Feasibility Study on Production of High-Purity Rhenium-185 by Nuclear Transmutation of Natural Tantalum
Abstract
1. Introduction
2. Analysis Methods
3. Results and Discussion
3.1. Production of Re-185 via the Transmutation of Natural Ta Compared to Natural W
3.2. Improving the Production Rate of Re-185 from Natural Ta Using a Moderator
3.2.1. Comparison of Re-185 Production Using ZrH1.7 or ZrD1.7 as a Moderator
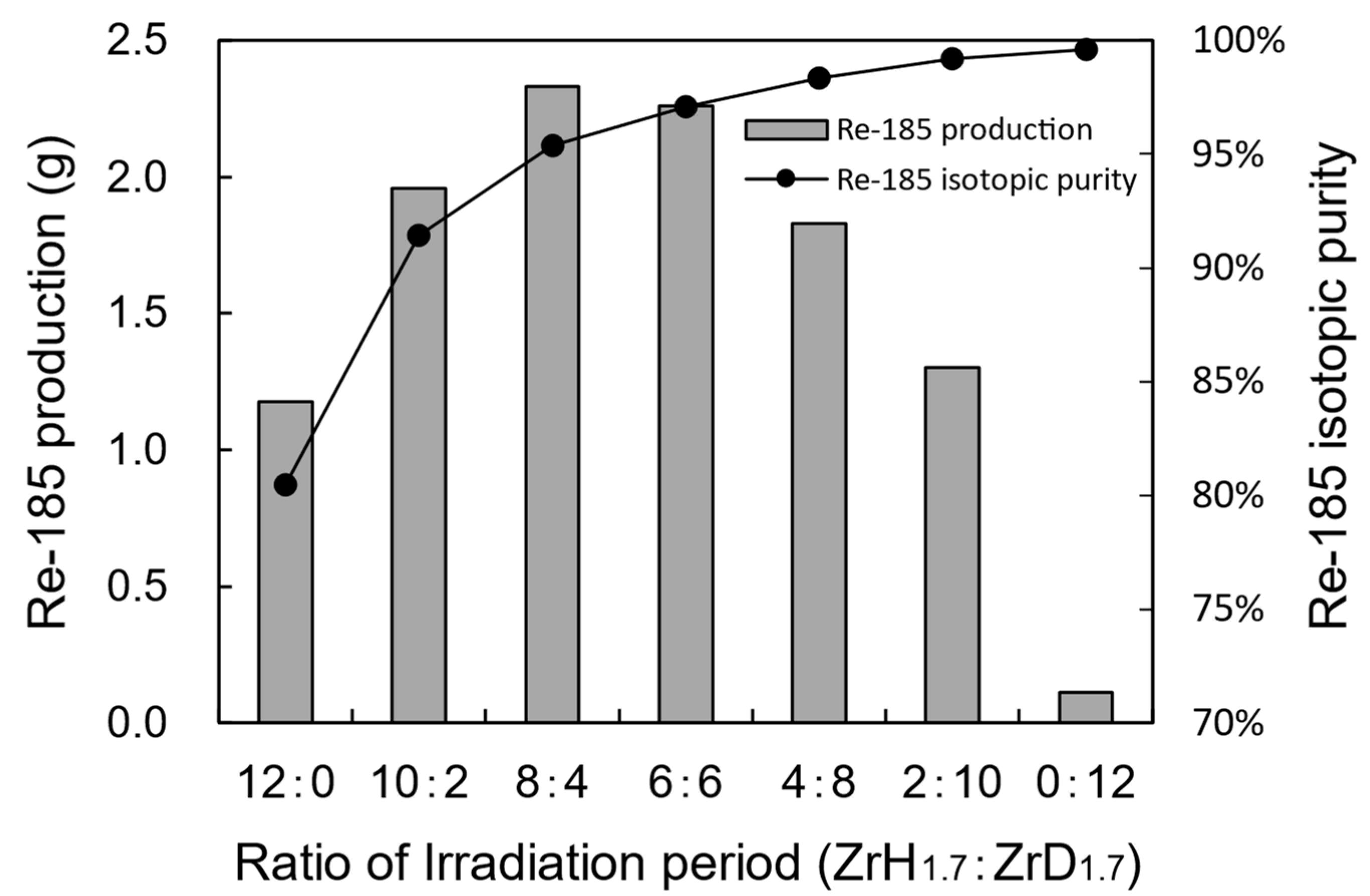
3.2.2. Improvement in the Re-185 Production Rate via Two-Step Irradiation with ZrH1.7 and ZrD1.7 Moderators
3.3. Estimated Doses of Medical Re-186 Produced from Re185
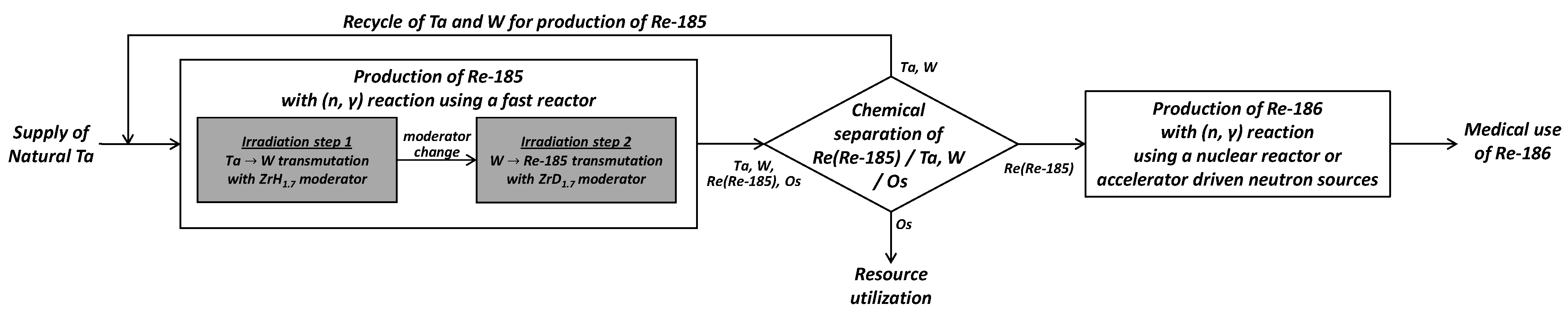
3.4. Scheme for the Production of Medical Re-186 Starting from the Transmutation of Natural Ta
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- John, D.A.; Seal, R.R., II; Polyak, D.E. Rhenium. In Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; U.S. Geological Survey Professional Paper 1802–P; Schulz, K.J., DeYoung, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; U.S. Geological Survey: Reston, VA, USA, 2017; pp. P1–P49. [Google Scholar] [CrossRef]
- Shen, L.; Tesfaye, F.; Li, X.; Lindberg, D.; Taskinen, P. Review of rhenium extraction and recycling technologies from primary and secondary resources. Miner. Eng. 2021, 161, 106719. [Google Scholar] [CrossRef]
- O’Sullivan, J.; McCready, V.; Flux, G.; Norman, A.R.; Buffa, F.M.; Chittenden, S.; Guy, M.; Pomeroy, K.; Cook, G.; Gadd, J.; et al. High activity Rhenium-186 HEDP with autologous peripheral blood stem cell rescue: A phase I study in progressive hormone refractory prostate cancer metastatic to bone. Br. J. Cancer 2002, 86, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Argyrou, M.; Valassi, A.; Andreou, M.; Lyra, M. Dosimetry and Therapeutic Ratios for Rhenium-186 HEDP. Int. Sch. Res. Not. 2013, 2013, 124603. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Manual for Reactor Produced Radioisotopes; IAEA-TECDOC-1340; International Atomic Energy Agency: Vienna, Austria, 2003; pp. 179–185. [Google Scholar]
- Khandaker, M.U.; Nagatsu, K.; Minegishi, K.; Zhang, M.-R.; Jalilian, A.R.; Bradley, D. production of no carrier added 186gRe radionuclide for theranostic applications. Appl. Radiat. Isot. 2020, 166, 109843. [Google Scholar] [CrossRef] [PubMed]
- Moustapha, M.E.; Ehrhardt, G.J.; Smith, C.J.; Szajek, L.P.; Eckelman, W.C.; Jurisson, S.S. Preparation of cyclotron-produced 186Re and comparison with reactor-produced 186Re and generator-produced 188Re for the labeling of bombesin. Nucl. Med. Biol. 2006, 33, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lapi, S.; Ruth, T.J.; Becker, D.W.; D’Auria, J.M. US20080241025–Method and Apparatus for ISO Lating The Radioisotope 186Rhenium. U.S. Patent US7708961B2, 4 May 2010. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US42294788 (accessed on 4 June 2023).
- Keim, C.P. Enriching Stable Isotopes Electromagnetically. J. Appl. Phys. 1953, 24, 1255–1261. [Google Scholar] [CrossRef]
- Love, L.O. Electromagnetic Separation of Isotopes at Oak Ridge. Science 1973, 182, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Egle, B.J.; Hart, K.J.; Aaron, W.S. Stable isotope enrichment capabilities at Oak Ridge National Laboratory. J. Radioanal. Nucl. Chem. 2014, 299, 995–999. [Google Scholar] [CrossRef]
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Weissleder, R. Radiotheranostics: A roadmap for future development. Lancet Oncol. 2020, 3, e146–e156. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Terashima, A.; Ozawa, M. Evaluation of Rhenium Production Rates in Tungsten Irradiated in Fast Reactors by Using Continuous Energy Monte Carlo Code MVP. In Proceedings of the 8th International Symposium on Technetium and Rhenium: Science and Utilization, Pornichet, France, 29 September–3 October 2014; pp. 526–532. [Google Scholar]
- Yokoyama, T.; Tanoue, Y.; Terashima, A.; Ozawa, M. Optimizing the Transmutation Rate of Tungsten to Rhenium by Using Zirconium Hydride Moderators in Fast Reactors. In Proceedings of the 3rd Japan-China Academic Symposium on Nuclear Fuel Cycle (ASNFC2015), Program and Abstracts, Tokyo, Japan, 2–5 December 2015; pp. 96–97. [Google Scholar]
- Yokoyama, T.; Tanoue, Y.; Terashima, A.; Ozawa, M. Production of Rhenium by Transmuting Tungsten Metal in Fast Reactors with Moderator. J. Energy Power Eng. 2016, 10, 159–165. [Google Scholar]
- Yokoyama, T.; Ozawa, M. Production of Low Activity Rhenium by Transmuting Tungsten Metal in Fast Reactors with Moderator. Int. J. At. Nucl. Phys. 2019, 4, 012. [Google Scholar]
- Aoyama, T.; Maeda, S.; Maeda, Y.; Suzuki, S. Transmutation of Technetium in the Experimental Fast Reactor “JOYO”. J. Nucl. Radiochem. Sci. 2005, 6, 279–282. [Google Scholar] [CrossRef][Green Version]
- Nagaya, Y.; Keisuke, O.; Sakurai, T.; Mori, T. MVP/GMVP Version 3: General Purpose Monte Carlo Codes for Neutron and Photon Transport Calculations Based on Continuous Energy and Multigroup Methods; JAEA-Data/Code--2016-018; Japan Atomic Energy Agency: Tokai, Japan, 2017. [Google Scholar]
- Okumura, K.; Mori, T.; Nakagawa, M.; Kaneko, K. Validation of a Continuous-Energy Monte Carlo Burn-up Code MVP-BURN and Its Application to Analysis of Post Irradiation Experiment. J. Nucl. Sci. Technol. 2000, 37, 128–138. [Google Scholar] [CrossRef]
- Iwamoto, O.; Iwamoto, N.; Kunieda, S.; Minato, F.; Nakayama, S.; Abe, Y.; Tsubakihara, K.; Okumura, S.; Ishizuka, C.; Yoshida, T.; et al. Japanese evaluated nuclear data library version 5: JENDL-5. J. Nucl. Sci. Technol. 2023, 60, 1–60. [Google Scholar] [CrossRef]
- Kotlyar, D.; Fridman, E.; Shwageraus, E. One-Group Cross-Section Generation for Monte Carlo Burnup Codes: Multigroup Method Extension and Verification. Nucl. Sci. Eng. 2015, 179, 274–284. [Google Scholar] [CrossRef]
- Chadwick, M.; Herman, M.; Obložinský, P.; Dunn, M.; Danon, Y.; Kahler, A.; Smith, D.; Pritychenko, B.; Arbanas, G.; Arcilla, R.; et al. ENDF/B-VII.1 Nuclear Data for Science and Technology: Cross Sections, Covariances, Fission Product Yields and Decay Data. Nucl. Data Sheets 2011, 112, 2887–2996. [Google Scholar] [CrossRef]
- Uccelli, L.; Martini, P.; Urso, L.; Ghirardi, T.; Marvelli, L.; Cittanti, C.; Carnevale, A.; Giganti, M.; Bartolomei, M.; Boschi, A. Rhenium Radioisotopes for Medicine, a Focus on Production and Applications. Molecules 2022, 27, 5283. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Uccelli, L.; Pasquali, M.; Duatti, A.; Taibi, A.; Pupillo, G.; Esposito, J. 188W/188Re Generator System and Its Therapeutic Applications. J. Chem. 2014, 2014, 529406. [Google Scholar] [CrossRef]

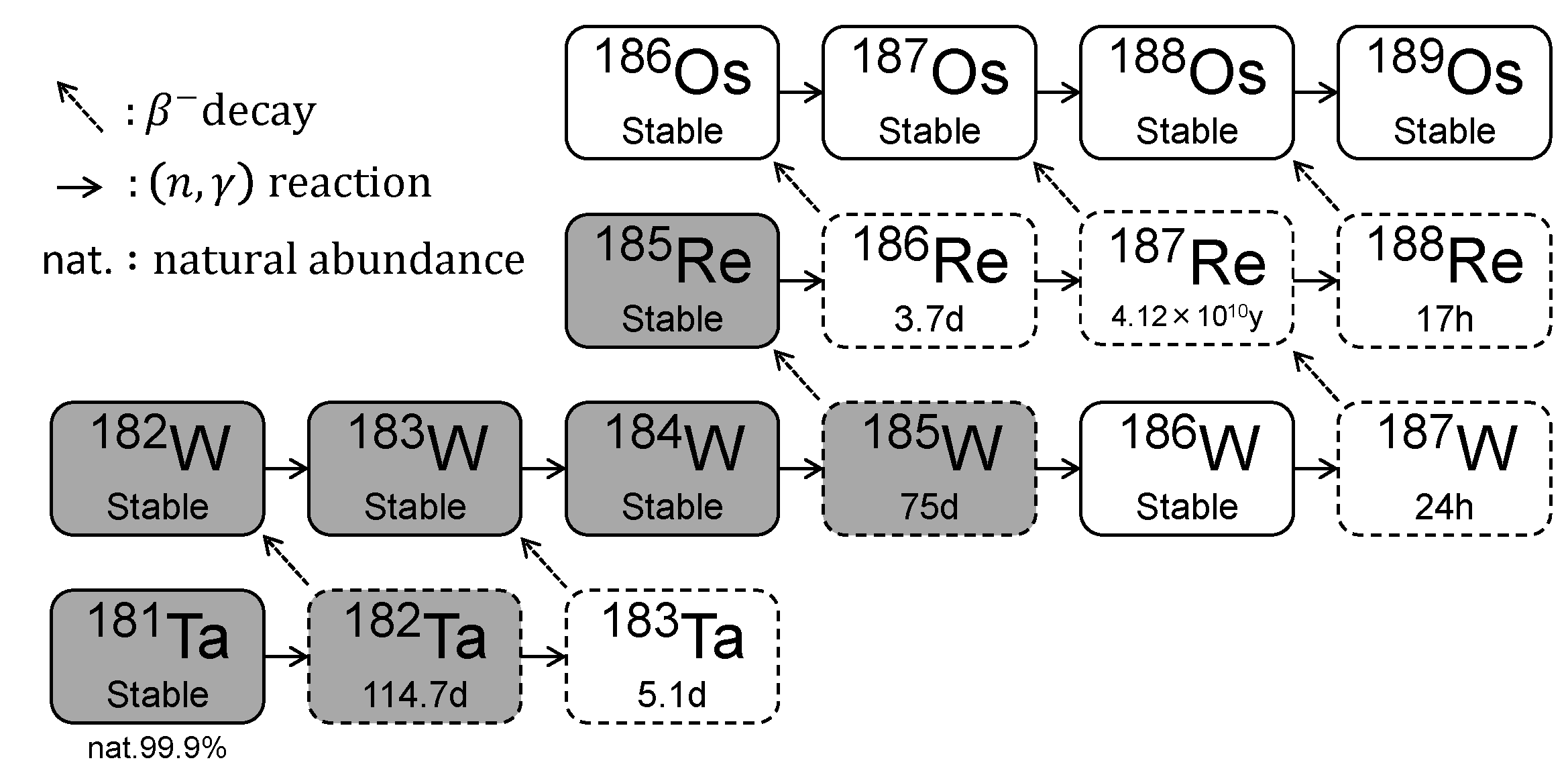
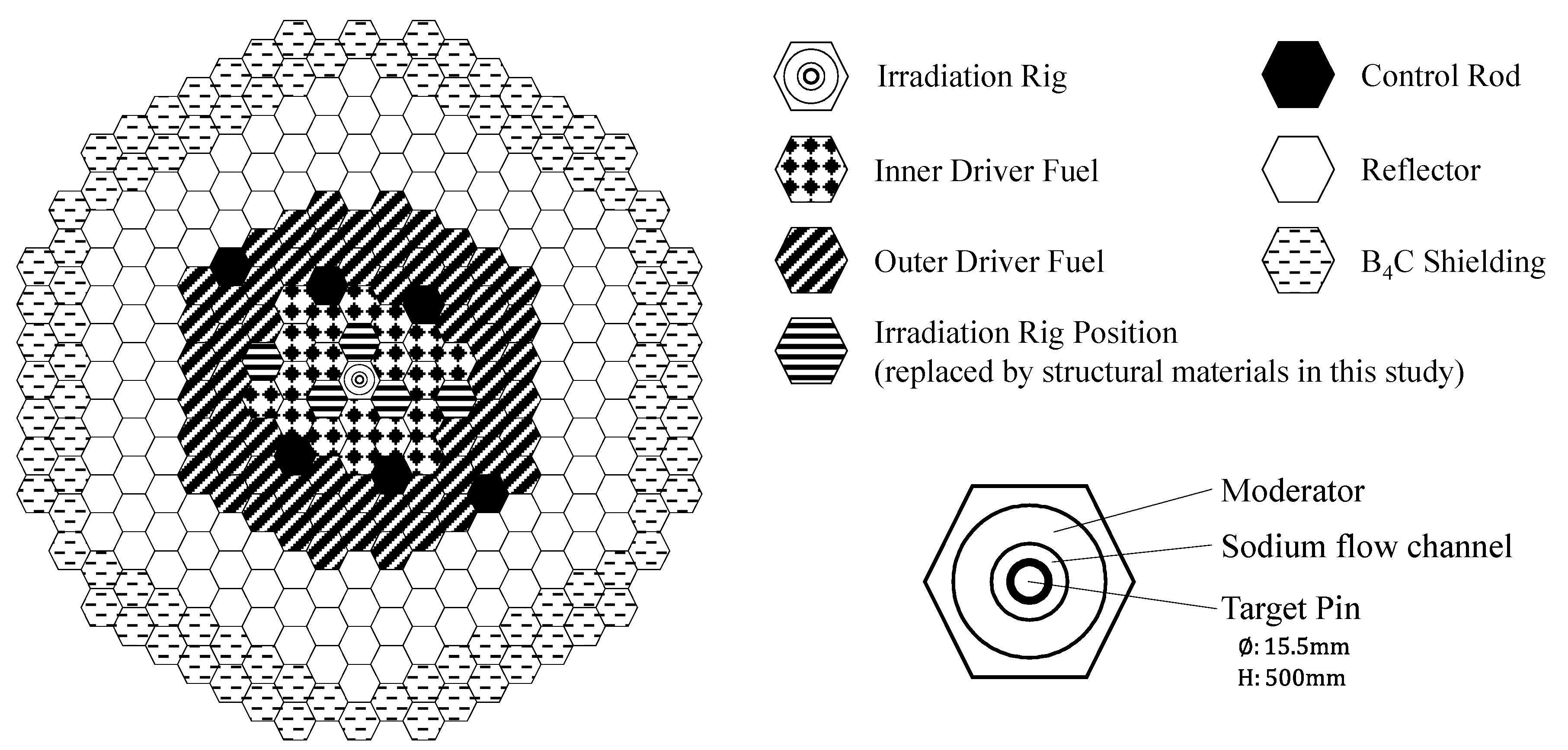
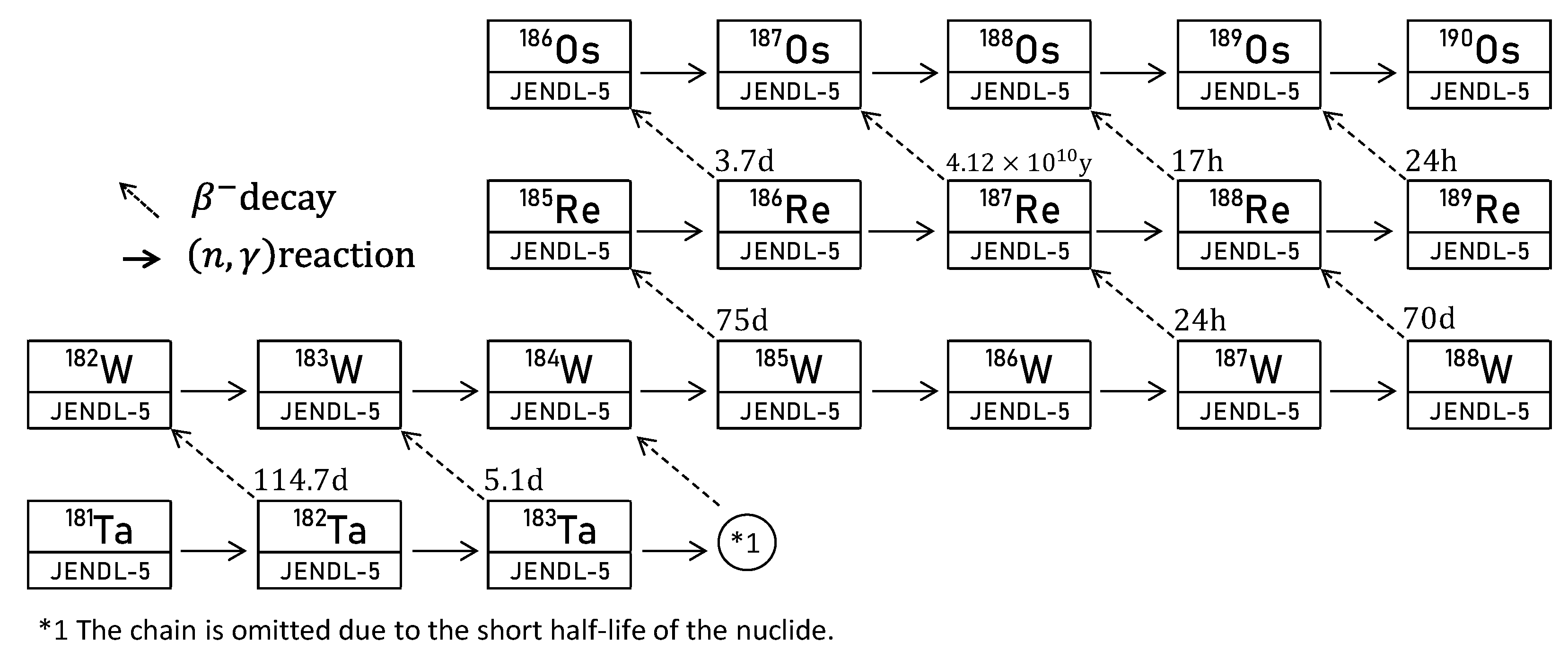
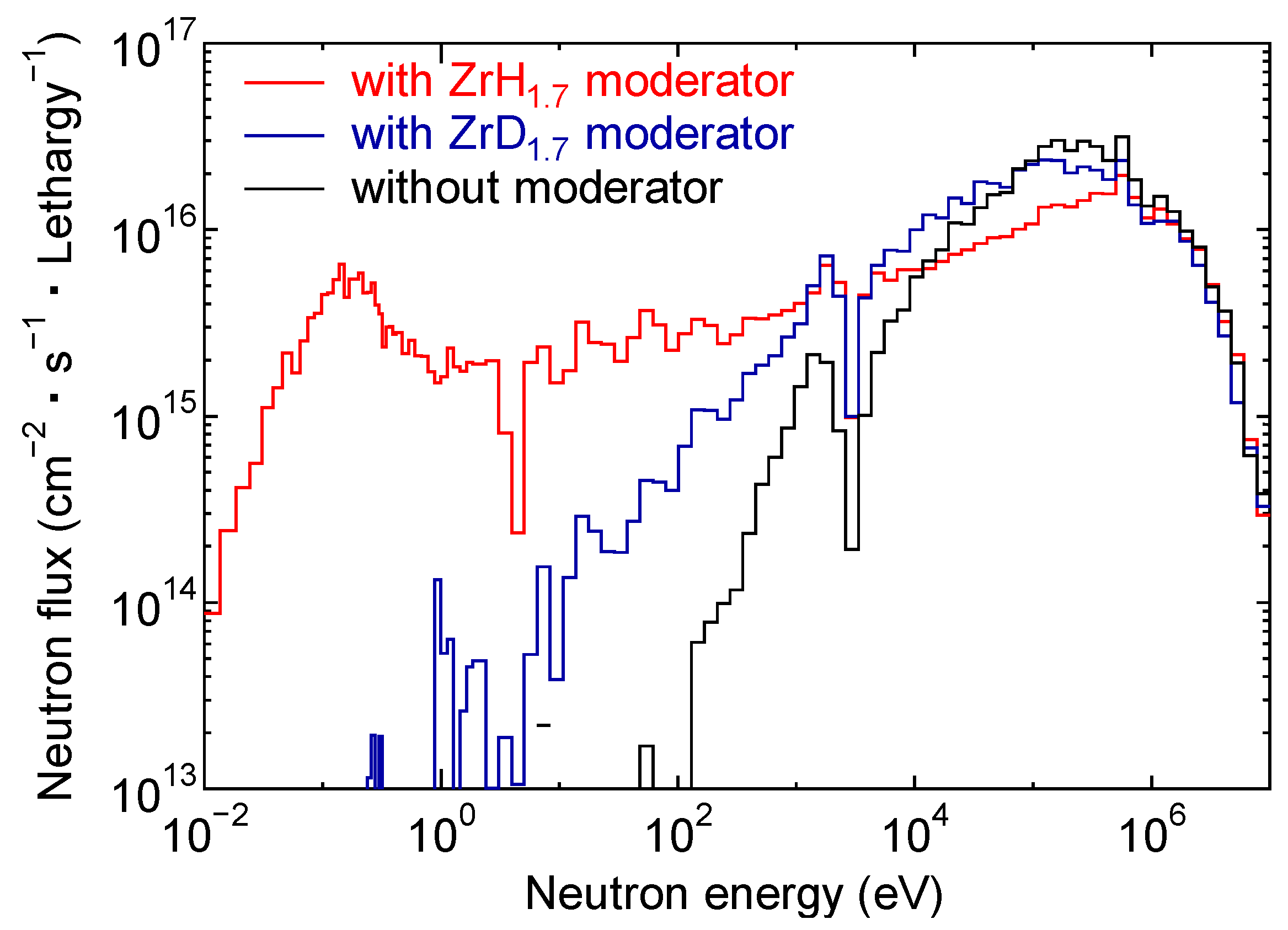
| Nuclide | Isotope Ratio | Half-Life |
|---|---|---|
| Re-185 | Stable | |
| Re-187 |
| Specification | Data | |
|---|---|---|
| Reactor Thermal Power | (MWt) | 100 |
| Number of driver fuel subassembly (Inner Driver Fuel and Outer Driver Fuel) | 77 | |
| Equivalent core diameter | (cm) | 80 |
| Core height | (cm) | 50 |
| 235U enrichment | (wt%) | 18 |
| Pu content: Pu/(Pu+U) | (wt%) | 16/21 * |
| Fissile Plutonium content: (239Pu + 241Pu)/(Pu+U) | (wt%) | 12/15 * |
| Reflector/shielding | Steel Special Use Stainless (SUS)/B4C |
| Nuclide | Irradiation to Natural Ta | Irradiation to Natural W | ||
|---|---|---|---|---|
| Initial (g) | after 1 Year of Irradiation and 30 Days of Cooling (g) | Initial (g) | after 1 Year of Irradiation and 30 Days of Cooling (g) | |
| Ta-181 | — | — | ||
| W-182 | — | |||
| W-183 | — | |||
| W-184 | — | |||
| W-186 | — | |||
| Re-185 | — | — | ||
| Re-186 | — | — | ||
| Re-187 | — | — | ||
| Os (total) | — | |||
| Nuclide | Initial (g) | After 1 Year of Irradiation and 30 Days of Cooling (g) | ||
|---|---|---|---|---|
| with ZrH1.7 Moderator | with ZrD1.7 Moderator | (without Moderator) * | ||
| Ta-181 | ||||
| W-182 | ― | |||
| W-183 | ― | |||
| W-184 | ― | |||
| W-186 | ― | |||
| W (Total) | ― | |||
| Re-185 | ― | |||
| Re-186 | ― | |||
| Re-187 | ― | |||
| Os (Total) | ― | |||
| Nuclide | The One-Group Effective Cross-Section (Barn) | ||
|---|---|---|---|
| with ZrH1.7 Moderator | with ZrD1.7 Moderator | without Moderator | |
| Ta-181 | |||
| Ta-182 | |||
| Ta-183 | |||
| W-182 | |||
| W-183 | |||
| W-184 | |||
| W-185 | |||
| W-186 | |||
| W-187 | |||
| W-188 | |||
| Re-185 | |||
| Re-186 | |||
| Re-187 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanoue, Y.; Yokoyama, T.; Ozawa, M. Feasibility Study on Production of High-Purity Rhenium-185 by Nuclear Transmutation of Natural Tantalum. J. Nucl. Eng. 2023, 4, 625-633. https://doi.org/10.3390/jne4030039
Tanoue Y, Yokoyama T, Ozawa M. Feasibility Study on Production of High-Purity Rhenium-185 by Nuclear Transmutation of Natural Tantalum. Journal of Nuclear Engineering. 2023; 4(3):625-633. https://doi.org/10.3390/jne4030039
Chicago/Turabian StyleTanoue, Yuki, Tsugio Yokoyama, and Masaki Ozawa. 2023. "Feasibility Study on Production of High-Purity Rhenium-185 by Nuclear Transmutation of Natural Tantalum" Journal of Nuclear Engineering 4, no. 3: 625-633. https://doi.org/10.3390/jne4030039
APA StyleTanoue, Y., Yokoyama, T., & Ozawa, M. (2023). Feasibility Study on Production of High-Purity Rhenium-185 by Nuclear Transmutation of Natural Tantalum. Journal of Nuclear Engineering, 4(3), 625-633. https://doi.org/10.3390/jne4030039






