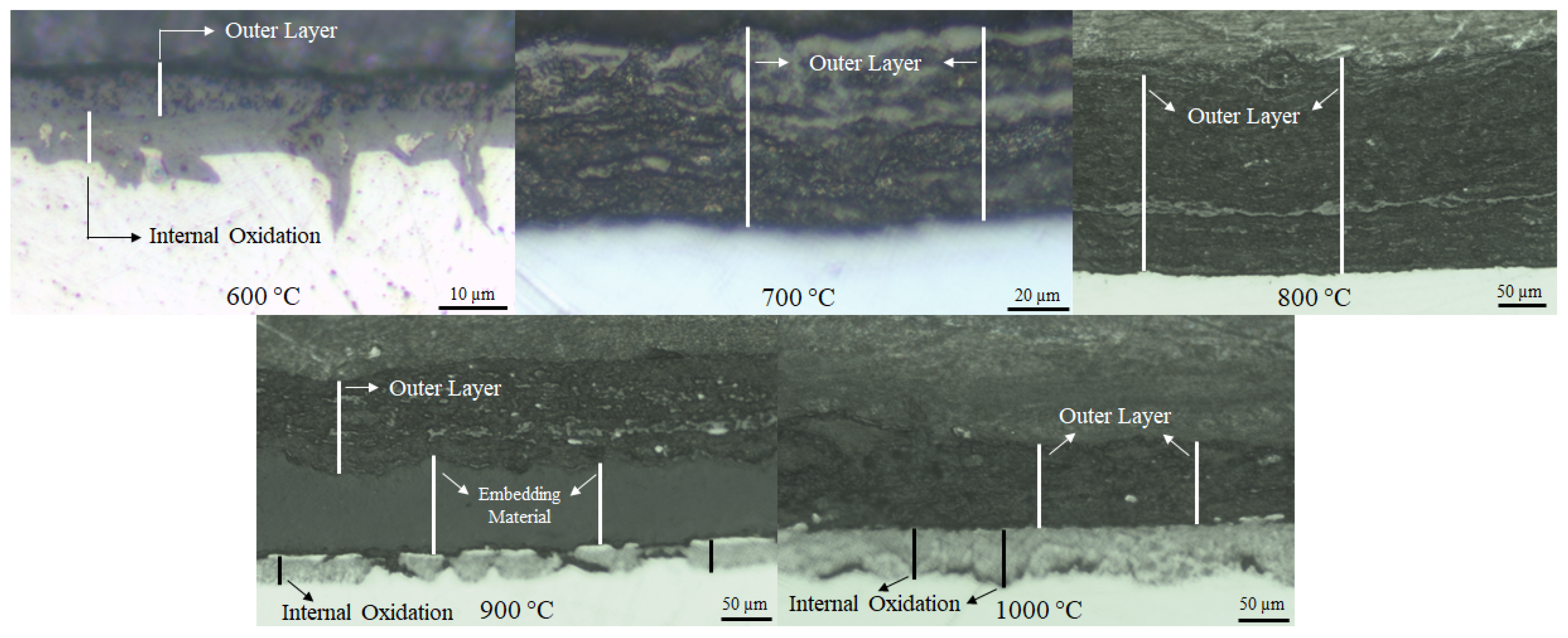
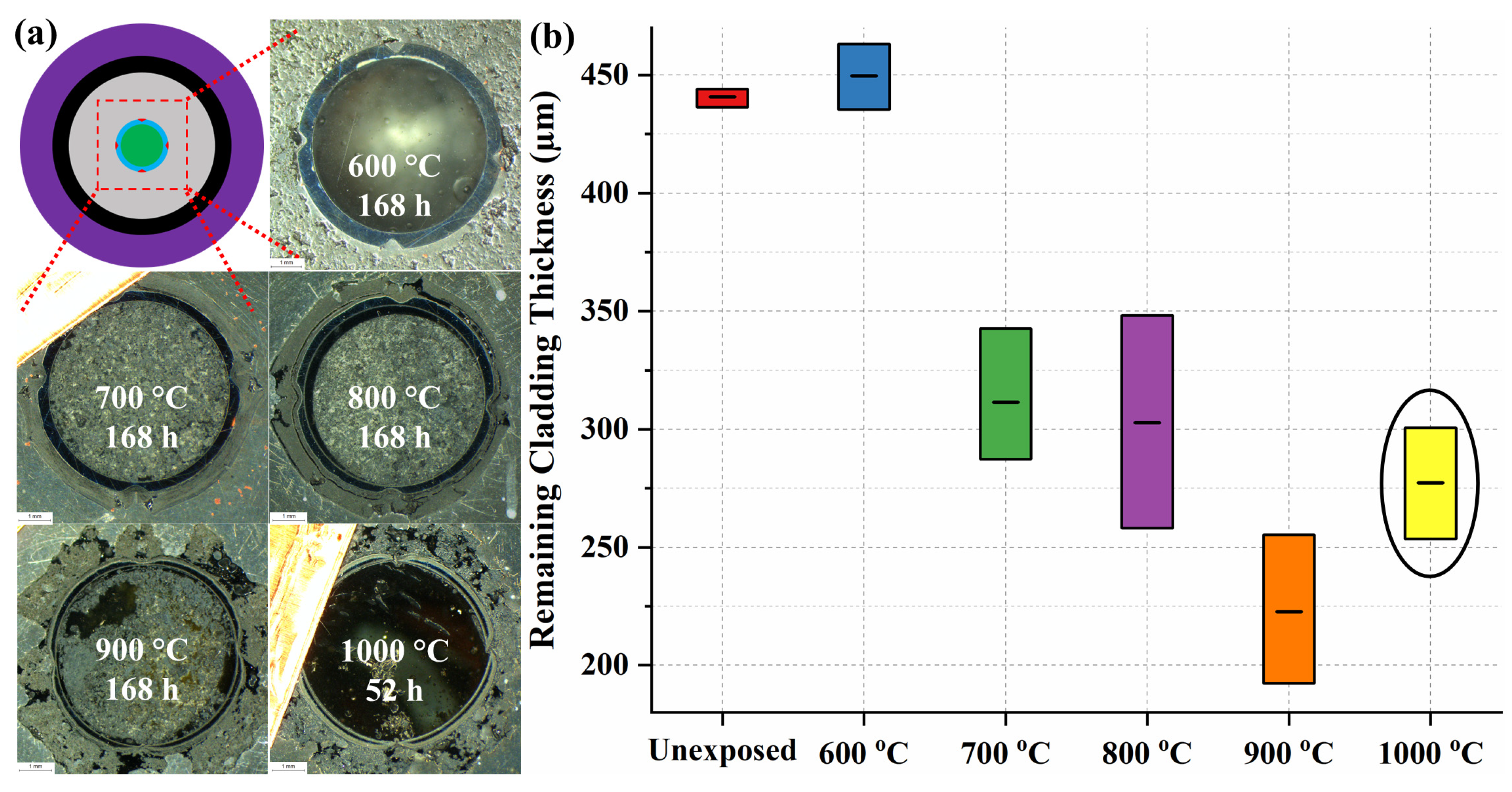
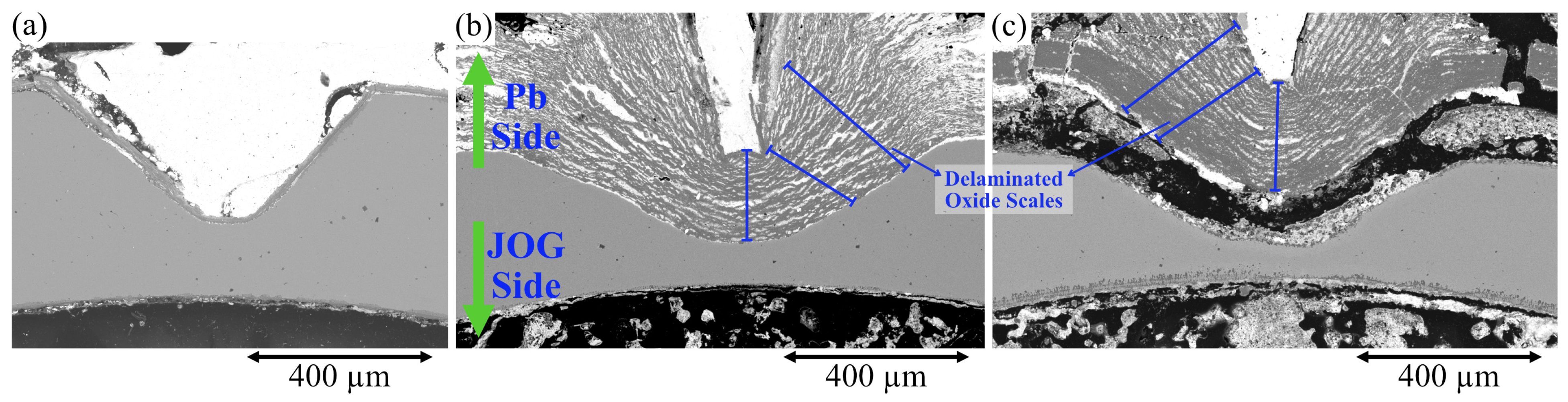



| Sample | Cr | Ni | Mo | Mn | Si | C |
| Swagelok (Alloy 316)—Advertised | 17.08–19.00 | 12.5–15.00 | 2.5–3.0 | Max 2.00 | Max 1.00 | Max 0.030 |
| Swagelok (Alloy 316)—EDX | 17.96 | 12.9 | 3.15 | 1.85 | 0.7 | n.q. |
| Cladding Tube (15-15Ti)—Ref. [8] | 15.08 | 15.04 | 1.21 | 1.83 | 0.56 | 0.10 |
| Cladding Tube (15-15Ti)—EDX | 15.56 | 14.84 | 1.58 | 2.1 | 1 | n.q. |
| Sample | S | Ti | P | Co | B | N |
| Swagelok (Alloy 316)—Advertised | Max 0.015 | n.s. | n.s. | n.s. | n.s. | n.s. |
| Swagelok (Alloy 316)—EDX | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cladding Tube (15-15Ti)—Ref. [8] | <0.001 | 0.49 | 0.013 | 0.02 | 0.0028 | 0.011 |
| Cladding Tube (15-15Ti)—EDX | n.d. | 0.41 | n.d. | n.d. | n.d. | n.d. |
| Sample | Cu | V | Ta | Ca | Fe | |
| Swagelok (Alloy 316)—Advertised | n.s. | n.s. | n.s. | n.s. | Bal. | |
| Swagelok (Alloy 316)—EDX | n.d. | n.d. | n.d. | n.d. | 63.3 | |
| Cladding Tube (15-15Ti)—Ref. [8] | 0.026 | 0.034 | <0.005 | <0.010 | Bal. | |
| Cladding Tube (15-15Ti)—EDX | n.d. | n.d. | n.d. | n.d. | 64.5 |
| Temperature (°C) | Time (h) | Remaining Hosting Tube Thickness (mm) | Internal Oxidation (µm) | Outer Inhomogeneous Layer (µm) |
|---|---|---|---|---|
| 600 | 168 | 1.92–2.02 | 5–18 | 4.2–6.1 |
| 700 | 168 | 1.89–1.90 | None | 50–64 |
| 800 | 168 | 1.82–1.88 | None | 216–277 |
| 900 | 168 | 1.36–1.72 | 15–32 | 80–114 |
| 1000 | 52 | 1.8–1.94 | 37–63 | 82–120 |
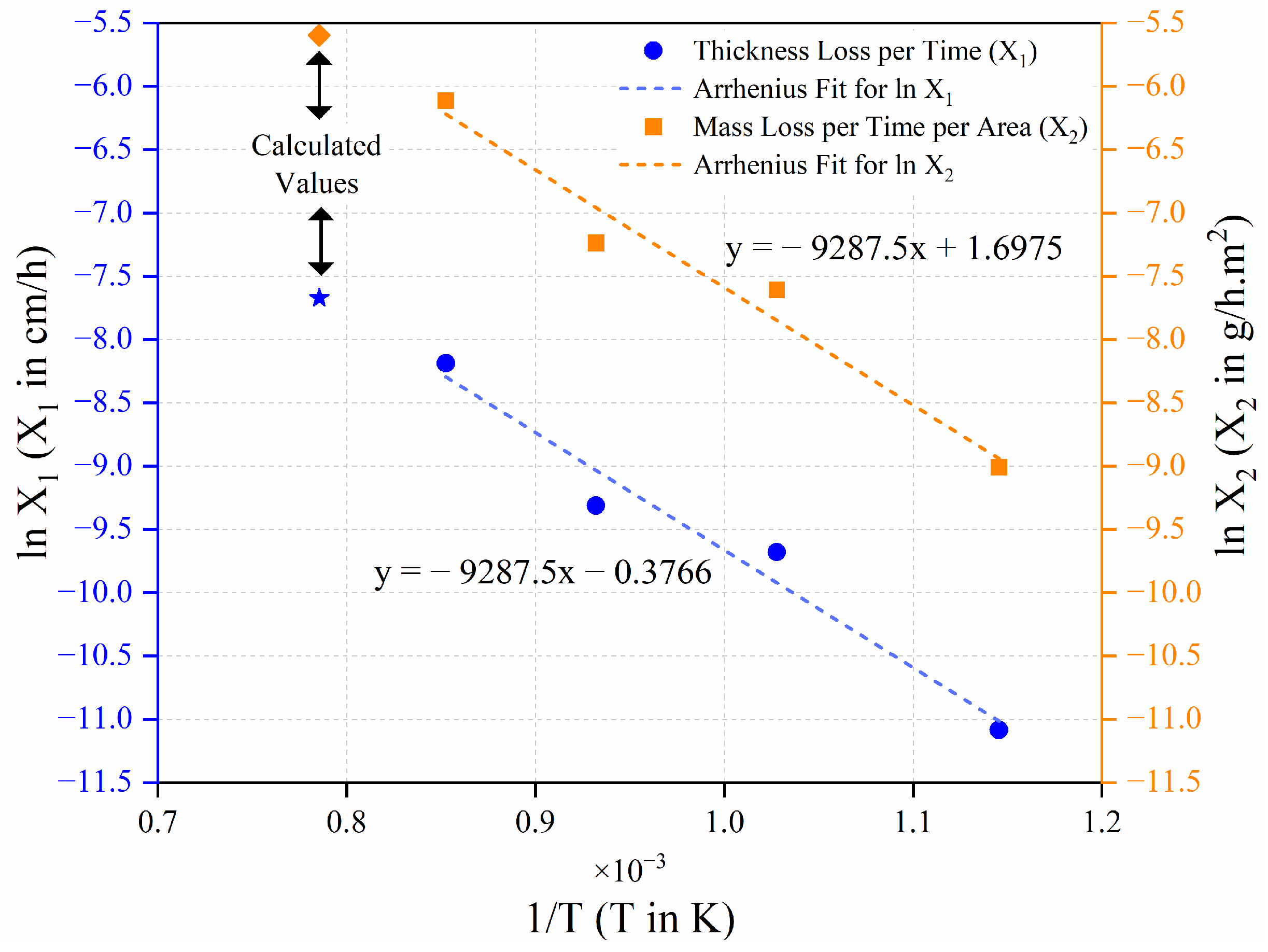
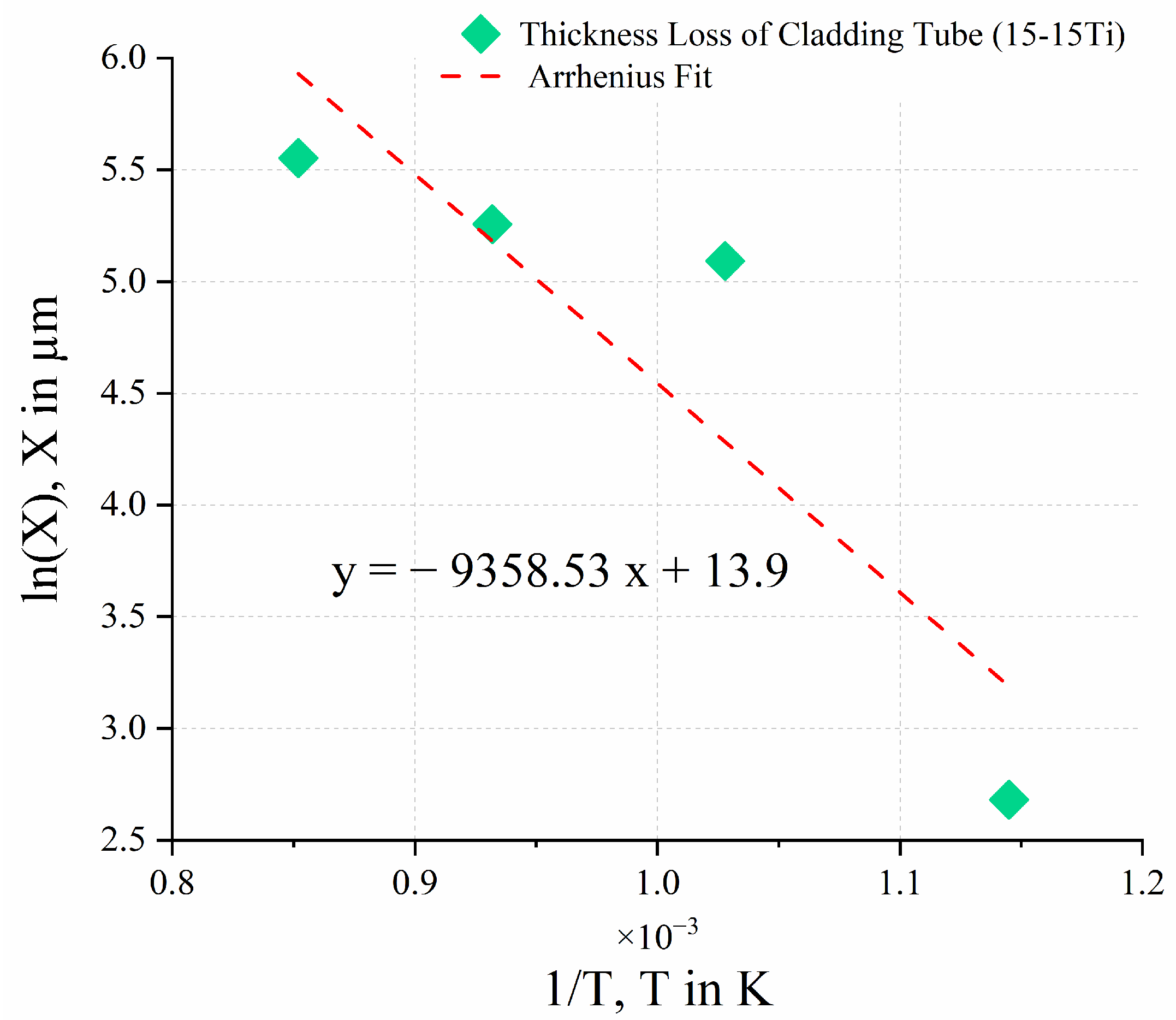
| T in °C | T in K | 1/T | in µm | |
|---|---|---|---|---|
| 600 | 873.15 | 0.001145 | 14.6 | 2.681 |
| 700 | 973.15 | 0.001028 | 162.7 | 5.092 |
| 800 | 1073.15 | 0.000932 | 191.8 | 5.256 |
| 900 | 1173.15 | 0.000852 | 257.7 | 5.552 |
| 928.09 | 1201.24 | 0.000833 | 450 | 6.109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarı, D.; Retegan Vollmer, T.; Geers, C. Reaction Capsule Design for Interaction of Heavy Liquid Metal Coolant, Fuel Cladding, and Simulated JOG Phase at Accident Conditions. J. Nucl. Eng. 2024, 5, 57-73. https://doi.org/10.3390/jne5010005
Tarı D, Retegan Vollmer T, Geers C. Reaction Capsule Design for Interaction of Heavy Liquid Metal Coolant, Fuel Cladding, and Simulated JOG Phase at Accident Conditions. Journal of Nuclear Engineering. 2024; 5(1):57-73. https://doi.org/10.3390/jne5010005
Chicago/Turabian StyleTarı, Doğaç, Teodora Retegan Vollmer, and Christine Geers. 2024. "Reaction Capsule Design for Interaction of Heavy Liquid Metal Coolant, Fuel Cladding, and Simulated JOG Phase at Accident Conditions" Journal of Nuclear Engineering 5, no. 1: 57-73. https://doi.org/10.3390/jne5010005
APA StyleTarı, D., Retegan Vollmer, T., & Geers, C. (2024). Reaction Capsule Design for Interaction of Heavy Liquid Metal Coolant, Fuel Cladding, and Simulated JOG Phase at Accident Conditions. Journal of Nuclear Engineering, 5(1), 57-73. https://doi.org/10.3390/jne5010005