Optimization of TEMPO-Mediated Oxidation of Chitosan to Enhance Its Antibacterial and Antioxidant Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chitosan Preparation
2.2. Preparation of TEMPO-Oxidized Chitosan
2.3. Determination of Carboxylate Content and Oxidation Degree
2.4. Preparation of the Films
2.5. Optimization of the Effects of the Operating Factors of Chitosan Oxidation Through Doehlert Design
- √
- Temperature (T): −5 ≤ T ≤ 40 °C with ΔvT = 22.5,
- √
- Duration (t): 48 ≤ t ≤ 282 min with Δvt = 135,
- √
- pH: 9 ≤ pH ≤ 11 with ΔvpH = 1.
2.6. Characterization Techniques
2.7. Antibacterial and Antifungal Assay
2.8. Antioxidant Activity
2.8.1. DPPH Radical Scavenging Assay
2.8.2. Ferrous Ion Chelating Assay
2.8.3. ABTS Radical Scavenging Assay
3. Results and Discussion
3.1. Structural Characterization of the Films
3.2. Evaluation of the Effects of the Studied Factors on the Degree of Oxidation of Chitosan
3.3. Comparison of Antioxidant Activities
3.4. Biological Evaluation
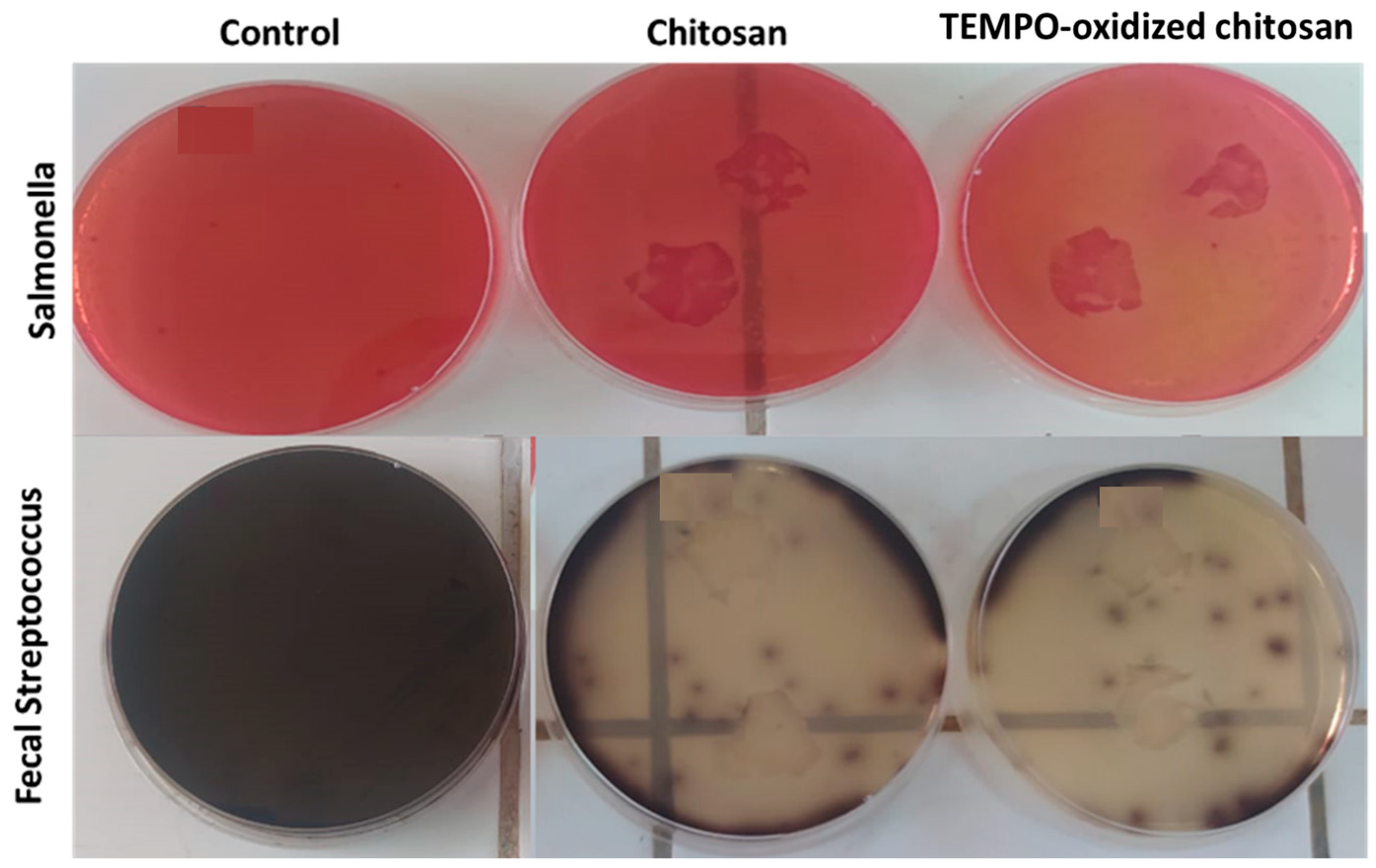
3.4.1. Antimicrobial Assay
3.4.2. Kinetics of the Growth Inhibition of the Bacteria and Antimicrobial Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TEMPO | 2,2,6,6-Tetramethylpiperidin-1-yl oxy |
| NaClO | Sodium hypochlorite |
| NaBr | Sodium bromide |
References
- Fadiji, T.; Rashvand, M.; Daramola, M.O.; Iwarere, S.A. A review on antimicrobial packaging for extending the shelf life of food. Processes 2023, 11, 590. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Lee, J. Recent Advances in Functional Biopolymer Films with Antimicrobial and Antioxidant Properties for Enhanced Food Packaging. Polymers 2025, 17, 1257. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U. Novel biopolymer-based sustainable composites for food packaging applications: A narrative review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Karnwal, A.; Kumar, G.; Singh, R.; Selvaraj, M.; Malik, T.; Al Tawaha, A.R.M. Natural biopolymers in edible coatings: Applications in food preservation. Food Chem. X 2025, 25, 102171. [Google Scholar] [CrossRef]
- Gupta, D.; Lall, A.; Kumar, S.; Patil, T.D.; Gaikwad, K.K. Plant based edible films and coatings for food packaging applications: Recent advances, applications, and trends. Sustain. Food Technol. 2024, 2, 1428–1455. [Google Scholar] [CrossRef]
- Ahmad Dar, S.; Abd Al Galil, F.M. Biodegradation, biosynthesis, isolation, and applications of chitin and chitosan. In Handbook of Biodegradable Materials; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–42. [Google Scholar]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Devi, N.; Bansal, N.; Sharma, S.; Dubey, S.K.; Kumar, S. Novel chitosan-based smart bio-nanocomposite films incorporating TiO2 nanoparticles for white bread preservation. Int. J. Biol. Macromol. 2024, 267, 131367. [Google Scholar]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and challenges in the use of chitosan and its derivatives in biomedical fields: A review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog. Polym. Sci. 2018, 86, 122–148. [Google Scholar] [CrossRef]
- Kartik, R.; Muthusamy, S.; Shelly, K.; Surendiran, M. Chemical Modifications of Chitin and Chitosan Fibers and Filaments: A Review. Macromol. Chem. Phys. 2025, 226, 2400422. [Google Scholar] [CrossRef]
- Kato, Y.; Kaminaga, J.; Matsuo, R.; Isogai, A. TEMPO-mediated oxidation of chitin, regenerated chitin and N-acetylated chitosan. Carbohydr. Polym. 2004, 58, 421–426. [Google Scholar] [CrossRef]
- Tolaimate, A.; Desbrieres, J.; Rhazi, M.; Alagui, A. Contribution to the preparation of chitins and chitosans with controlled physico-chemical properties. Polymer 2003, 44, 7939–7952. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; ArguÈelles-Monal, W.; DesbrieÁres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Rocchetti, R. Determination of the degree of acetylation of chitosans by first derivative ultraviolet spectrophotometry. Carbohydr. Polym. 1985, 5, 461–472. [Google Scholar] [CrossRef]
- da Silva, S.B.; Batista, G.L.; Santin, C.K. Chitosan for sensors and electrochemical applications. In Chitin and Chitosan: Properties and Applications; van den Broek, L.A.M., Boeriu, C.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 461–476. [Google Scholar]
- Delattre, C.; Rios, L.; Laroche, C.; Le, N.H.T.; Lecerf, D.; Picton, L.; Berthon, J.Y.; Michaud, P. Production and characterization of new families of polyglucuronic acids from TEMPO-NaOCl oxidation of curdlan. Int. J. Biol. Macromol. 2009, 45, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Sousa Filho, H.R.; Oliveira, D.M.; Lemos, V.A.; Bezerra, M.A. Evaluation of Two Statistical Tools (Least Squares Regression and Artificial Neural Network) in the Multivariate Optimization of Solid-Phase Extraction for Cadmium Determination in Leachate Samples. J. Braz. Chem. Soc. 2015, 26, 40–50. [Google Scholar] [CrossRef]
- Resendiz-Moctezuma, C.; Fonville, A.P.; Harsh, B.N.; Stasiewicz, M.J.; Miller, M.J. Use of Doehlert Matrix as a Tool for High-Throughput Screening of Organic Acids and Essential Oils on Miniaturized Pork Loins, Followed by Lab-Scale Validation That Confirmed Tested Compounds Do Not Show Synergistic Effects against Salmonella Typhimurium. Foods 2023, 12, 4034. [Google Scholar] [CrossRef]
- Muloiwa, M.; Nyende-Byakika, S.; Dinka, M. Comparison of unstructured kinetic bacterial growth models. S. Afr. J. Chem. Eng. 2020, 33, 141–150. [Google Scholar] [CrossRef]
- Mansouri, A.; Embarek, G.; Kokkalou, E.; Kefalas, P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem. 2005, 89, 411–420. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Antioxidant and free radical scavenging activities of whole wheat and milling fractions. Food Chem. 2007, 101, 1151–1157. [Google Scholar] [CrossRef]
- Re, R.; Nicoletta, P.; Anna, P.; Ananth, P.; Min, Y.; Catherine, R.-E. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pierre, G.; Salah, R.; Gardarin, C.; Traikia, M.; Petit, E.; Delort, A.-M.; Mameri, N.; Moulti-Mati, F.; Michaud, P. Enzymatic degradation and bioactivity evaluation of C-6 oxidized chitosan. Int. J. Biol. Macromol. 2013, 60, 383–392. [Google Scholar] [CrossRef]
- Korica, M.; Mihajlovski, K.; Mohan, T.; Kostić, M. Films based on TEMPO-oxidized chitosan nanoparticles: Obtaining and potential application as wound dressings. Carbohydr. Res. 2024, 542, 109203. [Google Scholar] [CrossRef] [PubMed]
- Boligon, A.A.; Machado, M.M.; Athayde, M.L. Technical Evaluation of Antioxidant Activity. Med. Chem. 2014, 4, 517–522. [Google Scholar] [CrossRef]
- Ouamnina, A.; Alahyane, A.; Elateri, I.; Abderrazik, M. Phenolic composition, antioxidant capacity, and antiglycation potential of select Moroccan date varieties: Promising sources for functional food development. Euro-Mediterr. J. Environ. Integr. 2024, 9, 745–760. [Google Scholar] [CrossRef]
- Ouamnina, A.; Alahyane, A.; Elateri, I.; Boutasknit, A.; Abderrazik, M. Relationship between Phenolic Compounds and Antioxidant Activity of Some Moroccan Date Palm Fruit Varieties (Phoenix dactylifera L.): A Two-Year Study. Plants 2024, 13, 1119. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent advances of chitosan and its derivatives for novel applications in food science. J. Food Process. Beverages 2013, 1, 13. [Google Scholar]
- Aouadi, A.; Saoud, D.H.; Rebiai, A.; Laouini, S.E.; Achouri, A.; Zaater, A.; Abdullah, J.A.A. Valorizing shrimp shell chitosan: A versatile biomaterial for fabricating effective antibacterial and antioxidant silver nanoparticles. J. Sol-Gel Sci. Technol. 2024, 112, 752–767. [Google Scholar] [CrossRef]
- Ivashkiva, L.N.; Salomatina, E.V.; Apryatina, K.V.; Markin, A.V.; Sologubov, S.S.; Mochalova, A.E. Terpolymers of chitosan with acrylamide and sodium salt of acrylic acid as prospects flocculants and sorbents. Polym. Bull. 2024, 82, 1–20. [Google Scholar] [CrossRef]
- da Silva, S.B.; Krolicka, M.; van den Broek, L.A.; Frissen, A.E.; Boeriu, C.G. Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO-laccase redox system. Carbohydr. Polym. 2018, 186, 299–309. [Google Scholar] [CrossRef] [PubMed]
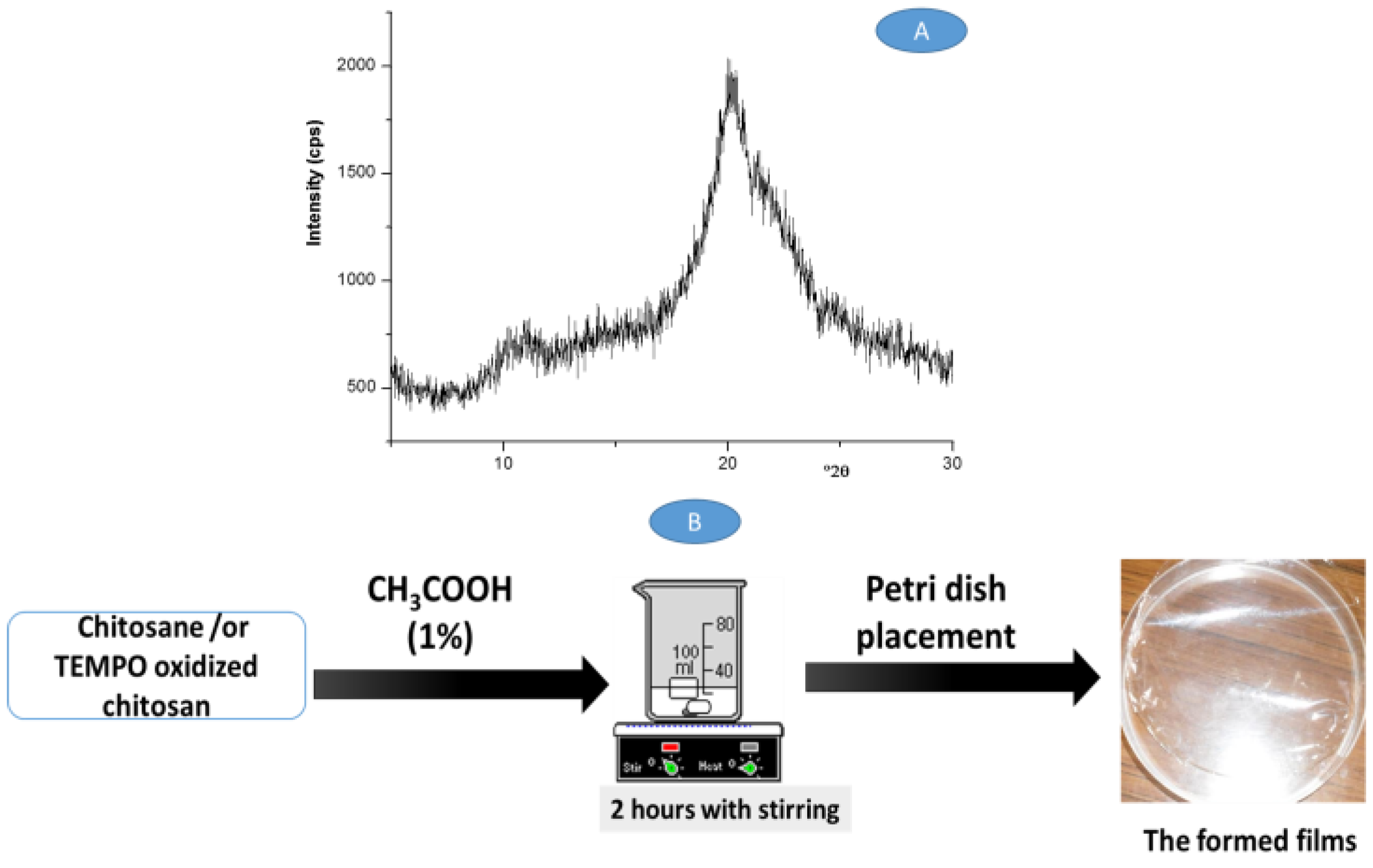



| Chitosan (CTS) | |||
|---|---|---|---|
| (DD) (%) | Crystallinity Index (%) | Molecular Weight (g/mol) | Degree of Polymerization (DP) |
| 95 ± 1.5 | 18 ± 2 | 165.000 | 980 |
| Run | X1 | X2 | X3 | T (°C) | t (min) | pH | OD (%) of Oxidized Chitosan |
|---|---|---|---|---|---|---|---|
| 1 | 1.00000 | 0.00000 | 0.00000 | 40 | 165 | 10 | 75.09 |
| 2 | −1.00000 | 0.00000 | 0.00000 | −5 | 165 | 10 | 72.90 |
| 3 | 0.50222 | 0.86667 | 0.00000 | 28.8 | 282 | 10 | 79.15 |
| 4 | −0.49778 | −0.86667 | 0.00000 | 6.3 | 48 | 10 | 74.63 |
| 5 | 0.50222 | −0.86667 | 0.00000 | 28.8 | 48 | 10 | 76.66 |
| 6 | −0.49778 | 0.86667 | 0.00000 | 6.3 | 282 | 10 | 78.10 |
| 7 | 0.50222 | 0.28889 | 1.00000 | 28.8 | 204 | 11 | 78.90 |
| 8 | −0.49778 | −0.28889 | −1.00000 | 6.3 | 126 | 9 | 76.33 |
| 9 | 0.50222 | −0.28889 | −1.00000 | 28.8 | 126 | 9 | 82.80 |
| 10 | 0.00000 | 0.57778 | −1.00000 | 17.5 | 243 | 9 | 75.66 |
| 11 | −0.49778 | 0.28889 | 1.00000 | 6.3 | 204 | 11 | 82.05 |
| 12 | 0.00000 | −0.57778 | 1.00000 | 17.5 | 87 | 11 | 74.35 |
| 13 | 0.00000 | 0.00000 | 0.00000 | 17.5 | 165 | 10 | 78.07 |
| 14 | 0.00000 | 0.00000 | 0.00000 | 17.5 | 165 | 10 | 78.07 |
| 15 | 0.00000 | 0.00000 | 0.00000 | 17.5 | 165 | 10 | 78.07 |
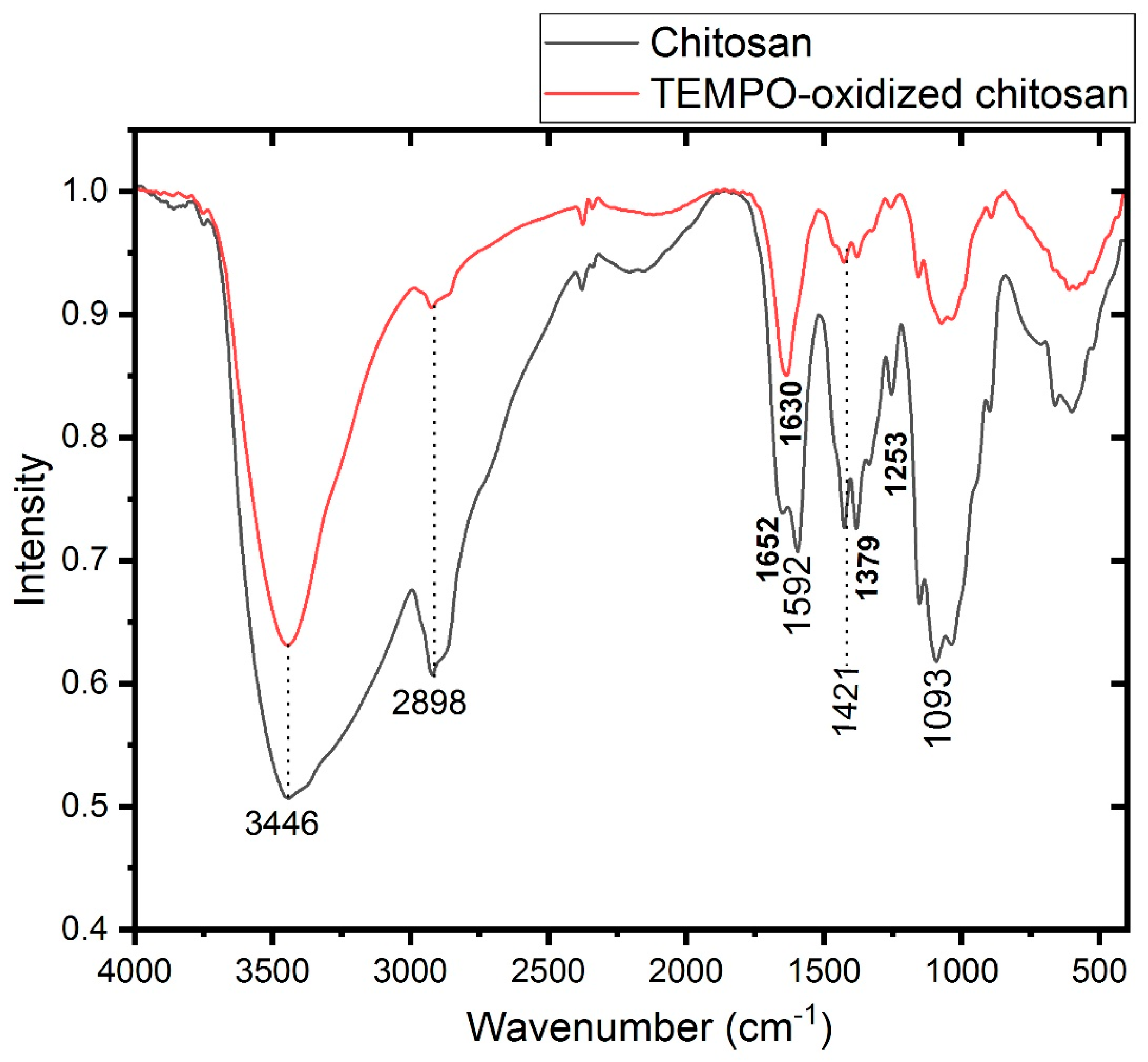
| Chitosan | Oxidized Chitosan | Assignment | ||
|---|---|---|---|---|
| 3446 | 3446 | νOH | ||
| 2898 | 2919 | νCH2 | ||
| 1652 | νCO (amide I) | |||
| 1626 | νCO(Acide) | |||
| 1592 | δ-NH3+ | |||
| 1421 | 1421 | δ CH2 (CH2OH) | ||
| 1379 | 1379 | δ CH3 (NHCOCH3) | ||
| 1253 | 1253 | NHCO group (amide III) | ||
| 1093 | 1093 | νCO (secondary OH group) | ||
| 1031 | 1031 | νCO (primary OH group) | ||
| 661 | 661 | δNH out of plane | ||
| 602 | 602 | δOH out of plane | ||
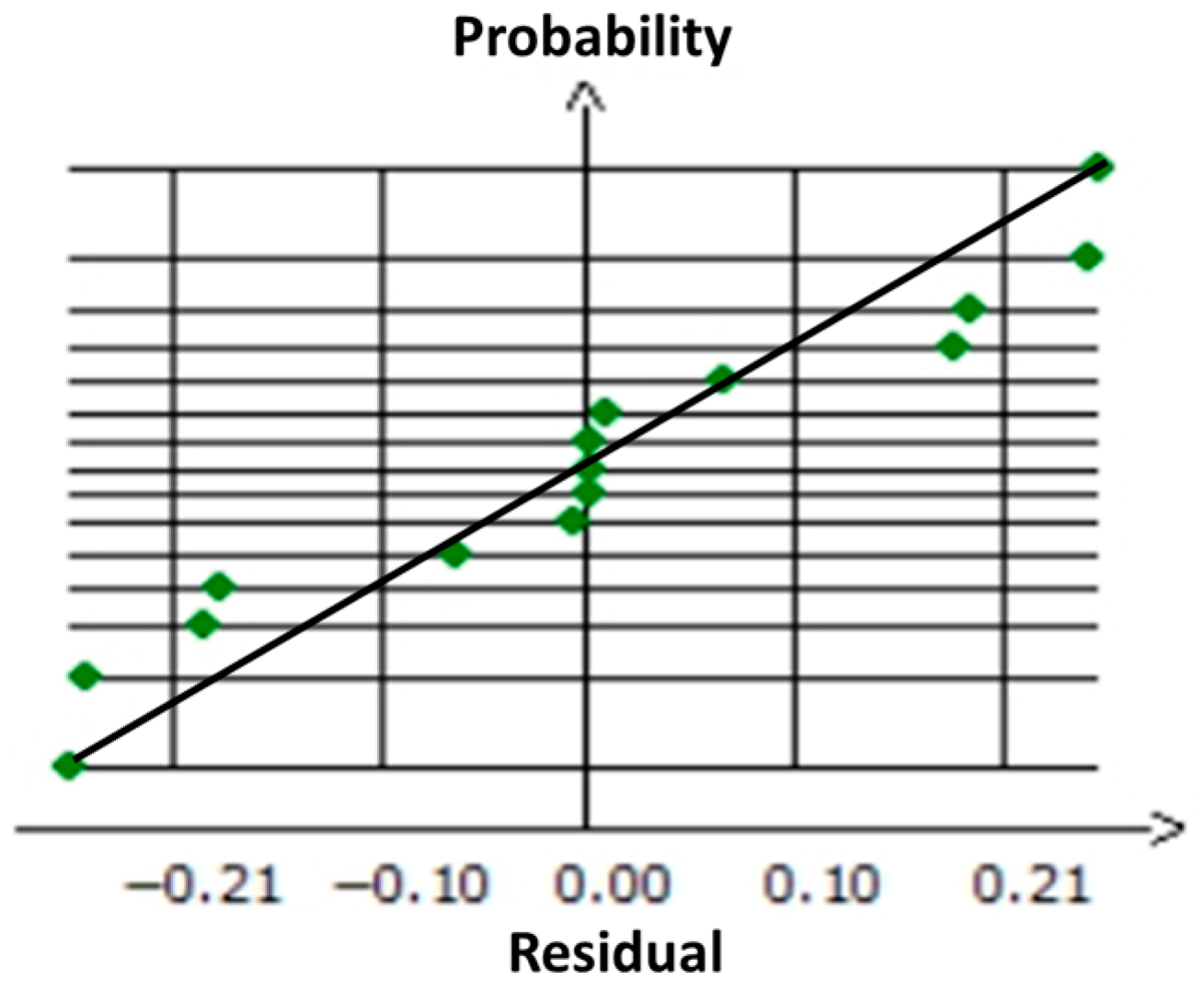
| Sources of Variation | Sum of Squares | Degrees of Freedom | Mean Square | F-Ratio | Significance | R2Adj | |
|---|---|---|---|---|---|---|---|
| OD | Regression | 104.7933 | 9 | 11.6437 | 139.2669 | <0.01 *** | 0.989 |
| Residues | 0.4180 | 5 | 0.0836 | ||||
| Total | 105.2114 | 14 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourak, A.; Ait-Karra, A.; Ouhammou, M.; Ouamnina, A.; Boutasknit, A.; Bouchari, M.E.H.; Elhadiri, N.; Alagui, A. Optimization of TEMPO-Mediated Oxidation of Chitosan to Enhance Its Antibacterial and Antioxidant Activities. Polysaccharides 2025, 6, 65. https://doi.org/10.3390/polysaccharides6030065
Mourak A, Ait-Karra A, Ouhammou M, Ouamnina A, Boutasknit A, Bouchari MEH, Elhadiri N, Alagui A. Optimization of TEMPO-Mediated Oxidation of Chitosan to Enhance Its Antibacterial and Antioxidant Activities. Polysaccharides. 2025; 6(3):65. https://doi.org/10.3390/polysaccharides6030065
Chicago/Turabian StyleMourak, Abdellah, Aziz Ait-Karra, Mourad Ouhammou, Abdoussadeq Ouamnina, Abderrahim Boutasknit, Mohamed El Hassan Bouchari, Najat Elhadiri, and Abdelhakim Alagui. 2025. "Optimization of TEMPO-Mediated Oxidation of Chitosan to Enhance Its Antibacterial and Antioxidant Activities" Polysaccharides 6, no. 3: 65. https://doi.org/10.3390/polysaccharides6030065
APA StyleMourak, A., Ait-Karra, A., Ouhammou, M., Ouamnina, A., Boutasknit, A., Bouchari, M. E. H., Elhadiri, N., & Alagui, A. (2025). Optimization of TEMPO-Mediated Oxidation of Chitosan to Enhance Its Antibacterial and Antioxidant Activities. Polysaccharides, 6(3), 65. https://doi.org/10.3390/polysaccharides6030065






