Abstract
FSH, estrogen and progesterone testing are widely utilized in clinical practice. Lateral flow assays (LFAs) are cost-effective tools used for diagnosing infectious diseases, pregnancy, and substance testing. The focus of this narrative review is the potential for the wider utilization of listed hormone LFAs. A search was conducted with PubMed, Google Scholar and Wiley online libraries using keywords without any limitation on the publication date; animal studies were excluded. Clinical guidelines for the related conditions were included. According to published data, E3G and PdG are used to determine ovulatory cycles and can be utilized for research purposes to establish the normal range of menstrual cycles, as there is currently disagreement among guidelines. FSH measurement in blood samples is utilized to predict oocyte yield in assisted cycles and to differentiate women with premature ovarian insufficiency from hypothalamic amenorrhea, and can be replaced with more convenient urine testing. PdG was tested to assess the risk of pregnancy complications, specifically miscarriage and ectopic pregnancy, and might become a screening tool for miscarriage in the future. PMS, PMDD and ovarian carcinogenesis could be extensively studied using LFAs to gain a better understanding of the biology behind these conditions. Before implementing these LFAs into clinical practice, the reproducibility of progesterone assays should be evaluated. The results are critical for treatment decisions, and universally recognized standards for estradiol measurement should be developed.
1. Introduction
Point-of-care (POC) diagnostics is a rapidly growing field that has revolutionized the way healthcare is delivered. Its growth started before and was enhanced by the COVID-19 pandemic. POC refers to medical tests with immediate results that can be performed at or near the site of patient care. Lateral flow assays (LFAs) are platforms made of paper that contain either antibody- or nucleic-acid-recognizing elements. They are widely used in POC diagnostics to detect various substances, such as allergens, infectious agents, antibiotics, cancer biomarkers, hormones and their metabolites among others, due to their affordability, simplicity and short development cycle. In 2022, LFAs’ market valuation was $8.75 billion with an expected growth up to $14.5 billion by 2032, according to Market Global Insights [1]. LFAs can measure hormonal levels qualitatively, semi-quantitatively and quantitatively [2]. Improved sensitivity, specificity and chemical stability scaled commercialization of such products, in addition to the current ability of smartphone utilization in reading LFA results is creating and maintaining a trend [3,4]. According to the International Data Corporation, smartphone market valuation will reach almost 100 billion by 2026 with a shipment of over 400 million units, which should stimulate even wider implementation [5].
Since this narrative review focuses on the measurement of reproductive hormones, specifically follicle stimulating hormone (FSH), estrogens and progesterone, the following paragraphs provide a brief description of each hormone. LH is included in the list for completeness, however, the other hormones are the focus of this review.
Follicle stimulating hormone is composed of α- and β-subunits. It is secreted by the pituitary gland and is a member of the glycoprotein hormone family [6]. It plays a crucial role in stimulating folliculogenesis and estrogen production in women, as well as converting androgens to estrogens through the activation of aromatase. Additional functions include the regulation of bone mass, adipose tissue, energy metabolism and cholesterol synthesis in both sexes [7]. To diagnose reproductive and developmental disorders, FSH levels are commonly measured using immunoassays due to their practical benefits (cost, time, safety) [6].
Luteinizing hormone is a glycoprotein hormone secreted by the anterior pituitary gland in response to gonadotropin-releasing hormone from the hypothalamus. It plays a critical role in regulating reproductive function by stimulating the production of estrogen, maturation of primordial follicles, triggering ovulation during the mid-cycle rise, contributing to the regulation of the menstrual cycle and inducing progesterone synthesis [8]. Most commonly, measuring LH utilizing LFAs is required for ovulation detection in subfertile couples for more precise intercourse timing.
Estrogens are a family of hormones primarily produced by the ovaries, but also in small amounts by the brain, skin, heart and liver, and converted from androgens by aromatase in adipose tissue. Estradiol (E2) is the main biologically active hormone in the estrogen family [9]. Estrone-3-glucuronide (E3G) is its urinary metabolite, which reflects the previous day’s serum estradiol level. Measuring estradiol levels is beneficial for diagnosing conditions such as hypogonadism, ovarian tumors, assisting in fertility treatment and evaluating the effectiveness of aromatase inhibitor therapy in women [10].
Progesterone is produced by the corpus luteum during the luteal phase of the menstrual cycle and by the placenta during pregnancy. Pregnanediol-3-glucuronide (PdG) is the major urine metabolite that reflects the prior day’s serum levels when measured in morning samples. Progesterone testing was implemented in assisted reproduction, for luteal phase deficiency diagnosis and pregnancy progression.
The primary role of FSH, estrogens and progesterone lies in the regulation of the female reproductive system, which means that hormonal testing is useful in the diagnosis and management of development issues and subfertility. However, the diagnosis and management of other conditions may benefit from understanding the objective picture of individual hormonal levels across menstrual cycle phases compared to established ranges and individual information gathered over a period of time. This literature review solely focuses on conditions related to the reproductive system, such as ovarian cysts, ovulatory disorders and infertility. It also includes conditions that are influenced by the menstrual cycle, such as premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD).
Questions to answer: 1. According to existing literature and guidelines, is the testing of FSH, estrogens and progesterone used for diagnostic purposes in chosen conditions? 2. If not, is there evidence that these hormones contribute to the pathophysiology of the mentioned conditions and can help in diagnosis, staging and/or exacerbation prediction? 3. What is the potential for LFA utilization?
2. Materials and Methods
A literature search for a narrative review was conducted using PubMed, Google Scholar and Wiley online libraries without any limit on the publication date. Animal studies were excluded. Keywords used: “reproductive hormones”, “sex hormones”, “FSH”, “estrogens”, “progesterone”, “ovarian cysts”, “infertility”, “PMS”, “PMDD” and “lateral flow assays”. Then, titles and abstracts were evaluated, duplicating papers were excluded and only manuscripts relevant to the topic were extracted. Reference lists of the selected manuscripts were checked, and any further relevant references were included. Additionally, clinical guidelines for related conditions were searched and included in the review.
3. Results
This manuscript contains a total of 81 papers, including observational studies, randomized controlled trials, reviews, practice bulletins, committee opinions, position statements, reports and classification standards.
3.1. Lateral Flow Immunoassays’ Performance
Studies from the past 20 years revealed that there is acceptable performance of LFAs for hormone testing in urine samples. In 2003, 60 ovulating women collected urine specimens and tested E3G and PdG over a six-month period. Then, the monitor for home testing was used to analyze the LFA results and compare them to radioimmunoassay results. The coefficient of correlation was 0.84 for 80% of cycles, which confirmed that the monitor for home testing was as accurate as laboratory measures [11]. Then in 2012, home testing results without controls were compared to the results obtained in the local center. Reliable hormone profiles were obtained both via home testing and laboratory measurements, which led to the conclusion that a lay person could accurately perform testing without supervision [12]. These results are in agreement with a 2015 prospective study that compared urinary FSH, LH, E3G and PdG metabolites to their corresponding blood concentrations, and ultrasound-observed ovulation during one cycle. Serum and urinary levels showed excellent agreement [13]. In addition, a recent study published in 2023 compared E2, progesterone and LH concentrations to E3G, PdG and LH in urine. A correlation of 0.96, 0.95 and 0.98 allowed researchers to conclude that the use of urine tests instead of blood is acceptable whenever testing is required [14]. These results indicate that LFAs are accurate alternatives to blood sampling that are more convenient, painless, eliminate the need for venipuncture, and can be completed at home on a regular basis.
3.2. Premenstrual Syndrome and Premenstrual Dysphoric Disorder
PMS is a commonly observed condition in adolescent girls and young women, with university students having prevalence rates ranging from 58.1% to 92.3% [15]. According to a recent meta-analysis, the global prevalence of PMS is 47.8% (95% CI: 32.6–62.9) [16]. PMDD is a severe form of PMS with a prevalence ranging from 2.2% to 17.6% that varies across countries, cultures and ethnic groups [17,18]. The difference in prevalence of these conditions could be due to different study designs used, age, ethnicity and may also be attributed to the different classification systems and definitions used for data collection and analysis.
While mild moliminal symptoms are a normal part of the menstrual cycle and are caused by fluctuating hormonal levels, these conditions could significantly impact quality of life due to physical symptoms such as lower abdominal pain, tender breasts, swelling, disturbed sleep and mental symptoms, for instance mood swings, aggression and depressive thoughts. The severity of these symptoms may vary depending on the classification system used. However, there are no biomarkers in use and the diagnosis is made based on reported symptoms, which lack objectivity.
In the International Classification of Diseases 11th Revision by the World Health Organization, the syndrome is referred to as Premenstrual Tension Syndrome [19]. It is characterized by behavioral, emotional and physical symptoms that interfere with daily life and are associated with the menstrual cycle [19]. The diagnosis requires evidence that a person is socioeconomically affected during this period and a prospective diary with documented symptoms where the link with the luteal phase is obvious. According to the gynecological guidelines from the American College of Obstetricians and Gynecologists (ACOG), PMS is recognized when disturbing symptoms appear five days before menstruation and disappear four days after, during at least three subsequent cycles [20].
There are a few differences in the PMDD definition by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition: women should have at least five out of 11 established symptoms that begin one week before menstruation, subside shortly after, and last for a minimum of 12 months [21]. It is also important that the symptoms are not caused by another condition and have a significant impact on social life. A prospective diary with documented symptoms for at least two months is sufficient to confirm the diagnosis. From a patient’s perspective, diagnosis could be frustrating and challenging. Without objective measures to confirm their symptoms, patients may feel like their experiences are not being taken seriously or that they are being dismissed as simply “overreacting”. This can lead to delays in receiving appropriate treatment and support and have a significant impact on their quality of life.
While the exact pathophysiological pathways of PMS and PMDD development are not fully understood, there are several hypotheses suggesting sex hormones may play a role. Some research has indicated that conditions associated with estrogen withdrawal and hypoestrogenic states may contribute to the precipitation or exacerbation of psychotic episodes [22]. Additionally, higher progesterone concentrations have been found to be significantly associated with an increase in subjective fatigue ratings [23]. Neuroimaging studies suggest that estrogens and progesterone impact the modulation of γ-aminobutyric acid and serotonin receptors, while other studies claim the central nervous system’s improper response to hormonal fluctuations results in premenstrual symptoms [24,25]. As a result, there is no agreement among the findings obtained during the past 40 years of research. This could be partially due to the different study populations, samples and methods of testing used, and possibly due to different kinetics of progesterone secretion and metabolism among the groups of participants [26]. Hantsoo et al. in their review suggest that PMDD may have biological subtypes, and that the use of a typing approach can aid in further research and understanding of the underlying biology of PMDD [25].
In the 1980s, studies confirmed that high plasma concentrations of estradiol in the luteal phase were associated with adverse PMS symptoms and correlated with their severity [27]. Later in 1998, researchers validated 30 PMS symptoms and collected blood samples from 30 participants for two consecutive months [28]. They showed that concentrations of estradiol and LH in the luteal phase were positively correlated with symptom severity. Another study on healthy individuals tested norepinephrine, estradiol, progesterone, LH and FSH [29]. Researchers found that during the late luteal phase, individuals with PMS symptoms had lower levels of estradiol compared to those without the condition. Additionally, a negative association was discovered between the area under the curve for estradiol during the luteal phase and the somatic and mental scores of PMS patients [29].
In 2012, Ziomkiewicz et al. measured morning progesterone levels in saliva and assessed mood during the luteal phase of the menstrual cycle in 122 healthy individuals [30]. Their results showed a negative effect of low progesterone levels on mood, aggressive behavior and fatigue. Researchers suggested a biphasic action of progesterone metabolites on the mood of healthy women of reproductive age.
In 2019, there were more attempts to establish objective criteria for diagnosing PMS and PMDD and to define severity stages. Roomruangwong et al. checked hormonal levels once a week, starting from day 7, then on days 14, 21 and 28 [15]. Based on their results, however insignificant, the hormonal levels did not correlate with the PMS score, but they were useful in predicting PMS symptoms during the luteal phase and their severity. The limitation of this study is its small sample size, with only 41 participants, which means that the results may not be generalizable. Testing these findings on a larger audience will enable researchers to determine the applicability of their findings. Ju-Yu Yen et al. analyzed serum levels of estrogen and progesterone in 63 women with PMDD and 53 control subjects during the early and late luteal phases [31]. Their findings demonstrated an association between lower estrogen and higher progesterone levels with PMDD severity, which requires further research and may assist in the development of effective hormone interventions for women with this condition.
The absence of biomarkers for PMS and PMDD diagnosis highlights the need for more research and resources to better understand these conditions. Larger decentralized studies using LFAs and urine samples have the potential to improve the study quality and convenience for participants. These studies could enhance the understanding of biology, staging of PMS and PMDD and the development of effective treatment interventions.
3.3. Ovarian Cysts
Benign cysts in the ovaries are common in women of reproductive age. However, it is difficult to accurately estimate their true incidence since not all types require intervention, and some cysts are discovered incidentally during physical examination and/or ultrasound [32]. It is estimated that up to 20% of women will develop an ovarian cyst at some point in their lives, with benign types predominating and only 1–4% being malignant [32,33].
Symptoms of a benign ovarian cyst vary depending on its size and location. Some women may not experience any symptoms at all, while others may experience pelvic pain, bloating, or a sensation of fullness in the abdomen. In some cases, a large cyst may cause the ovary to tort, leading to severe pain and requiring emergency surgical treatment.
There are several types of benign ovarian cysts, including functional (follicular and luteal), dermoid, endometriomas and cystadenomas, among others. Determining the appropriate management of ovarian cysts depends on several factors, such as medical history, age, risk of malignancy and characteristics of the lesion. Functional and benign cysts in women of reproductive age usually do not require surgery. However, complications such as blood loss and ovarian torsion may require immediate management [33].
As malignant cysts are often diagnosed in the later stages due to the absence of screening tests, this leads to poorer outcomes and urges the development of more effective diagnostic methods [33]. There are two main hypotheses related to the development of ovarian cancer. The first one suggests that the risk of ovarian cancer increases with the number of ovulatory cycles, as the ovarian epithelium undergoes trauma and recovery processes more frequently. The second one links excessive gonadotropin secretion to the subsequent proliferation and malignization of the ovarian epithelium [34].
In 1983, researchers analyzed the family and reproductive histories of 430 white women. They compared the test group, who had epithelial ovarian cancer, with healthy controls and found a protective effect of pregnancy. This effect was stronger with an increasing number of live births [35]. In 1989, an experiment conducted in a cell culture demonstrated increased proliferation in an epithelial ovarian cancer cell line when exposed to 17 beta-estradiol [36]. The effect was dose-dependent. One additional study conducted in cell culture provided further evidence to support the hypothesis that sex hormones have an impact on cancer development. Increasing levels of gonadotropins, estrogen and androgen showed a promoting effect on cancer development, while progesterone at high doses, similar to progesterone levels in pregnancy, had a protecting effect and stimulating effect in low doses [37].
In 1995, Helzlsouer et al. published the results of a prospective nested case-control study involving 31 individuals. In this study, they assessed the relationship between serum sex hormone levels and the risk of ovarian cancer [38]. According to their findings, the risk significantly increased with higher levels of androstenedione (4.5 nmol/L vs. 3.3 nmol/L). However, it was not associated with estradiol, estrone or dehydroepiandrosterone sulfate (DHEAS). According to their results, low levels of FSH after menopause were associated with an increased risk of ovarian cancer, which contradicts the gonadotropins theory. Similar results and conclusions were shared by McSorley et al. in 2009 [39]. They analyzed the data obtained from 1974 to 2000 and found that higher FSH levels during perimenopause and postmenopause were associated with a reduced risk of ovarian cancer.
However, in 1996, Schildkraut et al. found an increased risk of ovarian cancer among women with PCOS, which can partially support the hypothesis of excessive gonadotropin production [40]. Then, in 1998, a study detected that malignant cysts contained higher concentrations of FSH and LH in the cystic fluid, while benign cysts had low or undetectable concentrations [41]. Researchers suggested that FSH and LH may play a role in ovarian cancer. However, they did not provide a detailed explanation. Later, a study conducted in 2004 found statistically significant differences in LH and FSH levels in both serum and cyst fluid between malignant and borderline tumors, cystadenomas and non-neoplastic ovarian cysts [42]. In 2008, researchers found that malignant ovarian tumors had significantly higher concentrations of LH and FSH in cyst fluid compared to benign tumors. There was also a strong correlation between cyst fluid and serum hormone concentrations in cases of malignant tumors that supported the idea of vascular permeability within malignant cysts. Authors could not exclude a possibility of gonadotropin production by malignancy, which could be the cause of elevated levels of these hormones in cysts and has to be further explored [43].
A multicenter case-controlled study, published in 2003, did not find any clear association between ovarian cancer risk and circulating blood hormonal levels [34]. Researchers tested 132 women with ovarian cancer and compared them to a control group that was twice the size. They analyzed FSH, testosterone, DHEAS, androstenedione and sex-hormone-binding globulin. Estrone was measured only in postmenopausal women. The findings suggest that there may be a correlation between elevated levels of androstenedione in the blood of premenopausal women and an increased risk of cancer.
These findings are consistent with regards to exogenous hormone supplementation. A nested case-control study, utilizing data from the Women’s Health Initiative Observational Study, showed that estrogen/estrogen metabolite levels were not associated with ovarian cancer risk among menopausal hormone therapy users [44].
A high prevalence of benign cysts, absence of specific symptoms to detect them, absence of screening tests and diagnosis on advanced stages of malignant cysts urges the need for more research and discoveries in this area, and LFAs could be utilized to fulfill existing gaps.
3.4. Ovulatory Disorders
A regular menstrual cycle is associated with ovulation, and hormonal dysregulation can lead to anovulation, which results in changes in bleeding and interval patterns. Based on existing guidelines from the International Federation of Gynecology and Obstetrics, a regular menstrual cycle is considered to be between 24 and 38 days, while according to the ACOG practice bulletin #128, it is 21–35 days. However, there is even more inconsistency in published studies from different countries [45,46,47].
There are several major conditions associated with ovulatory dysfunction, including functional hypothalamic amenorrhea, polycystic ovarian syndrome and thyroid dysfunction. Other possible reasons are hyperprolactinemia, premature ovarian insufficiency, adrenal disorders and congenital malformations.
Functional hypothalamic amenorrhea is a condition that is often caused by low calorie intake, excessive exercise and high-level stress, or a combination of these factors. It results in anovulation and the absence of menstruation for more than three consecutive months or when a cycle consistently exceeds 45 days [48]. The Endocrine Society recommends testing serum levels of the thyroid-stimulating hormone (TSH), free thyroxine (T4), prolactin, LH, FSH, E2 and anti-Mullerian hormone for diagnostic purposes.
Polycystic ovary syndrome is an endocrine disorder that affects 4–20% of women of reproductive age worldwide. The condition was first described in 1935 by Stein and Leventhal, who noted the presence of enlarged ovaries with multiple small cysts in women experiencing amenorrhea or oligomenorrhea and hirsutism [49]. Since then, the diagnostic criteria for PCOS have evolved, with the most recent guidelines emphasizing the importance of hyperandrogenism, ovulatory dysfunction and the presence of polycystic ovaries on ultrasound for diagnosis.
The National Institute of Health established diagnostic criteria for PCOS in 1990, which include hyperandrogenism and oligoovulation, but exclude other conditions that may resemble PCOS, such as adult-onset congenital adrenal hyperplasia, hyperprolactinemia and androgen-secreting tumors [50]. In 1992, S. Robinson et al. conducted a study to determine which hormones should be used for diagnostic purposes. They tested serum levels of LH, FSH, LH/FSH ratio, total testosterone, derived free testosterone, sex-hormone-binding globulin, androstenedione and dehydroepiandrosterone (DHEA) [51]. They found a significantly elevated total testosterone concentration in 70% of the PCOS group. The mean LH, LH/FSH ratio and androstenedione were significantly higher in the PCOS group, but appeared less frequently than total testosterone. They concluded that in addition to the ovarian picture on ultrasound, the presence of hirsutism and oligoovulation, total testosterone is the only biomarker that should be tested.
In 2003, the Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group concluded that PCOS is a syndrome characterized by the most common clinical picture of irregular menstruation, hyperandrogenism and excessive weight. They also emphasized that a single criterion, such as an elevated testosterone level, is not sufficient to make a diagnosis [52]. The Endocrine Society revised the evidence in 2013 and suggested that a diagnosis of PCOS should be made in adult women who exhibit two of the following characteristics: excess androgen production, anovulation and an ultrasound picture of polycystic ovaries [53,54,55].
Primary ovarian insufficiency is a rare condition in which the ovarian follicle pool is diminished and ovaries cannot perform their endocrine function significantly earlier than the average age of menopause [56]. It is often associated with irregular menstrual cycles, menopausal symptoms and reduced fertility. The condition can only be diagnosed in women under 40 if they have elevated levels of FSH and low levels of estrogen from two separate blood tests taken at least one month apart, along with a history of irregular menstrual cycles or amenorrhea for at least four months. An ultrasound may also be performed to confirm the absence of ovarian follicles. Decreased levels of E2 through a negative feedback loop activate the release of FSH in the pituitary, which leads to elevated levels of FSH in the blood. Low levels of E2 indicate that the ovaries are not functioning properly. The sooner the condition is diagnosed, the better it is for patients, as assisted reproduction may be available if they have not yet completed their family.
Menstrual cycle length remains the main sign that reflects if ovulation is happening or not, so it is crucial to have established norms that are age and race specific and LFAs could assist in that aspect. Testing for ovulation prediction and confirmation will be covered in detail in the next section.
3.5. Infertility Management
Infertility is a growing concern worldwide, with an estimated 1 in 6 people experiencing difficulties conceiving at some point in their lives [57]. Infertility is defined as the inability to conceive after one year of unprotected intercourse. FSH, E3G and PdG’s primary goal of testing refers to this area.
In Figure 1, an example of a 29-day menstrual cycle is presented with an ovulation on day 15. The first rise in the FSH occurs during 2–4 days of the follicular phase with typical FSH testing on day 3 for predicting the fertility outcome during fertility treatment. An LH peak 1–2 days prior to ovulation represents an expected event, however it is clear from the graph that E2 and FSH also have preovulatory surges, thus their testing could also be utilized for that purpose. The progesterone rise during the luteal phase reaches its peak on day 6–8 after ovulation representing the end of testing, as following days will not add value to the obtained results. Testing for each situation is described in further detail below.
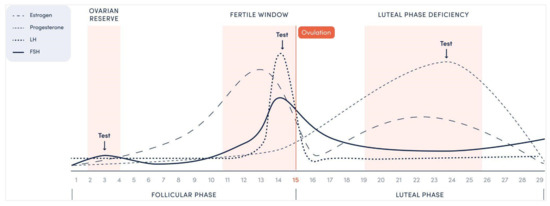
Figure 1.
Expected hormonal fluctuations during a menstrual cycle with recommended testing windows for three clinical questions.
3.5.1. Ovarian Reserve Testing
Ovarian reserve testing was first introduced in the 1990s as a way to predict a woman’s reproductive potential and determine the optimal timing for fertility treatments [58]. The tests include measuring levels of hormones such as FSH, estradiol and anti-Mullerian hormone concentrations, as well as ultrasound imaging of the ovaries to assess the quantity and quality of follicles. These tests are conducted during the early follicular phase, typically between menstrual cycle days 2–5. However, there are controversies surrounding the accuracy and usefulness of these tests. Some experts question their ability to accurately predict fertility outcomes, while others argue that they may lead to unnecessary anxiety and treatment. Additionally, there is a debate over which specific tests are the most effective for assessing ovarian reserve and when they should be performed.
The ACOG and the American Society for Reproductive Medicine (ASRM) recommend testing FSH levels in assisted cycles to predict oocyte yield, and to differentiate between women with primary ovarian insufficiency who may benefit from oocyte donation and those with hypothalamic amenorrhea who require exogenous gonadotropin stimulation for ovulation induction. Women with normal FSH and E2 levels in the setting of oligomenorrhea or anovulation should be evaluated for PCOS and non-classic adrenal hyperplasia. If serum FSH levels are higher than 10 IU/L, it may result in a weaker reaction to ovarian stimulation. Estradiol can be used as a guide to support the information obtained from FSH testing. Basal blood E2 levels should be lower than 60–80 pg/mL. If E2 levels are high, it may lower FSH levels as a temporary measure and indicate a decrease in ovarian reserve, which later results in high levels of FSH and low levels of E2 [59,60,61].
Testing FSH at home using LFAs in assisted reproduction can provide patients with a more convenient option and has shown positive results, as seen with the use of remote self-ultrasound monitoring, and these tests are present as consumer products [62,63].
3.5.2. Ovulation Testing
There are several tests available for this purpose, including urine-based ovulation predictor kits, basal body temperature monitoring and cervical mucus analysis.
LH testing. The first consumer kit for LH testing was proposed and has been widely used since the 1980s [64]. The LH urine test detects the presence of LH in the urine, which typically surges 1–2 days before ovulation [65]. The test line on the LH urine test should be as dark or darker than the control line to indicate that the LH surge has occurred and ovulation may occur within the next 24–48 h.
Estrogen testing. Typically, estradiol levels rise a few days before ovulation. However, they can change from cycle to cycle and vary among individuals. Urinary E3G has been tested against serum E2 and confirmed in several studies as being an appropriate method for predicting ovulation. Researchers also concluded that it can be used for monitoring in controlled ovarian hyperstimulation cycles [66,67]. The median levels of urinary peak E3G were detected half a day before ovulation (the 5th to 95th percentile: −2.5 to +9.5), and the median peak day for FSH levels was also half a day before ovulation (the 5th to 95th percentile: −2.5 to −0.5 days) [68]. The testing can be performed in combination with LH and FSH.
Progesterone testing. According to ASRM, a serum concentration of progesterone greater than 3 ng/mL is sufficient evidence of ovulation [60,69]. PdG showed a strong correlation with serum progesterone levels and can be utilized to confirm ovulation [13]. Barrett et al. proposed an assay for measuring PdG that required a 24-h urine collection. The amount of a hormone metabolite was calculated as a rate, regardless of the volume of urine [70]. It has been widely tested, and there are recognized values that are used to distinguish between fertile and anovulatory cycles. Typically, progesterone levels are measured 6–8 days after the expected day of ovulation. If the excretion rate of PdG exceeds 13.5 μmol/24 h within 6 days of the estrogen peak day, it is considered a fertile ovulatory cycle [67]. An excretion rate between 7–9 μmol/24 h is indicative of ovulation and can distinguish a luteinized unruptured follicle from an inadequate luteal phase. A rate of less than 7 μmol/24 h is a sign of anovulation [71]. However, first-morning urine was tested as an alternative to the 24-h collection, so the values obtained from other studies may differ from the ones mentioned [72].
In women with PCOS, the use of LH testing may be limited due to the fact that LH levels can be elevated throughout the menstrual cycle in these individuals. Therefore, other methods such as ultrasound monitoring of follicle development, predicting ovulation with urine E3G, FSH testing or a combination of these methods may be more effective. In addition, based on existing evidence from high-income countries, using ovulation predictor kits can improve fertility management among couples who are trying to conceive without adding stress or anxiety [73].
3.5.3. Luteal Phase Deficiency
Back in the 1940s, Jones studied and treated luteal phase deficiency (LPD), which occurs when the corpus luteum fails to secrete an adequate amount of progesterone [74,75]. In the BioCycle Study of 259 women, Schliep et al. defined “biochemical” LPD as <5 ng/mL measured in serum and “clinical” when the luteal phase is shorter than 10 days. They determined the prevalence of LPD by utilizing these two established criteria and examining their correlation with hormone concentrations. Based on their results, almost all participants with clinical LPD had midluteal progesterone levels less than 10 ng/mL [76]. According to ASRM, the combined testing of luteal progesterone and evaluation of the length of the luteal phase is a promising tool that requires further research [53].
With respect to the data obtained from research, PDG testing in the luteal phase can assist in identifying patients with LPD and patients at a high risk of miscarriage when tested in the first trimester, as well as those who may have an ectopic pregnancy [72,77,78].
3.5.4. Ovarian Stimulation
There are practical examples of LFA utilization in stimulation cycles where urinary tests have shown rapid and accurate results that are comparable to blood ones [79]. For instance, urine E3G monitoring during gonadotropin stimulation has been found to be comparable with serum E2 for predicting oocyte retrieval outcomes when measured on the day of trigger with coefficients of determination of 0.7066 and 0.6102. Daily samples that were matched confirmed a good correlation between urine E3G and serum E2 [79].
4. Discussion
Hormonal monitoring plays a pivotal role in diagnosis and monitoring reproductive conditions (e.g., ovulatory disorders, infertility). This review evaluated historical data related to the hormonal impact in disease pathophysiology and current utilization of testing in diagnostic purposes. The primary role of LFAs lies in the replacement of traditional testing, which can subsequently increase patient satisfaction and quality of life. Additionally, LFAs could be used in the discovery process and contribute to uncovering existing gaps in knowledge of disease development.
4.1. Replacement
As LFAs showed great agreement in ovulation detection comparable to serum testing and ultrasound, they could be widely used in subfertile couples for timing intercourse, as well as in stimulation cycles where they showed comparable results in predicting oocyte retrieval outcomes [13,73,79]
Conditions such as oligomenorrhea and amenorrhea can be present in women with primary ovarian insufficiency, hypothalamic amenorrhea and PCOS, making it important to distinguish the cause and establish a diagnosis. Physicians typically exclude pregnancy and then assess levels of FSH, LH, prolactin, androgens and TSH [48,56]. While all of these tests can be performed in the laboratory, utilizing LFAs can make it more convenient and aid in monitoring over a chosen period of time, providing a clearer picture of hormonal levels that can assist in decision making regarding reproductive goals and treatment efficacy.
Utilization in pregnancy requires more time to validate accuracy and safety standards, however, there are already available examples of beneficial PdG monitoring to estimate a risk of pregnancy and make a decision on progesterone supplementation, which could be further transformed into screening [72].
4.2. Discovery
Premenstrual syndrome. The limitations of current evidence include the heterogeneity of study designs, small sample sizes and insignificant results. Given the prevalence of PMS and PMDD within the population, there is potential for conducting larger decentralized studies using LFAs and urine samples. These tests might also increase the quality of the studies by improving convenience for participants and increasing compliance with study protocols. Such studies would contribute to the understanding of biology, staging of these conditions and the development of objective diagnostic criteria and effective treatment interventions.
Ovarian cysts. The role of estrogens, progesterone and gonadotropins in cyst formation and malignization is not clear to date, and there is limited knowledge about hormonal profiles among women with different types of cysts. Epidemiological studies suggest that the link exists, and there is evidence from in vitro studies on cell cultures. However, endogenous hormone levels and hormone replacement therapy with estrogens alone and in combination with progesterone were not found to increase the risk of developing ovarian cancer [44]. Taken altogether, there is a space for research using LFAs, as even multicenter studies are limited in terms of participant numbers and geographical representation. Additionally, even in some studies of peri/premenopausal women, blood samples analyzed are single measures, which are not sufficient due to the fluctuation of levels in cycle phases, intercycle variability and individual variability.
Normal ranges. In the context of ovulatory dysfunction, the primary potential of LFAs in combination with existing digital menstruation tracking tools is to establish a consensus on the normal range and parameters of menstrual cycles. As we have seen, there is a disagreement in the guidelines, which can impact clinical decision making [45,46,47].
4.3. Limitations
With all the improvements and great potential in the utilization of reproductive hormone monitoring, there are certain limitations.
Estrogens testing. The Endocrine Society has stated that there are no universally recognized ranges for estradiol measurements that are specific to the menstrual cycle phase, age and gender. Estradiol measurements are important for diagnosing and managing infertility, as well as identifying tumors that secrete estradiol. However, there are limitations to accurately measuring very low concentrations of estradiol. The Endocrine Society recommends establishing a universally recognized standard for estradiol measurements, as well as reference ranges that are age-, gender- and biologically specific for puberty/adolescence, menstrual cycle stage and menopause [80].
Progesterone testing. Lawrenz et al. conducted a retrospective observational study to compare progesterone assays and evaluate their reproducibility. Prior studies have indicated that high levels of progesterone during ovarian stimulation for IVF/ICSI can have a negative impact on the outcome. Additionally, different progesterone assays may show non-identical results. Researchers were focused on levels below 1.5 ng/mL, which are crucial for the early detection of a rise in progesterone during ovarian stimulation. The analysis showed that various assays have limited reproducibility, and the results depend on both the specific assay used and the range of progesterone levels. This may lead to different treatment decisions and requires critical interpretation of thresholds [81].
As the mentioned suggestions are general and primarily related to the results obtained in laboratories, further testing and standardization should be conducted before implementing LFA on a larger scale.
5. Conclusions
There are certain limitations in the utilization of LFA for diagnostic purposes when any of the described conditions are suspected. These limitations affect the areas where LFA can be implemented beneficially and its potential for research. Based on the aforementioned information, hormonal monitoring with LFAs is utilized and has the potential to establish the normal range of reproductive hormones at different stages of the menstrual cycle. It can also determine variation and phase parameters, as there is still disagreement in their definition. This affects the diagnostic criteria for conditions related to the menstrual cycle.
To conduct research and evaluate on a larger scale existing hypotheses behind PMS and PMDD with more detailed measurements of hormonal levels. This can help in gaining a better understanding of the biology of these conditions, and the role of estrogens and progesterone in them. In addition, in PMS and PMDD, testing can help predict symptoms and their severity. However, all aspects of utilization should be tested before wider implementation.
In fertility assistance, monitoring estrogen and progesterone levels is helpful for predicting and confirming ovulation, especially in cases with PCOS, as the LH tests may provide unreliable results. Serum FSH testing is currently used to predict oocyte yield in an assisted reproductive cycle and can potentially be fully replaced with a more convenient urine testing option. Progesterone testing shows promising results in estimating the risk of pregnancy complications, diagnosing luteal phase deficiency and identifying patients at a high risk of miscarriage and ectopic pregnancy.
Author Contributions
Conceptualization, A.T.; methodology, A.T.; writing—original draft preparation, A.T.; writing—review and editing, A.T. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
A.T. is affiliated with WLNess Science LTD. K.M. received consulting fee from WLNess Science LTD. The authors declare no conflict of interest.
Abbreviations
| ACOG | the American College of Obstetricians and Gynecologists |
| ASRM | the American Society of Reproductive Medicine |
| CI | confidence interval |
| COVID-19 | coronavirus disease |
| DHEAS | dehydroepiandrosterone sulfate |
| E2 | estradiol |
| E3G | estrone-3-glucuronide |
| ESHRE | the European Society of Human Reproduction and Embryology |
| FSH | follicle stimulating hormone |
| ICSI | intracytoplasmic sperm injection |
| IVF | in vitro fertilization |
| LFAs | lateral flow assays |
| LH | luteinizing hormone |
| LPD | luteal phase deficiency |
| PCOS | Polycystic ovarian syndrome |
| PdG | pregnanediol-3-glucuronide |
| PMS | premenstrual syndrome |
| PMDD | premenstrual dysphoric disorder |
| POC | point-of-care |
| POI | primary ovarian insufficiency |
| TSH | thyroid stimulating hormone |
References
- Lateral Flow Assays Market Share: Forecasts Report, 2032. Global Market Insights Inc. Available online: https://www.gminsights.com/industry-analysis/lateral-flow-assays-market (accessed on 11 May 2023).
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Ahmed, R.; Damayantharan, M.; Ünal, B.; Butt, H.; Yetisen, A.K. Lateral and Vertical Flow Assays for Point-of-Care Diagnostics. Adv. Healthc. Mater. 2019, 8, e1900244. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.V.; Lei, R.; Mohan, C.; Kourentzi, K.; Willson, R.C. Flash Characterization of Smartphones Used in Point-of-Care Diagnostics. Biosensors 2022, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- IDC Forecasts Nearly 415 Million Used Smartphones Will Be Shipped Worldwide in 2026 with a Market Value of $99.9 Billion. Available online: https://www.idc.com/getdoc.jsp?containerId=prUS50005523 (accessed on 21 June 2023).
- Rose, M.P.; Das, R.E.G.; Balen, A.H. Definition and Measurement of Follicle Stimulating Hormone. Endocr. Rev. 2000, 21, 5–22. [Google Scholar] [CrossRef]
- Targonskaya, A.; Maslowski, K. FSH, Estrogens, Progesterone effects on female bodies during reproductive stages and their utilization in clinical practice and research. Res. J. Womens Health 2023, 10, 1. [Google Scholar] [CrossRef]
- Filicori, M. The role of luteinizing hormone in folliculogenesis and ovulation induction. Fertil. Steril. 1999, 71, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Ketha, H.; Girtman, A.; Singh, R.J. Estradiol assays—The path ahead. Steroids 2015, 99, 39–44. [Google Scholar] [CrossRef]
- Blackwell, L.F.; Brown, J.B.; Vigil, P.; Gross, B.; Sufi, S.; D’arcangues, C. Hormonal monitoring of ovarian activity using the Ovarian Monitor, Part I. Validation of home and laboratory results obtained during ovulatory cycles by comparison with radioimmunoassay. Steroids 2003, 68, 465–476. [Google Scholar] [CrossRef]
- Blackwell, L.F.; Vigil, P.; Gross, B.; D’Arcangues, C.; Cooke, D.G.; Brown, J.B. Monitoring of ovarian activity by measurement of urinary excretion rates of estrone glucuronide and pregnanediol glucuronide using the Ovarian Monitor, Part II: Reliability of home testing. Hum. Reprod. 2011, 27, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; Johnson, S.; Weddell, S.; Godehardt, E.; Schiffner, J.; Freundl, G.; Gnoth, C. Monitoring the menstrual cycle: Comparison of urinary and serum reproductive hormones referenced to true ovulation. Eur. J. Contracept. Reprod. Health Care 2015, 20, 438–450. [Google Scholar] [CrossRef]
- Pattnaik, S.; Das, D.; Venkatesan, V.A.; Rai, A. Predicting serum hormone concentration by estimation of urinary hormones through a home-use device. Hum. Reprod. Open 2022, 2023, hoac058. [Google Scholar] [CrossRef] [PubMed]
- Roomruangwong, C.; Carvalho, A.F.; Comhaire, F.; Maes, M. Lowered Plasma Steady-State Levels of Progesterone Combined with Declining Progesterone Levels During the Luteal Phase Predict Peri-Menstrual Syndrome and Its Major Subdomains. Front. Psychol. 2019, 10, 2246. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Upadhyay, K.; Briggs, P.; Osborn, E.; Panay, N. Premenstrual disorders including premenstrual syndrome and premenstrual dysphoric disorder. Obstet. Gynaecol. 2023, 25, 38–46. [Google Scholar] [CrossRef]
- Hong, J.P.; Park, S.; Wang, H.-R.; Chang, S.M.; Sohn, J.H.; Jeon, H.J.; Lee, H.W.; Cho, S.-J.; Kim, B.-S.; Bae, J.N.; et al. Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc. Psychiatry Psychiatr. Epidemiol. 2012, 47, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, A.B.; Cardoso, T.d.A.; Mondin, T.C.; da Silva, R.A.; Souza, L.D.d.M.; Magalhães, P.V.d.S.; Jansen, K. Prevalence and factors associated with Premenstrual Dysphoric Disorder: A community sample of young adult women. Psychiatry Res. 2018, 268, 42–45. [Google Scholar] [CrossRef]
- ICD-11 for Mortality and Morbidity Statistics. Available online: https://icd.who.int/browse11/l-m/en (accessed on 21 June 2023).
- ACOG Committee on Practice Bulleteins. Practice Bulletin. Int. J. Gynecol. Obstet. 2001, 73, 183–191. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association, American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Mahe, V.; Dumaine, A. Oestrogen withdrawal associated psychoses. Acta Psychiatr. Scand. 2001, 104, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Noreika, D.; Griškova-Bulanova, I.; Alaburda, A.; Baranauskas, M.; Grikšienė, R. Progesterone and Mental Rotation Task: Is There Any Effect? BioMed Res. Int. 2014, 2014, 741758. [Google Scholar] [CrossRef]
- Poromaa, I.S.; Smith, S.; Gulinello, M. GABA receptors, progesterone and premenstrual dysphoric disorder. Arch. Women’s Ment. Health 2003, 6, 23–41. [Google Scholar] [CrossRef]
- Hantsoo, L.; Payne, J.L. Towards understanding the biology of premenstrual dysphoric disorder: From genes to GABA. Neurosci. Biobehav. Rev. 2023, 149, 105168. [Google Scholar] [CrossRef]
- Lovick, T.A.; Guapo, V.G.; Anselmo-Franci, J.A.; Loureiro, C.M.; Faleiros, M.C.M.; Del Ben, C.M.; Brandão, M.L. A specific profile of luteal phase progesterone is associated with the development of premenstrual symptoms. Psychoneuroendocrinology 2017, 75, 83–90. [Google Scholar] [CrossRef]
- Hammarbäck, S.; Damber, J.E.; Bäckström, T. Relationship between Symptom Severity and Hormone Changes in Women with Premenstrual Syndrome. J. Clin. Endocrinol. Metab. 1989, 68, 125–130. [Google Scholar] [CrossRef]
- Seippel, L.; Bäckström, T. Luteal-Phase Estradiol Relates to Symptom Severity in Patients with Premenstrual Syndrome. J. Clin. Endocrinol. Metab. 1998, 83, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Blum, I.; Lerman, M.; Misrachi, I.; Nordenberg, Y.; Grosskopf, I.; Weizman, A.; Levy-Schiff, R.; Sulkes, J.; Vered, Y. Lack of Plasma Norepinephrine Cyclicity, Increased Estradiol during the Follicular Phase, and of Progesterone and Gonadotrophins at Ovulation in Women with Premenstrual Syndrome. Neuropsychobiology 2004, 50, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ziomkiewicz, A.; Pawlowski, B.; Ellison, P.; Lipson, S.; Thune, I.; Jasienska, G. Higher luteal progesterone is associated with low levels of premenstrual aggressive behavior and fatigue. Biol. Psychol. 2012, 91, 376–382. [Google Scholar] [CrossRef]
- Yen, J.-Y.; Lin, H.-C.; Liu, T.-L.; Long, C.-Y.; Ko, C.-H. Early- and Late-Luteal-Phase Estrogen and Progesterone Levels of Women with Premenstrual Dysphoric Disorder. Int. J. Environ. Res. Public Health 2019, 16, 4352. [Google Scholar] [CrossRef] [PubMed]
- Demont, F.; Fourquet, F.; Rogers, M.; Lansac, J. Epidemiology of apparently benign ovarian cysts. J. Gynecol. Obstet. Biol. Reprod. 2001, 30, S8–S11. [Google Scholar]
- Terzic, M.; Aimagambetova, G.; Norton, M.; Della Corte, L.; Marín-Buck, A.; Lisón, J.F.; Amer-Cuenca, J.J.; Zito, G.; Garzon, S.; Caruso, S.; et al. Scoring systems for the evaluation of adnexal masses nature: Current knowledge and clinical applications. J. Obstet. Gynaecol. 2020, 41, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Lukanova, A.; Lundin, E.; Akhmedkhanov, A.; Micheli, A.; Rinaldi, S.; Zeleniuch-Jacquotte, A.; Lenner, P.; Muti, P.; Biessy, C.; Krogh, V.; et al. Circulating levels of sex steroid hormones and risk of ovarian cancer. Int. J. Cancer 2003, 104, 636–642. [Google Scholar] [CrossRef]
- Cramer, D.W.; Hutchison, G.B.; Welch, W.R.; Scully, R.E.; Ryan, K.J. Determinants of Ovarian Cancer Risk. I. Reproductive Experiences and Family History. JNCI J. Natl. Cancer Inst. 1983, 71, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.D.; Ozols, R.F.; Smyth, J.F.; Hamilton, T.C. Estrogen and Anti-Estrogen Effects on the Growth of Human Epithelial Ovarian Cancer In Vitro. Am. J. Obstet. Gynecol. 1989, 73, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Syed, V.; Ulinski, G.; Mok, S.C.; Yiu, G.K.; Ho, S.M. Expression of Gonadotropin Receptor and Growth Responses to Key Reproductive Hormones in Normal and Malignant Human Ovarian Surface Epithelial Cells. Cancer Res. 2001, 61, 6768–6776. [Google Scholar] [PubMed]
- Helzlsouer, K.J.; Alberg, A.J.; Gordon, G.B.; Longcope, C.; Bush, T.L.; Hoffman, S.C.; Comstock, G.W. Serum Gonadotropins and Steroid Hormones and the Development of Ovarian Cancer. JAMA 1995, 274, 1926–1930. [Google Scholar] [CrossRef] [PubMed]
- McSorley, M.A.; Alberg, A.J.; Allen, D.S.; Allen, N.E.; Brinton, L.A.; Dorgan, J.F.; Kaaks, R.; Rinaldi, S.; Helzlsouer, K.J. Prediagnostic circulating follicle stimulating hormone concentrations and ovarian cancer risk. Int. J. Cancer 2009, 125, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Schildkraut, J.M.; Schwingl, P.J.; Bastos, E.; Evanoff, A.; Hughes, C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet. Gynecol. 1996, 88, 554–559. [Google Scholar] [CrossRef]
- Krämer, S.; Leeker, M.; Jäger, W. Gonadotropin Levels in Ovarian Cyst Fluids: A Predictor of Malignancy? Int. J. Biol. Markers 1998, 13, 165–168. [Google Scholar] [CrossRef]
- Chudecka-Głaz, A.; Rzepka-Górska, I.; Kosmowska, B. Gonadotropin (LH, FSH) levels in serum and cyst fluid in epithelial tumors of the ovary. Arch. Gynecol. Obstet. 2003, 270, 151–156. [Google Scholar] [CrossRef]
- Thomas, C.M.G.; A Boss, E.; Boonstra, H.; Van Tienoven, D.; Sweep, C.G.J.; Massuger, L.F.A.G. Gonadotropins and female sex steroid hormones in cyst fluid and serum from patients with ovarian tumors. Eur. J. Gynaecol. Oncol. 2008, 29, 468–472. [Google Scholar]
- Trabert, B.; Coburn, S.B.; Falk, R.T.; Manson, J.E.; Brinton, L.A.; Gass, M.L.; Kuller, L.H.; Rohan, T.E.; Pfeiffer, R.M.; Qi, L.; et al. Circulating estrogens and postmenopausal ovarian and endometrial cancer risk among current hormone users in the Women’s Health Initiative Observational Study. Cancer Causes Control 2019, 30, 1201–1211. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.; Fraser, I.S.; The FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int. J. Gynecol. Obstet. 2018, 143, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 128: Diagnosis of Abnormal Uterine Bleeding in Reproductive-Aged Women. Obstet. Gynecol. 2012, 120, 197–206. [Google Scholar] [CrossRef] [PubMed]
- International Evidence Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. 2018. Available online: https://www.monash.edu/medicine/sphpm/mchri/pcos (accessed on 19 December 2022).
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef]
- Azziz, R.; Adashi, E.Y. Stein and Leventhal: 80 years on. Am. J. Obstet. Gynecol. 2016, 214, 247.e1–247.e11. [Google Scholar] [CrossRef]
- Mohammad, M.B.; Seghinsara, A.M. Polycystic Ovary Syndrome (PCOS), Diagnostic Criteria, and AMH. Asian Pac. J. Cancer Prev. 2017, 18, 17–21. [Google Scholar] [CrossRef]
- Robinson, S.; Rodin, D.A.; Deacon, A.; Wheeler, M.J.; Clayton, R.N. Which hormone tests for the diagnosis of polycystic ovary syndrome? Br. J. Obstet. Gynaecol. 1992, 99, 232–238. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Practice Committees of the American Society for Reproductive Medicine and the Society for Reproductive Endocrinology and Infertility. Diagnosis and treatment of luteal phase deficiency: A committee opinion. Fertil. Steril. 2021, 115, 1416–1423. [Google Scholar] [CrossRef]
- Sidra, S.; Tariq, M.H.; Farrukh, M.J.; Mohsin, M. Evaluation of clinical manifestations, health risks, and quality of life among women with polycystic ovary syndrome. PLoS ONE 2019, 14, e0223329. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-I.; Liou, T.-H.; Chou, S.-Y.; Chang, C.-Y.; Hsu, C.-S. Diagnostic criteria for polycystic ovary syndrome in Taiwanese Chinese women: Comparison between Rotterdam 2003 and NIH 1990. Fertil. Steril. 2007, 88, 727–729. [Google Scholar] [CrossRef]
- Chon, S.J.; Umair, Z.; Yoon, M.-S. Premature Ovarian Insufficiency: Past, Present, and Future. Front. Cell Dev. Biol. 2021, 9, 672890. [Google Scholar] [CrossRef]
- World Health Organization. Infertility Prevalence Estimates, 1990–2021. Available online: https://www.who.int/publications/i/item/978920068315 (accessed on 3 April 2023).
- Ulrich, N.D.; Marsh, E.E.M. Ovarian Reserve Testing: A Review of the Options, Their Applications, and Their Limitations. Clin. Obstet. Gynecol. 2019, 62, 228–237. [Google Scholar] [CrossRef]
- Infertility Workup for the Women’s Health Specialist. Obstet. Gynecol. 2019, 133, e377–e384. [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Fertility Evaluation of Infertile Women: A Committee Opinion. Fertil. Steril. 2021, 116, 1255–1265. [Google Scholar] [CrossRef]
- Penzias, A.; Azziz, R.; Bendikson, K.; Falcone, T.; Hansen, K.; Hill, M.; Hurd, W.; Jindal, S.; Kalra, S.; Mersereau, J.; et al. Testing and Interpreting Measures of Ovarian Reserve: A Committee Opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J.; D’hooghe, T.; Dancet, E.A.F.; Aurell, R.; Lunenfeld, B.; Orvieto, R.; Pellicer, A.; Polyzos, N.P.; Zheng, W. Self-Monitoring of Urinary Hormones in Combination with Telemedicine—A Timely Review and Opinion Piece in Medically Assisted Reproduction. Reprod. Sci. 2021, 29, 3147–3160. [Google Scholar] [CrossRef]
- Gerris, J.; Delvigne, A.; Dhont, N.; Vandekerckhove, F.; Madoc, B.; Buyle, M.; Neyskens, J.; Deschepper, E.; De Bacquer, D.; Pil, L.; et al. Self-operated endovaginal telemonitoring versus traditional monitoring of ovarian stimulation in assisted reproduction: An RCT. Hum. Reprod. 2014, 29, 1941–1948. [Google Scholar] [CrossRef]
- Yong, E.L.; Wong, P.C.; Wong, Y.C.; Goh, H.H.; Hagglund, L.; Ratnam, S. Simple Office Methods to Predict Ovulation: The Clinical Usefulness of a New Urine Luteinizing Hormone Kit Compared to Basal Body Temperature, Cervical Mucus and Ultrasound. Aust. N. Z. J. Obstet. Gynaecol. 1989, 29, 155–160. [Google Scholar] [CrossRef]
- Leiva, R.A.; Bouchard, T.P.; Abdullah, S.H.; Ecochard, R. Urinary Luteinizing Hormone Tests: Which Concentration Threshold Best Predicts Ovulation? Front. Public Health 2017, 5, 320. [Google Scholar] [CrossRef] [PubMed]
- Vladimirov, I.; Martin, V.; Desislava, T. P–670 Urine estrone–3-glucuronide (E3G) assay: Is there any place during ovarian stimulation for IVF cycles? Hum. Reprod. 2021, 36, deab130.669. [Google Scholar] [CrossRef]
- Tanabe, K.; Susumu, N.; Hand, K.; Nishii, K.; Ishikawa, I.; Nozawa, S. Prediction of the potentially fertile period by urinary hormone measurements using a new home-use monitor: Comparison with laboratory hormone analyses. Hum. Reprod. 2001, 16, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Weddell, S.; Godbert, S.; Freundl, G.; Roos, J.; Gnoth, C. Development of the first urinary reproductive hormone ranges referenced to independently determined ovulation day. Clin. Chem. Lab. Med. 2015, 53, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Wathen, N.C.; Perry, L.; Lilford, R.J.; Chard, T. Interpretation of single progesterone measurement in diagnosis of anovulation and defective luteal phase: Observations on analysis of the normal range. BMJ 1984, 288, 7–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barrett, S.A.; Brown, J.B. An evaluation of the method of cox for the rapid analysis of pregnanediol in urine by gas—Liquid chromatography. J. Endocrinol. 1970, 47, 471–480. [Google Scholar] [CrossRef]
- Blackwell, L.F.; Cooke, D.G.; Brown, S. The Use of Estrone-3-Glucuronide and Pregnanediol-3-Glucuronide Excretion Rates to Navigate the Continuum of Ovarian Activity. Front. Public Health 2018, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Beckley, A.; Klein, J.; Park, J.; Eyvazzadeh, A.; Levy, G.; Koudele, A. The Predictive Value of Urinary Progesterone Metabolite PdG Testing in Pregnancy Outcomes. Obstet. Gynecol. Res. 2022, 05, 194–198. [Google Scholar] [CrossRef]
- Yeh, P.T.; E Kennedy, C.; Van der Poel, S.; Matsaseng, T.; Bernard, L.; Narasimhan, M. Should home-based ovulation predictor kits be offered as an additional approach for fertility management for women and couples desiring pregnancy? A systematic review and meta-analysis. BMJ Glob. Health 2019, 4, e001403. [Google Scholar] [CrossRef]
- Astwood, E.B.; Jones, G.E.S. A Simple Method for The Quantitative Determination of Pregnanediol In Human Urine. J. Biol. Chem. 1941, 137, 397–407. [Google Scholar] [CrossRef]
- Jones, G.E.S. Some Newer Aspects of the Management of Infertility. JAMA 1949, 141, 1123–1129. [Google Scholar] [CrossRef]
- Schliep, K.C.; Mumford, S.L.; Hammoud, A.O.; Stanford, J.B.; Kissell, K.A.; Sjaarda, L.A.; Perkins, N.J.; Ahrens, K.A.; Wactawski-Wende, J.; Mendola, P.; et al. Luteal phase deficiency in regularly menstruating women: Prevalence and overlap in identification based on clinical and biochemical diagnostic criteria. J. Clin. Endocrinol. Metab. 2014, 99, E1007–E1014. [Google Scholar] [CrossRef]
- Deng, W.; Sun, R.; Du, J.; Wu, X.; Ma, L.; Wang, M.; Lv, Q. Prediction of miscarriage in first trimester by serum estradiol, progesterone and β-human chorionic gonadotropin within 9 weeks of gestation. BMC Pregnancy Childbirth 2022, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Chen, Z.-Y.; Zhang, J.; Xu, H.; Zhang, X.-M.; Huang, X.-F. Clinical utility of serum reproductive hormones for the early diagnosis of ectopic pregnancy in the first trimester. J. Obstet. Gynaecol. Res. 2012, 39, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Nakhuda, G.S.; Li, N.; Yang, Z.; Kang, S. At-home urine estrone-3-glucuronide quantification predicts oocyte retrieval outcomes comparably to serum estradiol. F&S Rep. 2023, 4, 43–48. [Google Scholar] [CrossRef]
- Rosner, W.; Hankinson, S.E.; Sluss, P.M.; Vesper, H.W.; Wierman, M.E. Challenges to the Measurement of Estradiol: An Endocrine Society Position Statement. J. Clin. Endocrinol. Metab. 2013, 98, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Lawrenz, B.; Sibal, J.; Garrido, N.; Abu, E.; Jean, A.; Melado, L.; Fatemi, H.M. Inter-assay variation and reproducibility of progesterone measurements during ovarian stimulation for IVF. PLoS ONE 2018, 13, e0206098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).