Fish Gelatin Edible Films with Prebiotics and Structuring Polysaccharides for Probiotic Delivery: Physicochemical Properties, Viability, and In Vitro Gastrointestinal Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Probiotic Suspension
2.3. Film-Forming Solution Formulation
2.4. Viability of Probiotic Bacteria
2.5. Film Characterization
2.5.1. Water Solubility and Water Vapor Permeability (WVP)
2.5.2. Thickness and Moisture Content
2.5.3. Water Contact Angle (WCA)
2.5.4. Tensile Strength (TS) and Elongation at Break (EB)
2.6. Differential Scanning Calorimetry (DSC)
2.7. In Vitro Gastrointestinal Digestion
2.8. Viability During Storage
2.9. Statistical Analysis
3. Results and Discussion
3.1. Cell Viability of the Film-Forming Solution and Films
3.2. Physical and Barrier Properties of the Films
3.3. Water Contact Angle (WCA)
3.4. Mechanical and Thermal Properties
3.5. Probiotic Viability During Simulated Gastrointestinal Digestion
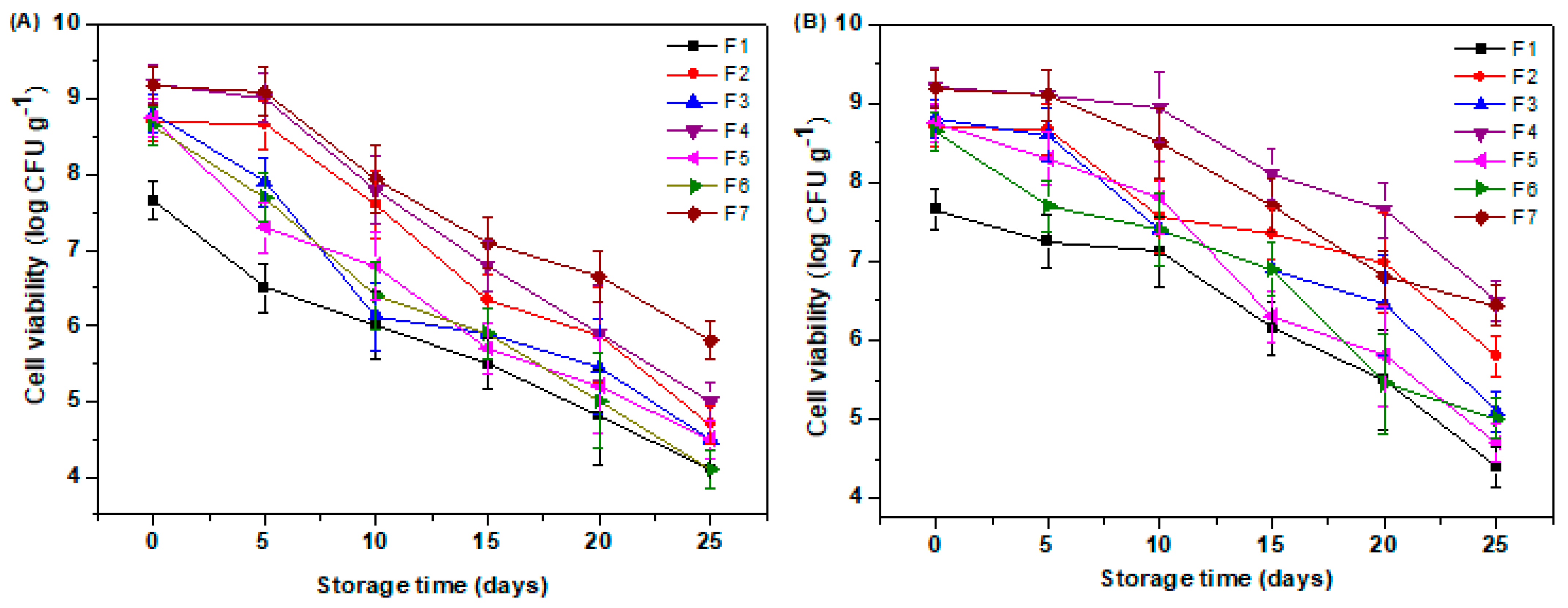
3.6. Cell Viability During Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huda, A.N.; Asfar, M.; Bastian, F.; Syarifuddin, A. Extraction of fish oil from Sardinella longiceps and pectin from Dillenia serrata fruit peel and theirs usage in gum Arabic edible film. Food Chem. Adv. 2025, 7, 101026. [Google Scholar] [CrossRef]
- Khalili, E.; Khaniki, G.J.; Shojaee-Aliabadi, S.; Shariatifar, N.; Aslani, R.; Mirmoghtadaie, L. Development and characterization of amaranth protein-based edible films incorporating Satureja khuzestanica essential oil. Appl. Food Res. 2025, 5, 100967. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; Albuquerque, J.C.; de Lima, T.L.B.; de Sousa, F.M.; de Alcântara Silva, V.M.; de Sousa, Á.B.B. Effect of Ultrasound and Freeze-Drying to Enhance the Extraction of Phenolic Compounds in Dragon Fruit Peels and Apply Them in Edible Starch-Based Films. Packag. Technol. Sci. 2024, 37, 901–916. [Google Scholar] [CrossRef]
- Jafari, M.; Afkhami, R.; Sedaghat, N. Preparation and characterization of active Cirish fructans–fish gelatin film: Physicochemical, antioxidant, and antimicrobial properties. Food Sci. Nutr. 2023, 11, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; Montero, P.; Fernández-Martín, F.; Gómez-Guillén, M.C. Physico-chemical and film-forming properties of bovine-hide and tuna-skin gelatin: A comparative study. J. Food Eng. 2009, 90, 480–486. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef]
- Sáenz, C.; Tapia, M.S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Jiménez, A.; Talens, P.; Chiralt, A. Properties of starch–hydroxypropyl methylcellulose based films obtained by compression molding. Carbohydr. Polym. 2014, 109, 155–165. [Google Scholar] [CrossRef]
- Lima, A.T.; Silva, H.A.; Rodrigues, T.J.A.; Santos, N.C.; Rocha, A.P.T.; de Araújo, G.T. Effect of chitosan concentration, glycerol and cashew peel extract on the physical, mechanical and morphological properties of starch-based films. Packag. Technol. Sci. 2024, 37, 1003–1015. [Google Scholar] [CrossRef]
- Costa, E.S.; dos Santos Moreira, I.; Gomes, J.P.; do Socorro Rocha Bastos, M.; Mattos, A.L.A.; Santos, N.C.; Matsui, K.N. Barrier, mechanical and antimicrobial properties of yam starch film with Cássia cinnamon essential oil and its application in strawberry storage. Packag. Technol. Sci. 2025, 38, 681–697. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG in prebiotic edible films. Food Chem. 2014, 159, 302–308. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Y.; Chang, C.; Wu, J. Novel probiotic fermented edible film based on gum arabic/whey protein isolate/isomalt/glycerol incorporated with Lactobacillus rhamnosus: Physical, antibacterial, and fresh pork preservation properties. Food Hydrocoll. 2025, 161, 110875. [Google Scholar] [CrossRef]
- Nguyen, N.H.K.; Bach, G.L.; Tran, T.T. Effects of film-forming components on the viability of probiotics and the application of synbiotic pectin film in preserving Da Xanh pomelo and Thai jackfruit fresh-cut. Food Sci. Biotechnol. 2024, 33, 3093–3104. [Google Scholar] [CrossRef] [PubMed]
- Todhanakasem, T.; Boonchuai, P.; Itsarangkoon Na Ayutthaya, P.; Suwapanich, R.; Hararak, B.; Wu, B.; Young, B.M. Development of bioactive Opuntia ficus-indica edible films containing probiotics as a coating for fresh-cut fruit. Polymers 2022, 14, 5018. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Almeida, R.L.; Santos, N.C.; Pereira, E.M.; Silva, A.P.; Oliveira, H.M.L.; Pasquali, M.A.D.B. New functional foods with cactus components: Sustainable perspectives and future trends. Foods 2023, 12, 2494. [Google Scholar] [CrossRef]
- Coimbra, P.; Alarico, S.; Empadinhas, N.; Braga, M.E.; Gaspar, M.C. Sustainable starch-based edible films with agrifood residues as potential carriers for the probiotic Lactobacillus rhamnosus. Innov. Food Sci. Emerg. Technol. 2023, 88, 103452. [Google Scholar] [CrossRef]
- Kalkan, S.; Çopur, Ş.Y.; Akben, S.B.; Otağ, M.R.; Engin, M.S. Effect of Incorporation of Mix Probiotic Culture in Sodium Alginate Film Characteristics: Antimicrobial, Physiochemical, Mechanical, and Barrier Properties. Probiotics Antimicrob. Proteins 2025, 1–16. [Google Scholar] [CrossRef]
- Sogut, E.; Filiz, B.E.; Seydim, A.C. Whey protein isolate-and carrageenan-based edible films as carriers of different probiotic bacteria. J. Dairy Sci. 2022, 105, 4829–4842. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Mohammadi, R.; Rouhi, M.; Mortazavian, A.M.; Shojaee-Aliabadi, S.; Koushki, M.R. Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT 2018, 87, 54–60. [Google Scholar] [CrossRef]
- Lian, W.C.; Hsiao, H.C.; Chou, C.C. Viability of microencapsulated bifidobacteria in simulated gastric juice and bile solution. Int. J. Food Microbiol. 2003, 86, 293–301. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Chin, J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef]
- Orozco-Parra, J.; Mejía, C.M.; Villa, C.C. Development of a Bioactive Synbiotic Edible Film Based on Cassava Starch, Inulin, and Lactobacillus casei. Food Hydrocoll. 2020, 104, 105754. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; Monteiro, S.S.; de Lima, T.L.B.; da Silva Lúcio, A.; da Silva Nogueira, L.P.; Rocha, A.P.T. Microencapsulating Lacticaseibacillus rhamnosus GG by spray drying using pea protein, pectin, and tapioca flour: Probiotic viability, digestibility and thermal stability. Food Bioprod. Process. 2025, 150, 207–2016. [Google Scholar] [CrossRef]
- Morais, S.K.Q.; Felipe, K.H.; de Souza Silva, J.D.; Santos, N.C.; de Farias Araújo, M.S.; Vieira, P.P.F.; Gouveia, D.S. Development of Nile tilapia gelatin films with grape pomace extract for fish fillet storage: Hydrophobic, functional properties, and biodegradability. Food Biosci. 2025, 68, 106675. [Google Scholar] [CrossRef]
- Krishna, M.; Nindo, C.I.; Min, S.C. Development of fish gelatin edible films using extrusion and compression molding. J. Food Eng. 2012, 108, 337–344. [Google Scholar] [CrossRef]
- Salimiraad, S.; Safaeian, S.; Basti, A.A.; Khanjari, A.; Nadoushan, R.M. Characterization of novel probiotic nanocomposite films based on nano chitosan/nano cellulose/gelatin for the preservation of fresh chicken fillets. LWT 2022, 162, 113429. [Google Scholar] [CrossRef]
- ASTM E96/E96M-16 Standard Test; Methods for Water Vapor Transmission of Materials. ASTM-American Society for Testing and Materials: West Conshohocken, PA, USA, 2016. [CrossRef]
- Yin, Y.C.; Yin, S.W.; Yang, X.Q.; Tang, C.H.; Wen, S.H.; Chen, Z.; Xiao, B.J.; Wu, L.Y. Surface modification of sodium caseinate films by zein coatings. Food Hydrocoll. 2014, 36, 1–8. [Google Scholar] [CrossRef]
- ASTM Designation D 828–97; Standard Test Method for Tensile Properties of Paper and Paperboard Using Constant-Rate-of-Elongation Apparatus. American Society for Testing and Materials: West Conshohocken, PA, USA, 2002.
- Zhang, Y.; Zhao, K.; Guo, Y.; Teng, A.; Li, S.; Ma, Y.; Wang, W. Effects of physical crosslinking methods on digestibility in vitro and safety of edible packaging: A primary study on collagen films. Food Mater. Res. 2023, 3, 1–7. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG incorporated in edible films: Impact of anionic biopolymers and whey protein concentrate. Food Hydrocoll. 2017, 70, 345–355. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z.; Lacroix, M. Gelatin and low methoxyl pectin films containing probiotics: Film characterization and cell viability. Food Biosci. 2020, 36, 100660. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Tavera-Quiroz, M.J.; Bertola, N.; Mobili, P.; Pinotti, A.; Gómez-Zavaglia, A. Edible methylcellulose-based films containing fructo-oligosaccharides as vehicles for lactic acid bacteria. Food Res. Int. 2014, 64, 560–566. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of cross-linking with calcium ions on the physical properties of alginate films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef]
- Correa, A.C.; Lopes, M.S.; Perna, R.F.; Silva, E.K. Fructan-type prebiotic dietary fibers: Clinical studies reporting health impacts and recent advances in their technological application in bakery, dairy, meat products and beverages. Carbohydr. Polym. 2024, 323, 121396. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; Brito, A.C.D.O.; Silva, V.M.D.A.; Albuquerque, J.C.; Saraiva, M.M.T.; Mota, M.M.D.A. Effect of pulse electric field (PEF) intensity combined with drying temperature on mass transfer, functional properties, and in vitro digestibility of dehydrated mango peels. J. Food Meas. Charact. 2023, 17, 5219–5233. [Google Scholar] [CrossRef]
- Mukaila, T.; Adeniyi, A.; Bello, I.; Sarker, N.C.; Monono, E.; Hammed, A. Optimizing film mechanical and water contact angle properties via PLA/starch/lecithin concentrations. Clean. Circular Bioecon. 2024, 8, 100095. [Google Scholar] [CrossRef]
- Zhai, X.; Ma, X.; Sun, Y.; Xue, Y.; Ban, W.; Song, W.; Shen, T.; Li, Z.; Huang, X.; Sun, Q.; et al. A Hydrophobic Ratiometric Fluorescent Indicator Film Using Electrospinning for Visual Monitoring of Meat Freshness. Foods 2025, 14, 2200. [Google Scholar] [CrossRef]
- Silva, K.C.G.; Sato, A.C.K. Biopolymer gels containing fructooligosaccharides. Food Res. Int. 2017, 101, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bertan, D.W.; da Silva Lima, L.R.; Benoso, P.; Lourenço, R.V.; Bittante, A.M.Q.B.; Gomes, A.; do Amaral Sobral, P.J. Impact of hydroethanolic extracts from Guaco leaves (Mikania glomerata Sprengel) on mechanical properties and bioactivities of gelatin-based bioactive films. Food Bioprod. Process. 2025, 151, 355–371. [Google Scholar] [CrossRef]
- Romo, I.; Abugoch, L.; Tapia, C. Soluble complexes between chenopodins and alginate/chitosan: Intermolecular interactions and structural-physicochemical properties. Carbohydr. Polym. 2020, 227, 115334. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Nam, J.; Yun, H.; Jin, H.J.; Kwak, H.W. Aquatic polymer-based edible films of fish gelatin crosslinked with alginate dialdehyde having enhanced physicochemical properties. Carbohydr. Polym. 2021, 254, 117317. [Google Scholar] [CrossRef] [PubMed]
- Tessaro, L.; Pereira, A.G.R.; Martelli-Tosi, M.; Sobral, P.J.D.A. Improving the Properties of Gelatin-Based Films by Incorporation of “Pitanga” Leaf Extract and Crystalline Nanocellulose. Foods 2024, 13, 1480. [Google Scholar] [CrossRef]
- Karaki, N.; Aljawish, A.; Muniglia, L.; Humeau, C.; Jasniewski, J. Physicochemical characterization of pectin grafted with exogenous phenols. Food Hydrocoll. 2016, 60, 486–493. [Google Scholar] [CrossRef]
- Zinina, O.; Merenkova, S.; Galimov, D. Development of biodegradable alginate-based films with bioactive properties and optimal structural characteristics with incorporation of protein hydrolysates. Sustainability 2023, 15, 15086. [Google Scholar] [CrossRef]
- Kozlov, P.V.; Burdygina, G.I. The structure and properties of solid gelatin and the principles of their modification. Polymer 1983, 24, 651–666. [Google Scholar] [CrossRef]
- Segtnan, V.H.; Isaksson, T. Temperature, sample and time dependent structural characteristics of gelatine gels studied by near infrared spectroscopy. Food Hydrocoll. 2004, 18, 1–11. [Google Scholar] [CrossRef]
- Long, T.; Tan, W.; Tian, X.; Tang, Z.; Hu, K.; Ge, L.; Li, D. Gelatin/alginate-based microspheres with sphere-in-capsule structure for spatiotemporal manipulative drug release in gastrointestinal tract. Int. J. Biol. Macromol. 2023, 226, 485–495. [Google Scholar] [CrossRef]






| Formulations | Gelatin (% m/m) | Glycerol (% w/w) | Prebiotic (2.0 g.100 g−1) | Probiotic (0.02 g.100 g−1) | Polysaccharide (% m/m) |
|---|---|---|---|---|---|
| F1 | 3.0 | 30 | No | Yes | No |
| F2 | 3.0 | 30 | Inulin | Yes | No |
| F3 | 3.0 | 30 | Inulin | Yes | Pectin (1.0%) |
| F4 | 3.0 | 30 | Inulin | Yes | Alginate (0.50%) |
| F5 | 3.0 | 30 | FOSs | Yes | No |
| F6 | 3.0 | 30 | FOSs | Yes | Pectin (1.0%) |
| F7 | 3.0 | 30 | FOSs | Yes | Alginate (0.50%) |
| Films | Solubility (%) | WVP (g·mm/m2·d·kPa) | Thickness (mm) | Moisture Content (%) |
|---|---|---|---|---|
| F1 | 41.37 ± 0.22 a | 11.52 ± 0.91 a | 0.085 ± 0.003 c | 17.50 ± 0.22 a |
| F2 | 38.20 ± 0.75 b | 10.24 ± 0.76 b | 0.091 ± 0.002 a,b | 15.23 ± 0.31 b |
| F3 | 32.51 ± 0.38 c,d | 9.65 ± 0.82 b,c | 0.088 ± 0.004 b | 15.10 ± 0.27 b |
| F4 | 28.73 ± 0.78 e | 8.30 ± 0.57 d | 0.095 ± 0.003 a | 13.47 ± 0.19 c |
| F5 | 34.08 ± 0.44 c | 11.75 ± 0.36 a | 0.089 ± 0.001 a,b | 15.36 ± 0.50 b |
| F6 | 31.88 ± 0.11 d | 9.08 ± 0.13 c | 0.090 ± 0.002 a,b | 14.72 ± 0.17 b |
| F7 | 27.50 ±0.27 e | 8.17 ± 0.28 d | 0.088 ± 0.003 b | 13.66 ± 0.24 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.M.d.; Santos, N.C.; Silva, L.A.d.; de Lima, T.L.B.; Leite, M.d.O.; Silva, V.M.d.A.; Oliveira, L.d.S.; Ribeiro, V.H.d.A.; Meira, A.S.; Felix, P.H.D.; et al. Fish Gelatin Edible Films with Prebiotics and Structuring Polysaccharides for Probiotic Delivery: Physicochemical Properties, Viability, and In Vitro Gastrointestinal Release. Polysaccharides 2025, 6, 79. https://doi.org/10.3390/polysaccharides6030079
Silva GMd, Santos NC, Silva LAd, de Lima TLB, Leite MdO, Silva VMdA, Oliveira LdS, Ribeiro VHdA, Meira AS, Felix PHD, et al. Fish Gelatin Edible Films with Prebiotics and Structuring Polysaccharides for Probiotic Delivery: Physicochemical Properties, Viability, and In Vitro Gastrointestinal Release. Polysaccharides. 2025; 6(3):79. https://doi.org/10.3390/polysaccharides6030079
Chicago/Turabian StyleSilva, Gabriel M. da, Newton Carlos Santos, Luanna A. da Silva, Thalis L. B. de Lima, Mateus de Oliveira Leite, Virgínia Mirtes de Alcântara Silva, Liandra de S. Oliveira, Victor Herbert de Alcântara Ribeiro, Ariadne Soares Meira, Poliana H. D. Felix, and et al. 2025. "Fish Gelatin Edible Films with Prebiotics and Structuring Polysaccharides for Probiotic Delivery: Physicochemical Properties, Viability, and In Vitro Gastrointestinal Release" Polysaccharides 6, no. 3: 79. https://doi.org/10.3390/polysaccharides6030079
APA StyleSilva, G. M. d., Santos, N. C., Silva, L. A. d., de Lima, T. L. B., Leite, M. d. O., Silva, V. M. d. A., Oliveira, L. d. S., Ribeiro, V. H. d. A., Meira, A. S., Felix, P. H. D., Dias, R. A. d. L., Gouveia, D., Gomes, J. P., & Rocha, A. P. T. (2025). Fish Gelatin Edible Films with Prebiotics and Structuring Polysaccharides for Probiotic Delivery: Physicochemical Properties, Viability, and In Vitro Gastrointestinal Release. Polysaccharides, 6(3), 79. https://doi.org/10.3390/polysaccharides6030079






