Abstract
This study aims to develop a modified starch with menthol (M) or sulfobetaine (S) using 1,6-hexamethyl diisocyanate (HMDI) as a linker to create biodegradable antibacterial materials for active packaging applications. The modification of potato starch is performed in a two-step reaction. First, the starch modifiers are synthesized through an equimolar reaction between HMDI and menthol or the sulfobetaine precursor. Next, the synthesized HMDI derivative is dissolved in a bio-based solvent (methyl-THF) with starch and K2CO3 (1:1 weight ratio) to chemically modify the starch. The chemical and thermal properties of the modified starch are analyzed. Starch films containing 25 wt.% glycerol and low amounts (0.5, 1, and 3% wt.) of M- or S-modified starch were successfully produced by extrusion. Although most film properties remain similar to the control, adding 3% of S-modified starch resulted in a 149% increase in Elastic Modulus and a 29% decrease in water vapor permeability. Additionally, just 0.5 wt.% of either M- or S-modified starch effectively inhibits S. aureus growth, indicating its potential as a bioactive compound for active packaging.
1. Introduction
Starch is a plentiful, biocompatible, biodegradable, and non-toxic polymer that naturally functions as the main storage polysaccharide in higher plants and serves as an appealing raw material for creating biodegradable plastics [1]. Native starches are primarily used in the paper and food industries as binding and thickening agents. However, their direct use as plastic substitutes is limited because starch-based polymers tend to be water-sensitive and have poor mechanical properties [2]. Therefore, the chemical modification of starch has emerged as an effective strategy to improve its functional and physical performance.
Starch is relatively easy to chemically modify, typically through hydroxyl substitutions at the C2, C3, and/or C6 positions, which alter the structures of amylose and amylopectin [3]. These modifications are generally achieved via derivatization (such as esterification, etherification, or cross-linking), degradation (acidic or enzymatic hydrolysis, or oxidation), or physical treatments like heat and moisture [4]. Adjusting the type, extent, and degree of modification can give a wide range of physicochemical properties. In particular, the characteristics of chemically derivatized starches are strongly influenced by the degree of substitution (DS), as well as the length and nature of the introduced substituent. For instance, when the substituent is an aliphatic chain, increasing the DS and chain length enhances the hydrophobicity of the starch [5]. Modification of starch through coupling reactions can significantly expand its application field, including food processing, packaging, pharmaceuticals, nutrient delivery, and environmental protection [6,7].
Among the reagents used for starch modification, diisocyanates stand out for their reactivity, which enables efficient coupling reactions under mild conditions [8]. Notably, 1,6-hexamethyl diisocyanate (HMDI) has a flexible structure with an aliphatic chain of six methylene groups. Previous studies have reported HMDI-modified polysaccharides via one-pot click reactions for environmental remediation [9], food packaging [10], and polymer functionalization [5]. The primary limitation lies in the high reactivity of isocyanates with moisture in starch or with the plasticizer, which reduces the overall efficiency of the process [11].
Recently, interest has grown in incorporating natural bioactive compounds into starch matrices. Menthol, a natural compound, is widely utilized in the medical and pharmaceutical fields for its diverse biological effects, including analgesic, antifungal, antipruritic, antibacterial, anticancer, and anti-inflammatory activities [12]. However, its practical applications and shelf-life are significantly limited by its extremely low water solubility, high volatility, low boiling point, and thermal instability, which make it prone to evaporation and degradation [13]. Therefore, most of menthol’s active packaging applications generally involve encapsulation to enable a controlled release over time, enhancing food shelf-life and minimizing limitations associated with direct application [14].
In parallel, zwitterionic materials have attracted attention for their exceptional biocompatibility, stemming from their biomimetic chemical structures, making them widely used in biomedical fields as efficient wound-dressing materials. Zwitterionic compounds exhibit excellent anti-fouling properties, as their opposing charges create a high hydration layer, thereby overcoming non-specific protein adsorptions and maintaining an overall neutral charge [15]. Among them, sulfobetaine is a compound with an anionic sulfonate group and a cationic trimethyl ammonium group, which is highly attractive for biomaterials due to its ease of synthesis, purification, and biomechanical properties [16]. Yi et al. [17] prepared monochloride-triazine sulfobetaine–chitosan films that exhibited excellent water absorption, degradability, and no cytotoxicity, and showed good bactericidal activity against S. Aureus and E. coli, making them a promising material. Similarly, sulfobetaine’s excellent biocompatibility, biodegradability, and strong resistance to nonspecific protein adsorption make it ideal for drug delivery [18].
Finally, economic and social pressures to adopt greener solvents are driving the use of bio-based alternatives. One such example is 2-methyltetrahydrofuran (methyl-THF), a biomass-derived cyclic ether. Owing to its volatility and solvent properties, methyl-THF has emerged as a sustainable substitute for conventional petrochemical solvents in chemical processes, such as THF or dichloromethane [19].
This work aims to develop starch-based materials with antimicrobial properties for future packaging applications. To the best of our knowledge, this is the first reported chemical modification of starch using sulfobetaine and menthol, using HMDI as a connecting agent and methyl-THF as a bio-based solvent. The modified starches were characterized by H-NMR, FTIR, TGA, and DSC—as well, the effects of varying the amount of M-/S-modified starch (0.5%, 1%, and 3%) added to native starch film properties were studied. Small incremental levels of modified starch were incorporated to enhance the active properties of the films, while minimizing disruption of the matrix. The characterization of the films included physicochemical, thermal, antimicrobial, and compostability properties. These active films have the potential to expand the shelf-life of packaged food while advancing the use of starch-based materials in the packaging sector.
2. Materials and Methods
2.1. Material
Commercial potato starch (approx. 21% amylose) was kindly provided by Lyckeby (Kristianstad, Sweden). Sulfobetaine (CAS 4727-41-7), Menthol (CAS 2216-51-5), Glycerol (purity ≥ 99%, CAS 56-81-5), hexamethyl diisocyanate (HMDI, CAS 822-06-0), 2-dimethylaminoethanol (CAS 108-01-0) and methyl-THF (CAS 96-47-9) were purchased from Sigma Aldrich (St. Louis, MO, USA). Potassium carbonate (CAS 584-08-7) was purchased from PanReac AppliChem (Barcelona, Spain).
2.2. Starch Functionalization
Potato starch was functionalized with an antibacterial agent (menthol or sulfobetaine) via urethane using HMDI as a linker, as seen in Figure 1. Firstly, a mixture of menthol 1.3 g (SM1) or 2-dimethylaminoethanol 0.8 g (SS1) and HMDI (1:1 weight) was mixed and heated to 65 °C for 10 h in a nitrogen atmosphere, yielding a waxy white product. The derived isocyanate was prepared according to the method described earlier [20] with slight modifications. The waxy product was then dissolved in 5 mL of methyl-THF and added to a mixture of starch powder and K2CO3 (1:1 weight) that had been previously dispersed in 10 mL of methyl-THF by sonication for 30 min in a nitrogen atmosphere. The mixture was heated to 65 °C and vigorously stirred for 48 h. Finally, the reaction mixture was washed first with methyl-THF and then with water, removing the unbound compound by filtration. The white residue was dried at 70 °C for 15 h, obtaining the product as a white powder (SM1 or SS1). In order to obtain the sulfobetaine modified starch, SS1 was mixed with 1.3 g of propanesultone and dispersed in 15 mL EtOH. The mixture was stirred for 48 h at 40 °C. Then, the reaction mixture was filtered, washed repeatedly with EtOH, and dried at 70 °C for 15 h, obtaining the product as a white powder (SS2).
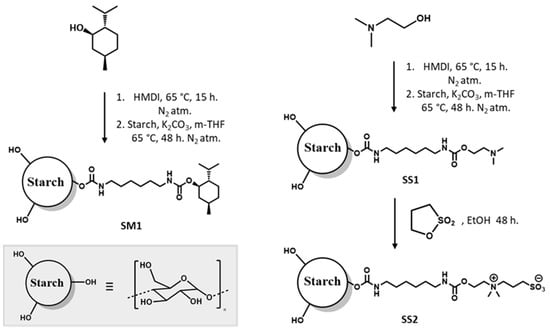
Figure 1.
Synthesis of menthol- and sulfobetaine-modified starch (SM1 and SS2, respectively).
2.3. Film Preparation
Samples were prepared in a lab micro-compounder Xplore MC15 HT (Xplore Instruments BV, Sittard, The Netherlands, 15 cm3 capacity). Potato starch with the corresponding (0–3 wt.%) amount of menthol-/sulfobetaine-modified starch (75 wt.% total) was manually mixed with 25 wt.% glycerol until a homogeneous mixture was achieved. The mixture was left overnight. The following day, the mixture was introduced into the extruder, which operated in recirculation mode to allow repeated processing and homogenization of the melt for 3 min. The co-rotating screw speed was set at 200 rpm, and the operating temperature was 110 °C. The resulting extrudate filament was pelletized. The pellets were introduced into the extruder operating in open mode, 110 °C and 100 rpm. Film extrusion was carried out using a 60 × 1 mm flat-sheet die attached directly to the extruder die. A total of 7 formulations were prepared: control film (P25), menthol-modified (M0.5, M1 and M3), and sulfobetaine-modified (S0.5, S1 and S3).
2.4. Starch Characterization & Equipment
The chemical structure of the samples was characterized by Fourier-transform infrared (FTIR) spectroscopy using a Spectrum One spectrometer (Perkin Elmer Instruments, Madrid, Spain). Measurements were performed in the range of 4000–400 cm−1 with a resolution of 4 cm−1, averaging four scans per sample, and employing an attenuated total reflectance (ATR) accessory with a diamond/ZnSe crystal.
1H NMR (400 MHz) spectra were recorded on a Bruker 400 MHz spectrometer in DMSO-d6. Chemical shifts (δ) are reported in parts per million (ppm) relative to tetramethylsilane, even though the signal of residual DMSO protons at 2.50 ppm was employed as an internal reference.
Thermal stability of native starch, modified potato starch, and starch-based films was assessed using thermogravimetric analysis (TGA, Q500, TA Instruments, Waters, Madrid, Spain). Approximately 10 mg of each sample was heated from 30 to 800 °C under a nitrogen atmosphere at a rate of 10 °C·min−1. The maximum degradation temperature (Tmax) was determined from the first derivative of the TGA curves. Differential scanning calorimetry (DSC) was performed with a DSC822e instrument (Mettler Toledo, Barcelona, Spain). Samples (~2 mg) were sealed in aluminum pans and heated between 25 and 100 °C at 10 °C·min−1 under nitrogen flow.
2.5. Film Characterization & Equipment
The color properties of the films were measured in a UV-VIS spectrophotometer equipped with an integrating sphere. Optical properties L* (100 = white to 0 = black), a* (− green to + red), and b* (− blue to + yellow) were expressed as average and standard deviation of three readings from aleatory points of the sample. The total color differences (ΔE) of the films brought by the addition of M-/S- modified starch were calculated as the variance from color coordinates between sample films and control, following:
where ΔL*, Δa*, and Δb* are the differences between the color of the samples and the reference film. The transparency of the films in terms of opacity was determined as the transmittance measured at a wavelength of 600 nm.
The surface morphology of the films was examined using field emission scanning electron microscopy (FESEM, Hitachi S8000, Hitachi Co., Tokyo, Japan) operated at an accelerating voltage of 1 kV and a working distance of 8 mm. Prior to imaging, the samples were cryo-fractured and sputter-coated with an Au–Pd alloy (80:20).
Mechanical performance was characterized with an Instron Universal Testing Machine (Barcelona, Spain). Tensile tests were performed on at least 5 dog-bone-shaped specimens per sample (2 mm width, 20 mm gauge length) at a crosshead speed of 10 mm·min−1. Stress–strain curves were used to determine Young’s modulus, tensile strength, and elongation at break.
Dynamic mechanical thermal analysis (DMTA) was conducted on a DMA Mettler-Toledo (Ohio, OH, USA) in clamp tension mode. Analyses were conducted at five frequencies simultaneously (0.5, 1, 3, 10 and 20 Hz) with a constant amplitude of 15 µm and a normal force of ±2 N. The temperature sweep was done between −80 °C and 130 °C at a heating rate of 2 °C min−1. The rectangular samples had approximate dimensions of 15.0, 5.0 and 0.5 mm in length, width, and thickness, respectively. As a result, the dynamic storage modulus (E′), loss modulus (E′′) and loss factor (tan δ) curves are reported as a temperature function.
The apparent Activation Energy (Ea) was calculated following the Arrhenius relationship (time–temperature superposition principles) by superimposing the tan δ peaks determined over the range of frequencies used [21]. The temperature dependence of the frequency of excitation can be expressed as:
Reformulations of this formula allow plotting of the natural logarithm of frequency (ln f) against the reciprocal of the temperature (1/T) given by the chosen peak maxima. Ea can be obtained as the product of the gradient of the resulting straight line and the value of the Universal Gas Constant (R = 8.314 × 10−3 kJ mol−1 K−1):
The water vapor permeability (WVP) of the samples was assessed by calculating the water vapor transmission rate (WVTR) by gravimetry based on the ASTM E96-00 procedure [22]. Circular pieces of the film were placed as lids of the permeability cups, with an exposed surface area (A) of 10 cm2. Silica gel (2 g per cup) was used as a desiccant to create a dry atmosphere (0% RH) inside the cups. The cups were placed in a desiccator at 23 ± 1 °C with a saturated potassium nitrate (KNO3) solution (75% RH) and weighed hourly for 8 h. WVTR was calculated using:
where mt represents the mass of the cup at time t, m0 is the initial mass of the cup, and A denotes the exposed film area. WVP was determined using the following equation:
where S is the saturation vapor pressure of water at 23 °C, R1 and R2 correspond to the relative humidity in the desiccator and inside the cup, respectively, and ε is the film thickness. Samples were tested in triplicate.
The antimicrobial effectiveness of the developed films was assessed based on the Japan Industrial Standard Z 2801 [23], with slight modifications. In brief, Staphylococcus aureus (Gram-positive, CECT 240, ATCC 6538P) was sourced from the Spanish Type Culture Collection (Valencia, Spain) and used due to its importance in food packaging applications. Film samples of 3 cm × 3 cm were used, inoculated with 200 µL of bacterial suspension, and covered with 2 cm × 2 cm polypropylene films to ensure uniform contact. The inoculated films were incubated at 36 °C for 24 h, after which the bacteria were recovered using 40 mL of neutralizing solution, followed by serial dilution and plating on PCA for enumeration.
where N is the number of colony-forming units counted per dilution, D is the dilution factor, V is the volume of solution used to recover the bacteria, and A is the contact area between the film and the bacteria. The value of R represents the logarithmic difference in colony-forming units per square centimeter (CFU/cm2) between untreated and treated samples, and it was calculated according to the following equation:
where Uₜ is the average number of viable bacteria recovered from untreated control films after 24 h, and Aₜ is the average number recovered from the treated films under the same conditions. The detection limit of the assay corresponded to a maximum measurable antimicrobial activity of R = 3, based on a control recovery of log Ut = 5.3 (CFU/cm2). Consequently, reductions exceeding 3-log (99.9%) could not be confirmed due to methodological constraints. Samples were tested in triplicate.
The disintegration of the films was assessed under laboratory-scale industrial composting conditions in accordance with ISO 20200 guidelines [24]. Film specimens (20 × 20 mm) were enclosed in mosquito nets and buried within a perforated plastic container filled with 1 kg of artificial compost. The mesh allowed intimate contact between the samples and the compost while facilitating the retrieval of partially degraded films. The artificial compost was prepared by manual mixing of approximately 40% sawdust, 30% rabbit feed, 10% mature compost, 10% corn starch, 5% sugar, 4% corn oil, and 1% urea (45 wt.% of the total mass). The remaining 55 wt. % of the compost soil consists of deionized water. The box was kept under aerobic conditions at 58 °C in an oven for 3 months. The degraded film samples were unburied after 1, 3, 7, 14, 28, 60 and 90 days, and dried in an oven at 37 °C for 24 h. Photographs of the recovered samples were taken for visual comparison. The disintegration degree of the sample was estimated as the variation in mass (m) after (t) days in contact with the compost soil:
2.6. Statistical Analysis
One-way analysis of variance (ANOVA) was performed to evaluate the effects of M- or S-modified starch concentration (0, 0.5, 1, and 3%) on the mechanical and WVP properties of the films. The control film (P25) was used as the baseline (0%) for both reinforcement types, which were analyzed separately. When the ANOVA showed statistically significant effects (p < 0.05), Tukey’s HSD post hoc test was applied to identify specific group differences. All statistical analyses were conducted using Origin 2023, and the results are reported as mean ± standard deviation.
3. Results
3.1. Functionalized-Starch Characterization
Yields for SM1 and SS2 were estimated at 76–80%, considering the amount of starch as starting material. 1H NMR spectra of commercial potato starch (red), the modified starch with menthol SM1 (green-left), and the modified starch with sulfobetaine SS2 (green-right) are shown in Figure 2. Also, spectra of menthol and the hydroxyl-sulfobetaine were added (purple-left and -right, respectively) as references. By modifying starch with a urethane group, the H6 protons shift to a higher ppm (5.70 ppm). Additionally, a peak appears at 6.95 ppm, typical of the urethane amine group, as well as signals corresponding to the HMDI methylenes at 2.95, 1.30, and 1.20 ppm. In the case of SM1, the peaks of the methylene groups from menthol appear between 2.0 and 0.6 ppm, and the CH-OH proton shifts from 3.15 to 4.40 ppm upon formation of the urethane group. For SS2, an intense peak appears at 3.10 ppm corresponding to the methyl groups of the ammonium group of the sulfobetaine, and the CH2-OH protons shift from 3.80 to 4.35 ppm upon urethane formation. In both cases, a 15% mol functionalization degree is obtained, normalizing to 1 the 1H band (5.25–5.00 ppm). The assignment of the proton signals in the chemical structure of native, SM1 and SM2 starches can be found in Supplementary Materials Figure S1.
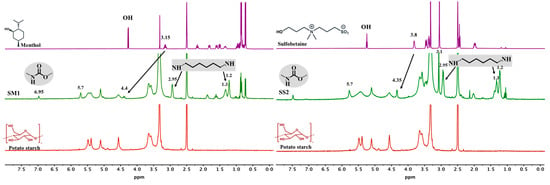
Figure 2.
1HNMR spectra of commercial potato starch (red), menthol (purple-left) and sulfobetaine (purple-right), and the modified starch with menthol SM1 (green-left), and with sulfobetaine SS2 (green-right).
The FTIR analysis is shown in Figure 3, where the potato starch (black) spectrum exhibits a wide band in the 3000–3600 cm−1 range that corresponds to the stretching vibration of the hydroxyl group. The vibration bands at 2923 cm−1, 1412 cm−1 and 1242 cm−1 correspond to the carbon-hydrogen (C-H), carbon-carbon (C-C) and the deformation of the methyl group (C-H) bonds of the methylene groups, respectively. The absorption bands at 1362 cm−1 and 1465 cm−1 are attributed to the plane O-H bending vibration in the glucose unit and 931 cm−1 to the O-H out-of-plane bending vibration [25]. Other characteristic bands were observed at 1640 cm−1, indicating the presence of bonded water, and 1145, 1075 and 996 cm−1 were associated with the stretching vibration of the C-O bond, C-O-H and C-O-C groups in the anhydrous glucose ring, respectively. Additionally, in the infrared spectra of the modified starch (SM1—red, and SS2—green), a band corresponding to the carbonyl groups due to the urethane motifs (NH-C=O) appears at 1688 cm–1, while the band corresponding to their amine groups (N-H) appears at 3325 cm–1. In addition, the presence of the sulfobetaine group is noted in the SS2 spectrum by a sharp band at 1035 cm–1, characteristic of the SO3- motifs.
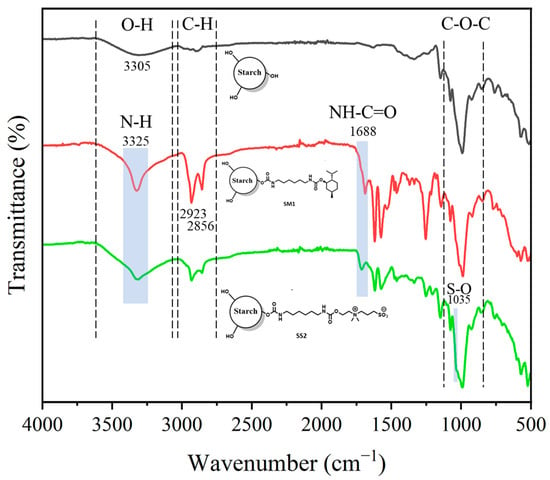
Figure 3.
FTIR spectrum of potato starch (black), sulfobetaine-modified starch SS2 (red) and menthol-modified starch SM1 (green).
TGA of the native and modified starches was carried out to assess their thermal properties (Figure 4—left). The TGA curves show three stages for the native starch and four for the modified ones. The initial weight reduction observed between 60 and 110 °C corresponds to the evaporation of water and the release of low-molecular-weight compounds, accounting for approximately 5% of the total mass. The major mass loss occurring in the range of 220–300 °C is attributed to the thermal decomposition of carbohydrates, primarily amylose and amylopectin (approx. 60–70% of weight). Above 300 °C, the native starch undergoes carbonization decomposition, which culminates in the formation of carbon black, leaving a final residue of 18–19%. The thermal degradation profile of the native starch matches the TGA data reported by other authors [26]. The modified starches exhibit an earlier onset of weight loss, starting around 240 °C, which is expected due to the introduction of thermally less stable urethane linkages and organic molecules, which begin to degrade earlier than the native starch backbone. HMDI, menthol, or sulfobetaine all undergo carbonization above 420 °C, resulting in SM1 and SS2 having similar residues to native starch (17–19%).
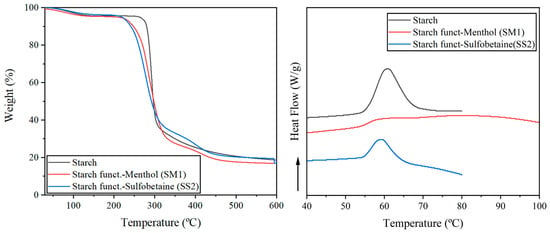
Figure 4.
TGA (left) and DSC (right) analysis of commercial potato starch (black), starch funct.-Menthol SM1 (red) and starch funct.-Sulfobetaine SS2 (blue).
Starch suffers gelatinization in excess water, in which an irreversible reaction occurs, destabilizing the hydrogen bonds of the amorphous regions, leading to the loss of their crystallinity and modification. The DSC analysis (Figure 4—right) showed a gelatinization curve of the native potato starch between 55–70 °C, with a maximum peak centered at 60.7 °C. Similarly, SS2, starch modified with sulfobetaine, showed a gelatinization process between 54–67 °C, centered at 59.0 °C. Choque-Quispe et al. [27] describe a similar behavior for another variety of potato starch: an initial temperature of 54.1 °C, a final temperature of 73.0 °C and a gelatinization peak of 61.8 °C. SS1, sulfobetaine modified starch, presented a comparable gelatinization profile with the peak centered at 59.0 °C. However, SM1, starch modified with menthol, exhibited a broad gelatinization process between 54–100 °C, which may be explained by the hydrophobic nature of the starch carrying menthol motifs may explain.
3.2. Film Characterization
The aspect of packaging can influence consumer decisions, making high transparency in food packaging films generally desired. The color parameters (L*, a*, b*, ∆E, YI, and WI) were therefore measured, and the results for the films are presented in Supplementary Materials Table S1 and Figure 5. As expected, the control TPS film (P25) yielded a colorless and transparent film. The incorporation of chemically modified starch slightly improved the optical properties of the films when compared to the control. All modified samples exhibited slightly higher lightness (L*) values, indicating a brighter appearance. The a* and b* coordinates remained near neutral, with modified films showing a slight reduction in b*, suggesting decreased yellowness. This trend was further reinforced by the reduced yellowness index (YI), particularly in the M1 sample (0.6) compared to the control (1.5). The whiteness index (WI) increased in all modified films, reaching a maximum in M1 (90.2), indicating improved visual brightness. Color difference values (ΔE) ranged from 0.9 to 1.9, with M1 again showing the most notable shift, though still within the range of only noticeable by an experienced observer (ΔE < 2).

Figure 5.
Visual comparison of extruded films.
All the films exhibited high transmittance (88–91%) across the visible region of the spectrum, spanning from 400 to 800 nm, as shown in Supplementary Materials Figure S1. Overall, the addition of M- or S-modified starch, especially at 1%, enhanced the optical properties of the films by marginally increasing lightness and whiteness while slightly diminishing yellow tones.
The cryo-fractured cross-sections of all formulations were examined by SEM, as shown in Figure 6. The control film displays a uniform morphology, indicating that the plasticizer, along with heat and shear stress during extrusion, effectively broke down the starch granules. Similarly, SEM micrographs of the other films confirm that they are homogeneous and that the M- and S-modified starches are evenly dispersed within the TPS matrix, even at 3% wt. (Figure 6b–g).
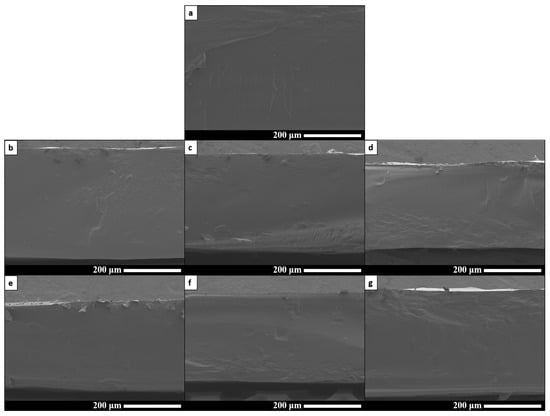
Figure 6.
FE-SEM micrographs of: (a) P25, (b) M0.5, (c) M1, (d) M3, (e) S0.5, (f) S1 and (g) S3.
The FTIR spectra of the samples presented the characteristic signal bands of TPS consisting of: hydroxyl (O-H) stretching (3500–3100 cm−1), C–H stretching from alkyl groups (2930 cm−1and 2890 cm−1), C–O and C–C stretching (1150 cm−1 and 1075 cm−1, respectively), and C–O–H bending (1100–900 cm−1) [28]. The major difference is evident in the S-modified films, where a signal at 1730 cm−1, likely associated with the previously identified carbonyl groups at 1688 cm–1 in SS2, gradually increases with the SS2 concentration, as shown in Figure 7.
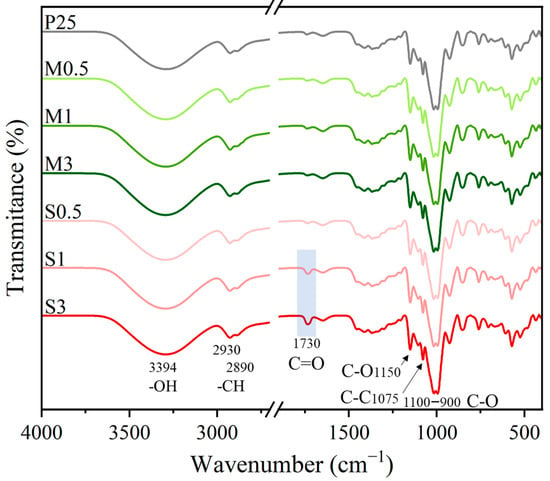
Figure 7.
FTIR spectra of films reinforced with menthol- and sulfobetaine-modified starch.
The thermal stability of the films was assessed using TGA under a nitrogen atmosphere. The main thermal parameters for this analysis are listed in Table 1, whereas the TGA and DTGA curves are presented in Supplementary Materials Figure S2. The thermal degradation of all the films happens in the same three stages. The first step (T < 250 °C) is a consequence of the release of adsorbed water and glycerol evaporation. The main stage takes place between 280–330 °C, which is associated with the degradation of starch and the hydrogen-bonded glycerol. The final step (T > 400 °C) results from the carbonization of the remaining mass.

Table 1.
Thermogravimetric analysis of the films.
The addition of modified starch increases the thermal stability of films as compared to the control film. Among all formulations, M1 exhibits the greatest improvement in thermal stability, with a T10% of 225.4 °C, which is an increase of over 40 °C compared to the control film. This could be due to a decrease in absorbed water in the film due to the hydrophobic nature of the M-modified starch. Tmax values are marginally higher for all modified films compared to P25, indicating a slight delay in the main decomposition phase, particularly in M-modified samples. Most samples exhibit comparable mass residue at 600 °C, slightly under 10% of the remaining mass. The lower residue values compared with the previous native and modified starch TGA patterns (17–19% mass remaining) are expected as a result of the addition of the 25% glycerol content, which completely degrades, and the evaporation of absorbed water.
The X-ray diffraction (XRD) patterns of all the extruded formulations are shown in Supplementary Materials Figure S3. All the films presented a broad amorphous halo with a main diffraction peak at 2θ = 19.5° and a small peak at 13.5°. This shape is typical of TPS, a semi-crystalline polymer with low crystallinity. This low crystallinity is associated with a smooth surface morphology, as confirmed by the FESEM analysis. Furthermore, films exhibiting amorphous structural characteristics tend to be flexible and easily processable, which is deemed suitable for potential application in food packaging [29].
The incorporation of chemically modified starch had a significant impact on the mechanical properties of the films compared to the control (P25), as seen in Table 2 and Supplementary Materials Figure S4. M-modified samples (M1 and M3) showed moderate increases in strength while preserving elongation, offering a balanced mechanical profile. However, these changes in Elastic Modulus and Tensile Strength were not significant (p < 0.05) according to the ANOVA test. On the other hand, films containing S-modified starch, particularly at 3% (S3), exhibited a marked increase in elastic modulus (225.3 MPa) and maximum tensile strength (7.7 MPa), indicating a reinforcement effect on the TPS matrix. Similarly, elongation at break decreased with higher concentrations of modified starch, with S3 showing the lowest elongation (51.6%).

Table 2.
Mechanical and barrier properties of the films.
DMTA measurements can provide information on the relaxation processes in viscoelastic materials. The evolution with temperature of the storage modulus (E′), along with the loss modulus (E”) and the loss factor (tan δ) at 10 Hz, is shown in Figure 8. Meanwhile, Supplementary Materials Tables S2–S6 gather the key thermomechanical information derived from these graphs. In general terms, the thermomechanical properties of all the films were quite common and similar to those obtained in other studies. As expected, the alteration in the vibrational frequency from 0.5 Hz to 20 Hz in the tan δ curve causes a rise in the temperature and amplitude of the relaxation peaks at lower and higher temperature regions (Tβ and Tα), as seen in Supplementary Materials Figure S5.
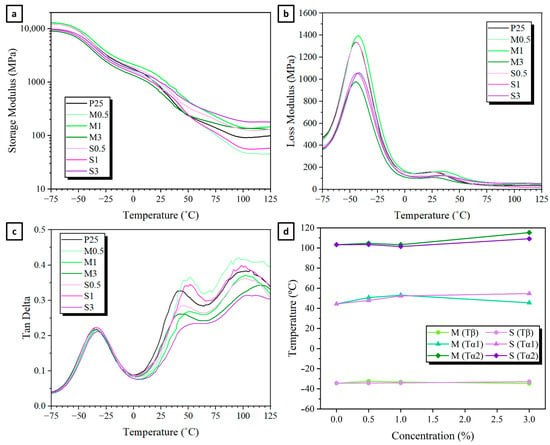
Figure 8.
(a) Storage modulus, (b) Loss modulus, and (c) Tan δ at 10 Hz of M-/S- modified starch-based films. (d) Influence of M-/S-modified starch on Tβ, Tα1 and Tα2 at 10 Hz of films.
The storage modulus (E′) reflects the maximum energy stored elastically in a material. In the low-temperature range (T < −60 °C), where the material remains in a glassy state, molecular mobility is highly restricted, resulting in minimal variation in E′. As the temperature increases, the storage modulus decreases significantly in a two-step process. The initial drop, observed at −50 °C, is attributed to the molecular mobility transition within the glycerol-rich phase. A second, more pronounced decline begins around 20 °C, corresponding to changes in the molecular dynamics of the starch-rich phase. At room temperature, E′ values of the extruded films are in the 500–1000 MPa range, as seen in Supplementary Materials Table S2, with the incorporation of modified starch generally increasing storage modulus. Above 105 °C, E′ of the films exhibits a small increase, attributed to the evaporation of water [30].
The temperatures at which the tan δ maxima are observed are related to the glass transitions or secondary transitions in the studied material. TPS systems typically exhibit a phase separation, due to the non-uniform distribution of plasticizer molecules in the film, resulting in the formation of plasticizer-rich and starch-rich domains [31]. All samples presented at least two distinct maxima in the loss modulus and tan δ, a narrow and high-intensity peak centered at −40 °C (Tβ), corresponding to the glycerol-rich phase, and a broad peak between 25–125 °C (Tα), corresponding to the starch-rich phase. Tβ generally occurs below 0 °C, owing to the low molecular weight and inherently low Tg temperature of plasticizers. In this phase, the mobility of starch chains is primarily dictated by localized motions of small molecular segments, primarily involving water and glycerol [32]. Since glycerol concentration is constant in all formulations (25 wt. %), incorporation of M-/S-modified starch results in minor variations, indicating that local molecular mobility remains largely unaffected by the modifications, as seen in Figure 8d.
In contrast, Tα (starch-rich phase) is influenced by the degree of interaction between starch chains and the plasticizer, as well as the water content, which facilitates chain mobility [33]. Tα typically occurs above room temperature and, in highly amorphous starches rich in amylopectin, may be observed at temperatures exceeding 100 °C [34]. When studying the 30–120 °C region DMTA data of the films, the presence of two types of relaxation processes inside the starch-rich domains can be seen. Other researchers have assigned these peaks to the linear amylose and branched amylopectin chains that form starch [35]. The lower shoulder in the 35–55 °C region (Tα1) has been allocated to amylose, due to its minor molecular weight, while the 98–110 °C peak (Tα2) is linked to starch-rich phase containing remnants of semicrystalline regions and/or amylose domains [36]. In the majority of the modified-starch films, Tα1 shifts to higher temperatures. This is probably a result of deterring in the segmental motions of the modified starch molecules, as seen in Figure 8d and Supplementary Materials Table S3. Interestingly, S3 presents the average biggest Tα1 increase (11.4 °C) and M3 the smallest (1.3 °C). The analysis of Tα2 was more challenging due to the amplified variability of tan δ values and decreased accuracy in identifying Tα peak positions, which is associated with the low storage and loss modulus values in this temperature range. Consequently, the regression coefficients (R2) of the lines used to determine the activation energy become lower as temperature increases (Tα2 < Tα1 < Tβ), as seen in Supplementary Materials Tables S3–S5. When comparing formulations, Tα2 values remain somewhat similar at 0.5 and 1 wt. % of modified starch, but increased at 3%, especially M3 with an average 9.7 °C increase, as seen in Supplementary Materials Table S5. The increase in Tα values of M3 and S3 could be related to the hydrophobic behavior of modified starches, promoting a phase separation, as previously mentioned.
The apparent activation energy (Ea) is calculated supposing that the relaxation processes follow Arrhenius behavior, as shown in Supplementary Materials Table S6. Ea values in the Tβ region range between 135–144 kJ/mol, while in the Tα1 region, they vary between 270–395 kJ/mol and 263–949 kJ/mol for Tα2, reflecting typical relaxation behavior of plasticized starch matrices. The control film (P25) exhibits similar values to the Tβ and Tα1 (139.2 and 234.2 kJ·mol−1, respectively) reported by Sreekumar et al. [37]. Pronounced differences are observed in the Tα1 and Tα2 transitions, which are associated with segmental motions and long-range macromolecular rearrangements, respectively. In Tα2 relaxation, the control film demonstrates a high activation energy of 949.0 kJ·mol−1, this value decreases substantially in most modified films. The most pronounced reduction is observed in the M3 and S3 formulations (263.4 and 417.9 kJ·mol−1), suggesting a substantial disruption in long-range molecular interactions and decreased thermal stability.
Water barrier properties are of immense relevance in packaging films intended to reduce or at least mitigate moisture transfer between the food and the surrounding environment, to preserve their quality and extend storage times. WVP results are shown in Table 2. Formulations containing 0.5 and 1% M- or S-modified starch are not statistically different from the control film (p < 0.05), as values remain similar. However, WVP was reduced in films with higher concentrations of modified starch, particularly in M3 (0.092 g·m/d·m2·kPa) and S3 (0.082 g·m/d·m2·kPa), indicating an improvement in the barrier properties. Probably the hydrophobic nature of the modified starch resulted in poor miscibility with native starch during extrusion, creating a tortuous path.
The antimicrobial activity of the extruded starch-based films was tested against the Gram-positive Bacterium S. Aureus, following the Japanese Industrial Standard JIS Z 2801:2000 [38]. S. Aureus was prioritized in this work due to its robust peptidoglycan structure and its sensitivity profile, which make it a suitable first indicator of antimicrobial activity [39]. According to the Japanese standard, a material demonstrates effective antimicrobial properties when the reduction in bacterial concentration is equal to or higher than 2 log units (R ≥ 2), corresponding to a reduction of at least 99% in viable microorganisms relative to an unmodified control surface.
In the case of menthol, the antimicrobial mechanism described in the literature is primarily associated with disruption of the bacterial membrane [40]. Sulfobetaine, on the other hand, has been reported to exhibit antifouling behavior, acting through resistance to microbial colonization by creating a non-adherent surface and potentially through contact-based interactions [41]. Given that the experimental setup in this study involved direct surface contact with the extruded films, it is important to highlight that the observed activity reflects the performance of the materials under conditions relevant to surface-associated applications.
The control film (P25) showed an average bacterial recovery of log CFU/cm2 = 5.3 ± 0.2. Based on the detection threshold of the plating methodology used, the minimum quantifiable bacterial concentration was established at log 2.3. Consequently, samples from which no bacterial colonies were recovered (i.e., S0.5, S1, and M0.5) are reported with R values exceeding this threshold (R > 3.0), acknowledging that their true antibacterial activity may surpass the quantification limit of the assay.
The log CFU/cm2 values and corresponding R values for each tested sample are presented in Figure 9. Samples S0.5, S1, M0.5, and M1 exhibited potent antibacterial activity, with R values ranging from 2.6 to greater than 3.0, thereby achieving reductions of 99.7% or more in bacterial load. These findings confirm effective antimicrobial behavior in accordance with JIS Z 2801 criteria. In contrast, samples S3 and M3, each containing the highest concentration of additive (3%), demonstrated negligible antimicrobial effects, with R values of 0.6 and 0.1, respectively. These reductions are substantially below the defined efficacy threshold and are statistically comparable to the control film. This observation may be attributed to a decrease in additive dispersion or compatibility within the film matrix at elevated concentrations. The hydrophobic nature of the modified starch, as evidenced by WVP data, may have contributed to phase separation or reduced contact with microbial fluids, ultimately limiting its antimicrobial effectiveness [42]. Future studies will include Gram-negative strains such as E. Coli to broaden the antimicrobial spectrum assessment.
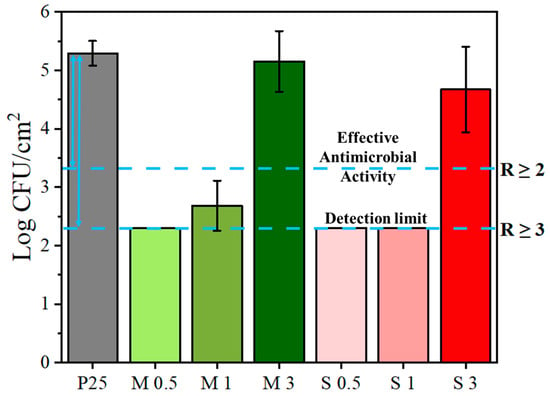
Figure 9.
Antimicrobial activity of modified starch films against S. Aureus, expressed as log CFU/cm2 and log reduction (R).
The biodegradable nature of TPS-based materials is one of the most outstanding qualities for packaging applications. The disintegrability of the films was assessed through the disintegration test following the ISO 20200 standard [24]. The test replicates, under lab-simulated circumstances, the heat (58 °C) and humidity (about 55% RH) typical of a commercial composting plant’s thermophilic phase. Figure 10 shows the disintegration profile of all the studied samples in terms of mass loss, whereas Figure 11 shows the visual evolution of the samples during the test. During the process, the films exhibit a sponge-like behavior, absorbing moisture from the compost and adhering sawdust to their surface. Moreover, the color of the films gradually changes from transparent to dark brown after prolonged contact with the dark compost soil.
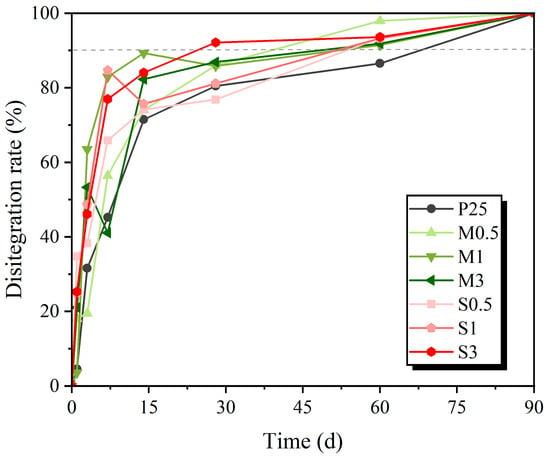
Figure 10.
Disintegration rate of the films.
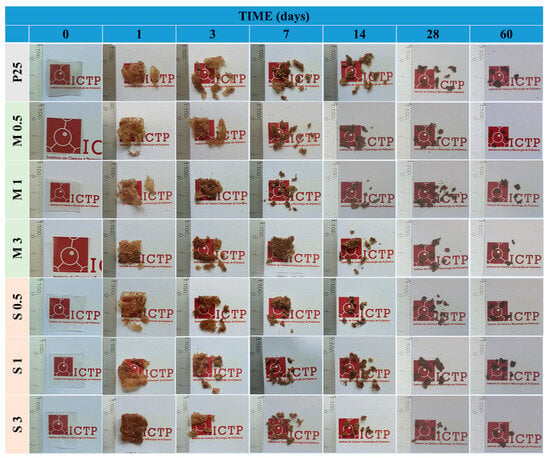
Figure 11.
Visual appearance of films during the disintegration test.
All compositions exhibit a similar disintegration pattern. Within the first 24 h, the films show initial fragmentation accompanied by a clear brown coloration. By day 3, most films break into 3–5 major fragments, and by day 7, they disintegrate into more than 10 smaller fragments. By day 14, the films are completely fragmented, with no visible resemblance to their original square shape. At this stage, the disintegration rate slows significantly, and the rate of mass loss decreases gradually beyond day 14. By day 60, all modified films reach a disintegration rate of 90%, which meets the commonly accepted criteria for compostability. Based on the mass loss values over time, the incorporation of modified starch slightly accelerates the disintegration process, as illustrated in Figure 10.
4. Discussion
A comparative evaluation of the key properties of the films was conducted to assess their overall suitability for food packaging applications, as shown in Figure 12. To allow both visual and quantitative comparison among the different formulations, all measured parameters were normalized to a 0–1 scale and displayed in a radar plot. For properties where higher values indicate better performance (such as mechanical strength or antioxidant activity), direct normalization was used, while water vapor permeability (WVP) was inversely scaled, since lower values signify improved barrier effectiveness.
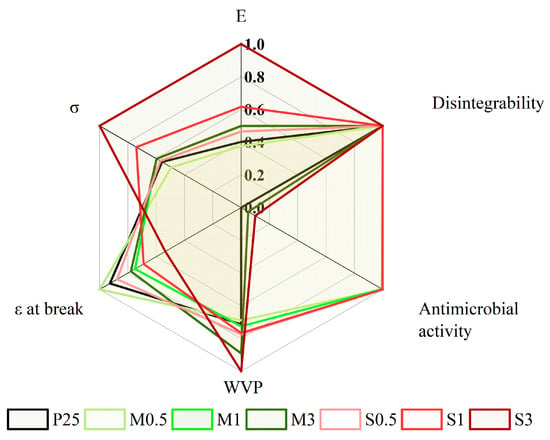
Figure 12.
Effect of the formulation on film properties.
At low concentrations of SM1 and SS2, starch films M0.5, M1, and S0.5 showed mechanical and barrier properties similar to those of the control film (P25), indicating that the polymeric matrix remained largely unaffected by the addition of small amounts of modified starch. However, these films demonstrated a significant antimicrobial effect, confirming the effectiveness of the menthol and sulfobetaine functionalities even at minimal loadings. In contrast, at higher concentrations, a greater impact on the film structure was observed, especially for the SS2-based samples. Films S1 and S3 exhibited higher E and σ values, along with a decrease in ε at break, suggesting stiffening. Additionally, M3 and S3 films showed reduced WVP and no detectable antimicrobial activity, likely because of the encapsulation or limited migration of the active hydrophobic compounds within the hydrophilic starch network. The biodegradability of all films remained unchanged, indicating that the chemical modification did not hinder the degradation of the starch matrix.
The proposed approach provides a straightforward and versatile method for the functionalization of polysaccharides with nucleophile-bearing bioactive compounds. The reaction proceeds under mild conditions, is readily scalable, and does not require chromatographic purification steps, making it suitable for the sustainable production of functional starch derivatives. The successful linking of menthol and sulfobetaine onto the starch backbone through one-pot reactions demonstrates the applicability of this strategy for introducing diverse functionalities into biodegradable matrices.
As a possible limitation, the modified starches showed poor miscibility in water, mainly due to the disruption of intermolecular interactions and the hydrophobic nature of menthol. Additionally, some crosslinking may occur during urethane formation, which could also reduce the solubility of the modified samples in water. Further optimization of reaction conditions and substituent ratios might improve control over the degree of substitution and help retain desirable processing properties.
5. Conclusions
Starch was chemically modified with menthol (M) or sulfobetaine (S) using 1,6-hexamethyl diisocyanate (HMDI) as a linker in a two-step reaction and a bio-based solvent (Methyl-THF). NMR and FTIR spectra confirmed the modification, which in both cases reached a 15% mol functionalization degree. The modified starches present a high hydrophobic behavior, especially M-modified starch, and a lower thermal stability. Films consisting of potato starch with 25 wt. % glycerol and different amounts of menthol M- or S-modified starch were prepared by extrusion. The incorporation improves the thermal stability without altering the visual properties of the films. The mechanical properties demonstrate that both types of chemical modification can enhance the performance of starch-based films, with 3% S-modified starch promoting the greatest mechanical reinforcement with an increase in elastic modulus from 90.6 MPa to 225.3 MPa. Similarly, this composition also presented the greatest increase in moisture resistance with a 29% decrease in WVP, followed by 3% Menthol with a 20% decrease. Films with 0.5% and 1% M- or S-modified starch present very high antimicrobial activity against S. Aureus. These results support the growing interest in developing sustainable bio-based products with active packaging that can reduce the dependence on fossil fuels.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polysaccharides6040103/s1, Figure S1: NMR spectra; Figure S2: UV-vis light transmission spectra of the films; Figure S3: TGA of the modified potato starch-based films; Figure S4: XRD of the modified potato starch-based films; Figure S5: Mechanical Properties of the films; Figure S6: Tan delta of films at 0.5, 1, 5, 10 and 20 Hz; Table S1: Optical properties of the films; Table S2: Storage Modulus of the films at room temperature and 1 Hz; Table S3: Tβ of the modified potato starch-based films; Table S4: Tα1 of the modified potato starch-based films; Table S5: Tα2 of the modified potato starch-based films; Table S6: Activation Energy of the modified potato starch-based films.
Author Contributions
Conceptualization, A.d.P., D.L. and L.P.; methodology, P.F.M.-G. and A.d.P.; software, P.F.M.-G.; validation, A.d.P., L.P. and D.L.; investigation, A.d.P., P.F.M.-G. and A.A.-G.; re-sources, D.L. and L.P.; writing—original draft preparation, P.F.M.-G. and A.d.P.; writing—review and editing, A.A.-G., A.d.P., D.L. and L.P.; supervision, D.L. and L.P.; project administration, D.L. and L.P., funding acquisition, D.L. and L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agencia Estatal de Investigación (AEI, MICINN, Spain), Fondo Europeo de Desarrollo Regional (FEDER, EU), NextGenerationEU MRR TED2021-129335B-C21, and MCIN/AEI/10.13039/501100011033/ by “ERDF A way of making Europe” for the PID2021-123753NB-C31 project.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANOVA | Analysis of variance |
| CFU | Colony-forming units |
| DMTA | Dynamic mechanical thermal analysis |
| DS | Degree of substitution |
| DSC | Differential Scanning Calorimetry |
| Ea | Apparent activation energy |
| EtOH | Ethanol |
| E. Coli | Escherichia coli |
| FESEM | Field emission scanning electron microscope |
| FTIR | Fourier-transform infrared spectroscopy |
| HMDI | Hexamethyl diisocyanate |
| M0.5/M1/M3 | Films reinforced with menthol-functionalized starch |
| Methyl-THF | 2-Methyltetrahydrofuran |
| NMR | Nuclear magnetic resonance |
| P25 | Control film |
| S0.5/S1/S3 | Films reinforced with sulfobetaine-functionalized starch |
| S. Aureus | Staphylococcus Aureus |
| SM | Functionalized starch with menthol |
| SS | Functionalized starch with sulfobetaine |
| THF | Tetrahydrofuran |
| TGA | Thermogravimetric analysis |
| TPS | Thermoplastic starch |
| WI | Whiteness index |
| WVP | Water vapor permeability |
| WVTR | Water vapor transmission rate |
| YI | Yellow index |
References
- Villar, M.A.; Barobsa, S.E.; García, M.A.; Castillo, L.A.; López, O.V. Starch-Based Materials in Food Packaging: Processing, Characterization and Applications; Academic Press: Cambridge, MA, USA, 2017; Volume 4, ISBN 978/0/12/809439/6/0128094397. [Google Scholar]
- Llanos, J.H.R.; Tadini, C.C. Preparation and Characterization of Bio-Nanocomposite Films Based on Cassava Starch or Chitosan, Reinforced with Montmorillonite or Bamboo Nanofibers. Int. J. Biol. Macromol. 2018, 107, 371–382. [Google Scholar] [CrossRef]
- Garcia, M.A.V.T.; Garcia, C.F.; Faraco, A.A.G. Pharmaceutical and Biomedical Applications of Native and Modified Starch: A Review. Starch-Stärke 2020, 72, 1900270. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Sharma, L.; Kaur, M. Effect of Granule Size on Physicochemical, Morphological, Thermal and Pasting Properties of Native and 2-Octenyl-1-Ylsuccinylated Potato Starch Prepared by Dry Heating under Different PH Conditions. LWT 2015, 61, 224–230. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Spychaj, T. Chemical Modification of Starch with Hexamethylene Diisocyanate Derivatives. Carbohydr. Polym. 2007, 70, 334–340. [Google Scholar] [CrossRef]
- Castro, L.M.G.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M. Impact of High Pressure on Starch Properties: A Review. Food Hydrocoll. 2020, 106, 105877. [Google Scholar] [CrossRef]
- Cao, Y.; Ding, S. Starch Functionalization and Coupling Reaction. J. Food Technol. Food Chem. 2022, 4, 1–24. [Google Scholar]
- Valodkar, M.; Thakore, S. Isocyanate Crosslinked Reactive Starch Nanoparticles for Thermo-Responsive Conducting Applications. Carbohydr. Res. 2010, 345, 2354–2360. [Google Scholar] [CrossRef]
- Okoli, C.P.; Adewuyi, G.O.; Zhang, Q.; Zhu, G.; Wang, C.; Guo, Q. Aqueous Scavenging of Polycyclic Aromatic Hydrocarbons Using Epichlorohydrin, 1,6-Hexamethylene Diisocyanate and 4,4-Methylene Diphenyl Diisocyanate Modified Starch: Pollution Remediation Approach. Arab. J. Chem. 2019, 12, 2760–2773. [Google Scholar] [CrossRef]
- Tabaght, F.E.; El Idrissi, A.; Aqil, M.; Benahemad, A.; El Barkany, S.; Bellaouchi, R.; Asehraou, A. Synthesis and Characterization of (Thio)Carbamates Based on Cellulose and Cellulose Acetate: Biodegradation and Solubility Studies. Cellul. Chem. Technol. 2020, 54, 207–223. [Google Scholar] [CrossRef]
- Schwach, E.; Six, J.L.; Avérous, L. Biodegradable Blends Based on Starch and Poly(Lactic Acid): Comparison of Different Strategies and Estimate of Compatibilization. J. Polym. Environ. 2008, 16, 286–297. [Google Scholar] [CrossRef]
- Abualhasan, M.N.; Zaid, A.N.; Jaradat, N.; Mousa, A. GC Method Validation for the Analysis of Menthol in Suppository Pharmaceutical Dosage Form. Int. J. Anal. Chem. 2017, 2017, 1728414. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, S.; Liang, R.; Ni, X.; Du, Y.; Wang, J.; Yang, C. Design and Characterization of Starch/Solid Lipids Hybrid Microcapsules and Their Thermal Stability with Menthol. Food Hydrocoll. 2021, 116, 106631. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, S.; Das, D. Sugarcane Bagasse Fiber Reinforced Active Starch Biocomposite Films with Menthol Encapsulated Powder for Fresh-Cut Fruit Packaging. Food Bioprocess Technol. 2025, 18, 6191–6212. [Google Scholar] [CrossRef]
- Dong, D.; Hao, T.; Wang, C.; Zhang, Y.; Qin, Z.; Yang, B.; Fang, W.; Ye, L.; Yao, F.; Li, J. Zwitterionic Starch-Based Hydrogel for the Expansion and “Stemness” Maintenance of Brown Adipose Derived Stem Cells. Biomaterials 2018, 157, 149–160. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, M.; Zhou, N.; Wu, F.; Sun, B.; Shen, J. Synthesis and Characterization of a Novel Antibacterial Material Containing Poly(Sulfobetaine) Using Reverse Atom Transfer Radical Polymerization. RSC Adv. 2018, 8, 33000–33009. [Google Scholar] [CrossRef]
- Yin, M.; Wang, Y.; Ren, X.; Huang, T.S. Development of a Biodegradable, Cytocompatible, Antibacterial, and Biofilm-Controlling Chitosan Sulfobetaine Derivative Film as a Biological Material. Engineering 2024, 35, 95–103. [Google Scholar] [CrossRef]
- Zhao, Z.; Ding, R.; Wang, Y.; Yuan, R.; Zhang, H.; Li, T.; Zheng, W.; Chen, E.; Wang, A.; Shi, Y. Sulfobetaine Modification of Poly (D, L-Lactide-Co-Glycolic Acid) Nanoparticles Enhances Mucus Permeability and Improves Bioavailability of Orally Delivered Liraglutide. J. Drug Deliv. Sci. Technol. 2024, 93, 105437. [Google Scholar] [CrossRef]
- Englezou, G.; Kortsen, K.; Pacheco, A.A.C.; Cavanagh, R.; Lentz, J.C.; Krumins, E.; Sanders-Velez, C.; Howdle, S.M.; Nedoma, A.J.; Taresco, V. 2-Methyltetrahydrofuran (2-MeTHF) as a Versatile Green Solvent for the Synthesis of Amphiphilic Copolymers via ROP, FRP, and RAFT Tandem Polymerizations. J. Polym. Sci. 2020, 58, 1571–1581. [Google Scholar] [CrossRef]
- Biyani, M.V.; Weder, C.; Foster, E.J. Photoswitchable Nanocomposites Made from Coumarin-Functionalized Cellulose Nanocrystals. Polym. Chem. 2014, 5, 5501–5508. [Google Scholar] [CrossRef]
- Karbhari, V.M.; Wang, Q. Multi-Frequency Dynamic Mechanical Thermal Analysis of Moisture Uptake in E-Glass/Vinylester Composites. Compos. Part B Eng. 2004, 35, 299–304. [Google Scholar] [CrossRef]
- ASTM E96-00; Standard Test Methods for Water Vapor Transmission of Materials. ASTM: West Conshohocken, PA, USA, 2002.
- Aragón-Gutiérrez, A.; Rosa, E.; Gallur, M.; López, D.; Hernández-Muñoz, P.; Gavara, R. Melt-Processed Bioactive Evoh Films Incorporated with Ferulic Acid. Polymers 2021, 13, 68. [Google Scholar] [CrossRef]
- ISO 20200:2015; Plastics—Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test. ISO. International Organization for Standardization: Geneva, Switzerland, 2015; pp. 1–8.
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.A.; Fontes, R.L.S.; Fontes-Sant’Ana, G.C.; Calado, V.; López, E.O.; Rocha-Leão, M.H.M. Extraction, Modification, and Chemical, Thermal and Morphological Characterization of Starch From the Agro-Industrial Residue of Mango (Mangifera Indica L) Var. Ubá. Starch/Staerke 2019, 71, 1800023. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Obregón Gonzales, F.H.; Carranza-Oropeza, M.V.; Solano-Reynoso, A.M.; Ligarda-Samanez, C.A.; Palomino-Ríncón, W.; Choque-Quispe, K.; Torres-Calla, M.J. Physicochemical and Technofunctional Properties of High Andean Native Potato Starch. J. Agric. Food Res. 2024, 15, 100955. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Famá, L.; Candal, R.; Goyanes, S. Bioactive Starch Nanocomposite Films with Antioxidant Activity and Enhanced Mechanical Properties Obtained by Extrusion Followed by Thermo-Compression. Food Hydrocoll. 2019, 96, 518–528. [Google Scholar] [CrossRef]
- Charles, A.L.; Motsa, N.; Abdillah, A.A. A Comprehensive Characterization of Biodegradable Edible Films Based on Potato Peel Starch Plasticized with Glycerol. Polymers 2022, 14, 3462. [Google Scholar] [CrossRef]
- Shi, R.; Liu, Q.; Ding, T.; Han, Y.; Zhang, L.; Chen, D.; Tian, W. Ageing of Soft Thermoplastic Starch with High Glycerol Content. J. Appl. Polym. Sci. 2007, 103, 574–586. [Google Scholar] [CrossRef]
- Averous, L.; Boquillon, N. Biocomposites Based on Plasticized Starch: Thermal and Mechanical Behaviours. Carbohydr. Polym. 2004, 56, 111–122. [Google Scholar] [CrossRef]
- Wang, N.; Yu, J.; Han, C. Influence of Citric Acid on the Properties of Glycerol-Plasticised Cornstarch Extrusion Blends. Polym. Polym. Compos. 2007, 15, 545–552. [Google Scholar] [CrossRef]
- Baran, A.; Vrábel, P.; Kovaľaková, M.; Hutníková, M.; Fričová, O.; Olčák, D. Effects of Sorbitol and Formamide Plasticizers on Molecular Motion in Corn Starch Studied Using NMR and DMTA. J. Appl. Polym. Sci. 2020, 137, 48964. [Google Scholar] [CrossRef]
- Pulido Díaz, A.; Lourdin, D.; Della Valle, G.; Fernández Quintero, A.; Ceballos, H.; Tran, T.; Dufour, D. Thermomechanical Characterization of an Amylose-Free Starch Extracted from Cassava (Manihot Esculenta, Crantz). Carbohydr. Polym. 2017, 157, 1777–1784. [Google Scholar] [CrossRef]
- Saparová, S.; Ondriš, L.; Kovaľaková, M.; Fričová, O.; Peidayesh, H.; Baran, A.; Hutníková, M.; Chodák, I. Effects of Glycerol Content on Structure and Molecular Motion in Thermoplastic Starch-Based Nanocomposites during Long Storage. Int. J. Biol. Macromol. 2023, 253, 126911. [Google Scholar] [CrossRef] [PubMed]
- Rana, L.; Kouka, S.; Gajdosova, V.; Strachota, B.; Konefał, M.; Pokorny, V.; Pavlova, E.; Stary, Z.; Lukes, J.; Patocka, M.; et al. Thermoplastic Starch with Maltodextrin: Preparation, Morphology, Rheology, and Mechanical Properties. Materials 2024, 17, 5474. [Google Scholar] [CrossRef]
- Sreekumar, P.A.; Gopalakrishnan, P.; Leblanc, N.; Saiter, J.M. Effect of Glycerol and Short Sisal Fibers on the Viscoelastic Behavior of Wheat Flour Based Thermoplastic. Compos. Part A Appl. Sci. Manuf. 2010, 41, 991–996. [Google Scholar] [CrossRef]
- JIS Z 2801 2000; Antimicrobial Products—Test for Antimicrobial Activity and Efficac. Japanese Standards Association: Tokyo, Japan, 2000.
- Zhou, X.; Cegelski, L. Nutrient-Dependent Structural Changes in S. Aureus Peptidoglycan Revealed by Solid-State NMR Spectroscopy. Biochemistry 2012, 51, 8143–8153. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Lo Schiavo, S.; Gulino, A.; Fragalà, M.E.; Mineo, P.; Nicosia, A.; Ali, R.H.; Calorenni, P.; Ferlazzo, A.; Nicolò, M.S.; De Leo, F.; et al. A Sulfobetaine Containing-Polymethylmethacrylate Surface Coating as an Excellent Antifouling Agent against Chlorella Sp. Prog. Org. Coatings 2025, 199, 108940. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, P.; Zhang, Z.; Chen, C.; Wang, X. The Choice of Antimicrobial Polymers: Hydrophilic or Hydrophobic? Chinese Chem. Lett. 2024, 35, 109768. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).