1. Introduction
Against the backdrop of growing concern for environmental protection and the steadily increasing consumer demand for sustainable food packaging solutions, the eco-friendly food packaging industry has experienced significant development in recent years. Current trends in food packaging materials are guided by the principles of sustainability, with a strong emphasis on the use and development of packaging derived from renewable, recyclable, biodegradable, and/or compostable resources [
1]. The growing interest in eco-friendly alternatives has driven the packaging industry to explore innovative solutions. In this context, paper stands out as an ideal packaging material, offering multiple advantages over synthetic materials, such as lower production costs from renewable sources, superior recyclability, biodegradability, and compostability [
2].
Due to their numerous advantages: availability, renewability, versatility, non-toxicity, biocompatibility, and biodegradability, the utilization of biopolymers, either as standalone materials or as coating layers for paper, in the production of food packaging has attracted significant interest [
3].
Polysaccharides are among the most promising biopolymers for replacing petroleum-based polymers currently employed in the coating of food packaging paper. Due to their excellent film-forming ability and strong affinity for cellulosic substrates, they significantly enhance the barrier properties of paper against gases and volatile compounds, while also improving its mechanical strength. In addition, polysaccharides are biodegradable, non-toxic, and can act as functional matrices for incorporating bioactive compounds, thus conferring antimicrobial and/or antioxidant properties to the packaging material.
From a chemical perspective, polysaccharides are carbohydrate polymers composed of hundreds or even thousands of monosaccharide units interconnected through glycosidic bonds, which result from the condensation of monosaccharide residues via hemiacetal or hemiketal linkages. These biopolymers can originate from a wide range of sources, including higher photosynthetic plants, marine biomass, bacteria, and fungi [
4].
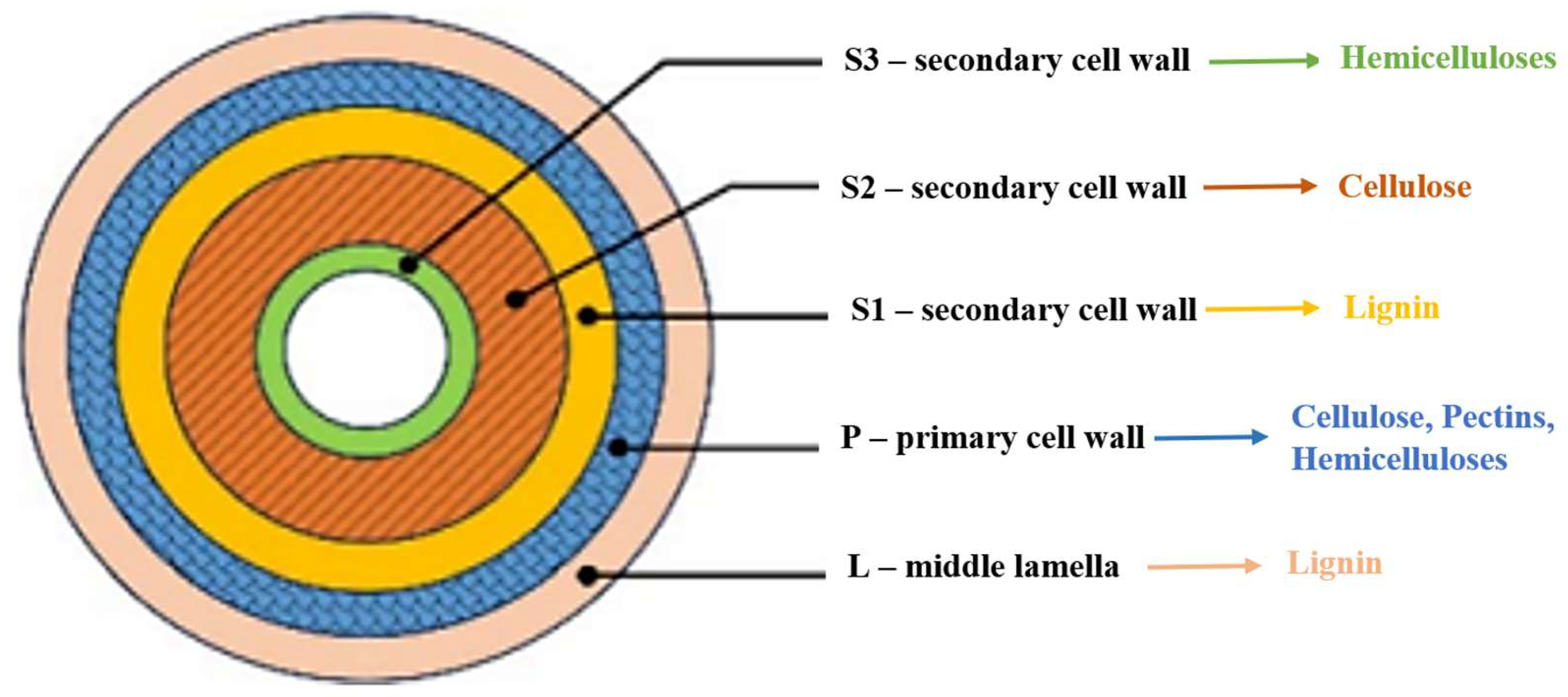
Hemicelluloses represent the second most abundant class of plant polysaccharides after cellulose and are essential components of the cell walls in lignocellulosic biomass (
Figure 1). They account for approximately 20–35% of the biomass composition, with the exact proportion varying depending on the plant source.
The use of hemicelluloses in industrial applications is limited by their highly hydrophilic nature, resulting from the extensive presence of hydroxyl groups in their structure. However, this same feature enables hemicelluloses to undergo various chemical modifications, such as oxidation, reduction, esterification (e.g., acetylation, quaternization), and etherification (e.g., carboxymethylation, alkylation) [
5,
6]. These modifications introduce hydrophobic groups into the hemicellulose structure, thereby enhancing their thermal stability, solubility in organic solvents, water resistance, and rheological properties, properties that are essential for their use in coating layers for packaging paper.
Moreover, the hydrophilic character of hemicelluloses can be adjusted through ionic interactions with other cationic, amphoteric, or amphiphilic biopolymers, such as chitosan or hemicellulose derivatives, forming complex polyelectrolyte systems with improved functional properties. Xylans represent the main hemicellulose component in hardwood, accounting for approximately 30% of the woody cell wall. They are also found in various annual plants, such as cotton and sugarcane, as well as in several agricultural by-products, including wheat and sorghum straw, corn stalks, and cobs. Additionally, xylans are present in secondary products derived from cellulose manufacturing, as well as in certain marine algae.
In 2021, the global xylan market was valued at USD 1.57 billion, with forecasts indicating an annual growth rate of approximately 6% between 2022 and 2029, reaching nearly USD 2.50 billion by the end of the forecast period [
7]. In the packaging industry, xylan is used as an additive in the production of plastic materials to enhance their mechanical strength and biodegradability [
8]. In the food packaging industry, xylan can be used in the development of edible films and coatings or as a component in coating formulations for food packaging paper, with the aim of enhancing barrier properties. The scientific literature indicates that most xylan applications in packaging focus on the production of edible films, while fewer studies address its use in paper-based food packaging. Research has shown that xylan-based films exhibit good oxygen and fat barrier properties, moderate antifungal activity, and a mild antibacterial effect against certain pathogenic bacteria (
E. coli,
S. aureus) [
9,
10]. However, films made from native xylan are brittle, moisture-sensitive, and exhibit low mechanical strength, which limits their use in the native form [
5]. The limitations of using xylan on a large scale for food packaging production are related, on one hand, to the availability of xylan hemicellulose in the raw materials market, and on the other hand, to the technical characteristics of native xylan hemicellulose in relation to the required properties of packaging materials intended for direct contact with food products.
The main limitation imposed by the technical characteristics of xylan-type hemicellulose lies in the fact that, in its native state, it cannot be used in high proportions for food packaging manufacturing due to its strongly hydrophilic nature, which results from the large number of hydroxyl groups present along the linear backbone and in the side chains of its structure [
11]. To reduce its hydrophilicity and improve the functional properties required for food packaging, structural modifications through various chemical reactions (etherification, esterification, oxidation, reductive amination, graft copolymerization, cross-linking, enzymatic modifications) are necessary [
12]. Xylan derivatives, obtained through such chemical modifications, exhibit enhanced hydrophobicity, mechanical strength, and barrier properties, making them more suitable for the development of food packaging films and coatings. Additionally, these biopolymers can form stable, flexible, and biodegradable films that better resist moisture and improve the shelf life of packaged food products. Consequently, research efforts have increasingly focused on optimizing the modification processes to tailor xylan-based materials for specific packaging applications while maintaining their environmental sustainability [
13].
Chitosan is a cationic polymer that, besides amino functional groups (-NH
2), contains numerous hydroxyl groups (-OH) capable of forming hydrogen bonds, allowing structural modification through various types of chemical reactions. Chitosan exhibits excellent film-forming ability, is biocompatible, non-toxic, biodegradable, and possesses antimicrobial properties effective against a wide range of microorganisms (bacteria, fungi). Therefore, its use as a coating layer on the surface of food packaging papers has been extensively investigated to develop coatings with gas and fat barrier properties, antimicrobial activity, as well as to produce smart packaging papers [
14,
15].
Chitosan is an important polymer for the food packaging industry due to its antimicrobial properties and film-forming abilities [
16]. Chitosan films exhibit selective gas permeability (to CO
2 and O
2), as well as good mechanical properties. The antifungal and antimicrobial activities of chitosan arise from its polycationic nature [
17]. The number of amino groups (-NH
2) present in chitosan increases with the degree of deacetylation (DD), which influences its antimicrobial activity. The bactericidal activity of chitosan is caused by the electrostatic interaction between the NH
3+ groups of chitosan and the phosphoryl groups of the phospholipid component of the cell membrane. The antibacterial and antifungal activity of chitosan depends on its molecular weight, degree of deacetylation, concentration, and the pH of the environment [
18]. Chitosan with a high degree of deacetylation is more effective at inhibiting bacterial growth than chitosan with lower degrees of deacetylation [
19].
Numerous studies have highlighted the potent antibacterial activity of zinc oxide nanoparticles (ZnO NPs) against
Salmonella aureus,
Bacillus atrophaeus, and
Escherichia coli spp. as well as their advantageous properties for food packaging applications [
20,
21,
22]. Research has consistently demonstrated that ZnO NPs exhibit minimal migration into food matrices, with concentrations well below the safety thresholds established for human consumption. In addition, their biocompatibility and reduced environmental persistence contribute to their appeal as sustainable alternatives for packaging materials [
23]. The integration of ZnO NPs into biopolymeric matrices to create bio-nanocomposites represents a promising strategy for the development of high-performance food packaging systems. Notably, ZnO NPs synthesized via green routes using plant extracts, such as
Capparis zeylanica,
Phoenix roebelenii,
Rubus fairholmianus, and
Amaranthus spinosus, have demonstrated remarkable antibacterial efficacy against both Gram-positive and Gram-negative bacteria. In some cases, these plant-derived ZnO NPs have exhibited superior antimicrobial performance compared to conventional antibiotics [
24]. The antimicrobial mechanism of ZnO NPs is primarily attributed to the release of Zn
2+ ions, which interact with bacterial membranes and generate reactive oxygen species—ROS, leading to oxidative stress. This stress causes damage to essential cellular components such as membrane proteins, DNA, and mitochondria, ultimately resulting in bacterial cell death [
24].
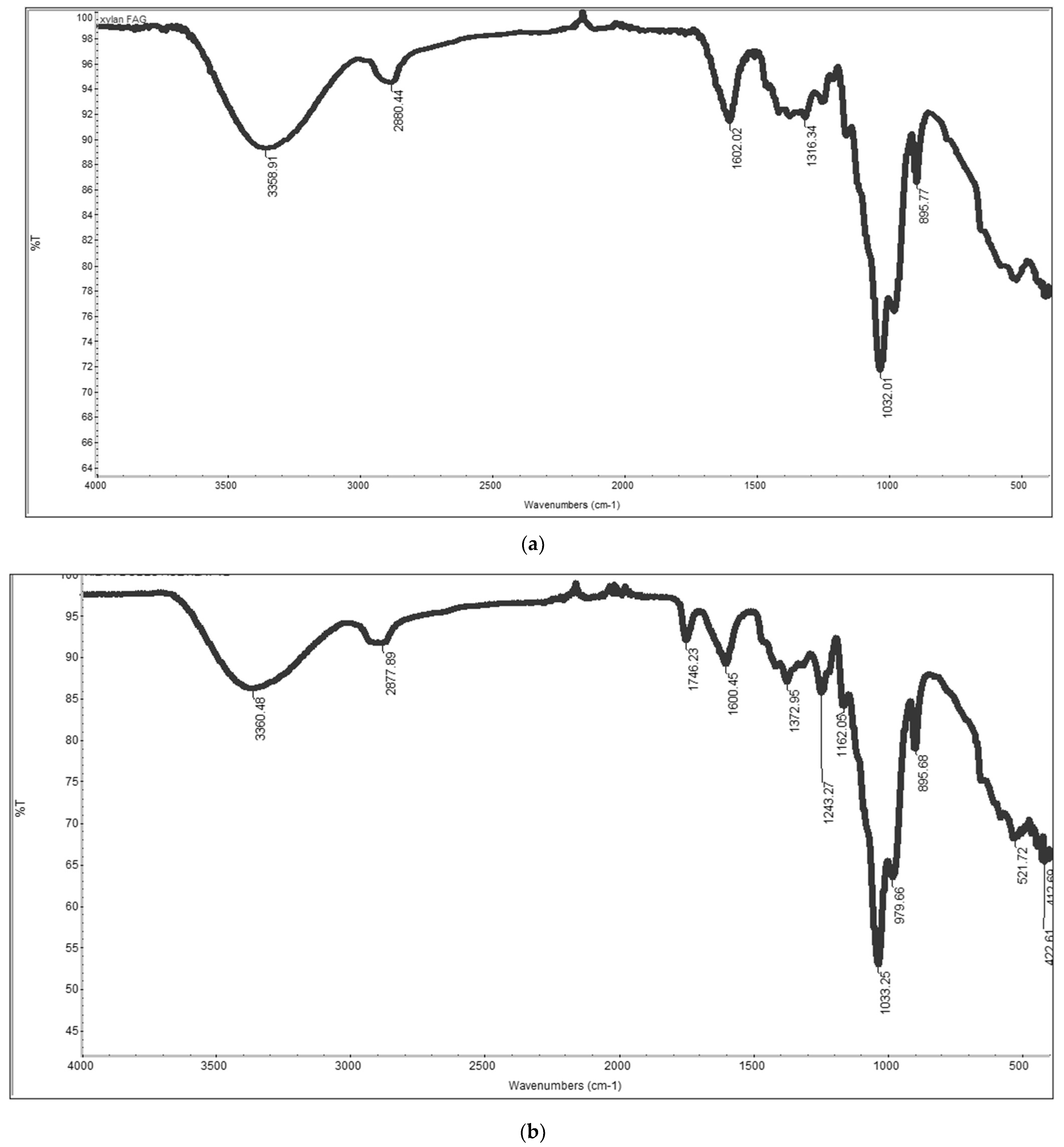
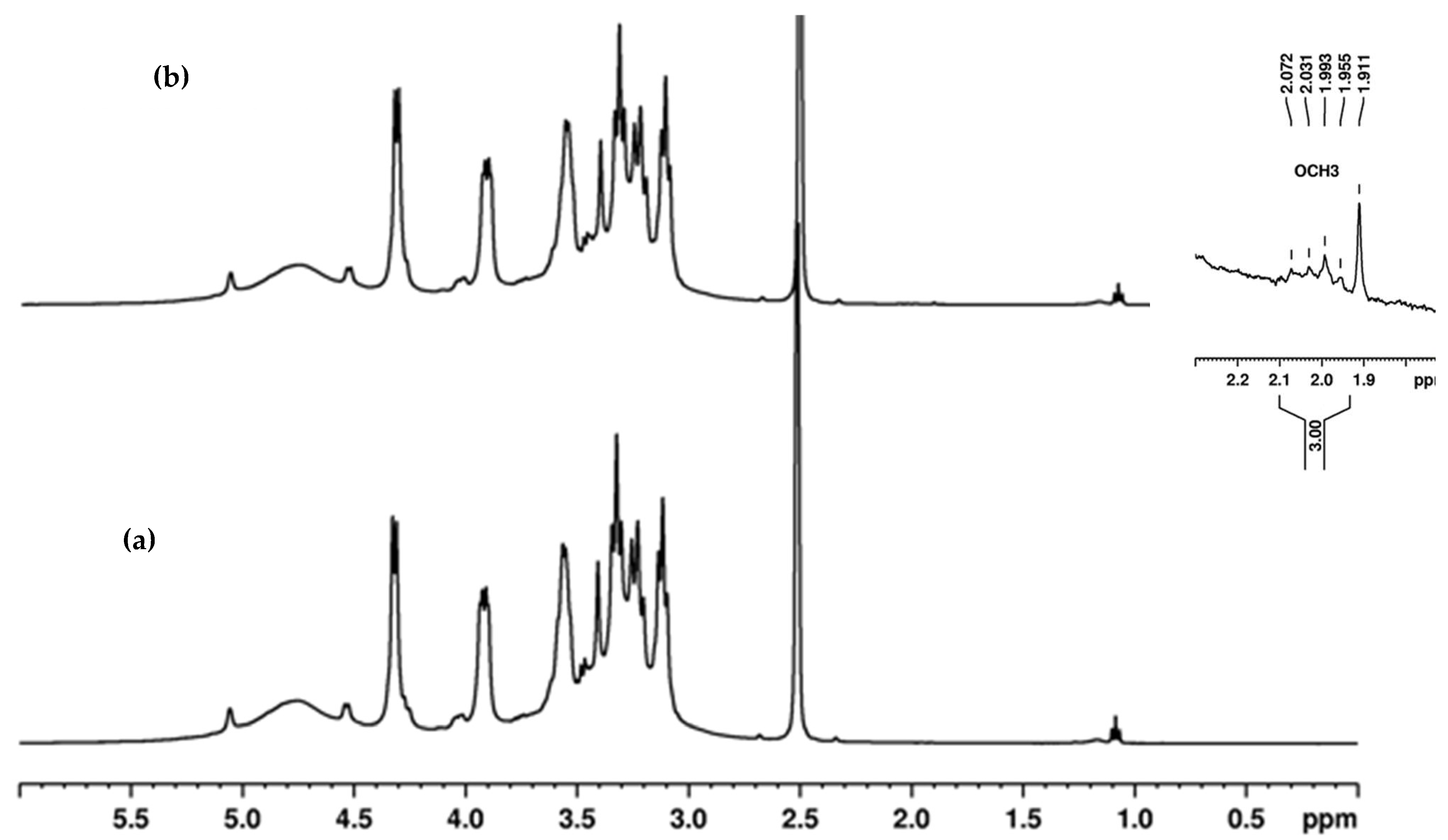
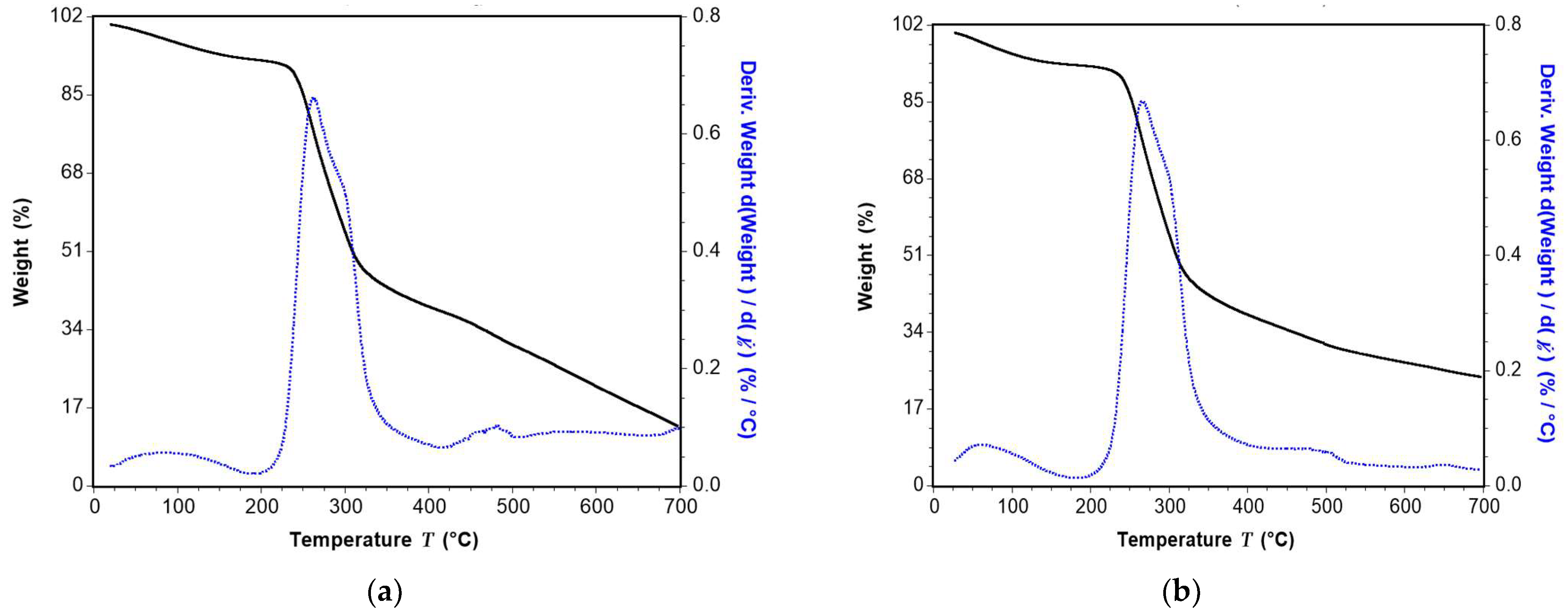
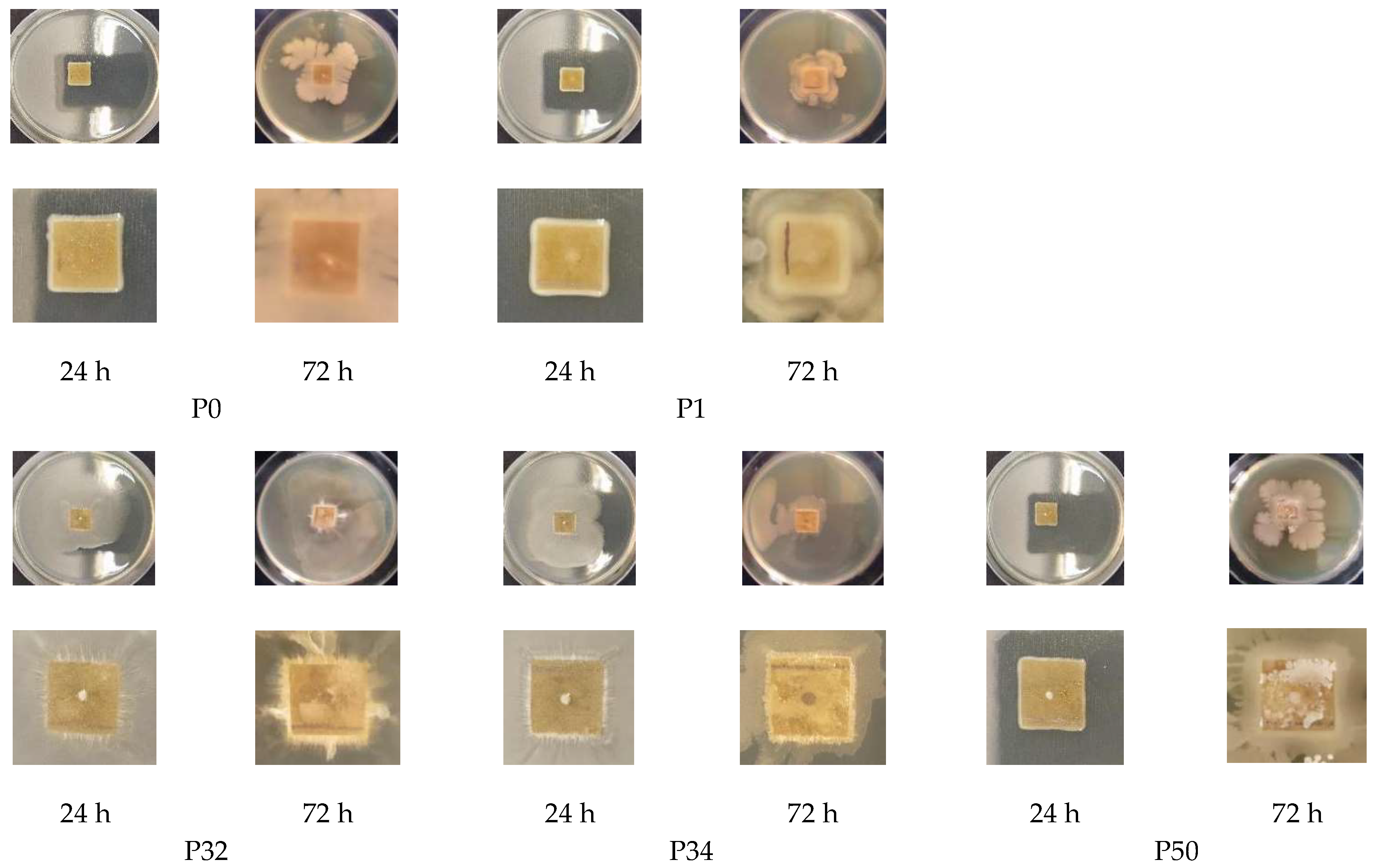
This study investigated the performance of acetylated xylan when used in composite coating formulations for food packaging paper. Acetylated xylan was synthesized in the laboratory, and colloidal dispersions containing acetylated xylan, chitosan, and zinc oxide nanoparticles (ZnONPs) were subsequently prepared. These dispersions were applied as single and double-layer coatings onto paper substrates intended for food packaging applications. Comprehensive analyses were conducted to characterize the structural and thermal stability of acetylated xylan using Fourier-transform infrared spectroscopy (FT-IR), proton nuclear magnetic resonance (1H-NMR), and thermogravimetric analysis (TGA). The functional properties of the coated papers were also evaluated, with particular focus on their mechanical strength and barrier performance against water, water vapor, oil, and grease, their resistance to microbial degradation, and their biodegradability, assessed through soil burial tests and quantification of CO2 evolution. For comparative purposes, packaging papers coated with native xylan-based dispersions, as well as uncoated papers, were included as control samples in the evaluation.