Abstract
The gastrointestinal tract harbors a densely populated community of microbes that exhibits sexual dimorphism. Dysbiosis of this community has been associated with chronic human disease states ranging from metabolic diseases to neuropsychiatric disorders (NPDs). The gut microbiota–gut–brain axis (GMGBA) is a bi-directional pathway that facilitates the interaction of the gut microflora with host physiological functions. Recently, research surrounding the potential roles of the GMGBA in the development of NPDs (e.g., depression, anxiety, and autism spectrum disorders (ASDs)) has increased. However, the role of the GMGBA in attention-deficit/hyperactivity disorder (ADHD), an NPD that affects an estimated 8.4% of children (5.1% of female and 11.5% of male children) and 4% of adults (with a male–female odds ratio of 1.6) in the United States, remains understudied. Herein, we synthesize the current literature regarding the GMGBA, ADHD, and the potentially relevant intersections between the GMGBA and ADHD. Recommendations are presented for pathways of future research into the role(s) of the GMGBA in ADHD etiology and symptomatology. Particular focus is given to the potential for the variable of host sex to act as an outcome modifier of the relationship between the GMGBA and ADHD.
1. Introduction
Throughout evolutionary history, organisms, including humans, have co-evolved as hosts for microbial symbionts. The human–microbiota interaction has developed over time largely because it is mutually beneficial. As reviewed in Godoy-Vitorino [1] and Yeoman et al. [2], the human body represents a stable, nutrient-rich environment for the microbiota and, in turn, the microbiota influence various facets of human health, including immunity, physiology, behavior, development, and predisposition to disease. Due to this close relationship, the human host and its microbiota have come to be collectively known as a holobiont or supraorganism [1,3]. Recent efforts to estimate the composition of the human body in terms of human cells and bacterial cells have been carried out using the “Reference Man” because most research regarding the quantification of human cells up until the present has been conducted using male subjects. From these calculations, it is estimated that the human body is composed of 3.0 × 1013 human cells and 3.8 × 1013 bacterial cells, with the bacterial cells contributing ca. 0.2 kg of weight. However, this estimate does not include the archaeal, viral, and fungal portions of the microbiota [4], which remain largely understudied. While the total number of microbial cells in the human body is roughly equivalent to the total number of human cells, the collective genome of the microbiota (i.e., the microbiome) is ca. 100–1000 × larger than the human genome, and the human gut microbiome alone harbors ca. 150-times the number of non-redundant genes found in the human genome [5]. Thus, the human microbiome has expanded the functional capacity of the host’s genome [6,7].
Over the past two decades, research into the human microbiome has accelerated [8]. The human microbiota inhabit the body’s internal and external surfaces, including the skin, gastrointestinal (GI) tract, oral cavity and oropharynx, conjunctiva, and vagina, with each of these niches exhibiting a distinctive microbial community composition [4,9]. In a 2012 assessment of the human microbiome using samples collected from female and male subjects, the oral and stool habitats exhibited the highest levels of species diversity, while the vagina was found to be the least diverse niche [9]. Data show that the structural variation of particular microbial species in the gut fluctuates between individuals based on variables including co-habitation and relatedness [10]. In this way, the large number of microbial genes in the gut are influenced by both the environment and genetics; thus, an immense amount of work remains to be done in identifying and understanding the functional significance of the human microbiome [8].
1.1. The Human Gut Microbiome
Among the most well-studied niches of the human body is the colon, or large intestine. There are an estimated 1014 bacterial cells in the colon, a number that represents the highest bacterial cell count across all habitats of the human body [4]. This density of microbes inhabiting the intestinal lumen and mucosal membrane is likely sustained by the nutrients found in the undigested and unabsorbed mass of food material present in the colon [1]. As reviewed in Yeoman et al. [2], the gut microbiota benefit the human host via functions such as the liberation of nutrients, production of vitamins, guidance of normal immune system development, and attenuation of the host inflammatory response.
The presence of two taxa, the Bacteroidetes and the Firmicutes, is most significant in defining this niche [9], as the Bacteroidetes and Firmicutes represent the two most abundant taxa in gut microbial samples across vertebrate lineages [11]. Bacteroidetes and Firmicutes play a large role in energy liberation within host species; members of these phyla ferment dietary fibers into short-chain fatty acids (SCFAs), such as butyrate, propionate, and acetate [12]. In addition to the Bacteroidetes and Firmicutes, numerous other taxa are also often present in the gut, including Bifidobacterium, Prevotella, Ruminococcus, Streptococcus, Enterobacteriaceae, Lactobacillus, and Akkermansia [1]. In a metagenomic study of European subjects (sex not specified), ca. 1000–1150 bacterial species were found to be appreciably abundant among the subjects. At least 160 of these species were found in each individual, but merely 57 species, or ca. 5% of total species, were common in >90% of the subjects [5]. These data highlight the presence of the rare biosphere in the human gut: species that are low in abundance, but cumulatively contribute substantial diversity to the gut microbial metagenome [13].
Despite some degree of similarity in species composition, various host characteristics influence the gut microbial composition to produce inter-individual, functionally specific variations in the gut microbiota [9]. Of pertinence to the topic of this review, host sex [14,15,16] (see Section 5) and clinical diagnoses of neuropsychiatric disorders (NPDs), including depression, anxiety, and autism spectrum disorders (ASDs) [17,18] (see Section 3), are examples of host characteristics that have been increasingly associated with gut microbial variation. However, attention-deficit/hyperactivity disorder (ADHD), an NPD that is comorbid to and exhibits symptom overlap with anxiety, depression, and ASDs, remains understudied with regard to its role as a host characteristic that might influence gut microbial composition.
1.2. Underrepresentation of Females in Research on ADHD and the Gut Microbiome
Several recently published reviews have begun the work of unravelling possible relationships between the gut microbiome and ADHD [19,20,21,22]. While these reviews address ADHD etiology and symptomatology, synthesize literature regarding the overlap of gut microbial metabolic activity and neuroactive compounds relevant to ADHD, and present recommendations for future research [19,20,21,22], there is little to no mention of the continued lack of research considering host sex as a potential outcome modifier of gut microbial composition, gut microbiome functioning, and ADHD etiology and symptomatology. Given the established knowledge of sex-based differences in the presentation and diagnosis of ADHD [23,24,25,26,27,28,29], it is puzzling to find this oversight. This sex-based gap in research is likely a relic from the past, as the implicit and explicit exclusion of female subjects from participation in U.S. medical research studies was the norm until the implementation of the NIH Revitalization Act of 1993 [30]. Although numerous health and disease factors are now known to differentially affect females and males over the lifespan, until the 1960s, female subjects were primarily relegated to studies regarding reproductive health [31]. Research-specific forms of sexism, such as “male bias (observer error caused by adopting a male perspective and habit of thought) and the male norm (the tendency to use males as the standard and to see females as deviant or problematic, even in studying diseases that affect both sexes)”, likely contributed to the problem [32].
Within the body of ADHD research, male-centric studies and/or studies neglecting to analyze patient sex as a potential outcome modifier continue to predominate [23]. This phenomenon bars progress toward generating a comprehensive understanding of the range of symptoms exhibited in patients with ADHD [23,24,25], particularly through perpetuating the “hyperactive boy” stereotype of ADHD while neglecting to consider the inattentive/internalizing ADHD symptoms that present more often in females [23,24]. The purpose of the current review is to expand upon this small body of work through identifying key points that must be considered within and between the fields of gut microbiome and ADHD research. Our review begins with a discussion of the gut microbiota–gut–brain axis (GMGBA), metabolites relevant to the GMGBA, and the GMGBA pathways through which relevant metabolites act. Then, the role of the GMGBA in the development and symptomatology of several NPDs that are closely related to ADHD—depression, anxiety, and ASDs—will be discussed in order to provide comparison points for the study of the roles of the GMGBA in ADHD. Next, an overview of the etiology and symptomatology of ADHD and research regarding the ADHD gut microbiome is presented, followed by an overview of preliminary research on sexual dimorphism in the gut microbiome. Finally, recommendations of pathways for future research are discussed, the focus of which rests heavily on bridging the gap in research surrounding host sex as a potential outcome modifier in gut microbial composition and activity, ADHD etiology and symptomatology, and the GMGBA–ADHD relationship.
2. The Gut Microbiota–Gut–Brain Axis
Hypotheses regarding potentially significant interactions between gut and brain functions have been around since the early 1900s [33]; however, acceptance of these interactions, termed the gut–brain axis (GBA), as critical to human health only began increasing in the 1990s due to accumulating evidence of the bottom-up regulation of neurological function, including emotion, by visceral afferents and the top-down regulation of GI function by neurological phenomena, such as stress and emotion (see Mayer’s [34] review). As evidence of the influence of the microbiota in human health and disease has mounted, the concept of the GBA has evolved to include the gut microbiota as a distinct entity within the axis, which is now more comprehensively referred to as the gut microbiota–gut–brain axis (GMGBA). Crucial to this expansion is the knowledge that the GMGBA plays roles in the development and symptomatology of many NPDs, including depression, anxiety, and ASDs, as a result of the influence of gut microbial metabolites at various points along nervous, endocrine, immune, and circulatory system pathways [18,33,35,36].
The GMGBA encompasses the complex bi-directional interaction of gut microbial metabolites with the central nervous system (CNS) and the enteric nervous system (ENS), via pathways including the vagus nerve of the autonomic nervous system (ANS), the hypothalamic–pituitary–adrenal (HPA) axis of the endocrine system, and stimulation of the immune system [17,18,33,35,37] (Figure 1). Through the GMGBA, neurological functions (the CNS component), such as cognition and emotion, are linked to GI functions (the ENS component), such as digestion, permeability, and motility, and to the body’s stress response (the HPA axis component), including cortisol release and stimulation of the immune system [33,35,37,38]. Due to the relationships between the nervous system functions of the brain and gut and maintenance of homeostasis during stress by the endocrine and immune systems—the relationships that define the GMGBA—there exists a high prevalence of co-morbidity of GI problems, including chronic abdominal pain, gut inflammation, and inflammatory bowel diseases (IBDs), with stress-related neurological conditions such as anxiety and depression [38,39,40]. As for the role of the microbiota in the GMGBA, gut microbial metabolites interact with the CNS via the ENS as mediated by the sympathetic and parasympathetic divisions, and neuroendocrine and neuroimmune components, of the ANS [18,33]. Additionally, the gut microbiome can alter the set point of the HPA axis, the primary regulatory axis of the human body’s stress response, and microbial metabolites may enter the blood stream through the intestinal epithelium and exert effects via receptors found on vagus and ANS nerves and on the brain epithelium [18,33,35]. Thus, the GMGBA encompasses an intricate array of interaction pathways and, as the microbial component of this axis has only recently been integrated, the roles and relationships of its components are yet to be fully elucidated.
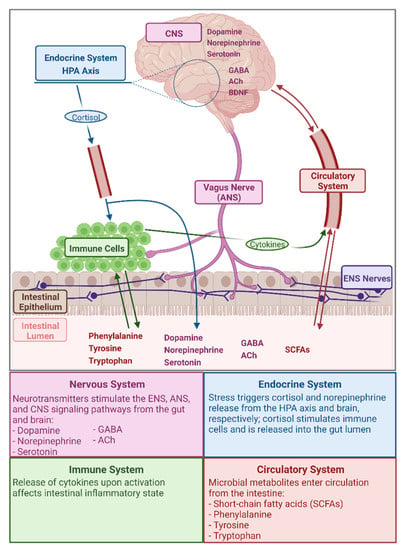
Figure 1.
Illustration and brief summary of gut microbiota–gut–brain axis pathways and several compounds relevant to this axis. Created with BioRender.com (figure inspired by Cryan and Dinan [33]).
Microbial Metabolites Relevant in the GMGBA
The gut microbiota produce and/or metabolize numerous compounds that interact with the human host, many of which are neurochemically significant [36]. Of particular relevance to the GMGBA are the amino acids phenylalanine, tyrosine, and tryptophan; the neurotransmitters norepinephrine, dopamine, serotonin, γ-aminobutyric acid (GABA), and acetylcholine (ACh); SCFAs; and the neurotrophin brain-derived neurotropic factor (BDNF). A summary of the functions of these metabolites and the role of the gut microbiota in the production and availability of these metabolites can be found in Table 1.
Given the various functions of microbial metabolites relevant within the GMGBA (Table 1), impaired or enhanced metabolite production has the potential to influence host physiology. For example, Desbonnet et al. [41] found that Bifidobacteria infantis administration in adult male rats was correlated with decreased interferon gamma (IFN-γ) production, which ultimately led to increased plasma tryptophan concentrations due to the decreased enzymatic diversion of tryptophan to the kynurenine pathway. As tryptophan is the precursor to serotonin, the concentrations of these two metabolites are directly related; thus, altered tryptophan metabolism in the face of gut dysbiosis may theoretically predispose the host to depressive symptoms [41,42]. In the case of dopamine and norepinephrine, these neurotransmitters are synthesized along a shared biochemical pathway by Bacillus and Escherichia species [18,33]; therefore, dysbiosis affecting these taxa could potentially play a role in altering neurological learning and memory functions [43,44,45]. The microbial metabolites that participate in the GMGBA (Table 1) do so via multiple pathways and mechanisms, namely the nervous, endocrine, immune, and circulatory components of the axis (Figure 1). The systems and pathways within the GMGBA are highly intricate and both affect, and are affected by, the composition of the gut microbiota and the function of the gut microbiome.

Table 1.
Microbial metabolites relevant to the gut microbiota–gut–brain axis, the function of these metabolites within the human host, and the role of the gut microbiota in the production and availability of these metabolites.
Table 1.
Microbial metabolites relevant to the gut microbiota–gut–brain axis, the function of these metabolites within the human host, and the role of the gut microbiota in the production and availability of these metabolites.
| Metabolite | Category | Metabolite Function | Role of Gut Microbiota | Relevant Microbial Taxa | Example Interaction |
|---|---|---|---|---|---|
| Phenylalanine and Tyrosine | Amino Acids | Precursors in biosynthetic pathway for norepinephrine and dopamine [43,44,46] | Metabolize to norepinephrine and dopamine [43,44,46]; deplete phenylalanine and tyrosine through use in other biosynthetic pathways [47] | Clostridia and Candida [47] | Clostridia and Candida decrease phenylalanine and tyrosine concentrations for neurotransmitter synthesis through use in non-neurotransmitter biosynthetic pathways [47] |
| Tryptophan | Amino Acid | Precursor in biosynthetic pathway for serotonin | Metabolize to serotonin [18,33,46]; indirectly alter blood plasma levels [41] | Bifidobacteria [41] | Bifidobacteria increase plasma tryptophan levels via suppression of IFN-γ production, a cytokine associated with activation of the enzyme that converts tryptophan to the compound kynurenine [41] |
| Norepinephrine | Neurotransmitter and Peripheral Hormone | Involved as a neurotransmitter in regulation of memory and attention during cognitive tasks, sleep/wake states, and stress reactions, and as a peripheral hormone in the “fight or flight” response of the sympathetic nervous system [44,45] | Biosynthesis | Escherichia, Bacillus, and Saccharomyces [18,33,48] | Escherichia, Bacillus, and Saccharomyces metabolize phenylalanine and tyrosine to norepinephrine [18,33] |
| Dopamine | Neurotransmitter | Involved in learning, memory consolidation, and reward and motivation pathways [43] | Biosynthesis | Bacillus and Escherichia [18,33,36] | Bacillus and Escherichia metabolize phenylalanine and tyrosine to dopamine [18,33,36] |
| Serotonin | Neurotransmitter | Involved in the regulation of a wide array of human behaviors and neurological processes, including mood, attention, memory, reward, perception, anger, aggression, and appetite, among other functions [42] | Biosynthesis; ca. 95% of serotonin in the human body is localized in the gut [18] | Candida, Streptococcus, Escherichia, and Enterococcus [18,33] | Candida, Streptococcus, Escherichia, and Enterococcus metabolize tryptophan to serotonin [18,33] |
| GABA | Neurotransmitter | Main inhibitory neurotransmitter in the brain and therefore affects many facets of neurological function [49] | Biosynthesis | Lactobacillus and Bifidobacterium [17,18,33,36] | Lactobacillus and Bifidobacterium harbor biosynthetic pathways for GABA production [17,18,33,36] |
| ACh | Neurotransmitter | Involved in regulation of attention, cue detection, attentional orienting (default to detection mode shift), and memory encoding [50] | Biosynthesis | Lactobacillus [18,33] | Lactobacillus harbor biosynthetic pathways for ACh production [18,33] |
| Short-Chain Fatty Acids (SCFAs—Butyric Acid, Propionic Acid, Acetic Acid, and Lactic Acid) | Fatty acids containing <6 carbon atoms | Energy source for human metabolic activity [12]; also involved in regulating learning and memory processes, enzymes in the biosynthetic pathway for norepinephrine and dopamine, gut inflammation, sympathetic nervous system stimulation, and gut mucosal serotonin release [17,18,33,35,46] | Liberate as byproducts of polysaccharide fermentation | Bacteroidetes and Firmicutes [12] and an incredibly expansive list of additional gut microbial taxa [18,51] | Metabolic activity of Bacteroidetes and Firmicutes liberates SCFAs from food sources, ultimately constituting ca. 10% of human host’s daily energy requirements [12] |
| BDNF | Neurotrophin | Involved in brain activity and function, including memory, learning, mood regulation, and neuronal growth and survival, specifically differentiation and survival of midbrain dopaminergic neurons [33,35,38,52] | Indirectly alter BDNF levels [21,38] | Preliminary evidence of generalized gut dysbiosis affecting BDNF levels in the brain [21,38] | Altered production of SCFAs by gut microbiota and germ-free and antibiotic-induced changes in gut microbiota composition are associated with changes in brain BDNF levels [21,38] |
3. Neuropsychiatric Disorders and the Gut Microbiota–Gut–Brain Axis
Given the myriad roles of the gut microbiome in maintaining human host health, many chronic disease states have been associated with dysbiosis of the gut. Due to the complexity of the human microbiome, many of these studies have been, and continue to be, correlative in design. With this context in mind, alterations in gut microbial composition have been linked with IBDs [53], type II diabetes [54], obesity [55], and metabolic syndrome [56], among many other conditions (see Sherwin et al.’s [17] and Spor et al.’s [57] reviews). In addition to these physiological disorders, gut microbial influence in the development and symptomatology of several NPDs, including depression, anxiety, and ASDs, has become increasingly apparent (see Sherwin et al.’s [17] and Rea et al.’s [18] reviews), and preliminary studies regarding gut dysbiosis associated with NPDs, including obsessive-compulsive disorder (OCD), bipolar disorder, schizophrenia, Alzheimer’s disease, and ADHD, are appearing more frequently in the literature (Table 2).
NPDs are characterized by altered cognitive, emotional, and executive functioning in the brain and, in addition to depression, anxiety, and ASDs, include conditions such as OCD, bipolar disorder, schizophrenia, Alzheimer’s disease, and ADHD [17,19,58]. Recently, the impacts of the gut microbiota on symptoms of depression, anxiety, and ASDs have been of particular interest to researchers (see Table 2 for summary of potentially relevant microbial taxa). This interest stems from observations that GI issues are highly comorbid with both stress-related NPDs, including anxiety and depression [39,40], and ASDs [59,60], and from evidence that the gut microbiota synthesize compounds relevant to the dysfunction(s) seen in many NPDs [33] (Table 1). Recently, this research interest has carried over to the study of potential interactions between ADHD and the gut microbiome. Given that ADHD exhibits symptom overlap with, and is commonly comorbid with, depression, anxiety, and ASDs, it is somewhat surprising that the relationship between gut dysbiosis and ADHD currently remains understudied.

Table 2.
Disease states associated with dysbiosis of the gut microbiota and the microbial taxa that have been found to exhibit altered abundance associated with the disease states.
Table 2.
Disease states associated with dysbiosis of the gut microbiota and the microbial taxa that have been found to exhibit altered abundance associated with the disease states.
| Disease State(s) | Relevant Microbial Taxa | Citation(s) |
|---|---|---|
| Depression and anxiety | Bifidobacterium infantis, Lactobacillus helveticus, Bifidobacterium longum, Lactobacillus rhamnosus | Desbonnet et al. [41], Sudo et al. [61], Messaoudi et al. [62], Arsenault-Bréard et al. [63], Bravo et al. [64] |
| ASDs | L. rhamnosus, Ruminococcus, Bacteroidetes, Firmicutes, Bacteroides, Parabacteroides, Dehalobacterium, Prevotella, Coprobacillus, Sutterella, Akkermansia, Desulfovibrionaceae, Enterobacteraceae, Oscillospira, Rikenellaceae, Saccharibacteria, Lactobacillus, Desulfovibrio, and Helicobacteraceae | Correti et al. [65], Pärtty et al. [66], Mudd et al. [67] |
| Obsessive-compulsive disorder (OCD) | Oscillospira, Odoribacter, and Anaerostipes | Turna et al. [68] |
| Bipolar disorder | OTU0003 Faecalibacterium, OTU00025 unidentified (Ruminococcaceae family), OTU00024 Anaerostipes, and OTU00022 unidentified (Enterobacteriaceae family) | Evans et al. [69] |
| Schizophrenia | Ruminococcus, Roseburia, and Veillonella | Li et al. [70] |
| Alzheimer’s disease | 82 operational taxonomic units (OTUs), including species within the Bacteroidetes, Firmicutes, and Actinobacteria phyla | Vogt et al. [71] |
| ADHD | Actinobacteria, Firmicutes (order Clostridiales and family Veillonellaceae), Neisseriaceae, Alcaligenaceae, Peptostreptococcaceae, Selenomonadaceae, and additional genera and species (see Section 4.2) | Pärtty et al. [66], Aarts et al. [72], Jiang et al. [73], Prehn-Kristensen et al. [74], Wang et al. [75], Szopinska-Tokov et al. [76], Richarte et al. [77] |
3.1. Depression and Anxiety
Depression and anxiety are stress-related conditions, and the effects of stress on the gut microbiota and microbiome have become clear over the past few decades [38,46]. Ultimately, early gut microbiota–immune system–HPA axis interactions affect individuals’ predisposition to developing depression and/or anxiety later in life [18,35,38,61]. Results from studies of male mice have shown that exposure to stress alters HPA axis activation and the expression of BDNF in germ-free (GF) mice [61]; the administration of Bifidobacterium infantis ameliorates increased stress responsiveness in mice [61] and is associated with increased levels of plasma tryptophan [41]; and the administration of Lactobacillus rhamnosus (JB-1) in healthy mice is associated with reduced anxiety- and depression-related behavior, reduced corticosterone release in response to stress, and altered regional expression of receptors in the GABAergic system [64]. A study of rats (sex not specified) with post-myocardial infarction depression showed that the administration of Lactobacillus helveticus and B. longum attenuated symptoms of behavioral despair, impaired processing of emotional memory, and abnormal social behaviors [63], while a study of male rats and healthy, white female and male humans found that the administration of L. helveticus and B. longum exerted beneficial effects on signs of anxiety and depression [62]. Thus, gut microbial composition and metabolite production are intertwined with the neurological symptoms of depression and anxiety via the complex bi-directional signaling of the GMGBA.
3.2. Autism Spectrum Disorders
In addition to depression and anxiety, the role of the GMGBA in ASDs has become increasingly apparent [17,33,59]. ASDs include autism, Asperger’s disorders, and what is referred to as pervasive developmental disorder not otherwise specified. Common amongst all ASDs are symptoms including repetitive behaviors and interests and social impairments during communication and interactions [59]. Several recent studies have produced evidence of the GMGBA’s influence in ASDs; a longitudinal study of female and male human subjects found that the administration of L. rhamnosus GG within the first 6 months of life affected rates of development of Asperger’s syndrome and ADHD by 13 years of age [66]. A study of male piglets showed a relationship between cortisol (see Figure 1 for illustration of role within the GMGBA), n-acetylaspartate (a neurometabolite marker of neuronal health, known to be decreased in autistic patients), and fecal Ruminococcus content [67], while a study of autistic female and male mice found dysbiosis of Bacteroidetes and Firmicutes as compared to control mice and sexual dimorphism of the gut microbiota in the autistic mice [65]. At present, autism is one of the most well-studied NPDs in relation to the gut microbiome, and many studies provide evidence that specific instances of gut dysbiosis are correlated with the development and symptom severity of autism (see Sherwin et al. [17] for a comprehensive list).
Interestingly, depression, anxiety, and ASDs are all NPDs, and all happen to share many features with ADHD. In a 2019 study of female and male subjects between 18 and 36 years old, including 145 controls, 121 drug-naïve ADHD patients, and 93 drug-treated ADHD patients, ADHD subjects were found, overall, to be more likely to exhibit comorbid psychiatric disorders such as generalized anxiety disorder (GAD), anxiety disorders, major depressive disorder (MDD), OCD, and mood disorders, among others [78]. Further, ADHD shares many symptoms with autism, namely inattention, behavior difficulties, and social impairments [79], and GI dysfunction in patients with ADHD has also been reported [80,81]. Despite many commonalities between ADHD and GMGBA-associated conditions, however, the potential roles of the GMGBA in ADHD etiology and symptomatology have been understudied.
4. Attention-Deficit/Hyperactivity Disorder and the Gut Microbiota–Gut–Brain Axis
ADHD is an NPD that begins in childhood and is characterized by persistent symptoms of inattention and/or hyperactivity–impulsivity that negatively impact individuals’ functioning and development. Inattention refers to behaviors such as difficulty in maintaining focus and deviation from a task, hyperactivity refers to symptoms including excessive motor activity and talkativeness, and impulsivity refers to rash actions and/or behaviors, ranging from those that could cause bodily harm to the excessive interruption of others during conversation [79]. Based on the prominence of certain symptoms, ADHD has been divided into three subtypes: (1) primarily inattentive (ADHD-I); (2) primarily hyperactive–impulsive (ADHD-HI); and (3) combined inattentive and hyperactive–impulsive (ADHD-C) [82]. Within the United States (U.S.), ADHD is highly prevalent. Data indicate that 8.4% of children and adolescents, including 5.1% of females and 11.5% of males, in the U.S. are diagnosed with ADHD [83]. Prevalence estimates for adults are more difficult to obtain due to sociodemographic biases in diagnosis, such as sex, race, and education level [26,27]; therefore, data vary, with estimates that ca. 1% of adults, with a female–male odds ratio of 0.943 [26], to ca. 4% of adults, with a male–female odds ratio of 1.6 [27], have ADHD. In terms of subtype prevalence, the ADHD-C subtype was found to be the most prevalent in a study of white female and male ADHD patients between 6 and 17 years old, but females were found to be 2.2-times more likely to be diagnosed with ADHD-I than were males [24].
4.1. Etiology of ADHD
The symptoms seen in ADHD are currently hypothesized to result from alterations in neurotransmitter systems and in brain structure and connectivity (see Table 1 for an overview of neurotransmitter functions). One proposed mechanism of neurotransmitter system alteration involves an imbalance in dopaminergic and noradrenergic systems that promotes hyperactivity and inattention [44]. Altered functioning of dopamine receptors, transporters, and enzymes has also been implicated in ADHD [84,85]. Along with differential functioning of neurotransmitter systems, alterations in brain structure and connectivity have been identified in ADHD. Relevant findings include structural differences seen in magnetic resonance imaging (MRI) brain images of 8–17-year-old ADHD-I, ADHD-C, and control subjects [82]; disparate patterns of brain activation during working memory (WM) tasks in adult female compared to adult male ADHD subjects despite similar symptom levels and behavioral tendencies across ADHD subjects [86]; and disparate patterns of brain activation in adolescent female ADHD subjects relative to control subjects during a WM task [87]. In 2018, Rubia [25] published a review that aggregates what is currently known about brain structure and connectivity in ADHD from fMRI studies. The review concludes that consistent evidence exists for executive functioning alterations in brain networks for inhibitory control, attention functions, WM, and timing functions, while emerging evidence exists for alterations in brain networks involved in motivation control, as well as for an impaired ability to suppress default mode network activity, a network that is typically deactivated during cognitive tasks. However, the author indicates that the majority of fMRI studies to date have been performed on males with ADHD-C and, thus, the findings of these studies should be considered with this caveat in mind [25].
As of the present, ADHD diagnosis has not been definitively linked to a genetic cause [79], and structural and connectivity alterations, as discussed in the previous paragraph, have been identified as varying with sex and subtype amongst individuals with ADHD. Therefore, given that both the underlying mechanisms of ADHD development and the disparities seen in symptom severity and presentation appear to be multifactorial, and that ADHD shares many features with GMGBA-associated NPDs, it is plausible that the gut microbiome may play a role in ADHD etiology. Indeed, there has been a recent increase in interest in this area of research: since 2015, several review articles [19,20,21,22] and preliminary studies [66,72,73,74,75,76,77] have been published regarding the relationship of the GMGBA and ADHD.
4.2. The ADHD Gut Microbiome
A first step in elucidating the possible roles of the GMGBA in ADHD etiology and symptom severity is the characterization of the gut microbial composition in individuals with ADHD compared to healthy age- and sex-matched control subjects. A 2018 study of German males between 9 and 15 years old, including 14 ADHD subjects and 17 control subjects, found that ADHD subjects had a significantly different gut microbial composition and diversity relative to control subjects. Compositional differences were attributed to a higher abundance of Prevotellaceae in control subjects and a higher abundance of Neisseriaceae in ADHD subjects. Further, results indicated that several genus-level biomarkers of ADHD may exist, including Prevotella as indicative of controls and Neisseria and two species of Bacteroides as markers of ADHD [74]. The authors posit that evidence of ADHD-like symptoms seen in patients following Neisseria meningitides infection [88] and the secretion of metabolites that affect the CNS and BBB structure by Bacteroides species [89] may be relevant to ADHD etiology and symptomatology.
In a 2017 study of majority-male adolescents and adults, including 6 female ADHD subjects, 13 male ADHD subjects, and 77 control subjects, results indicated that ADHD subjects exhibited an increased abundance of Actinobacteria at the expense of Firmicutes abundance, and that no significant differences were found between ADHD and control subjects in the abundance of Bacteroidetes. The genus Bifidobacterium (phylum Actinobacteria) was significantly increased in subjects with ADHD, while the order Clostridiales (phylum Firmicutes) was decreased in ADHD subjects. Additionally, a predicted increase in the abundance of the cyclohexadienyl dehydratase (CDT) enzyme of the phenylalanine synthesis pathway in the gut microbiome of ADHD compared to control subjects was found, with the predicted CDT increase correlated negatively with dopamine-modulated brain responses to reward anticipation. The authors propose that CDT abundance in the gut microbiome could potentially affect proper dopamine signaling in subjects with ADHD [72]. Of note regarding the data from both Prehn-Kristensen et al. [74] and Aarts et al. [72], dysbiosis of Bacteroidetes and Firmicutes, the two predominant SCFA-producing taxa in the vertebrate gut [11], may ultimately affect norepinephrine and dopamine biosynthesis, and learning and memory processes, through alterations in SCFA levels (Table 1).
A 2020 study of 6–10-year-old subjects receiving care at a Taiwanese hospital, including 7 female ADHD subjects, 23 male ADHD subjects, and 30 control subjects, found that subjects with ADHD had significantly more diverse gut microbial compositions than controls. Fusobacterium were more abundant in ADHD subjects, and Lactobacillus were more abundant in controls. Significant differences in the abundances of Bacteroides uniformis, Bacteroides ovatus, Sutterella stercoricanis, and Bacteroides coprocola between ADHD and control subjects were described as possible biomarkers of ADHD. Specifically, B. uniformis, B. ovatus, and S. stercoricanis were positively correlated with ADHD symptoms [75]. Given that members of the Bacteroides genus have been associated with healthy development of the hippocampus, frontal lobe, and cerebellum [90], the authors suggest that dysbiosis of Bacteroides species may affect brain development and functioning in ways that contribute to ADHD symptomatology [75]. In another study of 6–10-year-old, majority-male subjects, including 13 female ADHD subjects, 38 males ADHD subjects, and 32 control subjects, in this case receiving care at a Chinese hospital, no significant differences were found in gut microbial diversity between the ADHD and control subjects. However, the families Alcaligenaceae and Peptostreptococcaceae showed decreased and increased abundance, respectively, in ADHD subjects, while the genera Lachnoclostridium, Dialister, and Faecalibacterium showed decreased abundance in ADHD subjects [73]. The authors note that Faecalibacterium have been found to exhibit anti-inflammatory properties [91], and that increased levels of pro-inflammatory markers (such as cytokines) have been associated with numerous NPDs, including ADHD [92]. Further, neuroinflammation has been correlated with ADHD (see Cenit et al.’s [21] review) and, thus, alterations in Faecalibacterium abundance may be important to the etiology and/or symptomatology of ADHD.
A 2020 study of Dutch adolescents and adults, including 41 ADHD subjects (39% female), 14 subthreshold ADHD subjects (64% female), and 48 control subjects, found no significant differences in alpha and beta diversity measures between the three subject groups [76]. The phyla Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, and Verrucomicrobia were found to be the most abundant in ADHD and control subjects, with no differences in abundance found between the ADHD and control groups. At the genus level, however, Intestinibacter exhibited higher relative abundance in ADHD subjects, while Coprococcus_2 and Prevotella_9 exhibited higher relative abundance in control subjects. Further, results showed that the relative abundance of Coprococcus_2 tended toward a negative association with subjects’ inattention scores, and the use of ADHD medication reduced the relative abundance of Ruminococcaceae_g_, Ruminococcaceae_UCG.014, Lactobacillus, and Lachnospiraceae_ND3007_group in ADHD compared to control subjects [76].
More recently, in 2021, a study of adult subjects from Catalonia, including 100 ADHD subjects (49% female) and 100 control subjects, found no differences in gut microbial alpha and beta diversity measures between ADHD and control subjects [77]. As with Szopinska-Tokov et al. [76], the phyla Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, and Verrucomicrobia were found to be the most abundant in ADHD and control subjects, with no differences in abundance found between the ADHD and control groups. At the family and genus levels, ADHD subjects showed increased relative abundances of the Selenomonadaceae and Veillonellaceae families and the genera Dialister and Megamonas; control subjects exhibited increased relative abundances of the Gracilibacteraceae family and the genera Anaerotania and Gracilibacter [77]. The authors suggest that the increased relative abundance of the Dialister genus in ADHD subjects is particularly interesting given prior evidence that Dialister abundance correlates positively with activity levels in toddlers [93].
In addition to the multiple studies focused on characterization of the gut microbiota in children, adolescents, and adults with ADHD, at least one investigation into the effect of gut microbial alteration in ADHD development has been carried out. In this longitudinal study of 75 children visiting a Finnish hospital, 40 subjects were administered a L. rhamnosus GG probiotic before 6 months of age, while 35 subjects received a placebo during this time, in order to assess the probiotic’s protective effects against ADHD and AS diagnosis by 13 years of age. The researchers selected L. rhamnosus GG as the probiotic due to accumulating evidence of this species’ modulation of neurological functioning [64,94]. Of the 6 subjects ultimately diagnosed with ADHD and/or AS, all subjects were male and part of the placebo group [66]. Overall, the results of the preliminary studies referenced in this review indicate that significant alterations in gut microbial composition are apparent in individuals with ADHD relative to their neurotypical counterparts.
4.3. Potential Interactions of ADHD and the GMGBA
Within the past five years, several reviews regarding the hypothesized roles of the GMGBA in ADHD development have been published [19,20,21,22]. All four reviews provide a comprehensive overview of the bi-directional signaling that occurs in the GMGBA and the ways in which this signaling may affect the etiology and symptomatology of ADHD. The most prominent among the hypothesized mechanisms of GMGBA–ADHD interactions throughout the four review papers involve alterations in the gut microbial metabolism of compounds including tryptophan, phenylalanine, dopamine, norepinephrine, serotonin, GABA, SCFAs, and BDNF [19,20,21,22,72] (Table 3). However, none of the reviews consider human host sex as a prospective outcome modifier or mediator of the hypothesized GMGBA–ADHD relationship(s) [19,20,21,22], which represents a problem given the increasing evidence of sexual dimorphism in the human gut microbiome.

Table 3.
Relevant findings related to neuroactive gut microbial metabolites and hypothesized mechanisms of metabolite influence in GMGBA–ADHD interactions.
5. Host Sex and the Gut Microbiome
As discussed in the Introduction, the Bacteroidetes:Firmicutes ratio is of near-universal importance to the gut microbiome across vertebrate host lineages [11]. Other taxa within the gut microbial composition, however, show indications of sexual dimorphism in some host species (i.e., wild fish species [107], humans [107,108,109,110,111]). To date, few studies have focused specifically on unravelling the effects of host sex, including the effects of sex hormones, on the human gut microbiome (see Kim et al.’s [14], Jašarević et al.’s [15], and Valeri and Endres’ [16] reviews for summaries of current research). Where research has occurred on this front, the results are intriguing. In 2021, a study of Ukrainian children, adolescents, and adults, including 1515 female and 786 male subjects, showed that the relative abundances of Firmicutes and Actinobacteria were significantly increased, and the relative abundance of Bacteroidetes significantly decreased, in female compared to male subjects. Consequently, the Bacteroidetes:Firmicutes ratio was significantly increased in females compared to males [109].
Results from a 2019 study of nearly 9000 adults in cohorts from the United Kingdom (U.K.), U.S., Colombia, and China indicated that scores of gut microbial richness were significantly higher in young-adult females than in young-adult males in the U.K. and Colombian cohorts. Scores of richness in young-adult females in the U.S. cohort were moderately increased compared to those of young-adult males, while no sex differences in gut microbial richness were found in the Chinese cohort [110]. Similarly, a 2021 study of 1741 Han Chinese adults and three validation cohorts, including adults from China, the Netherlands, and Israel, found that premenopausal female subjects had significantly higher scores of gut microbial alpha diversity compared to male subjects across all four cohorts. This difference was attributed to the enrichment of Erysipelotrichaceae bacterium 3_1_53, Clostridiales bacterium 1_7_47FAA, Clostridium symbiosum, Anaerotruncus colihominis, Pseudoflavonifractor capillous, Blautia hydrogenotrophica, Eggerthella lenta, Akkermansia muciniphila, Alistipes sp. HGB5, and Alistipes shahii in the gut microbiomes of premenopausal female subjects in the Han Chinese cohort, with at least two of the validation cohorts exhibiting similar results for all ten taxa. Nine out of the ten taxa also showed significant, negative associations with measures of metabolic health (i.e., BMI, etc.) in premenopausal female Han Chinese subjects. Further, premenopausal female subjects in this cohort showed higher abundances of the two predominate gut microbial taxa, Firmicutes, including Clostridium, Eubacterium, and Ruminococcus, and Bacteroidetes, including Bacteroides spp. [111]. Given both the inverse correlations between female-associated gut microbial taxa and metabolic health presented in Zhang et al. [111] and the critical role of Bacteroidetes and Firmicutes in energy liberation within host species [11], it seems possible that sexual dimorphism of the gut microbiome may play a part in the differential health outcomes of females and males.
In addition to these preliminary studies focused on characterizing sex differences in the gut microbiome, several studies have correlated gut microbial sexual dimorphism to variables including dietary intake and sex hormone levels. In a 2014 comparative study of two wild fish species (Gasterosteus aculeatus and Perca fluviatilis), laboratory mice, and human subjects, dietary intake was found to affect the abundance of gut microbes differentially based on host sex in both of the fish species and in humans, indicating that human host sex acts as an outcome modifier in the relationship between dietary intake and gut microbial composition [107]. Results from a 2019 study of 57 subjects by Shin et al. [108] showed significantly higher scores of gut microbial diversity and evenness in female subjects with high serum estradiol levels and in male subjects with high serum testosterone levels in comparison to sex-matched subjects with lower serum hormone levels. In female subjects, as serum estradiol levels increased, the relative abundance of Bacteroidetes significantly increased and the relative abundance of Firmicutes significantly decreased. Both female and male subjects showed significant alterations in the relative abundances of various taxa associated with varying serum estradiol or serum testosterone levels [108].
Taken together, data from the few preliminary studies of sex differences in the gut microbiome indicate that sexual dimorphism is apparent within this community [107,108,109,110,111]. While it is clear that the gut microbial composition exhibits overarching similarities between subjects and species [9,11], compositional variation, particularly that of the rare biosphere, can reflect environmentally relevant, functionally specific, and potentially sexually dimorphic, physiological requirements of the host. Thus, there remains much to uncover regarding the mechanisms through which variables such as host sex affect the gut microbiome.
Throughout this review, it has been made clear that (1) deficits in the understanding of both ADHD etiology and symptomatology and sexual dimorphism of the human gut microbiome abound, (2) extant research on the ADHD gut microbiome indicates potential significance of gut microbial dysbiosis in ADHD, and (3) research into the roles of the gut microbiome in ADHD and ADHD-related NPDs supplies preliminary evidence for plausible GMGBA–ADHD interaction pathways. However, human host sex has been disregarded as a potentially relevant variable within this context. With this in mind, we suggest a pathway forward for research that addresses the consequential gap in research surrounding host sex as a potential outcome modifier of the gut microbiome, ADHD etiology and symptomatology, and the GMGBA–ADHD relationship.
6. A Pathway for Future Research
6.1. Foundational Holes in ADHD and GMGBA Research
Male-centric studies, as well as studies that neglect to consider patient sex as a potential outcome modifier, continue to predominate the field of ADHD research [23]. This research trend not only perpetuates the “hyperactive boy” stereotype of ADHD, but stifles progress toward more comprehensively understanding the symptomatology specific to all three ADHD subtypes, especially the symptoms of ADHD-I that are more commonly seen in females [23,24,25]. The lack of female representation in research studies and the concomitant “hyperactive boy” stereotype of ADHD have real consequences for females. In 2019, Mowlem et al. [28] found that hyperactive–impulsive externalizing problems were more strongly predictive of ADHD diagnosis and pharmacological treatment in females than in males. The authors posit that this female–male disparity is due in large part to the greater degree of contrast between female gender norms and externalizing symptoms than between male gender norms and these symptoms: females exhibiting hyperactive–impulsive symptoms are more likely to be seen by society as “having a problem” than are males with the same symptoms [28]. Therefore, this finding points to the continued generalization of ADHD as an NPD characterized by hyperactive–impulsive externalizing symptoms, and, in doing so, illuminates an unfortunate reality for females with ADHD: if females are diagnosed with ADHD-I 2.2× more often than are males [24], and females are more likely to receive proper clinical attention for ADHD if they exhibit symptoms seen in the ADHD-HI and ADHD-C subtypes [23], then the difficulty of receiving an ADHD diagnosis as a consequence of being female becomes strikingly apparent.
The current male-centric view of ADHD can be further understood as problematic when considering the relatively high prevalence of ADHD in the U.S.: ca. 8.4% of children [83] and ca. 1–4% [26,27] of adults are estimated to have ADHD. While the disparity between ADHD prevalence in childhood versus adulthood stems, in part, from the fact that sociodemographic biases in diagnosis render prevalence estimates for adult ADHD difficult to obtain [26,27], shifting frequencies of clinical referrals for ADHD over the lifespan and the phenomenon of symptom persistence may also contribute to this disparity. As reviewed in Young et al. [23] and Simon et al. [29], children are referred more frequently to clinics for ADHD symptoms than are adults, with boys being referred far more often than girls. The disparity in ADHD prevalence in childhood versus adulthood then may be explained by the lower persistence of ADHD symptoms in males compared to females, combined with similar rates of clinical referral for ADHD in adult females and males due to increased self-reporting (as opposed to parental reporting). The variance of symptom persistence, however, raises interesting questions.
The symptoms seen in ADHD are currently hypothesized to result from alterations in neurotransmitter systems and brain structure and connectivity, with both genetic and environmental factors exerting an influence. Overall, the knowledge of ADHD subtype-related differences in the anatomical connectedness of the brain [82], sex-based differences in some aspects of brain activation in ADHD patients [86], and the increased likelihood of ADHD-I diagnosis [24] and ADHD symptom persistence [23,29] in females compared to males indicates that female underrepresentation in research may constitute the confluence of many unknowns related to ADHD etiology and symptomatology. Thus, increasing female representation in research studies, both in terms of the research design process and as study participants, is crucial to fully understanding ADHD.
In addition to holes in ADHD research, there is room to add breadth and depth to the field of gut microbial research, as the gut microbial composition is known to vary based on factors ranging from the introduction of a western diet [112] to circumstances of host socioeconomic status (SES) [113] and lifestyle [8]. As for the ADHD-focused gut microbial characterization studies detailed in Section 4.2, the results of all but one study describe all- or majority-male subject samples that are ethnically and racially homogenous [72,73,74,75,76,77]. Therefore, future studies must include subject samples that are truly representative of the demographic breadth of the population of people with ADHD (Alexander Prehn-Kristensen, personal communication).
Overall, among the possible relationships between the GMGBA and ADHD etiology and symptomatology, perhaps the most understudied avenue of research lies in the potential for host sex to act as a modulating variable in these relationships. As sexual dimorphism has been observed in both the gut microbial composition [14,15,16,65,107,108,109,110,111,114] and the presentation of ADHD symptoms [24,86,87], what can be thought of as both “ends” of a relationship mediated by the GMGBA, it is plausible that host sex may constitute an outcome modifier in the roles that the GMGBA may play in ADHD etiology and symptomatology. Thus, as the pace of research aimed at providing insight into the relationship between the GMGBA and ADHD begins to increase, investigators should design future studies that include demographically representative subject samples and position the consideration of host sex as an outcome modifier firmly at the forefront of research questions and hypotheses.
6.2. Characterizing the ADHD Gut Microbiome
As future research projects are developed to delineate the relationship between the GMGBA and ADHD, it will first be necessary to characterize the gut microbiota of female and male subjects with and without ADHD [115]. Recent studies have indicated that significant differences exist between gut microbial alpha and beta diversity, and the relative abundances of specific taxa, in children and adolescents with and without ADHD [73,74,75] and adolescents and adults with and without ADHD [72,76,77]; however, most of these data describe samples comprising all- or majority-male, racially homogenous subjects who share a common nationality [72,73,74,75,76,77]. Further, the 2021 study by Richarte et al. [77] focused on characterizing the gut microbial compositions of adults with ADHD is one of few, and currently exhibits the largest sample size for a study of its kind. As for subjects without ADHD, evidence of sexual dimorphism of some taxa within the gut microbiome (see Section 5) has been presented in recent years [14,15,16,107,108,109,110,111]. Thus, characterizing the gut microbiota of adults with ADHD in studies with subject samples that are demographically representative of the worldwide ADHD population will fill a gap in the existing literature.
In designing future studies, methodological approaches including longitudinal study design and shotgun metagenomic sequencing should be among the primary considerations of investigators. Longitudinal study design is defined by sampling at multiple points in time and serves the purpose of gut microbiota characterization more comprehensively than cross-sectional study design, a method defined by sampling at a single point in time. Though cross-sectional data have been foundational to our understanding of the human microbiome and remain useful, the gut microbial composition is highly volatile [116], so data from cross-sectional studies can only be taken as a mere snapshot of the gut microbial profile at one point in time. For example, a recent study by Levy et al. [117], in which three fecal samples were taken from human subjects over the course of nine months, found that gut microbial states dominated by either Prevotella or Bacteroides comprised an inflammation- and cholesterol marker-associated compositional gradient in the gut microbiota, with the transition between states occurring after a period of relative depletion of both Prevotella and Bacteroides. In building upon preliminary data from cross-sectional studies, longitudinal study design such as that seen in Levy et al. [117] will provide a more comprehensive profile of the ADHD gut microbiome. Additionally, longitudinal design will allow for a more thorough understanding of two of the largest drivers of volatility in gut microbial composition—medication use and hormonal fluctuations.
Any full assessment of the human gut microbiota must account for two important variables. First, many non-antibiotic drugs are known to alter the gut microbial composition through antibiotic-like side effects [118]. Second, hormone cycling in females, and, to a lesser extent, males, along with the use of hormonal contraceptives, anabolic steroids, and exogenous androgens, can potentially influence the gut microbial composition [119] (Eve Valera, personal communication). Proper consideration of hormonal effects will be key in delineating host sex as a potential outcome modifier in the GMGBA–ADHD relationship because sexual dimorphism of the gut microbiota is partly attributed to the presence of different sex hormones in females and males [15,107,108]. In general, controlling for the effects of female hormone cycling continues to present challenges for research design and, thus, female-centric research is also lacking with regard to the gut microbiome [15,16]. However, caution should be used when considering female hormonal cycling as a confounding variable because this view positions the male sex as the standard for research. Thus, future longitudinal studies should position female hormonal cycling as a normal physiological process, the effects of which on gut microbial composition must be determined in order to reach a comprehensive understanding of the human gut microbiota.
Along with the use of longitudinal study design in future research, shotgun metagenomic sequencing will provide the exhaustive taxonomic characterization of gut microbial composition that will be necessary to move away from correlative studies. While data from studies using 16S rRNA gene sequencing and PCR amplification of the V3 and V4 hypervariable regions are undoubtedly useful, these sequencing methods allow for taxonomic classification of the gut microflora, but do not produce an assessment of an entire microbial metagenome. Moreover, 16S rRNA data may be coupled with methods such as PICRUSt2 or HUMAnN3 (see below) to generate predictive hypotheses regarding the functional capacity of the gut microbiota, but do not on their own catalog the functional diversity of the gut microflora. In order to move beyond a purely predictive capacity, shotgun metagenomic sequencing should be employed. In shotgun metagenomic sequencing, DNA is sequenced in small fragments before reconstruction via an algorithm to provide the entire genome of microbial species present in a community [120]. Thus, data from shotgun metagenomic sequencing studies describe sequences known to be present within a sample and can be used in the identification of gut–brain modules (GBMs), or microbial metabolic pathways that produce neuroactive metabolites [121]. In particular, shotgun metagenomic sequencing can be coupled with longitudinal study design, metabolomics, and other -omics techniques to facilitate the characterization of the ADHD gut microbiome, as well as elucidate potentially relevant metabolic/functional alterations in the ADHD gut microbiome compared to the non-ADHD gut microbiome.
In building upon data from ADHD gut microbiota characterization research, it will be crucial for investigators to include metabolomics in the design of studies, because ADHD-associated gut dysbiosis may alter the capacity of the gut microbiome to metabolize compounds that are potentially relevant to ADHD etiology and symptomatology, including tryptophan, phenylalanine, dopamine, norepinephrine, serotonin, GABA, SCFAs, and BDNF [19,20,21,22,72]. While many studies have employed PICRUSt2 or HUMAnN3 methodologies—two computational approaches that reconstruct the estimated functional potential of microbial communities using OTU and ASV sequence reads [122,123,124,125,126]—data generated from these methods are solely predictive of the metabolic potential of the gut microbiome. Metabolomics methods used in combination with shotgun metagenomic sequencing will allow for the elucidation of all metabolites present within a biological specimen [127]. The use of metabolomics methods will go beyond the prediction of the functional potential of the ADHD gut microbiome to produce data on the observed translational activity of this microbial community under different environmental conditions. In particular, data on the presence of compounds from GBMs, microbial metabolic pathways that produce neuroactive metabolites [121], could form the foundation of hypotheses for mouse model studies. Metabolic pathways that might be potentially relevant to ADHD etiology and symptomatology include those in which SCFAs are byproducts [115], vitamin B6 is synthesized [95,97], and the neurotransmitters dopamine and serotonin are synthesized [128].
Overall, the transition from correlative to causal research will require a comprehensive understanding of the temporal fluctuations in species presence and abundance in the gut microbiota of subjects with ADHD, as well as the effect(s) of such fluctuations on the metabolic capacity and/or activity of the gut microbiome (John Cryan, personal communication). Obtaining a combination of genomic and metabolomic data will be an important first step in identifying potentially relevant gut dysbiosis and/or biomarker species and any pertinent modification(s) of these two variables by host sex and life phase (i.e., perinatal period, childhood, adolescence, adulthood). Gathering these data will then inform research studies that move away from correlation towards identifying the underlying microbiome-related factors that drive the relationship(s) between host sex, the GMGBA, and ADHD. For example, mouse model studies that employ the inoculation of GF lab mice with microbial samples could be used to determine whether the development of a target phenotype is affected by the gut microbial composition [56,129] (Susan Campbell, personal communication).
7. Conclusions
The abundance of correlative evidence regarding gut microbial dysbiosis, the bi-directional activity of relevant metabolites within the GMGBA, and the etiology and symptomatology of various chronic diseases and NPDs, a small collection of which has been presented in this review, indicates that further investigation into the role of the GMGBA in the etiology of ADHD is warranted. A deeper understanding of the physiological underpinnings of ADHD etiology will be facilitated through characterization of the gut microbiota and potentially associated gut dysbiosis in subjects with ADHD. Along with taxonomic characterization, delineating alterations in the functional capacity of the ADHD gut microbiome will help to inform future testable hypotheses.
The ultimate goal of research conducted in these areas is to better inform best clinical practices for the treatment of individuals diagnosed with ADHD. At present, frontline treatments for ADHD include behavior management and/or medication, with the most suitable medication for a patient generally being prescribed in a trial-and-error manner due to the lack of neurobiological understanding of ADHD [130]. Unfortunately, though treatment of ADHD with medication is known to decrease symptom severity, several negative side effects, including decreased appetite, sleep disturbance, increased blood pressure and/or heart rate, tics, seizures, and psychotic symptoms, can make pharmacologic treatment undesirable [130].
Hopefully, the elucidation of relevant GMGBA–ADHD relationships will lead to clinical practices that include the cultivation of a gut microbiome within ADHD patients that promotes the increased efficacy of pharmacologic treatments and/or mitigates symptom severity without, or in addition to, pharmacologic treatments. For example, in a study of BTBR mouse models of autism, the ketogenic diet-associated mitigation of autism symptoms was suggested to result from the diet’s effect of reconfiguring the gut microbial composition of the mouse models [131]. In a similar manner, patients with ADHD may one day reap the benefits of GMGBA–ADHD research through the incorporation of diet manipulation, probiotic or prebiotic therapy, etc., into frontline clinical practices for the treatment of ADHD.
Author Contributions
H.V.S. conceived of the presented ideas, carried out research, and led in writing the manuscript. M.J.C. supervised this work, discussed ideas with H.V.S., and contributed to editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Virginia Foundation for Independent Colleges/Carilion Clinic Research Fellowship to H.V. Schleupner.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank John F. Cryan (APC Microbiome Ireland), Eve M. Valera (Harvard Medical School), Alexander Prehn-Kristensen (Christian-Albrechts-University Kiel), and Susan Campbell (Virginia Tech) for thoughtful conversations in their respective areas of expertise. Hannah V. Schleupner was financially supported by the Hollins University Department of Biology and through a Virginia Foundation for Independent Colleges/Carilion Clinic Research Fellowship.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Godoy-Vitorino, F. Human microbial ecology and the rising new medicine. Ann. Transl. Med. 2019, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, C.J.; Chia, N.; Yildirim, S.; Miller, M.E.B.; Kent, A.; Stumpf, R.; Leigh, S.R.; Nelson, K.E.; White, B.A.; Wilson, B.A. Towards an Evolutionary Model of Animal-Associated Microbiomes. Entropy 2011, 13, 570–594. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.R.; Schluter, J.; Coyte, K.Z.; Rakoff-Nahoum, S. The evolution of the host microbiome as an ecosystem on a leash. Nature 2017, 548, 43–51. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar]
- Zeevi, D.; Korem, T.; Godneva, A.; Bar, N.; Kurilshikov, A.; Lotan-Pompan, M.; Weinberger, A.; Fu, J.; Wijmenga, C.; Zhernakova, A.; et al. Structural variation in the gut microbiome associates with host health. Nature 2019, 568, 43–48. [Google Scholar] [CrossRef]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008, 6, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.T.; Bender, K.S.; Buckley, D.H.; Sattley, W.M.; Stahl, D.A. Brock Biology of Microorganisms, 15th ed.; Pearson: London, UK, 2017. [Google Scholar]
- Sogin, M.L.; Morrison, H.G.; Huber, J.A.; Welch, D.M.; Huse, S.M.; Neal, P.R.; Arrieta, J.M.; Herndl, G.J. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl. Acad. Sci. USA 2006, 103, 12115–12120. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Unno, T.; Kim, B.-Y.; Park, M.-S. Sex Differences in Gut Microbiota. World J. Mens Health 2020, 38, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Morrison, K.E.; Bale, T.L. Sex differences in the gut microbiome–brain axis across the lifespan. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150122. [Google Scholar] [CrossRef]
- Valeri, F.; Endres, K. How biological sex of the host shapes its gut microbiota. Front. Neuroendocr. 2021, 61, 100912. [Google Scholar] [CrossRef]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the social brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef]
- Rea, K.; Dinan, T.G.; Cryan, J.F. Gut Microbiota: A Perspective for Psychiatrists. Neuropsychobiology 2020, 79, 50–62. [Google Scholar] [CrossRef]
- Mathee, K.; Cickovski, T.; Deoraj, A.; Stollstorff, M.; Narasimhan, G. The gut microbiome and neuropsychiatric disorders: Implications for attention deficit hyperactivity disorder (ADHD). J. Med. Microbiol. 2020, 69, 14–24. [Google Scholar] [CrossRef]
- Boonchooduang, N.; Louthrenoo, O.; Chattipakorn, N.; Chattipakorn, S.C. Possible links between gut–microbiota and attention-deficit/hyperactivity disorders in children and adolescents. Eur. J. Nutr. 2020, 59, 3391–3403. [Google Scholar] [CrossRef]
- Cenit, M.C.; Nuevo, I.C.; Codoñer-Franch, P.; Dinan, T.G.; Sanz, Y. Gut microbiota and attention deficit hyperactivity disorder: New perspectives for a challenging condition. Eur. Child Adolesc. Psychiatry 2017, 26, 1081–1092. [Google Scholar] [CrossRef]
- Sandgren, A.M.; Brummer, R.J.M. ADHD-originating in the gut? The emergence of a new explanatory model. Med. Hypotheses 2018, 120, 135–145. [Google Scholar] [CrossRef]
- Young, S.; Adamo, N.; Ásgeirsdóttir, B.B.; Branney, P.; Beckett, M.; Colley, W.; Cubbin, S.; Deeley, Q.; Farrag, E.; Gudjonsson, G.; et al. Females with ADHD: An expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention-deficit/hyperactivity disorder in girls and women. BMC Psychiatry 2020, 20, 404. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Mick, E.; Faraone, S.V.; Braaten, E.; Doyle, A.; Spencer, T.; Wilens, T.E.; Frazier, E.; Johnson, M.A. Influence of Gender on Attention Deficit Hyperactivity Disorder in Children Referred to a Psychiatric Clinic. Am. J. Psychiatry 2002, 159, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K. Cognitive Neuroscience of Attention Deficit Hyperactivity Disorder (ADHD) and Its Clinical Translation. Front. Hum. Neurosci. 2018, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.; Jiang, S.-F.; Paksarian, D.; Nikolaidis, A.; Castellanos, F.X.; Merikangas, K.R.; Milham, M.P. Trends in the Prevalence and Incidence of Attention-Deficit/Hyperactivity Disorder among Adults and Children of Different Racial and Ethnic Groups. JAMA Netw. Open 2019, 2, e1914344. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Adler, L.; Barkley, R.; Biederman, J.; Conners, C.K.; Demler, O.; Faraone, S.V.; Greenhill, L.; Howes, M.J.; Secnik, K.; et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am. J. Psychiatry 2006, 163, 716–723. [Google Scholar] [CrossRef]
- Mowlem, F.D.; Rosenqvist, M.A.; Martin, J.; Lichtenstein, P.; Asherson, P.; Larsson, H. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur. Child Adolesc. Psychiatry 2019, 28, 481–489. [Google Scholar] [CrossRef]
- Simon, V.; Czobor, P.; Bálint, S.; Mézáros, Á.; Bitter, I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: Meta-analysis. Br. J. Psychiatry 2009, 194, 204–211. [Google Scholar] [CrossRef]
- NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research. Available online: https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm (accessed on 21 October 2020).
- Pinn, V.W. Sex and Gender Factors in Medical Studies: Implications for Health and Clinical Practice. JAMA 2003, 289, 397. [Google Scholar] [CrossRef]
- Mastroianni, A.; Faden, R.; Federman, D. Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies; National Academies Press: Washington DC, USA, 1994; Volume 1. [Google Scholar]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Lyte, M. Microbial endocrinology. Gut Microbes 2014, 5, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar]
- Berrill, J.W.; Gallacher, J.; Hood, K.; Green, J.T.; Matthews, S.B.; Campbell, A.K.; Smith, A. An observational study of cognitive function in patients with irritable bowel syndrome and inflammatory bowel disease. Neurogastroenterol. Motil. 2013, 25, 918-e704. [Google Scholar] [CrossRef]
- Söderquist, F.; Syk, M.; Just, D.; Kurbalija Novicic, Z.; Rasmusson, A.J.; Hellström, P.M.; Ramklint, M.; Cunningham, J.L. A cross-sectional study of gastrointestinal symptoms, depressive symptoms and trait anxiety in young adults. BMC Psychiatry 2020, 20, 535. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Arias-Carrión, O.; Stamelou, M.; Murillo-Rodríguez, E.; Menéndez-González, M.; Pöppel, E. Dopaminergic reward system: A short integrative review. Int. Arch. Med. 2010, 3, 24. [Google Scholar] [CrossRef]
- Hussain, L.S.; Reddy, V.; Maani, C.V. Physiology, Noradrenergic Synapse. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Schwarz, L.A.; Luo, L. Organization of the Locus Coeruleus-Norepinephrine System. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef] [PubMed]
- Clayton, T.A. Metabolic differences underlying two distinct rat urinary phenotypes, a suggested role for gut microbial metabolism of phenylalanine and a possible connection to autism. FEBS Lett. 2012, 586, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Ernst, S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992, 50, 203–212. [Google Scholar] [CrossRef]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef] [PubMed]
- Hasselmo, M.E.; Sarter, M. Modes and Models of Forebrain Cholinergic Neuromodulation of Cognition. Neuropsychopharmacology 2011, 36, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Liu, D.-Y.; Shen, X.-M.; Yuan, F.-F.; Guo, O.-Y.; Zhong, Y.; Chen, J.-G.; Zhu, L.-Q.; Wu, J. The Physiology of BDNF and Its Relationship with ADHD. Mol. Neurobiol. 2015, 52, 1467–1476. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M.; et al. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-Like Receptor 5. Science 2010, 328, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Spor, A.; Koren, O.; Ley, R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290. [Google Scholar] [CrossRef]
- Hyman, S.E. A glimmer of light for neuropsychiatric disorders. Nature 2008, 455, 890–893. [Google Scholar] [CrossRef] [PubMed]
- de Theije, C.G.M.; Wu, J.; da Silva, S.L.; Kamphuis, P.J.; Garssen, J.; Korte, S.M.; Kraneveld, A.D. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur. J. Pharmacol. 2011, 668, S70–S80. [Google Scholar] [CrossRef]
- Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the Intestinal Barrier in Patients with Autism Spectrum Disorders and in Their First-degree Relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Arseneault-Bréard, J.; Rondeau, I.; Gilbert, K.; Girard, S.-A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012, 107, 1793–1799. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Coretti, L.; Cristiano, C.; Florio, E.; Scala, G.; Lama, A.; Keller, S.; Cuomo, M.; Russo, R.; Pero, R.; Paciello, O.; et al. Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Sci. Rep. 2017, 7, 45356. [Google Scholar] [CrossRef] [PubMed]
- Pärtty, A.; Kalliomäki, M.; Wacklin, P.; Salminen, S.; Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr. Res. 2015, 77, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Berding, K.; Wang, M.; Donovan, S.M.; Dilger, R.N. Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes 2017, 8, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Turna, J.; Grosman Kaplan, K.; Anglin, R.; Patterson, B.; Soreni, N.; Bercik, P.; Surette, M.G.; Van Ameringen, M. The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age- and sex-matched controls: A pilot study. Acta Psychiatr. Scand. 2020, 142, 337–347. [Google Scholar] [CrossRef]
- Evans, S.J.; Bassis, C.M.; Hein, R.; Assari, S.; Flowers, S.A.; Kelly, M.B.; Young, V.B.; Ellingrod, V.E.; McInnis, M.G. The Gut Microbiome Composition Associates with Bipolar Disorder and Illness Severity. J. Psychiatr. Res. 2017, 87, 23–29. [Google Scholar] [CrossRef]
- Li, S.; Song, J.; Ke, P.; Kong, L.; Lei, B.; Zhou, J.; Huang, Y.; Li, H.; Li, G.; Chen, J.; et al. The gut microbiome is associated with brain structure and function in schizophrenia. Sci. Rep. 2021, 11, 9743. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, Y.; Zhou, G.; Li, Y.; Yuan, J.; Li, X.; Ruan, B. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behav. Brain Res. 2018, 347, 408–413. [Google Scholar] [CrossRef]
- Prehn-Kristensen, A.; Zimmermann, A.; Tittmann, L.; Lieb, W.; Schreiber, S.; Baving, L.; Fischer, A. Reduced microbiome alpha diversity in young patients with ADHD. PLoS ONE 2018, 13, e0200728. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yang, C.-Y.; Chou, W.-J.; Lee, M.-J.; Chou, M.-C.; Kuo, H.-C.; Yeh, Y.-M.; Lee, S.-Y.; Huang, L.-H.; Li, S.-C. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2020, 29, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Szopinska-Tokov, J.; Dam, S.; Naaijen, J.; Konstanti, P.; Rommelse, N.; Belzer, C.; Buitelaar, J.; Franke, B.; Bloemendaal, M.; Aarts, E.; et al. Investigating the Gut Microbiota Composition of Individuals with Attention-Deficit/Hyperactivity Disorder and Association with Symptoms. Microorganisms 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Richarte, V.; Sánchez-Mora, C.; Corrales, M.; Fadeuilhe, C.; Vilar-Ribó, L.; Arribas, L.; Garcia, E.; Rosales-Ortiz, S.K.; Arias-Vasquez, A.; Soler-Artigas, M.; et al. Gut microbiota signature in treatment-naïve attention-deficit/hyperactivity disorder. Transl. Psychiatry 2021, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.-J.; Tseng, W.-L.; Yang, L.-K.; Gau, S.S.-F. Psychiatric comorbid patterns in adults with attention-deficit hyperactivity disorder: Treatment effect and subtypes. PLoS ONE 2019, 14, e0211873. [Google Scholar] [CrossRef] [PubMed]
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Duel, B.P.; Steinberg-Epstein, R.; Hill, M.; Lerner, M. A Survey of Voiding Dysfunction in Children with Attention Deficit-Hyperactivity Disorder. J. Urol. 2003, 170, 1521–1524. [Google Scholar] [CrossRef] [PubMed]
- McKeown, C.; Hisle-Gorman, E.; Eide, M.; Gorman, G.H.; Nylund, C.M. Association of Constipation and Fecal Incontinence with Attention-Deficit/Hyperactivity Disorder. Pediatrics 2013, 132, e1210–e1215. [Google Scholar] [CrossRef] [PubMed]
- Saad, J.F.; Griffiths, K.R.; Kohn, M.R.; Clarke, S.; Williams, L.M.; Korgaonkar, M.S. Regional brain network organization distinguishes the combined and inattentive subtypes of Attention Deficit Hyperactivity Disorder. NeuroImage Clin. 2017, 15, 383–390. [Google Scholar] [CrossRef]
- Danielson, M.L.; Bitsko, R.H.; Ghandour, R.M.; Holbrook, J.R.; Kogan, M.D.; Blumberg, S.J. Prevalence of Parent-Reported ADHD Diagnosis and Associated Treatment Among U.S. Children and Adolescents, 2016. J. Clin. Child Adolesc. Psychol. 2018, 47, 199–212. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, H.; Sun, H.; Zou, L.; Zhu, L.-Q. Role of Dopamine Receptors in ADHD: A Systematic Meta-analysis. Mol. Neurobiol. 2012, 45, 605–620. [Google Scholar] [CrossRef]
- Bellgrove, M.A.; Mattingley, J.B. Molecular Genetics of Attention. Ann. N. Y. Acad. Sci. 2008, 1129, 200–212. [Google Scholar] [CrossRef]
- Valera, E.M.; Brown, A.; Biederman, J.; Faraone, S.V.; Makris, N.; Monuteaux, M.C.; Whitfield-Gabrieli, S.; Vitulano, M.; Schiller, M.; Seidman, L.J. Sex Differences in the Functional Neuroanatomy of Working Memory in Adults with ADHD. Am. J. Psychiatry 2010, 167, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.A.; Hinshaw, S.; D’Esposito, M. Efficiency of the Prefrontal Cortex During Working Memory in Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Trollfors, B.; Hugosson, S.; Fernell, E.; Svensson, E. Long-term follow-up of children with bacterial meningitis with emphasis on behavioural characteristics. Eur. J. Pediatr. 2002, 161, 330–336. [Google Scholar] [CrossRef]
- Lukiw, W.J. The microbiome, microbial-generated proinflammatory neurotoxins, and Alzheimer’s disease. J. Sport Health Sci. 2016, 5, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Tillisch, K.; Mayer, E.; Gupta, A.; Gill, Z.; Brazeilles, R.; Le Nevé, B.; van Hylckama Vlieg, J.E.T.; Guyonnet, D.; Derrien, M.; Labus, J. Brain structure and response to emotional stimuli as related to gut microbial profiles in healthy women. Psychosom. Med. 2017, 79, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, M.; Yang, X.; Hong, N.; Yu, C. Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J. Crohn’s Colitis 2013, 7, e558–e568. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.H.B.; Goldstein, B.I. Inflammation in Children and Adolescents with Neuropsychiatric Disorders: A Systematic Review. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 274–296. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.M.; Galley, J.D.; Hade, E.M.; Schoppe-Sullivan, S.; Kamp-Dush, C.; Bailey, M.T. Gut microbiome composition is associated with temperament during early childhood. Brain Behav. Immun. 2015, 45, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Enticott, P.G.; Rinehart, N.J.; Tonge, B.J.; Bradshaw, J.L.; Fitzgerald, P.B. A preliminary transcranial magnetic stimulation study of cortical inhibition and excitability in high-functioning autism and Asperger disorder. Dev. Med. Child Neurol. 2010, 52, e179–e183. [Google Scholar] [CrossRef]
- Dolina, S.; Margalit, D.; Malitsky, S.; Rabinkov, A. Attention-deficit hyperactivity disorder (ADHD) as a pyridoxine-dependent condition: Urinary diagnostic biomarkers. Med. Hypotheses 2014, 82, 111–116. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, E.; Nandagopal, K. Does serotonin deficit mediate susceptibility to ADHD? Neurochem. Int. 2015, 82, 52–68. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, I.E.; Ferreira, J.G.; Tellez, L.A.; Ren, X.; Yeckel, C.W. The Gut-Brain Dopamine Axis: A Regulatory System for Caloric Intake. Physiol. Behav. 2012, 106, 394–399. [Google Scholar] [CrossRef]
- Howells, F.M.; Stein, D.J.; Russell, V.A. Synergistic tonic and phasic activity of the locus coeruleus norepinephrine (LC-NE) arousal system is required for optimal attentional performance. Metab. Brain Dis. 2012, 27, 267–274. [Google Scholar] [CrossRef]
- Edden, R.A.E.; Crocetti, D.; Zhu, H.; Gilbert, D.L.; Mostofsky, S.H. Reduced GABA Concentration in Attention-Deficit/Hyperactivity Disorder. Arch. Gen. Psychiatry 2012, 69, 750–753. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- MacFabe, D.; Cain, D.; Rodriguezcapote, K.; Franklin, A.; Hoffman, J.; Boon, F.; Taylor, A.; Kavaliers, M.; Ossenkopp, K. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 2007, 176, 149–169. [Google Scholar] [CrossRef]
- Corominas-Roso, M.; Ramos-Quiroga, J.A.; Ribases, M.; Sanchez-Mora, C.; Palomar, G.; Valero, S.; Bosch, R.; Casas, M. Decreased serum levels of brain-derived neurotrophic factor in adults with attention-deficit hyperactivity disorder. Int. J. Neuropsychopharmacol. 2013, 16, 1267–1275. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.I.; Snowberg, L.K.; Hirsch, P.E.; Lauber, C.L.; Org, E.; Parks, B.; Lusis, A.J.; Knight, R.; Caporaso, J.G.; Svanbäck, R. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat. Commun. 2014, 5, 4500. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Park, Y.-H.; Sim, M.; Kim, S.-A.; Joung, H.; Shin, D.-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 2019, 170, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Moseiko, V.; Romanenko, M.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Vaiserman, A. Sex differences in the phylum-level human gut microbiota composition. BMC Microbiol. 2021, 21, 131. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Kelley, S.T.; Chen, Y.; Escobar, J.S.; Mueller, N.T.; Ley, R.E.; McDonald, D.; Huang, S.; Swafford, A.D.; Knight, R.; et al. Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults. mSystems 2019, 4, e00261-19. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, H.; Li, Y.; Shi, Z.; Ren, H.; Zhang, Z.; Zhou, X.; Tang, S.; Han, X.; Lin, Y.; et al. Sex- and age-related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nat. Aging. 2021, 1, 87–100. [Google Scholar] [CrossRef]
- Amato, K.R.; Yeoman, C.J.; Cerda, G.A.; Schmitt, C.; Cramer, J.D.; Miller, M.E.B.; Gomez, A.; Turner, T.R.; Wilson, B.A.; Stumpf, R.M.; et al. Variable responses of human and non-human primate gut microbiomes to a Western diet. Microbiome 2015, 3, 53. [Google Scholar] [CrossRef]
- Miller, G.E.; Engen, P.A.; Gillevet, P.M.; Shaikh, M.; Sikaroodi, M.; Forsyth, C.B.; Mutlu, E.; Keshavarzian, A.; Driks, A. Lower Neighborhood Socioeconomic Status Associated with Reduced Diversity of the Colonic Microbiota in Healthy Adults. Driks A, editor. PLoS ONE 2016, 11, e0148952. [Google Scholar]
- Kaliannan, K.; Robertson, R.C.; Murphy, K.; Stanton, C.; Kang, C.; Wang, B.; Hao, L.; Bhan, A.K.; Kang, J.X. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 2018, 6, 205. [Google Scholar] [CrossRef]
- Schleupner, H.V. Attention-Deficit/Hyperactivity Disorder Etiology and Symptomatology: The Potential Role of the Gut Microbiota-Gut-Brain Axis. Bachelor’s Thesis, Hollins University, Roanoke, VA, USA, 2021. [Google Scholar]
- Bastiaanssen, T.F.S.; Gururajan, A.; van de Wouw, M.; Moloney, G.M.; Ritz, N.L.; Long-Smith, C.M.; Wiley, N.C.; Murphy, A.B.; Lyte, J.M.; Fouhy, F.; et al. Volatility as a Concept to Understand the Impact of Stress on the Microbiome. Psychoneuroendocrinology 2021, 124, 105047. [Google Scholar] [CrossRef]
- Levy, R.; Magis, A.T.; Earls, J.C.; Manor, O.; Wilmanski, T.; Lovejoy, J.; Gibbons, S.M.; Omenn, G.S.; Hood, L.; Price, N.D. Longitudinal analysis reveals transition barriers between dominant ecological states in the gut microbiome. Proc. Natl. Acad. Sci. USA 2020, 117, 13839–13845. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Kaiser, U.B.; Chen, G.L.; Manson, J.E.; Goldstein, J.M. Sex and Gender Differences Research Design for Basic, Clinical, and Population Studies: Essentials for Investigators. Endocr. Rev. 2018, 39, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S. Shotgun DNA sequencing using cloned DNase I-generated fragments. Nucleic Acids Res. 1981, 9, 3015–3027. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Abubucker, S.; Segata, N.; Goll, J.; Schubert, A.M.; Izard, J.; Cantarel, B.L.; Rodriguez-Mueller, B.; Zucker, J.; Thiagarajan, M.; Henrissat, B.; et al. Metabolic Reconstruction for Metagenomic Data and Its Application to the Human Microbiome. Eisen JA, editor. PLoS Comput. Biol. 2012, 8, e1002358. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Franzosa, E.A.; McIver, L.J.; Rahnavard, G.; Thompson, L.R.; Schirmer, M.; Weingart, G.; Lipson, K.S.; Knight, R.; Caporaso, J.G.; Segata, N.; et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat. Methods 2018, 15, 962–968. [Google Scholar] [CrossRef]
- Langille, M.G.I. Exploring Linkages between Taxonomic and Functional Profiles of the Human Microbiome. mSystems 2018, 3, e00163-17. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Ge, W.-R.; Zhang, S.; Sun, Y.-L.; Wang, B.; Yang, G. Case-Control Study of the Effects of Gut Microbiota Composition on Neurotransmitter Metabolic Pathways in Children with Attention Deficit Hyperactivity Disorder. Front. Neurosci. 2020, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, A.C.; Franklin, C.L. Manipulating the Gut Microbiota: Methods and Challenges: Figure 1. ILAR J. 2015, 56, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S. Pharmocologic Treatment of Attention Deficit-Hyepractivity Disorder. N. Engl. J. Med. 2020, 383, 1050–1056. [Google Scholar] [CrossRef]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol. Autism. 2016, 7, 37. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).